Abstract
Second-line chemotherapy has been proved to be effective on patients with relapsed or refractory small cell lung cancer (SCLC). Although topotecan has been approved by many countries for the monotherapy with an acknowledged efficacy, its efficacy of low response rate and short median survival time is disappointing. Considering the optimal regimen of second-line therapy is yet uncertain, we conducted this meta-analysis to provide theoretical basis for making clinical decisions. A comprehensive electronic search was performed to identify eligible studies. The ending points included response, overall survival (OS), and adverse events. Odds ratios and 95% confidence interval were calculated to compare the effects. Six trials with 1369 patients were included. With regard to response rate, only amrubicin showed a significant improvement compared with topotecan. Irinotecan and etoposide did not show any advantages. When targeted on OS, neither of these monotherapy regimens exhibited any advantage when compared to topotecan. When aimed at toxicity, amrubicin showed a better effect on reducing hematologic toxicity, but a worse outcome on increasing the nonhematologic toxicity, whereas others showed equal efficacy. There is no strong evidence that any advantage for second-line treatment of SCLC when compared with topotecan, except amrubicin. And amrubicin seems to be superior to topotecan in terms of response rates, with a lower toxicity than topotecan, which is of high value in clinical application, and may be the direction of second-line monotherapy in the future.
Keywords: Small cell lung cancer, second-line treatment, single-drug chemotherapy, meta-analysis
Introduction
Although falling as a percentage of all lung cancers with the decreasing of the number of smokers, small cell lung cancer (SCLC) still constitutes approximately 13% of all cases with lung cancer [1]. Without any treatment, the patients with SCLC only have a median survival of 2 to 4 months [2]. Unlike other types of lung cancer, SCLC has an aggressive nature with early regional or distant metastases, and is more chemosensitive to initial systemic cytotoxic drugs. Combination chemotherapy is standard first-line therapy for patients with SCLC, and the most recommended regimen is platinum plus etoposide [3]. Although 50% to 80% of patients with SCLC can achieve a rapid high response rate, but complete response are seen in a minority of patients, duration of response is short, and overall survival is still very dismal because of early occurrence of chemotherapy resistant disease [4]. If suffering from tumor recurrence or progression, patients with SCLC only have a median survival period of 4 to 7 months, and even patients with a therapeutic effect of complete response also may relapse [5]. Therefore, most of patients with SCLC need second-line treatment.
Although various chemotherapeutic regimens have been evaluated either singly or combination in clinical trials, and some have shown promising antitumor activity, no standard chemotherapy had been established for second-line treatment of SCLC until recently [6,7]. According to a study by von Pawel et al. [8] compared topotecan with a traditional regimen of cyclophosphamide, doxorubicin, and vincristine (CAV) for patients with recurrent SCLC, topotecan can reduced symptoms to a greater degree than CAV and was associated with less toxicity. The results of this phase III trial have made topotecan the only drug with regulatory approval by the US Food and Drug Administration for the treatment of relapsed SCLC due to the lack of alternative drugs [9]. However, topotecan failed to provide any survival benefit, and it proved that single-drug regimen is much safer than multi-drug schedule in management of patients who receive second-line treatment of SCLC [10]. Thus, looking for a more effective and safer monotherapy regimen for second-line treatment of SCLC is necessary.
Recently, reports have surfaced on several drugs that were used singly in second-line chemotherapy of SCLC. But the results are contradictory, and the optimal chemotherapy regimen is not yet understood. Therefore, we conducted a meta-analysis to compare the efficacy and safety between the different drug regimens in the second-line treatment for SCLC, for the sake of providing a theoretical basis for making clinical decisions.
Subjects and methods
Search strategy
We searched PubMed, Embase, the Cochrane Register of Controlled Trials, and China National Knowledge Infrastructure via the Internet retrieval system. The following search terms were used: small cell lung cancer, second-line treatment, single drug, topotecan, previously treated, and randomized. We also handily examined reference lists of relevant primary studies, previous meta-analyses and reviews for additional publications. No language limitation was indicated, and the cut-off date of the included articles was 1 August, 2015. When multiple publications with the same institution were identified, only the published report with the largest series was included. This meta-analysis was reported following the Preferred Reporting Items for Systematic Review and Meta-Analysis statement [11].
Inclusion and exclusion criteria
The studies were manually selected carefully, and were eligible if they met the following criteria: (1) the trials were designed by prospective phase II and III randomized controlled trials (RCTs); (2) patients with pathologically confirmed as SCLC had been previously treated with first-line platinum-based chemotherapy, and suffered from tumor recurrence or progression after the treatment; (3) patients were divided into two groups at least, and one group was treated with single-drug regimen of second-line treatment for SCLC with topotecan, but another group used a non-topotecan regimen (e.g., amrubisin, irinotecan, or etoposide); (4) the studies reported at least one primary outcome to measure the effect of the treatments. Studies considered ineligible were as follows: reviews, conference abstracts, editorials, or case reports; researches on SCLC treated by multi-drug regimen of second-line treatment; articles with a cohort study design; and studies with incorrect analysis method or unavailable data.
Data extraction
All data were extracted by two authors and checked by a third reviewer. Consensus was reached through discussion of discrepancies. The following data were collected from the included studies: the first author’s name, publication years, locations, study design, sample size, disease stage, first- and second-line chemotherapy regimens, and radiotherapy; The clinical outcomes, including: (1) response rate, defined as the sum of complete and partial response rates according to the Response Evaluation Criteria in Solid Tumors [12]; (2) six-month and one-year overall survival rate (OS), defined as the time from random assignment to death from any cause, censoring patients who had not died at the date last known alive; (3) toxicity, defined as Grade 3 and 4 adverse events caused by chemotherapy according to the National Cancer Institute Common Terminology Criteria for Adverse Events [13].
Qualitative evaluation
We used the Newcastle-Ottawa Quality Assessment Scale (NOS) to evaluate the quality of the randomized controlled trials that were included in our meta-analysis [14,15]. According to the NOS, the studies were accessed in four broad aspects: selection (four criteria, one star for each), comparability (one criteria, one star), exposure (one criterion, two stars), and outcome (two criteria, one star for each). The total score ranged from 0 to 9. Articles that garnered five stars or more were considered high-quality studies, and only these papers were included in our meta-analysis.
Statistical analysis
The meta-analysis was spontaneously performed using STATA 11.0 (STATA Corporation, College Station, TX, USA). The results of each randomized controlled trial were treated as dichotomous frequency data. For all the outcomes of interest, event numbers were extracted from each individual study, and odds ratios (ORs) and 95% confidence interval (CI) were calculated before data pooling. For time-to-event data, the method proposed by Parmar et al. [16] were using to calculate event numbers from the survival curves when they were not reported. The pooled ORs and 95% CI were used to estimate the targeted events of other single-drug schedules compared with topotecan regimen.
Between-study heterogeneity was determined by the χ 2-based Q statistic and I 2 statistic inconsistency, P < 0.05 for χ 2 or I 2 value of 50% or more represented substantial heterogeneity [17]. A random-effect model based on the method of DerSimonian and Laird was applied when significant heterogeneity existed; otherwise, a fixed-effect model based on the Mantel-Haenszel method was used [18].
Sensitivity analysis was conducted by removing one individual study each time to estimate the robustness of the meta-analysis outcomes. Visual inspection of a funnel plot, Egger’s linear regression test, and Begg’s adjusted rank correlation test were performed to assess publication bias [19]. Two-sided P < 0.05 was considered statistically significance.
Results
Eligible studies and main characteristics
A total of 1369 articles were identified from our network retrieval, and only six trials [20-25] with a total sample of 893 patients were eligible for our meta-analysis according to the inclusion and exclusion criteria. The detailed steps of our literature search are shown in Figure 1.
Figure 1.
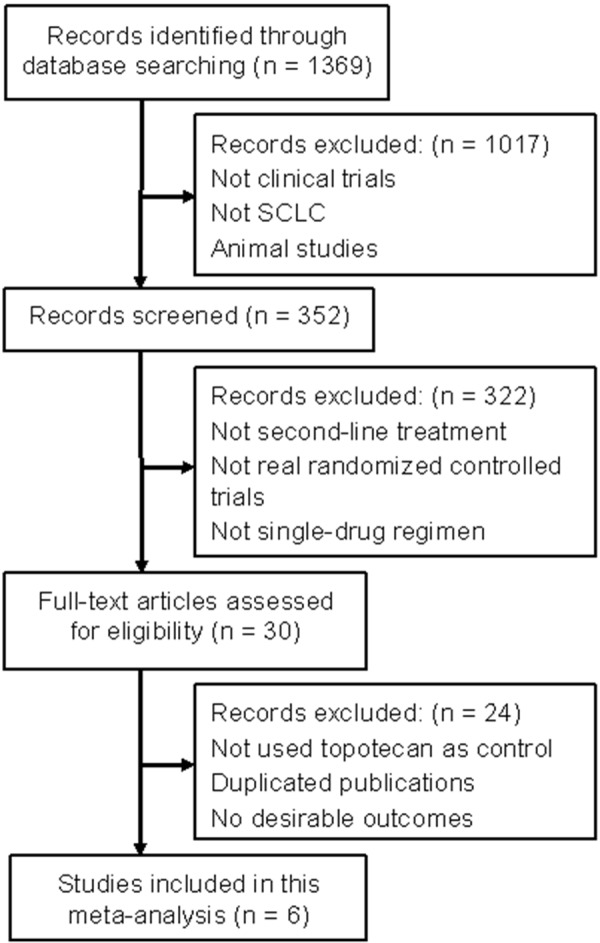
Flow diagram of study selection process and specific reasons for exclusion in the meta-analysis.
All the six articles with a sample size ranged from 32 to 637 were published from 2008 to 2014. Three of them were originated in China [23-25], and the rest in Netherlands [20], America [21], and Japan [22]. Five of them were designed in a phase II study [21-25], and the other one was in phase III [20]. Four of the 6 eligible studies contained both extensive- and limited-disease SCLC [20-22,25], and the other two only focused on extensive-disease SCLC [23,24]. All patients in the six studies had been treated with first-line platinum-based chemotherapy, and part of them had experienced radiotherapy. Three trials used armubisin in the observation group as second-line treatment [20-22], two used irinotecan [23,24], and only one used etoposide [25]. According to the quality assessment, all the trials were of high quality (had scores of five or more).
The main characteristics of the included studies are listed in Table 1, and the main outcomes of the studies through stratification by different drug schemes are showed in Table 2.
Table 1.
Main characteristics of the included studies
| Author (year) | Location | Study design | No. pts (exp/ctr) | Disease stage | SLT schedules | Course of treatment | FLT schedules | Prior to RT (%) | Score |
|---|---|---|---|---|---|---|---|---|---|
| von Pawel J (2014) | Netherlands | Phase III | 424/213 | extensive, limited | AMR 40 mg/m2 IV d1~3, TPT 1.5 mg/m2 IV d1~5, 21 day for cycle | 6 cycles or until PD | platinum-based CT | 48.0% | 7 |
| Jotte R (2011) | America | Phase II | 50/26 | extensive, limited | AMR 40 mg/m2 IV d1~3, TPT 1.5 mg/m2 IV d1~5, 21 day for cycle | until PD | platinum-based CT | NR | 6 |
| Inoue A (2008) | Japan | Phase II | 29/30 | extensive, limited | AMR 40 mg/m2 IV d1~3, TPT 1.0 mg/m2 IV d1~5, 21 day for cycle | At least 3 cycles or until PD | platinum + VP-16 or CPT-11 | 52.5% | 6 |
| Zhu Z (2013) | China | Phase II | 24/22 | extensive | CPT-11 300mg/m2 IV d1, TPT 1.5 mg/m2 IV d1~5, 21 day for cycle | 2~6 cycles | VP-16 + cisplatin | NR | 5 |
| Zhao ML (2011) | China | Phase II | 22/21 | extensive | CPT-11 300 mg/m2 IV d1, TPT 1.25~1.5 mg/m2 IV d1~5, 21 day for cycle | 2~6 cycles | VP-16 + platinum | 37.2% | 6 |
| Liu YY (2013) | China | Phase II | 18/14 | extensive, limited | VP-16 75 mg/m2 IV d1~5, TPT 1.20 mg/m2 IV d1~5, 21 day for cycle | 2 cycles | VP-16 + platinum | NR | 5 |
NR: none reported; exp: experimental group; ctr: contral group; SLT: second line treatment; FLT: first line treatment; IV: intravenously; PD: progressive disease; CT: chemotherapy; RT: radiotherapy; AMR: amrubixin; TPT: topotecan; CPT-11: irinotecan; VP-16: etoposide.
Table 2.
Main outcomes of the eligible studies
| Strategy | Study (year) | Response rate (events/total) (exp vs. ctr) | Six-month OS (events/total) (exp vs. ctr) | One-year OS (events/total) (exp vs. ctr) |
|---|---|---|---|---|
| AMR vs. TPT | von Pawel J (2014) | 132/424 vs. 36/213 | 254/424 vs. 128/213 | 115/424 vs. 53/213 |
| Jotte R (2011) | 22/50 vs. 4/26 | 26/44 vs. 10/19 | 16/44 vs. 6/19 | |
| Inoue A (2008) | 11/29 vs. 4/30 | 18/29 vs. 20/30 | 7/29 vs. 11/30 | |
| CPT-11 vs. TPT | Zhu Z (2013) | 8/24 vs. 6/22 | 3/24 vs. 2/22 | 2/24 vs. 0/22 |
| Zhao ML (2011) | 7/22 vs. 6/21 | 13/22 vs. 11/21 | 0/22 vs. 1/21 | |
| VP-16 vs. TPT | Liu YY (2013) | 3/18 vs. 2/14 | 17/18 vs. 12/14 | 16/18 vs. 11/14 |
OS: overall survival rate; exp: experimental group; ctr: control group; AMR: amrubixin; TPT: topotecan; CPT-11: irinotecan; VP-16: etoposide.
Pooled outcomes of response rate
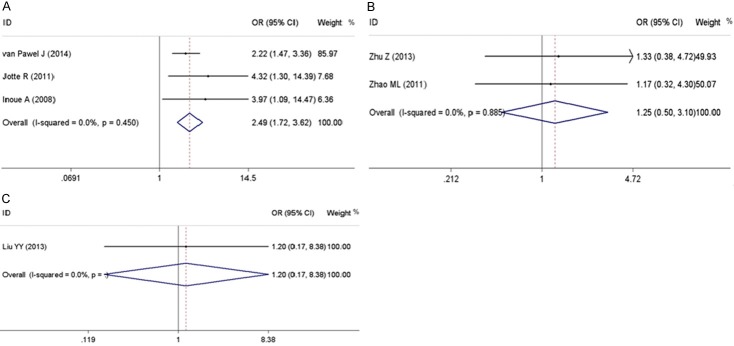
The response rates were reported by all of the six included trials, which were ranged from 16.7% to 44.0% in the non-topotecan group, and 13.3% to 28.6% in the topotecan group. After stratification by different chemotherapy regimens, only amrubisin revealed a better response rate than topotecan (OR = 2.50; 95% CI, 1.72 to 3.62, P = 0.001; fixed-effect model; Table 3) with data from 3 trials on 772 patients. When irinotecan and etoposide were taken into comparison with topotecan, there was no statistical significance found on the response rate (Table 3). No heterogeneity existed among the studies for these outcomes. A forest plot for response rate in different comparisons of non-topotecan regimens versus topotecan is shown in Figure 2.
Table 3.
Main results of the meta-analysis for response rate, six-month OS, and one-year OS
| Categories | Outcome | No. (cases) | OR (95% CI) | Z | P | I2 (%) | P h |
|---|---|---|---|---|---|---|---|
| AMR vs. TPT | Response rate | 3 (772) | 2.50 (1.72~3.62) | 4.80 | 0.001 | 0.0 | 0.450 |
| Six-month OS | 3 (759) | 1.00 (0.74~1.36) | 0.01 | 0.989 | 0.0 | 0.834 | |
| One-year OS | 3 (759) | 0.95 (0.68~1.34) | 0.28 | 0.778 | 0.0 | 0.490 | |
| CPT-11 vs. TPT | Response rate | 2 (89) | 1.25 (0.50~3.10) | 0.48 | 0.630 | 0.0 | 0.885 |
| Six-month OS | 2 (89) | 0.97 (0.37~2.53) | 0.06 | 0.955 | 42.4 | 0.188 | |
| One-year OS | 2 (89) | 0.70 (0.11~4.36) | 0.38 | 0.705 | 33.3 | 0.221 | |
| VP-16 vs. TPT | Response rate | 1 (32) | 1.20 (0.17~8.38) | 0.18 | 0.854 | - | - |
| Six-month OS | 1 (32) | 0.35 (0.03~4.35) | 0.81 | 0.416 | - | - | |
| One-year OS | 1 (32) | 0.46 (0.07~3.21) | 0.79 | 0.432 | - | - |
All pooled ORs were derived from fixed-effect model except for cells marked with (randomR). AMR: amrubixin; TPT: topotecan; CPT-11: irinotecan; VP-16: etoposide; OS: overall survival rate; OR: odds rate; P: P value for statistical significance based on Z test; P H: P value for heterogeneity based on Q test; -: unable to calculate.
Figure 2.
Forest plots of meta-analysis results for response rates. A: Forrest plot to assess the response rate when amrubicin vs. topotecan. B: Forrest plot to assess the response rate when irinotecan vs. topotecan. C: Forrest plot to assess the response rate when etoposide vs. topotecan.
Pooled outcomes of OS
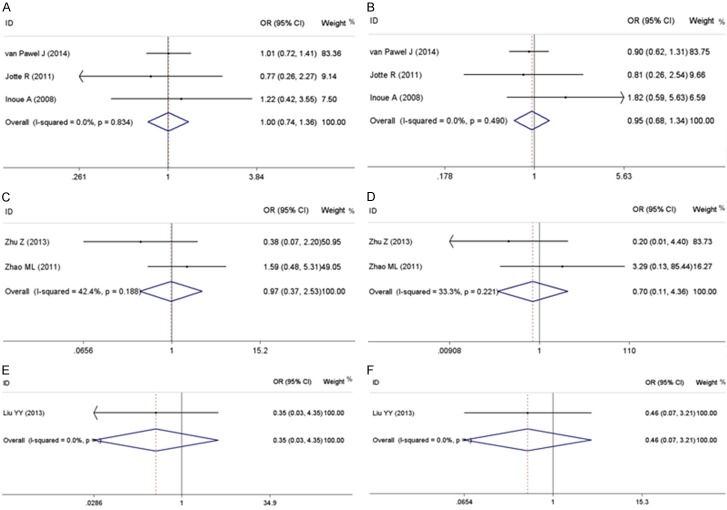
When the studies targeted on six-month or one-year OS, no significant advantage was shown when the other single-drug regimen was used in the second-line chemotherapy compared with topotecan. No extreme heterogeneity existed among these studies for these indexes. The main results for these outcomes are revealed in Table 3, and forest plots for these outcomes in different schedules are shown in Figure 3.
Figure 3.
Forest plots of meta-analysis results for overall survival rate. A: Forrest plot to assess the six-month overall survival rate when amrubicin vs. topotecan. B: Forrest plot to assess the one-year overall survival rate when amrubicin vs. topotecan. C: Forrest plot to assess the six-month overall survival rate when irinotecan vs. topotecan. D: Forrest plot to assess the one-year overall survival rate when irinotecan vs. topotecan. E: Forrest plot to assess the six-month overall survival rate when etoposide vs. topotecan. F: Forrest plot to assess the one-year overall survival rate when etoposide vs. topotecan.
Pooled outcomes of toxicity
Considerable variability of grade III/IV toxicity during the second-line chemotherapy was found among the included trials. In summary, armubisin showed a significant advantage in reducing anemia (OR = 0.48; 95% CI, 0.33 to 0.68, P < 0.001; fixed-effect model; Table 4), neutropenia (OR = 0.63; 95% CI, 0.45 to 0.86, P = 0.004; fixed-effect model; Table 4), and thrombocytopenia (OR = 0.26; 95% CI, 0.19 to 0.37, P < 0.001; fixed-effect model; Table 4). However, it explored some faults in promoting febrile neutropenia (OR = 3.10; 95% CI, 1.49 to 6.42, P = 0.002; fixed-effect model; Table 4), pneumonia (OR = 2.39; 95% CI, 1.04 to 5.49, P = 0.040; fixed-effect model; Table 4), and infections (OR = 1.94; 95% CI, 1.18 to 3.20, P = 0.009; fixed-effect model; Table 4). When irinotecan was used in the second-line treatment of SCLC, it protected against leucopenia (OR = 0.39; 95% CI, 0.15 to 0.98, P = 0.044; fixed-effect model; Table 4). When etoposide was taken into consideration, there was no significant difference between the incidences of toxicity. No between-study heterogeneity was found in the toxicity analyses. A summary of WHO grade III or greater drug-related toxicities is shown in Table 4.
Table 4.
Main results of the meta-analysis for Grade 3~4 toxicity
| Strategy | Outcomes | No. of studies (patients) | OR (95% CI) | P | I2 (%) | PH |
|---|---|---|---|---|---|---|
| AMR vs. TPT | Anemia | 3 (736) | 0.48 (0.33~0.68) | <0.001 | 0.0 | 0.607 |
| Leukopenia | 2 (677) | 0.69 (0.46~1.02) | 0.065 | 0.0 | 0.447 | |
| Neutropenia | 3 (736) | 0.63 (0.45~0.86) | 0.004 | 37.7 | 0.201 | |
| Thrombocytopenia | 3 (736) | 0.26 (0.19~0.37) | <0.001 | 40.3 | 0.187 | |
| Febrile neutropenia | 3 (736) | 3.10 (1.49~6.42) | 0.002 | 0.0 | 0.496 | |
| Dyspnea | 2 (677) | 0.74 (0.37~1.48) | 0.400 | 0.0 | 0.363 | |
| Fatigue | 3 (736) | 0.92 (0.57~1.48) | 0.727 | 0.9 | 0.365 | |
| Hyponatremia | 2 (677) | 0.96 (0.47~1.96) | 0.912 | 0.0 | 0.719 | |
| Pneumonia | 3 (736) | 2.39 (1.04~5.49) | 0.040 | 0.0 | 0.804 | |
| Infections | 3 (736) | 1.94 (1.18~3.20) | 0.009 | 0.0 | 0.656 | |
| CPT-11 vs.TPT | Leukopenia | 2 (89) | 0.39 (0.15~0.98) | 0.044 | 0.0 | 0.974 |
| Anemia | 2 (89) | 0.95 (0.06~16.28) | 0.973 | 0.0 | 0.751 | |
| Vomiting | 2 (89) | 0.61 (0.10~3.80) | 0.591 | 0.0 | 0.683 | |
| Delayed diarrhea | 2 (89) | 1.59 (0.20~12.47) | 0.660 | 0.0 | 0.588 | |
| Fatigue | 2 (89) | 0.61 (0.10~3.84) | 0.597 | 0.0 | 0.715 | |
| VP-16 vs. TPT | Neutropenia | 1 (32) | 0.71 (0.14~3.56) | 0.681 | - | - |
| Myelosuppression | 1 (32) | 0.31 (0.05~2.03) | 0.223 | - | - | |
| Gastrointestinal reaction | 1 (32) | 4.39 (0.20~99.23) | 0.352 | - | - |
All pooled ORs were derived from fixed-effect model except for cells marked with (randomR). AMR: amrubixin; TPT: topotecan; CPT-11: irinotecan; VP-16: etoposide; PH: P value for heterogeneity based on Q test; P: P value for statistical significance based on Z test; -: unable to calculate.
Sensitivity analysis and publication bias
Sensitivity analyses were performed by individually removing each trial in one time and estimating the effects of the remaining studies to evaluate the robustness of the results. No individual study dominated the overall OR estimate for response rate, six-month OS or one-year OS. The results of Begg’s and Egger’s test provided negligible evidence of publication bias for all the pooled outcomes. The shapes of funnel plots also did not show any obvious asymmetry, which indicated that there was no publication bias in each test for each endpoint analysis.
Discussion
Although SCLC is sensitive to chemotherapy or radiotherapy, most patients suffer from relapse within 2 years after the completion of first-line therapy because of the emergence of drug-resistant cancer cells during the induction therapy or the existence of such cells before chemotherapy [26]. About 80% of limited-disease patients and nearly all patients with extensive-stage disease will develop disease relapse or progression, and long-term survival is quite uncommon [27]. Without any second-line therapy, most SCLC patients relapse with relatively resistant disease share a short OS of only 2-3 months. Although a large number of chemotherapy was evaluated in clinical trials and some have shown a promising activity, no evidence-based standard treatment has been established for second line chemotherapy in this setting [28]. Furthermore, the results of second-line chemotherapy against SCLC are disappointing, with relatively low response rates, brief remissions, and a short survival time [29,30]. Against this background, there is a desperate need for the development of novel active drugs. Therefore, we conducted a meta-analysis which is useful to integrate results from independent studies for a specified outcome [31].
Topotecan is a semi-synthetic, water-soluble analog of camptothecin with specific targeting to the nuclear enzyme topoisomerase I. Inhibition of topoisomerase I produces irreversible DNA damage during the course of DNA replication, which could not be effectively repaired in the cells of mammals, and ultimately suppress tumor cell proliferation [32]. In 1999, Von Pawel et al. [8] conducted the first phase I I III study in patients with recurrent SCLC, in which CAV regimen were compared. This study showed the patients who experienced a symptoms improvement in the topotecan arm rather than in the CAV arm for four of eight symptoms estimated, including dyspnea, anorexia, hoarseness and fatigue, as well as interference with daily activity. Since then, topotecan is used more and more widely all over the world, and is proved to the drug with acknowledged efficacy in the treatment of SCLC. At present, topotecan is approved by more than 30 countries for the usage in second-line treatment of SCLC, including America, Canada, Swiss, and China, with a recommended dose of 1.5 mg/(m2.d), intravenous d1~d5, 21 days for a cycle [33]. However, according to the results of current researches, topotecan seems to have limited efficacy in relapse or refractory disease, producing overall response rate of 4% to 12%, and yields a minimal survival benefit with a median survival of 3~5 months [34,35]. Therefore, some other chemotherapy drugs have been researched to seek an alternative.
In this meta-analysis, 6 trials that covered three chemotherapeutics were studied as a comparison with topotecan. Based on the pooled outcomes of the published studies, no advantage was found in response rate, six-month and one-year OS, except for amrubicin in the response rate. This result indicated that amrubicin had demonstrable activity and showed a higher response rate comparable to that of topotecan in patients with relapse or refractory SCLC. Although amrubicin did not show any superiority in survival according to our meta-analysis, but it achieved a high response rate of 46% to 51% and a relatively long median OS of 9.2 to 10.3 months in some cohort studies, which were noteworthy [26,36,37]. Therefore, amrubicin is a promising topoisomerase II inhibitor, and may be a potential substitute medication for topotecan that is active in second-line therapy of SCLC.
Irinotecan is a potent inhibitor of DNA topoisomerase I, and has been widely investigated in the second-line management of patients with extensive stage SCLC [38]. In some phase II trials, irinotecan has been reported to be active in recurrent patients [38,39]. However, these studies were mainly designed in cohort study, and irinotecan was used in combination with platinum compounds. Although two RCTs were included in our meta-analysis, and the pooled outcomes show no significant difference between irinotecan and topotecan, the results should be interpreted by caution, because of the small sample size and lack of evidence of phase III study. Etoposide was not a common therapeutics in second-line treatment of SCLC, of which the published reports were rare. At present, there was only one RCT reported its clinical effect, so we were failed to conduct data synthesis. Therefore, further prospective RCTs are warranted to explore its potential efficacy and toxicity in second-line therapy of SCLC.
Toxicity is an important indicator for evaluating the safety and tolerance of pharmaceuticals. It is acknowledged that the dose-limited toxicity of topotecan is mainly myelosuppression [7]. From the comparison collected from the eligible cases, some toxicity was found to be more severe in patients who received the single-topotecan treatment when compared with amrubicin, mainly on hematologic toxicity. By contract, amrubicin may increase the incidence of nonhematologic toxicity (e.g. febrile neutropenia, pneumonia, infection), which is rarely happened in the topotecan therapy. However, they can be reversed by related symptomatic treatment (e.g. prophylactic growth factor, early use of antibiotics), and did not lead to a higher mortality, which was also supported by the composite survival results of our meta-analysis [20,21]. In addition, when irinotecan and etoposide were taken into comparison, they did not show any advantage in controlling the adverse events of monotherapy. To sum up, amrubicin showed a safety profile on reducing the risk of hematologic toxicity caused by cytotoxic drugs.
There were 6 RCTs included in this meta-analysis, which might with high probability of bias. But due to the specificity of tumor chemotherapy, it is hard to adopt hiding and blinding method of randomization designed study. Therefore, for the clinical researches on treating cancer with chemotherapeutics, these RCTs can still be regarded as high quality. In addition, some limitations exist in this meta-analysis. First, due to the small sample size of only six studies with a total of 893 patients, the results should be interpreted cautiously. Second, our analysis is not based on individual patient data (IPD) which would give more reliable estimation than one based on abstracted data, but is not always practical [40]. Therefore, this meta-analysis based on published group data may lead to an overestimation of treatment effects. Third, the therapeutic efficacy of second-line treatment for SCLC should be regarded as a comprehensive response of various factors, including response to first-line treatment, and time interval between the end of first-line treatment and tumor recurrence, residual toxicity to first-line therapy and performance status. Unfortunately, we were unable to explore this information.
In conclusion, although the second-line treatment of SCLC has been developed for several decades, there is still no approved treatment option for it currently. From our data of meta-analysis based on the current evidence, amrubicin seems to be superior to topotecan in terms of response rates, with a lower toxicity than topotecan, which indicated that amrubicin is of high value in clinical application, and may be the direction of second-line monotherapy in the future. However, a single-drug regimen is not “one size fits all” due to the complexity and diversity of the clinical patients. Thus, more studies are needed to identify the best scheme from which specific patients could get the most benefit.
Disclosure of conflict of interest
None.
References
- 1.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United State over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt JR, Bentsion DL, Lipatov ON, Polyakov IS, MacKintosh FR, Karlin DA, Baker GS, Breitz HB. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J. Clin. Oncol. 2009;27:2046–2051. doi: 10.1200/JCO.2008.19.3235. [DOI] [PubMed] [Google Scholar]
- 3.Kallianos A, Rapti A, Zarogoulidis P, Tsakiridis K, Mpakas A, Katsikogiannis N, Kougioumtzi I, Li Q, Huang H, Zaric B, Perin B, Courcoutsakis N, Zarogoulidis K. Therapeutic procedure in small cell lung caner. J Thorac Dis. 2013;5:S420–424. doi: 10.3978/j.issn.2072-1439.2013.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thatcher N, Faivre-Finn C, Lorigan P. Management of small-cell lung cancer. Ann Oncol. 2005;16(Suppl 2):ii235–239. doi: 10.1093/annonc/mdi700. [DOI] [PubMed] [Google Scholar]
- 5.Miao Q, Jiang LY. The study progress of second-line chemotherapy of small cell lung cancer. Chin J Lung Cancer. 2008;11:591–594. doi: 10.3779/j.issn.1009-3419.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Owonikoko TK, Behera M, Chen ZJ, Bhimani C, Curran WJ, Khuri FR, Ramalingam SS. A systematic analysis of efficacy of second line chemotherapy in sensitive and refractory small cell lung cancer. J Thorac Oncol. 2012;7:866–872. doi: 10.1097/JTO.0b013e31824c7f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang AM, Cai X, Liu JW. Advance in the second-line treatment of small cell lung cancer. Chin Clin Oncol. 2011;16:1134–1138. [Google Scholar]
- 8.von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, Carmichael J, Krebs JB, Ross G, Lane SR, Gralla R. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J. Clin. Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: small cell lung cancer (version 2, 2013) http://www.nccn.org/professional/physician_gls/f_guidelines.asp.
- 10.Song Z, Shao L, Lin B, Zhang Y. Single-agent chemotherapy compared with combination chemotherapy as second-line treatment in extensive-stage small cell lung cancer: a retrospective analysis. Clin Transl Oncol. 2013;15:843–848. doi: 10.1007/s12094-013-1013-5. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauser EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP, Bruner DW, Cleeland CS, Sloan JA, Chilukuri R, Baumgartner P, Denicoff A, St Germain D, O’Mara AM, Chen A, Kelaghan J, Bennett AV, Sit L, Rogak L, Barz A, Paul DB, Schrag D. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute Wed Site. 2012 [Google Scholar]
- 15.Maxwell L, Santesso N, Tugwell PS, Well GA, Judd M, Buchbinder R. Method guidelines for Cochrane Musculoskeletal Group systematic reviews. J Rheumatol. 2006;33:2304–2311. [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel E. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 19.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Pawel J, Jotte R, Spigel DR, O’Brien ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts K, Trigo JM, Clingan P, Schütte W, Lorigan P, Reck M, Domine M, Shepherd FA, Li S, Renschler MF. Randomized phase III trial of amrubisin versus topotecan as second-line treatment for patients with small-cell lung cancer. J. Clin. Oncol. 2014;32:4012–9. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]
- 21.Jotte R, Conkling P, Reynolds C, Galsky MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF, Oliver JW. Randomized phase II trial of single-agent amrubisin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J. Clin. Oncol. 2011;29:287–293. doi: 10.1200/JCO.2010.29.8851. [DOI] [PubMed] [Google Scholar]
- 22.Inoue A, Sugawara S, Yamazaki K, Maemondo M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, Watanabe H, Tsukamoto T, Saijo Y, Ishimoto O, Hommura F, Nukiwa T. Randomized phase II trial comparing amrubisin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J. Clin. Oncol. 2008;26:5401–5406. doi: 10.1200/JCO.2008.18.1974. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Ni BQ, Chen RX, Xu YA. Comparative study on the efficacy of irinotecan or topotecan as a second-line monotherapy for small-cell lung cancer. Jinlin Med. 2013;34:5367–5369. [Google Scholar]
- 24.Zhao ML, Bi Q, Ren HX, Tian Q, Bao ML. Clinical observation of irinotecan or topotecan as second-line chemotherapy on treating 43 patients with small-cell lung cancer. Chin Oncol. 2011;21:156–158. [Google Scholar]
- 25.Liu YY, Xie XD, Zheng ZD, Zhang GJ. Clinical efficacy of etoposide compared with topotecan as second-line treatment on elder patients with small-cell lung cancer. Chin J Geron. 2013;33:2000–2001. [Google Scholar]
- 26.Onoda S, Masuda N, Seto T, Eguchi K, Takiguchi Y, Isobe H, Okamoto H, Ogura T, Yokoyama A, Seki N, Asaka-Amano Y, Harada M, Tagawa A, Kunikane H, Yokoba M, Uematsu K, Kuriyama T, Kuroiwa Y, Watanabe K Thoracic Oncology Research Group Study 0301. Phase II trial of amrubicin for treatment of refractory or relaosed small-cell lung cancer: thoracic oncology research group study 0301. J. Clin. Oncol. 2006;24:5448–5453. doi: 10.1200/JCO.2006.08.4145. [DOI] [PubMed] [Google Scholar]
- 27.Tiseo M, Ardizzoni A. Current status of second-line treatment and novel therapies for small cell lung cancer. J Thorac Oncol. 2007;2:764–772. doi: 10.1097/JTO.0b013e3180986262. [DOI] [PubMed] [Google Scholar]
- 28.Sgambato A, Casaluce F, Maione P, Rossi A, Sacco PC, Panzone F, Ciardiello F, Gridelli C. Medical treatment of small cell lung cancer: state of the art and new development. Expert Opin Pharmacother. 2013;14:2019–2031. doi: 10.1517/14656566.2013.823401. [DOI] [PubMed] [Google Scholar]
- 29.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 30.Eckard JR. Second-line treatment of small-cell lung cancer: the case for systemic chemotherapy. Oncology. 2003;17:181–188. 191. discussion 191-192. [PubMed] [Google Scholar]
- 31.Wu JY, Hu LR, Wu FP, He TP. Prognostic value of rsf-1/hbxap in human solid tumors: a meta-analysis of cohort studies. Int J Clin Exp Med. 2015;8:1944–1955. [PMC free article] [PubMed] [Google Scholar]
- 32.von Pawel J. The role of topotecan in treating small cell lung cancer: second-line treatment. Lung Cancer. 2003;41(Suppl 4):S3–8. doi: 10.1016/s0169-5002(03)90519-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang XS, Wu TX, Hou M. Topotecan for small cell lung cancer: a systematic review. Chin J Evid-based Med. 2007;7:190–203. [Google Scholar]
- 34.Schmittel A. Second-line therapy for small-cell lung cancer. Expert Rev Anticancer Ther. 2011;11:631–637. doi: 10.1586/era.11.7. [DOI] [PubMed] [Google Scholar]
- 35.Ettinger DS, Jotte R, Lorigan P, Gupta V, Garbo L, Alemany C, Conkling P, Spigel DR, Dudek AZ, Shah C, Salgia R, McNally R, Renschler MF, Oliver JW. Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J. Clin. Oncol. 2010;28:2598–2603. doi: 10.1200/JCO.2009.26.7682. [DOI] [PubMed] [Google Scholar]
- 36.Igawa S, Yamamoto N, Ueda S, Ono A, Nakamura Y, Tsuya A, Murakami H, Endo M, Takahashi T. Evaluation of the recommended dose and efficacy of amrubicin as second- and third-line chemotherapy for small cell lung cancer. J Thora Oncol. 2007;2:740–744. doi: 10.1097/JTO.0b013e31811f46f0. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Mio T, Masago K, Irisa K, Sakamori Y, Mishima M. Retrospective analysis of Japanese patients with relapse or refractory small-cell lung cancer treated with amrubicin hydrochloride. Oncol Lett. 2010;1:569–572. doi: 10.3892/ol_00000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sevinc A, Kalender ME, Altinbas M, Ozkan M, Dikilitas M, Camci C Anatolian Society of Medical Oncology (ASMO) Irinotecan as a second-line monotherapy for small cell lung cancer. Asian Pac J Cancer Prev. 2011;12:1055–1059. [PubMed] [Google Scholar]
- 39.Morise M, Niho S, Umemura S, Matsumoto S, Yoh K, Goto K, Ohmatsu H, Ohe Y. Low-dose irinotecan as a second-line chemotherapy for recurrent small cell lung cancer. Jpn J Clin Oncol. 2014;44:846–851. doi: 10.1093/jjco/hyu094. [DOI] [PubMed] [Google Scholar]
- 40.Pignon JP, Hill C. Meta-analyses of randomized clinical trials in oncology. Lancet Oncol. 2001;2:475–482. doi: 10.1016/S1470-2045(01)00453-3. [DOI] [PubMed] [Google Scholar]