Abstract
Primary anterior mediastinum undifferentiated high grade pleomorphic sarcoma is rare that most physician including thoracic surgeon would not experience this disease. Huge anterior mediastinum tumor pressed the heart and it invaded into the left pulmonary arterial trunk. We did the mediastinum tumor resection, left pneumonectomy and left phrenic, sympathetic and vagus nerve merger excision. But the recurrence lesions were detected in the right lung, the left thoracic cavity and thoracic vertebrae within one month after operation. We reported a case of aggressively progressed undifferentiated high grade pleomorphic sarcoma originated from the anterior mediastinum.
Keywords: Anterior mediastinal tumor, undifferentiated high grade pleomorphic sarcoma
Introduction
Primary anterior mediastinum undifferentiated high grade pleomorphic sarcoma is extremely rare. High grade pleomorphic malignant tumors of soft tissue which don’t differentiate to specific histologic features are classified as undifferentiated high grade pleomorphic sarcoma. It was known that the prognosis of the sarcoma is poor and the growth of them is sometimes so fast. We experienced a case of so rapid growing undifferentiated high grade pleomorphic sarcoma originated from anterior mediastinum.
Case report
A 56 year-old man was detected the enlarged mediastinum tumor protruded in the left lung field by the annual medical chest roentgenogram (Figure 1A) which was not indicated one year ago. A chest contrast-enhanced computed tomography (CT) scan revealed huge tumor at the anterior mediastinum. The tumor was suspected high grade sarcoma or sarcomatoid thymic carcinoma by the CT guided biopsy. No metastatic lesions were detected by the positron emission tomography/CT (PET-CT) and head magnetic resonance imaging (MRI), so he was introduced and admitted to our hospital to receive surgical resection. The tumor markers were normal range, including CYFRA, CEA, and ProGRP. We took chest CT (Figure 1B, 1C) and MRI to compare the tumor status with the previous chest CT which taken one month ago. The tumor size was increased from 93 × 82 × 116 mm to 110 × 90 × 126 mm in just one month. Doubling time of the tumor was 92 Days. By the echocardiography, no obvious invasion to pericardium was detected, but the tumor pushed right ventricular and left arterial trunk. Because the tumor was aggressively increasing, to save his life and cure the disease, anterior mediastinum tumor resection, left pneumonectomy, partial resection of the pericardium, and lymph node resection had been done by mediastenotomy and left 4th lateral incision in spiral position. The tumor invaded into the hilum of the left lung, pericardium and left lung parenchyma. Merger resection of the left phrenic, sympathetic and vagus nerve was done because of the invasion to them. The operation time was 5 hr and 20 min and the total bleeding amount was 1,617 ml. Macroscopically, the tumor was originated from anterior mediastinum (probably thymus) and invaded into the hilum of the lung (including left pulmonary arterial trunk), pericardium, phrenic, sympathetic and vagus nerve (Figure 2A). The tumor was elastic soft, and the cut surface was white-yellowish with little viscosity. The tumor size was 130 × 145 × 85 mm and lymphoid metastasis was indicated at #2L, #3a and #5 LN.
Figure 1.
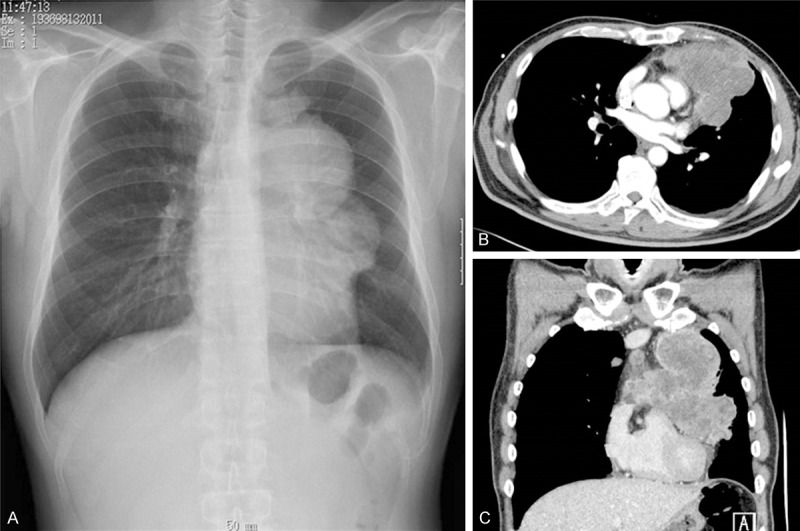
(A) Preoperative chest roentgenogram showed a widened mediastinum protruded to the left lung field. (A) Preoperative chest computed tomography (CT) showed a large tumor located at anterior mediastinum (B, C).
Figure 2.
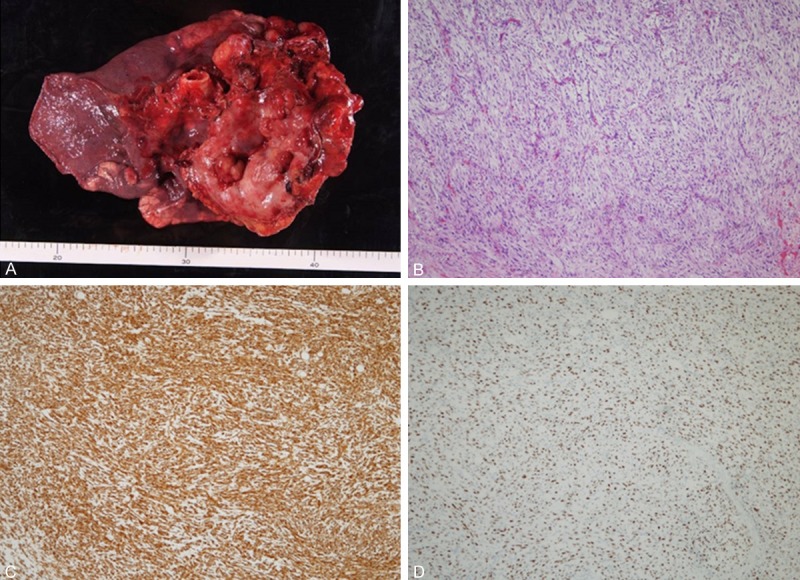
Resected specimen was 130 × 145 × 85 mm in size invaded to the left lung. (A) Microscopy with Hematoxylin and Eosin stain. (B, ×40) Immunohistochemical stain for vimentin (C, ×40), and MIB-1 (D, ×40).
Microscopy with Hematoxylin and eosin (HE) stain revealed polymorphic variant cells with increased nuclear chromatin proliferating and invading in diffuse (Figure 2B). Immunohistochemical staining for vimentin (Figure 2C), WT-1 and NSE protein were positive. But epithelial marker, cytokeratin AE1/AE3, Cam5.2, Calretinin, Mesotelin and EMA was negative, vessel and lymphoid marker, D2-40, CD31 and CD34 was negative, muscle marker, desmin, SMA and myogenin was negative, nervous marker, S-100 protein and neurofila was negative, and c-kit was negative. MIB-1 index was 54.7% (Figure 2D), so the malignancy of this tumor was extremely high. The result of the pathological examination revealed no differentiation to mature tissues, so the final diagnosis was undifferentiated high grade pleomorphic sarcoma originated from anterior mediastinum.
There was no complication after surgery. He discharged on postoperative 18 days. Before leaving the hospital, chest CT was taken and metastatic lesions of right lung parenchyma, left thoracic cavity and 10th thoracic vertebra were indicated. (Figure 3A, 3B) The patient and his family didn’t hope to receive additional therapy. Postoperative 3 months, the recurrence lesion aggressively increased and he vomited fresh blood. By the gastrointestinal fiberscope (GIF), the para-aortic metastatic lymph node directly invaded to the duodenum and the continuous bleeding was detected from that lesion (Figure 3C, 3D). Postoperative 4 months, he died. We experienced so rapid increasing undifferentiated high grade pleomorphic sarcoma originated from the anterior mediastinum.
Figure 3.
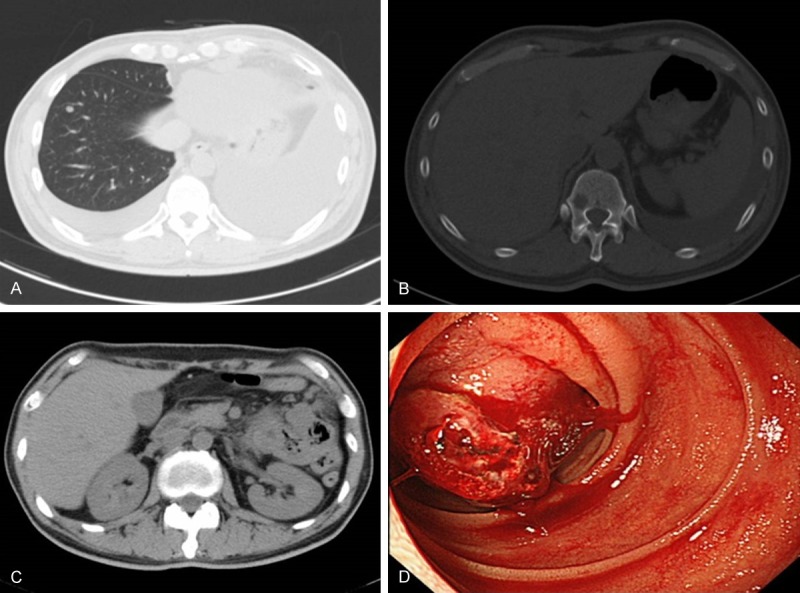
Postoperative chest computed tomography (CT) after one month indicated right lung metastasis (A) and vertebral metastasis (B). Postoperative CT after three month indicated para-aortic lymph node metastasis directly invaded to the duodenum (C). Postoperative gastro-intestinal fiber (GIF) after three months showed invasion of para-aortic lymph node to the duodenum with active bleeding (D).
Discussion
Undifferentiated high grade pleomorphic sarcoma was previously categorized mesenchymal fibrous histiocytosis (MFH), and extremely appeared at the arms and legs and post-peritoneum [1,2]. The definition of the category of soft tissue sarcoma were changed by the 2013 WHO classification and those tumors that cannot be classified into any of the other categories due to lack a specific line of differentiation or lack of distinguishing histologic, immunohistochemical or genetic features were categorized undifferentiated sarcoma [3,4]. The prognosis of the mediastinum undifferentiated high grade pleomorphic sarcoma is generally poor, because surgical resection is often incomplete and chemotherapy and/or radiotherapy often yield only temporary improvement [5].
The overwhelming factor determining survival was the ability to completely resect the tumors and the most ideal therapy for undifferentiated high grade pleomorphic sarcoma is intensive therapy including complete surgical resection, chemotherapy (mainly used doxorubicin, ifosfamide, dacarbazine and cyclophosphamide) and radiation therapy. In our case, the growth speed was so rapid that we think it is difficult to select chemotherapy and/or radiotherapy as preoperative therapy. Because there was no obvious metastatic lesion by the PET-CT and head MRI, we decided to do complete surgical resection and we planned to add adjuvant chemotherapy and/or radiation therapy after operation. But the tumor growth was so aggressive and the metastasis of right lung, left thoracic cavity and thoracic vertebra were detected within one month after resection. In our case, the patient refused to take chemotherapy and/or radiotherapy, we just did best supportive care after surgery.
Recently, molecular targeted therapy was proceeded in variety of malignant tumor, including sarcoma, and some of oncogenic driver translocation was found and connected to the specific targeted therapy [5,6]. The novel therapeutic targeted gene mutations or translocations associated with sarcoma [7] were hoped to be found and connected to the specific therapeutic strategy for the each patient.
In conclusion, we experienced a case of anterior mediastinum undifferentiated high grade pleomorphic sarcoma. The tumor growth of the anterior mediastinum sarcoma was sometimes so rapid that it is necessary for us to diagnose and start the adequate therapy as soon as possible.
Disclosure of conflict of interest
None.
References
- 1.Lawrence W Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349–59. doi: 10.1097/00000658-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher CDM, Unni KK, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 3rd edition. Lyon, France: IARC Press; 2002. [Google Scholar]
- 3.Fletcher CD, Hogendoorn P, Mettens F, Bridge J. WHO Classification of Tumors of Soft Tissue and Bone. 4th edition. Lyon, France: IARC Press; 2013. [Google Scholar]
- 4.Doyle LA. Sarcoma classification: an update on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120:1763–74. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 5.Burt M, Ihde JK, Hajdu SI, Smith JW, Bains MS, Downey R, Martini N, Rusch VW, Ginsberg RJ. Primary sarcomas of the mediastinum: Results of therapy. J Thorac Cardiovasc Surg. 1998;115:671–80. doi: 10.1016/S0022-5223(98)70333-2. [DOI] [PubMed] [Google Scholar]
- 6.Choi EY, Thomas DG, McHugh JB, Patel RM, Roulston D, Schuetze SM, Chugh R, Biermann JS, Lucas DR. Undifferentiated small round cell sarcoma with t(4;19)(q35;q13.1) CIC-DUX4 fusion: a novel highly aggressive soft tissue tumor with distinctive histopathology. Am J Surg Pathol. 2013;37:1379–86. doi: 10.1097/PAS.0b013e318297a57d. [DOI] [PubMed] [Google Scholar]
- 7.Pierron G, Tirode F, Lucchesi C, Reynaud S, Ballet S, Cohen-Gogo S, Perrin V, Coindre JM, Delattre O. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44:461–6. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]


