Abstract
Objective: We discussed the correlation between SNP loci (rs198389 and rs198388) in brain natriuretic peptide gene (NPPB) and susceptibility to congenital heart diseases (CHD). Method: Multiplex SNaPshot technique was adopted for profiling of SNP genotypes at loci rs198389 and rs198388 in NPPB gene among 150 cases of CHDand 150 normal controls. Results: The distribution frequency of 3 genotypes (AA, AG and GG) at locus rs198389 was 40.7%, 36.0% and 23.3% in CHD group, respectively, showing significant differences compared with the normal controls (P<0.001). Gallele was associated with higher risk of CHD (OR=2.48, 95% CI=1.77-3.48). The distribution frequency of CC, CTand TT genotypes at locus rs198388 was 60.7%, 17.3% and 22.0% in CHD group, respectively, also showing significant differences compared with the normal controls (P<0.001). C allele could increase the risk of CHD (OR=1.92, 95% CI=1.48-2.48). Conclusion: SNP loci rs198389 and rs198388 in NPPB gene were correlated with genetic susceptibility to CHD.
Keywords: Brain natriuretic peptide (NPPB), single nucleotide polymorphism (SNP), congenital heart disease (CHD), genetic susceptibility
Introduction
Congenital heart disease (CHD) is a defect in the structure of heart and great vessels that is present at birth due to developmental abnormalities at fetal stage. CHD is divided into various types, most of which have a high mortality rate. As the most common birth defect, CHD has an incidence of about 0.8% in China with new annual cases amounting to over 130 thousand [1,2]. Except for a few CHD cases presenting with single gene mutation and chromosomal aberrations, CHD is largely a polygenic disease involving multiple genes under hereditary and environmental influences [3,4]. NPPB consisting of 32 amino acids is a neurohormone synthesized and secreted by ventricular muscles and can serve as an indicator of ventricular functions [5]. Single nucleotide polymorphism (SNP) is a DNA sequence variation caused by the changes of bases of a single nucleotide. Human genome contains about 3 million SNP, and SNP occurs for every 1000 bases. SNP is another important genetic marker besides microsatellites with the highest density in human genome. Studies show that the polymorphism of NPPB gene is closely related to diabetes, hypertension, myocardial infarction and heart failure. So NPPB is now considered one candidate gene for the genetic susceptibility to cardiovascular diseases [6,7].
We aimed to study the correlation between SNP loci rs198389 and rs198388 in NPPB gene and susceptibility to CHD among China’s Han people. By highlighting the loci correlated with susceptibility to CHD, we can hope to provide an objective and reliable basis for the diagnosis of CHD during early screening, treatment and prevention of high-risk groups.
Materials and methods
Materials
The case group consisted of 150 CHD patients treated at Department of Cardiology at our hospital from October 2003 to December 2014. All recruited cases met to the diagnostic criteria for CHD. As control subjects, 150 normal cases which were matched for age, gender and examination time were selected. The two groups did not show significant differences in gender, age and general conditions (Table 1). The recruited cases had nokinship with each other. Informed content was signed and general information inquiry form was filled before blood sampling. 2 ml of fasting peripheral blood was collected into the tube containing citrate as anticoagulant and preserved at -70°C prior to genome DNA extraction.
Table 1.
Characteristics of participants in the case and control groups
| Characteristics | CHD group | Control group | P |
|---|---|---|---|
| Age (year) | 34.4±11.2 | 35.1±11.4 | 0.448 |
| Gender (M/F) | 98/52 | 94/56 | 0.753 |
| Height (m) | 1.68±0.13 | 1.67±0.11 | 0.475 |
| Weight (Kg) | 63.4±18.2 | 64.3±17.3 | 0.201 |
| BMI (kg/m2) | 23.2±6.3 | 23.6±6.6 | 0.282 |
Polymorphism detection of NPPB gene using multiplex SNaPshot technique
Genome DNA extraction
Genome DNA was extracted and purified using TIANamp B1ood DNA Kit (Tiangen).
Primer design and synthesis
Primers for PCR amplification and extension were designed using Sequenom Assay Design 3.1 software for SNP loci rs198389 and rs198388 in NPPB gene. Three primers were designed for each SNP, and two were used for the amplification of the target fragment with length of 200-500 bp and Tm of about 60 degrees. The other was designed in the upstream or downstream from the target SNP loci for the extension of ddNTP (Table 2). The primers were synthesized by Shanghai Invitrogen Biotechnology Co., Ltd (Shanghai, China).
Table 2.
The primer sequences of the two loci in BNP gene
| Locus | Primer 1 | Primer 2 | Primer 3 |
|---|---|---|---|
| Rs198388 | 5’-GAGTTTTTAATGCTCTGAAATGTTAATT-3’ | 5’-TAGCTGAGACCTGAAAGCAATAATC-3’ | 5’-TTTTTTTTAATATETEAAGCACTTGAGG-3’ |
| Rs198389 | 5’-GTCTCCCGCTTCTTCCTTTCC-3’ | 5’-CAGGAAGGAAAGCGCCAACCTA-3’ | 5’-TTTTTTTTAAATGTCCAGGTGTCC-3’ |
PCR amplification
The 15 ul PCR reaction system consisted of the following: 10× buffer 1.5 ul, dNTP mix (10 mmol/L) 0.3 ul, MgCl2+ = (25 mmol/L) 0.9 ul, Taq DNA polymerase 0.1 ul, PCR primers; DNA template (20 mg/L) 1 ul, DNA template (20 mg/L) 1 ul.
Multiplex PCR procedures: Denaturation at 95°C for 15 min, 94°C for 40 s, annealing at 63°C for 1 min, temperature reduction of 0.5°C for each cycle; extension at 72°C for 1.5 min, 15 cycles; denaturation at 94°C for 40 s, annealing at 56°C for 40 s, extension at 72°C for 1.5 min, 25 cycles; final extension at 72°C for 8 min.
Purification of PCR products: 5 U SAP’ and 2UExoI were added into 15 u1 PCR products and oscillated. The enzymes were deactivated by preservation at 37°C for 1 h then at 75°C for 15 min. The purified products were preserved at 4°C for 24 h or at -20°C for a longer period of time.
SNaPshot analysis
The reaction system consisted of purified PCR products, SNaPshot primers (0.2 umol/L) and SNaPshot fluorescent mixture.
SNaPshot procedures: Denaturation at 96°C for 10 s, 96°C for 10 s, annealing at 53°C for 5 s, extension at 60°C for 30 s, 25 cycles; final extension at 60°C for 30 s. SNaPshot PCR products were purified by these procedures.
Sequencing
The purified SNaPshot products were diluted by 20 times. The reaction system consisted of Hi-Di Formamide 8.6 ul, GeneScan-120LIZ Size Standard 0.9 ul and SNaPshot product 0.5 ul. After denaturation at 95°C for 5 min, the system was rapidly cooled for 4 min. Capillary electrophoresis was carried out using AI3I 3730XL DNA sequencer, with the addition of LIZ-12 as internal reference into each sample. The length of the extended fragment was accurately determined, and the sequencing results were analyzed by using GeneMappersoftware 4.0.
Statistical analysis
The correlation between polymorphism of NPPB gene and susceptibility to CHD was analyzed by calculating the distribution frequency of SNP genotypes in CHD group and control group. Whether the distribution frequency of SNP genotypes and alleles was representative of the population was examined by Hardy-Weinberg equilibrium test. The statistical processes were performed using SPSS13.0 software. The differences in distribution frequency of SNP genotypes between the two groups were compared by X2 test. Binary logistic regression was performed for different genetic models to identify the hereditary mode of the target loci. Odds ratio (OR) was calculated for different genotypes. All tests were two-sided, and P<0.05 was considered as statistically significant.
Results
Sequencing of SNP loci in NPPB gene
After processing with GeneMapper 4.0 software, the genotyping results of SNP loci rs98389 and RS198388 are shown in Figure 1.
Figure 1.
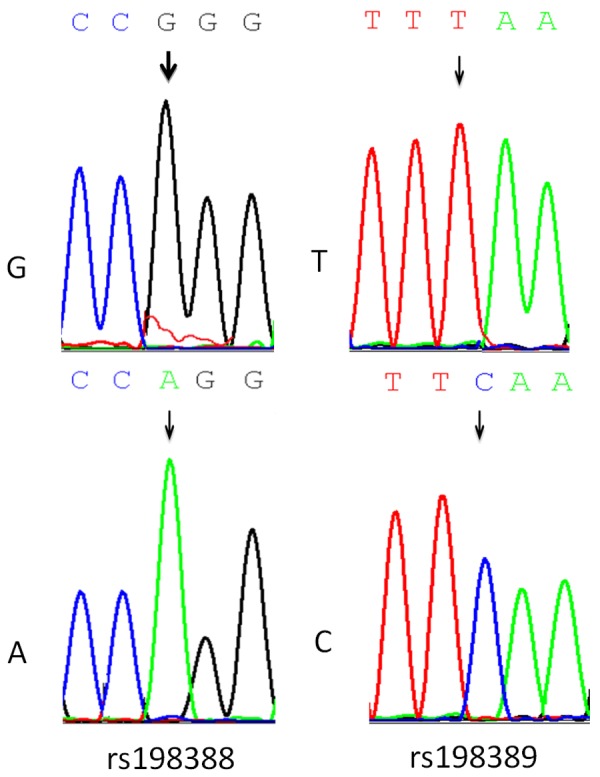
Sequence results of NPPB gene polymorphism.
Correlation between polymorphism of NPPB gene and susceptibility to CHD
As shown in Table 3, the distribution frequency of AA, AG and GG genotypes of locus rs198389 was 40.7%, 36.0% and 23.3% in CHD group, respectively. Significant differences were noted from the controls (73.4%, 15.3% and 11.3%, P<0.001). The distribution frequency of A and G alleles in CHD group was 79.3% and 20.7%, respectively, showing significant differences compared with the controls (90.5% and 9.5%).Compared with A allele, G allele increased 1.48-fold risk of CHD (OR=2.48, 95% CI=1.77-3.48).
Table 3.
The distributions of genotypes and alleles frequency of rs198388 and 198389
| SNP | Group | n | Genotype | P | Allele | P | OR* (95% CI) | OR** (95% CI) | |||
|
|
|
||||||||||
| CC | CT | TT | C | T | |||||||
|
| |||||||||||
| Rs198388 | Case | 150 | 91 (60.7) | 26 (17.3) | 33 (22.0) | <0.001 | 208 (34.7) | 392 (65.3) | 0.002 | 1.92 (1.48-2.48) | 2.01 (1.55-3.01) |
| Control | 150 | 43 (28.7) | 44 (29.3) | 63 (42.0) | 130 (21.7) | 470 (78.3) | |||||
|
| |||||||||||
| SNP | Group | n | Genotype | Allele | P | OR* (95% CI) | OR** (95% CI) | ||||
|
|
|
||||||||||
| GG | AG | AA | G | A | |||||||
|
| |||||||||||
| Rs198389 | Case | 150 | 35 (23.3) | 54 (36.0) | 61 (40.7) | <0.001 | 124 (20.7) | 476 (79.3) | <0.001 | 2.48 (1.77-3.48) | 2.33 (1.76-3.54) |
| Control | 150 | 17 (11.3) | 23 (15.3) | 110 (73.4) | 57 (9.5) | 543 (90.5) | |||||
unadjusted OR;
adjusted OR.
The distribution frequency of TT, TG and GG genotypes of locus RS198388 was 22.0%, 17.3 and 60.7% in CHD group, respectively. A significant difference was found between CHD group and control group (P<0.001). The distribution frequency of C allele and T allele in CHD group was 34.7% and 65.3%, respectively, which indicated significant difference from the control group (P<0.001). C allele had a higher risk of CHD than A allele (OR=1.92, 95% CI=1.48-2.48).
Discussion
CHD is the major cause of death among non-infectious diseases in children. Early diagnosis of CHD is crucial for improving the prognosis, survival rate and life quality of patients. It has been generally recognized that gene polymorphism is closely related to CHD. Thus the study on their correlations provides a solid basis for understanding the pathogenesis of CHD and for early diagnosis, genetic intervention and prognosis evaluation of CHD [8,9].
Recent researches have established the correlations between SNP of NPPB gene and various diseases. NEWTON-CHEH et al. investigated NPPA and NPPB genes among 14473 European people and found close connections between the polymorphism of loci rs5068, rs198358 and RS632793 of NPPA-NPPB gene and the serum levels of ANP and BNP and blood pressure [10,11]. Chen et al. showed that the polymorphism of loci RS198389 and RS198388 of NPPB gene was correlated with serum NT-proBNP in 164 typeI diabetes patients [12].
We performed multiplex SNaPshot technique to screen genotypes and alleles whose distribution frequency was representative of the population. SNP loci in NPPB gene showing significant differences in distribution frequency between CHD group and control group were identified along with the fittest genetic model. OR values for different genotypes were calculated (95% confidence level), and therefore the genotypes associated with high risk of CHD were determined. The case-control design was adopted for the genotyping of two SNP loci (rsl98389 and rs198388) in NPPB gene among 150 CHD cases and 150 normal controls. We found that G allele increased the risk of CHD; CC genotype at locus rs198388 increased the risk of CHD. Thus it was concluded that the polymorphism of loci rs198389 and rs198388 in NPPB gene was correlated with susceptibility to CHD among Chinese Han people.
A new light is shed on the molecular mechanism of CHD by investigating the correlation between polymorphism of NPPB gene and susceptibility to CHD. SNPs of NPPB gene can be used as the markers in screening, individualized prevention and diagnosis of CHD. Large-sample, multi-regional and multi-gene studies are required for verification.
Disclosure of conflict of interest
None.
References
- 1.Yu M, Ping Z, Zhang S, He Y, Dong R, Guo X. The survey of birth defects rate based on birth registration system. Chin Med J (Engl) 2015;128:7–14. doi: 10.4103/0366-6999.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Wang XF, Wang Y, Wang HW, Liu Y. The incidence rate, high-risk factors, and short- and long-term adverse outcomes of fetal growth restriction: a report from Mainland China. Medicine (Baltimore) 2014;93:e210. doi: 10.1097/MD.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Da M, Feng Y, Xu J, Hu Y, Lin Y, Ni B, Qian B, Hu Z, Mo X. Association of aminoacyl-tRNAsynthetases gene polymorphisms with the risk of congenital heart disease in the Chinese Han population. PLoS One. 2014;9:e110072. doi: 10.1371/journal.pone.0110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Yu D, Chen T, Liu J, Tong X, Yang L, Da M, Shen S, Fan C, Wang S, Mo X. Maternal parity and the risk of congenital heart defects in offspring: a dose-response meta-analysis of epidemiological observational studies. PLoS One. 2014;9:e108944. doi: 10.1371/journal.pone.0108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos-Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012;60:2140–9. doi: 10.1016/j.jacc.2012.02.092. [DOI] [PubMed] [Google Scholar]
- 6.Pereira NL, Tosakulwong N, Scott CG, Jenkins GD, Prodduturi N, Chai Y, Olson TM, Rodeheffer RJ, Redfield MM, Weinshilboum RM, Burnett JC. Circulating atrial natriuretic peptide genetic association study identifies a novel gene cluster associated with stroke in whites. Circ Cardiovasc Genet. 2015;8:141–9. doi: 10.1161/CIRCGENETICS.114.000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedorowski A, Franceschini N, Brody J, Liu C, Verwoert GC, Boerwinkle E, Couper D, Rice KM, Rotter JI, Mattace-Raso F, Uitterlinden A, Hofman A, Almgren P, Sjögren M, Hedblad B, Larson MG, Newton-Cheh C, Wang TJ, Rose KM, Psaty BM, Levy D, Witteman J, Melander O. Orthostatic hypotension and novel blood pressure-associated gene variants: Genetics of Postural Hemodynamics (GPH) Consortium. Eur Heart J. 2012;33:2331–41. doi: 10.1093/eurheartj/ehs058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blue GM, Kirk EP, Giannoulatou E, Dunwoodie SL, Ho JW, Hilton DC, White SM, Sholler GF, Harvey RP, Winlaw DS. Targeted next-generation sequencing identifies pathogenic variants in familial congenital heart disease. J Am Coll Cardiol. 2014;64:2498–506. doi: 10.1016/j.jacc.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Sun L, Du W, Song S, Wang S, Jiang W, Huang T, Li H. The association of the MTHFR c. 1625A>C genetic variant with the risk of congenital heart diseases in the Chinese. Genet Test Mol Biomarkers. 2015;19:44–7. doi: 10.1089/gtmb.2014.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis KL, Newton-Cheh C, Wang TJ, Frampton CM, Doughty RN, Whalley GA, Ellis CJ, Skelton L, Davis N, Yandle TG, Troughton RW, Richards AM, Cameron VA. Association of genetic variation in the natriuretic peptide system with cardiovascular outcomes. J Mol Cell Cardiol. 2011;50:695–701. doi: 10.1016/j.yjmcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Chen X, Guo Y, Shi R, Zhang G. Brain natriuretic peptide rs198388 polymorphism and essential hypertension in Hunan Han people. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1207–13. doi: 10.3969/j.issn.1672-7347.2010.12.001. [DOI] [PubMed] [Google Scholar]


