Abstract
The aim of the present study was to analyze the relationship between aberrant human mutL homolog 1 (hMLH1) expression and clinicopathological parameters of patients with sporadic colorectal cancer, and to explore the prognostic effect of aberrant hMLH1 expression in these patients. The relationship was measured by chi-square test and Fisher’s exact test. Survival analysis was performed with Kaplan-Meier analysis and Cox regression model to measure 5-year disease-free survival (DFS) and 5-year overall survival (OS) rates. Totally 17.13% of the patients with sporadic colorectal cancer showed aberrant nuclear staining for hMLH1 expression. Aberrant hMLH1 expression was related with tumor pathologic types, tumor location and TNM staging (P<0.05) in the patients with sporadic colorectal cancer. Cox regression analysis indicated important prognostic factors were age (RR: 1.021, 95% CI: 1.003-1.039, P=0.023), mucinous adenocarcinoma (RR: 2.603, 95% CI: 1.705-3.974, P<0.0001), TNM staging (RR: 2.071, 95% CI: 1.170-3.666, P=0.012), lymphangion invasion (RR: 2.013, 95% CI: 1.227-3.303, P=0.006) and aberrant hMLH1 expression (RR: 0.414, 95% CI: 0.216-0.791, P=0.008). Consequently, hMLH1 expression level is related with some clinicopathologic features. Aberrant hMLH1 expression plays a significant part in prognosis for patients with sporadic colorectal cancer and it will promisingly become an independent prognostic factor.
Keywords: hMLH1, sporadic colorectal cancer, prognosis, correlation
Introduction
Currently, colorectal cancer is one of the most common malignancies, accounting for the third most common malignancy and second leading cause of cancer-related death worldwide [1]. Therefore, it becomes particularly important for the deep research on its pathogenesis and prognostic factors. It has been established that most colorectal cancers are sporadic (85%) while only in around 15% a hereditary component can be detected [2]. Nearly all hereditary non-polyposis colorectal cancer (HNPCC) and 10%~20% of sporadic colorectal cancer occurs gene mutation of mismatch repair (MMR) gene [3], or inactivation gene promoter of MMR by hypermethylation. It can also lower body mismatch repair function, instable of the entire genome, rapidly accumulate mutations of certain oncogenes and tumor suppressor genes in vivo, and finally make colorectal cancer occur.
Recent studies have found that human mutL homolog 1 (hMLH ) expression can well predict function aberrant MMR gene and microsatelite instability (MSI) presence [4,5]. One important feature of MMR dysfunction is positive MSI. Compared with normal cells, gene mutation rate of microsatellite sequences in tumor cells with dysfunction of MMR gene is from 100 to 1000 times higher than normal cells. MSI can be found in a variety of tumors, of which colorectal cancer has been researched more. MSI performs positive expression in 15% of sporadic colorectal cancer, but its mechanism is not the same as HNPCC, and MSI occurred in sporadic colorectal cancer is mainly related with high hypermethylation of hMLH1 promoter [6,7]. Some other studies showed, there were significant different clinicopathological features between the patients with MSI-high (MSI-H) of colorectal cancer and normal expression of MMR, such as the tumors with MSI-H appeared more in female patients, in proximal colon, poorly differentiated and mucinous adenocarcinoma [8,9]. However, the majority of findings revealed that, higher MSI patients with colorectal cancer had a better prognosis [10].
Presently, there are few reports of the clinical significance of hMLH1 expression effect on sporadic colorectal cancer, especially for prognosis. In the study, by collecting clinicopathologic data and follow-up data of 327 patients with sporadic colorectal cancer in our hospital and detecting the protein expression level of hMLH1 by immunohistochemistry, we were to reveal the relationship of aberrant hMLH1 expression and clinicopathological parameters of patients with sporadic colorectal cancer, to investigate its function in pathogenesis and to explore its effect on prognosis.
Materials and methods
Study subjects and reagent
Clinicopathologic data and postoperative samples of 327 patients with colorectal cancer from January 1st 2005 to January 1st 2008 in our hospital were collected. Data regarding age at diagnosis, the gender, nationality, tumor size, histological type, TNM stage, tumor location, lymphangion invasion and peripheral nerve infiltration (detailed showing in Table 1). Diagnosis criterions are as follow: for hereditary non-polyposis colorectal cancer, HNPCC, according to Amsterdam II; for TNM stage, according to American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) TNM staging system of colorectal cancer (2010, Seventh Edition). Cases were excluded as followings, HNPCC diagnosis (23 cases), classical familial adenomatous polyposis, CFAP (4 cases), unknown-caused positive family history (3 cases), preoperative chemoradiotherapy (14 cases), preoperative radiotherapy (2 cases), preoperative chemotherapy (8 cases) or data lack (5 cases).
Table 1.
Univariate analysis results between hMLH1 protein expression and clinicopathological features
| Normal hMLH1 (n=271) | Aberrant hMLH1 (n=56) | Total (n=327) | χ2 | P-value | |
|---|---|---|---|---|---|
| Age (years) | 0.439 | 0.507 | |||
| <50 | 66 | 16 | 82 | ||
| ≥50 | 205 | 40 | 245 | ||
| Gender | 0.184 | 0.668 | |||
| Male | 168 | 33 | 201 | ||
| Female | 103 | 23 | 126 | ||
| Nationality | 1.109 | 0.775 | |||
| Han | 226 | 49 | 275 | ||
| Uyghur | 26 | 4 | 30 | ||
| Hui | 11 | 1 | 12 | ||
| Others | 8 | 2 | 10 | ||
| Tumor location | 14.486 | 0.001 | |||
| Right hemicolon | 46 | 22 | 68 | ||
| Left hemicolon | 88 | 11 | 99 | ||
| Rectal | 137 | 23 | 160 | ||
| Tumor size (cm) | 1.906 | 0.386 | |||
| <4 | 100 | 19 | 119 | ||
| 4-6 | 103 | 18 | 121 | ||
| ≥6 | 68 | 19 | 87 | ||
| Tissue type | 10.487 | 0.005 | |||
| Glandular (well/moderately) | 191 | 27 | 218 | ||
| Glandular (poorly) | 33 | 11 | 44 | ||
| Mucous gland/signet cell | 47 | 18 | 65 | ||
| TNM staging | 12.508 | 0.006 | |||
| I | 26 | 5 | 31 | ||
| II | 79 | 29 | 108 | ||
| III | 130 | 20 | 150 | ||
| IV | 36 | 2 | 38 | ||
| T staging | 1.620 | 0.655 | |||
| T1 | 15 | 2 | 17 | ||
| T2 | 32 | 6 | 38 | ||
| T3 | 62 | 17 | 79 | ||
| T4 | 162 | 31 | 193 | ||
| N staging | 6.834 | 0.033 | |||
| N0 | 114 | 34 | 148 | ||
| N1 | 90 | 11 | 101 | ||
| N2 | 67 | 11 | 78 | ||
| Distant metastasis | 4.263 | 0.039 | |||
| M0 | 235 | 54 | 289 | ||
| M1 | 36 | 2 | 38 | ||
| Lymphangion invasion | 0.006 | 0.578 | |||
| Yes | 30 | 6 | 36 | ||
| No | 241 | 50 | 291 | ||
| Peripheral nerve infiltration | 0.254 | 0.615 | |||
| Yes | 14 | 2 | 16 | ||
| No | 257 | 54 | 311 |
Concentration mouse anti-human MLH1 gene monoclonal antibody, DAB chromogenic reagent kit and polylysine were bought from Beijing Zhongshan Biotechnology Co., LTD. PV-9000 two step method assay kit was from American Golden Bridge (GBI) international co., LTD.
Postoperative therapy
Chemotherapy scheme FOLFOX6 was carried out to the patients with rectal cancer of stage III and stage II with high-risk factors received chemotherapy, including poor differentiation, large lumps, T4, less than 1 cm of tumor resection margin, fewer than 12 lymph nodes for postoperative biopsy. Oxaliplatin (L-OHP) injection, 130 mg/m2, intravenously infused for 3 hours, on the first day; calcium folinate (CF) injection, 300 mg/m2, intravenously infused, on the first day; 5-FU injection, 400 mg/m2, intravenously injected, on the first day; and 5-FU 2400 mg/m2, continuously intravenously infusion by micro pump for 48 hours. 14 days a cycle, 12 cycles in total. For recurrence therapy, FOLFORI scheme was performed. Irinotecan 180 mg/m2, intravenously infused, on the first and fourth day, two weeks a therapeutic circle, until dose intolerable or invalid. For the patients with drug resistance, chemotherapy scheme XELOX was applied, as followings, oxaliplatin (L-OHP) injection, 130 mg/m2, intravenously infused for 3 hours, on the first day; capecitabine tablets, 1000 mg/m2, po, twice a day, from first day to fourth day, 3 weeks a cycle, 9 cycles in total.
Follow-up
All patients above enrolled in our hospital were registered, and complete personal follow-up files of the patients with explicit pathological diagnosis were established. After surgery, the patients were followed up once every three months for two years, and then once every 6 months. Two follow-up ways were used, outpatient or inpatient review and telephone follow-up, including start time of postoperative chemotherapy, chemotherapy regimens, chemtherapy course count, side effects of chemotherapy, recurrence and survival time.
Immunohistochemical method
The neutral formalin-fixed(with concentration of 40 g/L), paraffin-embedded specimens were serially sectioned by thickness of 5 μm, and two-step method of PV-9000 was performed, using mouse anti-human monoclonal antibody of MLH1 as primary antibodies with working concentration of 1:150. Universal two-step method (HRP) detection kit was utilized. PBS was instead of primary antibody as negative control, while normal colorectal mucosa and/or infiltrating lymphocytes were used as positive control. Normal expression of MLH1 was in nucleus. Result judgement standard was according to Liangzhong Xu’s immunohistochemical method criterion [11], which was that microscopic tumor cells showing positive nuclear staining was combined with staining intensity and percentage of positive cells, to determine normal expression levels. 5 high-power fields with more cancer cells were selected from each slice by light microscope and each field counted 100 cells per field. According to grading of staining intensity, no coloring is 0 points, light yellow is 1 point, yellow is 2 points and brown is 3 points; according to grading of positive cell percentage, no positive cell is o point, Less than or equal 10% is 1 point, from 11% to 50% is 2 points, from 51% to 75% is 3 points and more than 75% is 4 points. If the result of two scores above multiplying is more than or equal 2 points, it will be judged as a normal expression case, meanwhile if less than 2 points, it will be judged as an aberrant expression case. Normal control was positive nuclei of normal colorectal mucosa and/or infiltrating lymphocytes. However, aberrant is judged in case of nucleus normal expression of normal control and tumor cell nuclei missing staining. Each judgement was finished by two pathology experts.
Statistical analysis
Univariate analysis between hMLH1 protein expression and clinicopathologic features was performed with chi-square test and Fisher’s exact test, and multivariate correlation analysis between the two above was made with Logistic regression test. Univariate survival analysis was carried out by Kaplan-Meier survival curves, and Log-rank test was used for comparison between the groups. Multivariate survival analysis was performed by COX regression model. All above were carried out via SPSS for Windows Version 18 (SPSS Inc., Chicago, IL, USA). P values of less than or equal 0.05 were considered to be statistically significant.
Results
Immunohistochemical measurement results of hMLH1
In the total of 327 cases with sporadic colorectal cancer, 56 cases (17.13%) showed aberrant nuclear staining and 271 cases (82.87%) indicated normal nuclear staining of hMLH1 expression (Table 1). Normal colorectal mucosa tissue showed normal nuclear staining of hMLH1 protein expression is observed in stromal cells and epithelial tumor cells. Colorectal cancer tissue indicated that hMLH1 protein expression is only observed in stromal cells, not in epithelial tumor cells (Figure 1).
Figure 1.
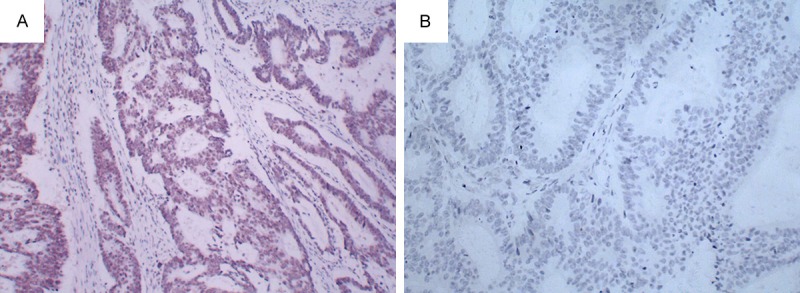
hMLH1 protein expression showing by Immunohistochemistry (magnification, ×100) A: Normal colorectal mucosa tissue showed normal nuclear staining of hMLH1 protein expression is observed in stromal cells and epithelial tumor cells. B. Colorectal cancer tissue indicated that hMLH1 protein expression is only observed in stromal cells, not in epithelial tumor cells. hMLH1, human mutL homolog 1.
Univariate analysis of hMLH1 expression and clinicopathologic features
By univariate analysis, hMLH1 aberrant expression of sporadic colorectal cancer was closely related with tumor location, tissue type, TNM staging, N staging, distant metastasis with significant statistically difference (P<0.05). There were no significant statistical difference between hMLH1 protein aberrant expression and age, gender, nationality, tumor size, T staging, lymphangion invasion and peripheral nerve infiltration (P>0.05; Table 1).
Multivariate analysis of hMLH1 expression and clinicopathologic features
By Logistic regression test, independent risk factors of hMLH1 aberrant expression were histological types (OR: 0.378, 95% CI: 0.205-0.695, P=0.002), tumor location (OR: 0.307, 95% CI: 0.161-0.587, P<0.0001) and TNM staging (OR: 1.626, 95% CI: 1.124-2.352, P=0.01) (Table 2).
Table 2.
Multivariate analysis results between hMLH1 protein expression and clinicopathologic features
| Variable | B value | OR | 95% confidence interval | P value | |
|---|---|---|---|---|---|
|
| |||||
| Lower bound | Upper bound | ||||
| Histological type | |||||
| Poor/mucinous adenocarcinoma vs good/moderate adenocarcinoma | -0.973 | 0.378 | 0.205 | 0.695 | 0.002 |
| Tumor location | |||||
| Right hemicolon vs left hemicolon/rectal | -1.180 | 0.307 | 0.161 | 0.587 | <0.0001 |
| TNM staging | |||||
| I, II vs III, IV | 0.486 | 1.626 | 1.124 | 2.352 | 0.01 |
Survival analysis of normal and aberrant expression groups of hMLH1
All patients were followed up for 5 years with 20 cases in lost midway. By survival analysis, for total patients, 5-year survival rate in group of hMLH1 aberrant expression was higher than that of normal group, with statistical significant difference (P<0.05). For staging II patients, 5-year survival rate of the group with aberrant expression of hMLH1 was a little higher than that of normal expression group, with no statistical significant difference (P>0.05). However, for staging III patients, 5-year survival rate of aberrant expression group was higher than that of normal group, with statistical significant difference (P<0.05) (Figure 2).
Figure 2.
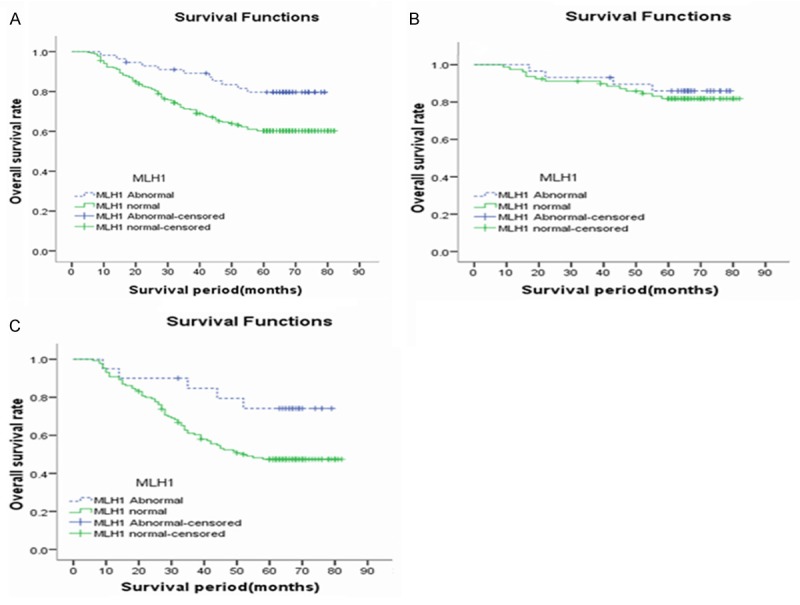
Comparison of 5-year survival rate between the groups of normal and aberrant hMLH1 expression. A. Represents total patients: 5-year overall survival rate in group of aberrant hMLH1 expression was higher than normal group, with significant statistical difference (Ptotal=0.007); B. Stands for staging II patients: 5-year overall survival rate in group of aberrant hMLH1 expression was higher than normal group, with no statistical difference (PII=0.595); C. Represents staging III: 5-year overall survival rate in group of aberrant hMLH1 expression was higher than normal group, with significant statistical difference (PIII=0.036).
Cox regression analysis indicated independent prognostic factors included age (RR: 1.021, 95% CI: 1.003-1.039, P=0.023), mucinous adenocarcinoma (RR: 2.603, 95% CI: 1.705-3.974, P<0.0001), TNM staging (RR: 2.071, 95% CI: 1.170-3.666, P=0.012), lymphangion invasion (RR: 2.013, 95% CI: 1.227-3.303, P=0.006) and aberrant hMLH1 expression (RR: 0.414, 95% CI: 0.216-0.791, P=0.008) (Table 3).
Table 3.
Multivariate survival analysis on prognosis of COX regression
| Variable | B value | RR | 95% confidence interval | P value | |
|---|---|---|---|---|---|
|
| |||||
| Lower bound | Upper bound | ||||
| Age | 0.020 | 1.021 | 1.003 | 1.039 | 0.023 |
| Tumor size | 0.038 | 1.039 | 0.947 | 1.139 | 0.417 |
| Glandular (well/moderately) | — | — | — | — | 0.000 |
| Glandular (poorly) | 0.313 | 1.368 | 0.731 | 2.562 | 0.328 |
| Mucinous adenocarcinoma | 0.957 | 2.603 | 1.705 | 3.974 | <0.0001 |
| Left hemicolon | — | — | — | — | 0.945 |
| Right hemicolon | 0.089 | 1.093 | 0.622 | 1.920 | 0.757 |
| Rectum | 0.062 | 1.064 | 0.679 | 1.668 | 0.786 |
| T staging | 0.008 | 1.008 | 0.788 | 1.289 | 0.949 |
| N staging | 0.260 | 1.297 | 0.923 | 1.822 | 0.134 |
| TNM staging | 0.728 | 2.071 | 1.170 | 3.666 | 0.012 |
| Distant metastasis | 0.271 | 1.311 | 0.578 | 2.972 | 0.517 |
| Lymphangion invasion | 0.700 | 2.013 | 1.227 | 3.303 | 0.006 |
| Peripheral nerve infiltration | 0.309 | 1.362 | 0.668 | 2.776 | 0.396 |
| hMLH1 expression | -0.883 | 0.414 | 0.216 | 0.791 | 0.008 |
Literature retrospection of hMLH1 effect on prognosis in sporadic colorectal cancer
Detailed showing in Table 4.
Table 4.
Literature retrospection of hMLH1 effect on sporadic colorectal cancer
| Country | n | MMR | Aberrant rate (%) | Selection of patients | Prognostic factor | |
|---|---|---|---|---|---|---|
| Cawkwell. 1999 | UK | 99 | hMLH1 hMSH2 | 15.15 | sporadic colorectal cancers | Yes |
| Kruschewski. 2002 | Germany | 127 | hMLH1 hMSH2 | 11.81 | sporadic colorectal cancers | No |
| Smyth. 2004 | UK | 111 | hMLH1 | 28.83 | right hemicolon cancer | Yes |
| Lanza. 2006 | Italy | 718 | hMLH1 hMSH2 | 15.88 | stageII, IIIcolorectal cancer | Yes |
| Park. 2010 | Korea | 318 | hMLH1 hMSH2 | 11.32 | sporadic colorectal cancers | Yes |
| Yoon. 2011 | Korea | 2028 | hMLH1 hMSH2 | 10.21 | sporadic colorectal cancers | DFS, Yes; OS, No |
| Aparicio. 2013 | France | 91 | MMR | 28.57 | colorectal cancer, ≥75 y, staging II | Yes |
Discussion
At present, a number of reports have confirmed immunohistochemical method is reliable for MMR gene measurement. The method has been put to use in vast majority of hospitals and research institutions. With stability and low cost, immunohistochemical method in detecting MMR gene expression and MSI of tissue specimens, has shown high sensitivity (77-100%) and specificity (98-100%) [12], so that recently immunohistochemical method has been suggested as the preferred method of MMR gene mutation analysis [13]. Immunohistochemistry PV-9000 two-step method belongs to enzymatic biotin method. Monovalent Fab fragments of second antibody molecules polymerize with enzyme instead of traditional method of secondary and third antibody. Consequently, the antigen-antibody binding signal is directly amplified. Compared with traditional SP third step method, it shows simple, fast and sensitive. Meanwhile, it also avoids background staining due to no biotin. Thus, immunohistochemistry PV-9000 two-step method is popularly used in clinical work. Thus, in the study immunohistochemistry PV-9000 two-step method was performed to measure the situation of hMLH1 expression in 327 postoperative pathologic specimens.
Recently, MMR system includes genes MLH1, MSH2, MSH6, PSM1, PSM2, MSH3 and MSH5 [14]. Their expression product is a nucleic acid enzymes to correct mismatched basic group in DNA replication process for keeping more fidelity in copy process. Genes MLH1 and MSH2 have been researched more in the world [15], while the studies of genes MSH6 and PSM2 are relatively less. Plenty of researchers have confirmed the deficiency rate of gene MLH1 is higher than MSH2 [16]. The reason of the occurrence of the majority of sporadic tumor with MSI-H may be inactivation of gene MLH1 in somatic cell [10]. CpG islands of MLH1 gene promoter region is hypermethylated, which causes barriers of gene transcription, translation and protein expression. And deficiency of MLH1 finally appears [17]. In the study, MLH1 was chosen for study. 17.13% patients showed aberrant nuclear staining of hMLH1.
Some studies showed that it turned out higher deficiency rate when tumor located in the right colon, belonged to histological type of mucus glands or signet ring cell carcinoma, or was poorly differentiated [10,18]. In the study, tumor in right colon, poorly differentiated and mucous glands suggested poor prognosis, with its histopathological features of MSI-H. MSI occurrence of sporadic colorectal cancer was mostly caused by MMR gene aberrant. However, hMLH1 owned the highest proportion in MMR system, so the high aberrant rate of hMLH1 was mostly found in right colon, poorly differentiated and mucinous adenocarcinoma. As to biological and molecule features, it was different between proximal and distal colon [19,20]. Most current studies indicated that there was more aberrant MMR in right-sided colon carcinoma than that in left-sided colon and rectum carcinoma. And no statistical difference in left colon and rectum was showed, which suggested pathopoiesia effect of aberrant MMR gene mainly existed in tumor incidence stage and was closely related with organizational molecular biology. There may exist higher proportion of gene promoter hypermethylation which could cause MSI occurrence. Meanwhile, this phenomenon might also explain the fact that most malignant tumor with high MSI were mainly caused by aberrant hMLH1 or sporadic promoter hypermethylation [21,22]. In colorectal cancer, promoter methylation mostly occurs in hMLH1 gene, consequently showing hMLH1 deficiency. For colorectal cancer patients, the factors of cancer tunica externa breakthrough, peripheral nerve and vascular invasion, regional lymph node metastasis and distant metastasis suggest advanced stage and poor prognosis. Park reported that aberrant MMR rate was very low in the patients with advanced stage factors of positive regional lymph node metastasis, distant metastasis, positive lymphatic invasion, TNM staging III and IV [23]. However, Kruschewski indicated that the patients with colorectal cancer of lymphatic invasion and right colon cancer showed a higher aberrant MMR rate, with no statistical difference of the rest clinicopathologic features [24]. Therefore, prognostic influence of aberrant MMR in patients with colorectal cancer hasn’t come to consistency.
In our study, the patients with negative regional lymph node metastasis, no distant metastasis, TNM staging I and II had a higher aberrant hMLH1 rate, which was consistent with Park’s study. Presumable reasons of the above may be the followings. The clinicopathologic features of negative regional lymph node metastasis, no distant metastasis, TNM staging I and II mostly indicate early stage of tumor development; aberrant hMLH1 mainly occur in initial stage of tumor, so the patients with the several features above had a higher aberrant hMLH1 rate; the higher aberrant hMLH1 rate was accompanied by MSI-H, which could indicate a good prognosis. Thus, the phenomenon that the higher aberrant hMLH1 rate was mainly in earlier stage was observed in patients with colorectal cancer. In contrast, the clinicopathologic features of tumor invasion through membrane, larger tumor, peripheral nerves invasion and lymphatic invasion indicated late tumor performance. Although the results suggested that aberrant hMLH1 rate was relatively low, the statistical results didn’t show significant statistical difference. Therefore, we speculated aberrant hMLH1 expression was mostly involved in occurrence of early-staging colorectal cancer and the patients with higher aberrant hMLH1 rate had a better prognosis.
Dieumegard reported that MSI can be measured in approximate 90% of patients with HNPCC, accompanied by aberrant mismatch repair genes MLH1 and MSH2 [25]. Although these patients were mostly young and low tissue differentiation, they often owned a better prognosis than those of sporadic colorectal cancer [26]. The reason may be that clinicopathologic features in the patients with HNPCC were similar to those of the patients with MSI-H in sporadic colorectal cancer, and the two types of patients had similar genetic background. The patients with colorectal cancer and positive MSI had some specific clinicopathological features, such as poorer differentiation, mucinous adenocarcinoma, young individuals and positive regional lymph node metastasis [27]. Characteristics of those were similar for HNPCC. Sankila reported that five year survival rate in the patients with HNPCC was 65%, while that of sporadic colorectal cancer was 44% [28]. The result was supported by the majority of other studies [29].
As the above mentioned, aberrant hMLH1 expression can be a good predictor of MSI presence. MSI highly expressed in the patients with HNPCC, and approximate 15% of the patients with sporadic colorectal cancer had MSI-H. A growing number of studies have been showing that MSI-H indicates good prognosis. Popat found that the patients with MSI showed better prognosis than microsatellite stability (MMS) by researching the prognosis of 7642 patients with colorectal cancer [10]. Wang XF reported survival analysis results of 146 patients with sporadic colorectal cancer were that 5-year survival rate of the patients with MSI was 92.3%, significantly higher than MMS showing of 63.5% [30]. The conclusion above had also been confirmed by most other studies [31,32]. The reason might be that MSI-H limited tumor growth. Although the studies above confirmed the patients with MSI had good prognosis, some other studies remained contrary views [33]. hMLH1 methylation of in sporadic colorectal cancer mostly causes MSI which is closely related to prognosis. Consequently, predicting better prognosis of aberrant hMLH1 can be inferred. However, some researchers gave different viewpoints. By researching 318 cases and the relation of the two groups of different aberrant hMLH1 and hMSH2 in sporadic colorectal cancer, Park pointed out that the group of the patients with aberrant MMR had better prognosis than normal MMR and staging III patients showed higher survival rate. As we all know, the later TNM staging of colorectal cancer patients are, the poorer prognosis they own. If there is better homogeneity that the patients with colorectal cancer in the same TNM staging, effect will be better by researching prognosis impact of a certain factor. By studying 718 patients with colorectal cancer in staging II and III, Lanza reported 6-year survival rates of the two groups of the patients with aberrant and normal expression in the same staging II were 97% and 82% respectively. And similarly those were 78% and 56% respectively in the same staging III [34]. Consequently, the difference was statistically significant. Up to now, there have been few researches on MMR expression and prognosis. Most studies showed that the patients with aberrant MMR had better prognosis [35], but the conclusion hasn’t yet form a final conclusion [36].
For the arguments above, in our study, by immunohistochemistry PV-9000 two step method hMLH1 expression of postoperative specimens of 327 patients with sporadic colorectal cancer were detected and all of them were postoperatively followed up. The results indicated the group of the patients with hMLH1 deletion had a higher 5-year survival rate than normal group (80.36% vs 61.25%). Simultaneously, to keep better homogeneity of different TNM staging, the patients with staging II and III were separately underwent statistical analysis. And the result showed that 5-year survival rate of aberrant hMLH1 group were both higher than normal group in the same staging II and III respectively. However, statistical difference only showed in staging III patients (P<0.05). Why do aberrant hMLH1 patients have better prognosis? Malesci reported that the reason was partly dependent on early cancer diagnosis [37], with consistency with Park’s study. Although we didn’t detect MSI expression of specimens in our study, the above mentioned MSI expression was mainly caused by aberrant MMR in sporadic colorectal cancer, and MSI expression closely related with hMLH1 methylation [38-40]. Therefore, the reason why the patients with aberrant hMLH1 had higher survival rate might be that the lack of hMLH1 accompanied by the occurrence of MSI and MSI-H in colorectal cancer limited tumor growth. Meanwhile, COX regression was performed on possible prognostic factors and the result suggested that hMLH1 deficiency was a better independent prognostic factor.
In conclusion, it indicated aberrant hMLH1 was closely correlated to some clinicopathologic features in patients with sporadic colorectal cancer. Its deletion was correlated with higher survival rate and could hopefully be regarded as a prognostic independent risk factor, especially for staging III patients. However, its detailed mechanism still requires deeper research.
Acknowledgements
The authors thank the patients for their informed consent prior to their inclusion in the study; Ethical approval is supported by Medical Ethics Committee of The Third Xiangya Hospital of Central South University. This work was supported by National Nature Scientific Foundation of China (81472286, 81272736, 81472287).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herfarth KK, Kodner IJ, Whelan AJ, Ivanovich JL, Bracamontes JR, Wells SA Jr, Goodfellow PJ. Mutations in MLH1 are more frequent than in MSH2 in sporadic colorectal cancers with microsatellite instability. Genes Chromosomes Cancer. 1997;18:42–49. doi: 10.1002/(sici)1098-2264(199701)18:1<42::aid-gcc5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du C, Zhao J, Xue W, Dou F, Gu J. Prognostic value of microsatellite instability in sporadic locally advanced rectal cancer following neoadjuvant radiotherapy. Histopathology. 2013;62:723–730. doi: 10.1111/his.12069. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, Thibodeau SN. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–3460. [PubMed] [Google Scholar]
- 7.Des Guetz G, Lecaille C, Mariani P, Bennamoun M, Uzzan B, Nicolas P, Boisseau A, Sastre X, Cucherousset J, Lagorce C, Schischmanoff PO, Morere JF. Prognostic impact of microsatellite instability in colorectal cancer patients treated with adjuvant FOLFOX. Anticancer Res. 2010;30:4297–4301. [PubMed] [Google Scholar]
- 8.Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Fam Cancer. 2004;3:93–100. doi: 10.1023/B:FAME.0000039849.86008.b7. [DOI] [PubMed] [Google Scholar]
- 9.Kaur G, Masoud A, Raihan N, Radzi M, Khamizar W, Kam LS. Mismatch repair genes expression defects & association with clinicopathological characteristics in colorectal carcinoma. Indian J Med Res. 2011;134:186–192. [PMC free article] [PubMed] [Google Scholar]
- 10.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 11.Xu LZ, Yang WT. Interpretation criteria of immunohistochemical reaction results. Chin J Cancer. 1996;6:229–231. [Google Scholar]
- 12.Ruszkiewicz A, Bennett G, Moore J, Manavis J, Rudzki B, Shen L, Suthers G. Correlation of mismatch repair genes immunohistochemistry and microsatellite instability status in HNPCC-associated tumours. Pathology. 2002;34:541–547. doi: 10.1080/0031302021000035965-2. [DOI] [PubMed] [Google Scholar]
- 13.Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, Gerald WL, Ellis NA. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29:96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- 14.Jiricny J, Nyström-Lahti M. Mismatch repair defects in cancer. Curr Opin Genet Dev. 2000;10:157–161. doi: 10.1016/s0959-437x(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 16.Plevová P, Sedláková E, Zapletalová J, Krepelová A, Skýpalová P, Kolár Z. Expression of the hMLH1 and hMSH2 proteins in normal tissues: relationship to cancer predispositionin hereditary non-polyposis colon cancer. Virchows Arch. 2005;446:112–119. doi: 10.1007/s00428-004-1139-5. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa T, Konishi F, Masubuchi S, Shitoh K, Nagai H, Tsukamoto T. Densely methylated MLH1 promoter correlates with decreased mRNA expression in sporadic colorectal cancers. Genes Chromosomes Cancer. 2002;35:1–10. doi: 10.1002/gcc.10100. [DOI] [PubMed] [Google Scholar]
- 18.Laghi L, Malesci A. Microsatellite instability and therapeutic consequences in colorectal cancer. Dig Dis. 2012;30:304–309. doi: 10.1159/000337003. [DOI] [PubMed] [Google Scholar]
- 19.Poynter JN, Siegmund KD, Weisenberger DJ, Long TI, Thibodeau SN, Lindor N, Young J, Jenkins MA, Hopper JL, Baron JA, Buchanan D, Casey G, Levine AJ, Le Marchand L, Gallinger S, Bapat B, Potter JD, Newcomb PA, Haile RW, Laird PW Colon Cancer Family Registry Investigators. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler JM, Loukola A, Aaltonen LA, Mortensen NJ, Bodmer WF. The role of hypermethylation of the hMLH1 promoter region in HNPCC versus MSI+ sporadic colorectal cancers. J Med Genet. 2000;37:588–592. doi: 10.1136/jmg.37.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wani M, Afroze D, Makhdoomi M, Hamid I, Wani B, Bhat G, Wani R, Wani K. Promoter Methylation Status of DNA Repair Gene (hMLH1) in Gastric Carcinoma Patients of the Kashmir Valley. Asian Pacific J Cancer Prev. 2012;13:4177–4181. doi: 10.7314/apjcp.2012.13.8.4177. [DOI] [PubMed] [Google Scholar]
- 23.Park JW, Chang HJ, Park S, Kim BC, Kim DY, Baek JY, Kim SY, Oh JH, Choi HS, Park SC, Jeong SY. Absence of hMLH1 or hMSH2 expression as a stage-dependent prognostic factor in sporadic colorectal cancers. Ann Surg Oncol. 2010;17:2839–2846. doi: 10.1245/s10434-010-1135-8. [DOI] [PubMed] [Google Scholar]
- 24.Kruschewski M, Noske A, Haier J, Runkel N, Anagnostopoulos Y, Buhr HJ. Is reduced expression of mismatch repair genes MLH1 and MSH2 in patients with sporadic colorectal cancer related to their prognosis? Clin Exp Metastasis. 2002;19:71–77. doi: 10.1023/a:1013853224644. [DOI] [PubMed] [Google Scholar]
- 25.Dieumegard B, Grandjouan S, Sabourin JC. Extensive molecular screening for hereditary non-polyposis colorectal cancer. Br J Cancer. 2000;82:871–880. doi: 10.1054/bjoc.1999.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brueckl WM, Jung A, Wein A, Brabletz T, Guenther K, Nusko G, Hahn EG. Microsatellite instability in colorectal adenomas: relevance and clinical importance. Int J Colorectal Dis. 2000;15:189–196. doi: 10.1007/s003840000241. [DOI] [PubMed] [Google Scholar]
- 27.Feeley KM, Fullard JF, Heneghan MA, Smith T, Maher M, Murphy RP, O’Gorman TA. Microsatellite instability in sporadic colorectal carcinoma is not an indicator of prognosis. J Pathol. 1999;188:14–17. doi: 10.1002/(SICI)1096-9896(199905)188:1<14::AID-PATH323>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Sankila R, Aaltonen LA, Järvinen HJ, Mecklin JP. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology. 1996;110:682–687. doi: 10.1053/gast.1996.v110.pm8608876. [DOI] [PubMed] [Google Scholar]
- 29.Percesepe A, Benatti P, Roncucci L, Sassatelli R, Fante R, Ganazzi D, Bellacosa A, Genuardi M, Neri G, Viel A, Ponz de Leon M. Survival analysis in families affected by hereditary non-polyposis colorectal cancer. Int J Cancer. 1997;71:373–376. doi: 10.1002/(sici)1097-0215(19970502)71:3<373::aid-ijc12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Wang XF, Jin HY, Ding YJ, Fan ZM, Liu XF, Gen JX. Influence of microsatellite instability on survival of patients with sporadic colorectal cancer in China. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:520–523. [PubMed] [Google Scholar]
- 31.Buecher B, Cacheux W, Rouleau E, Dieumegard B, Mitry E, Lièvre A. Role of microsatellite instability in the management of colorectal cancers. Dig Liver Dis. 2013;45:441–449. doi: 10.1016/j.dld.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Fenjvesi A. Prognostic significance of macrosatellite instability in patients uder 50 years of age suffering from colorectalcancer. Med Pregl. 2009;62:217–223. doi: 10.2298/mpns0906217f. [DOI] [PubMed] [Google Scholar]
- 33.Deschoolmeester V, Van Damme N, Baay M, Claes K, Van Marck E, Baert FJ, Wuyts W, Cabooter M, Weyler J, Vermeulen P, Lardon F, Vermorken JB, Peeters M. Microsatellite instability in sporadic colon carcinomas has no independent prognostic value in a Belgian study population. Eur J Cancer. 2008;44:2288–2295. doi: 10.1016/j.ejca.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Lanza G, Gafà R, Santini A, Maestri I, Guerzoni L, Cavazzini L. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectalcancer patients. J. Clin. Oncol. 2006;24:2359–2367. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 35.Aparicio T, Schischmanoff O, Poupardin C, Soufir N, Angelakov C, Barrat C, Levy V, Choudat L, Cucherousset J, Boubaya M, Lagorce C, Guetz GD, Wind P, Benamouzig R. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis. 2013;45:245–250. doi: 10.1016/j.dld.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Yoon YS, Yu CS, Kim TW, Kim JH, Jang SJ, Cho DH, Roh SA, Kim JC. Mismatch repair status in sporadic colorectal cancer: immunohistochemistry and microsatellite instabilityanalyses. J Gastroenterol Hepatol. 2011;26:1733–1739. doi: 10.1111/j.1440-1746.2011.06784.x. [DOI] [PubMed] [Google Scholar]
- 37.Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, Carnaghi C, Doci R, Rosati R, Montorsi M, Roncalli M, Gennari L, Santoro A. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831–3839. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 38.Arnold CN, Goel A, Compton C, Marcus V, Niedzwiecki D, Dowell JM, Wasserman L, Inoue T, Mayer RJ, Bertagnolli MM, Boland CR. Evaluation of microsatellite instability, hMLH1 expression and hMLH1 promoter hypermethylation in defining the MSI phenotype of colorectal cancer. Cancer Biol Ther. 2004;3:73–78. doi: 10.4161/cbt.3.1.590. [DOI] [PubMed] [Google Scholar]
- 39.Merok MA, Ahlquist T, Røyrvik EC, Tufteland KF, Hektoen M, Sjo OH, Mala T, Svindland A, Lothe RA, Nesbakken A. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274–1282. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012;18:1506–1512. doi: 10.1158/1078-0432.CCR-11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]


