Abstract
Azole resistance in Candida albicans is frequently caused by the overexpression of multi-drug efflux pump genes MDR1, CDR1, and CDR2 due to gain-of-function mutations in the zinc cluster transcription factors Mrr1p and Tac1p. In this study, we performed a comparative proteomic analysis to identify proteins whose expression level is influenced by these transcription factors. Both 2-DE and PMF were used to examine the expression profiles of six pairs of matched C. albicans isolates carrying gain-of-function mutations in either MRR1 or TAC1 resulting in the overexpression of either MDR1 or CDR1 and CDR2. Using this approach, 17 differentially expressed proteins were identified in the MDR1-overexpressing isolates, while 14 were identified in the isolates that overexpress CDR1 and CDR2. Furthermore, we found that the expression of many of these proteins was increased in a wild-type strain of C. albicans after the introduction of a gain-of-function allele of MRR1 or TAC1. Moreover, disruption of MRR1 and TAC1 in isolates carrying gain-of-function mutations resulted in decreased expression of these proteins, confirming their regulation by Mrr1p or Tac1p. Several proteins involved in heat shock and carbohydrate metabolism were differentially expressed in all clinical isolate sets, but these proteins were not dependent upon either Tac1p or Mrr1p.
Keywords: Azole resistance, Candida albicans, drug efflux pumps, fluconazole
1. Introduction
The opportunistic human fungal pathogen C. albicans is present on mucosal surfaces of the gastrointestinal and urogenital tracts in many healthy individuals with no clinical symptoms; however, this fungus is also a major cause of both superficial and systemic infections, including oropharyngeal candidiasis (OPC), the most frequently observed infection among patients with acquired immune deficiency syndrome (AIDS) [1, 2]. Treatment of these infections with the antifungal agent fluconazole is effective; however, prolonged exposure to this drug can lead to the emergence of azole-resistant strains of C. albicans and ultimately therapeutic failure [3–5].
The azole antifungals specifically inhibit the biosynthesis of ergosterol, the major sterol within the fungal membrane, by irreversibly binding to the heme group in the active site of the ERG11 gene product and azole target, 14α-lanosterol demethylase [6]. To date several mechanisms of azole resistance have been characterized in C. albicans [7–13]. First, point mutations in ERG11 can cause reduced affinity of Erg11p to azoles [8, 13]. Additionally, overexpression of ERG11 results in the increased production of 14α-lanosterol demethylase, requiring more azole for inhibition of its activity [14]. Moreover, azole resistance is also caused in part by insufficient levels of effective antifungal concentrations within the cell due to the constitutive overexpression of multi-drug efflux pump genes, such as the major facilitator superfamily gene, MDR1, as well as the ATP-binding cassette (ABC) transporter genes CDR1 and CDR2 [9–12, 14]. The up-regulation of MDR1 confers an azole-resistant phenotype specific for fluconazole, whereas overexpression of CDR1 and CDR2 causes azole resistance by mediating efflux of a variety of structurally diverse azoles out of the cell [7]. Furthermore, it has been shown that resistant strains of C. albicans isolated from patients with OPC possess a combination of distinct azole resistance mechanisms that can function synergistically, resulting in high-levels of fluconazole resistance [15].
Recent studies have implicated the zinc cluster transcription factors Mrr1p and Tac1p in the regulation of antifungal resistance genes MDR1, CDR1, and CDR2 in clinical isolates of C. albicans, respectively [16, 17]. These transcription factors both have zinc binuclear cluster Zn(2)-Cys(6) DNA-binding motifs and each presumably binds sequence specific response elements in the promoters of their target genes for transcriptional activation. Recent studies have shown that gain-of-function mutations in these transcription factors cause constitutive, hyperactive transcription of their target genes and confer multi-drug resistance [17, 18], but the specific mechanisms by which these mutations constitutively activate the function of these transcription factors is unknown. Moreover, while gene expression and location profiling studies have identified sets of genes that are targeted by these transcriptional regulators, their influence on protein expression has yet to be examined [17, 19].
In an effort to identify proteins that are coregulated with the multi-drug efflux pump genes MDR1, CDR1, and CDR2 upon activation of their respective transcriptional regulators, we examined six pairs of matched clinical isolates of C. albicans originally obtained from AIDS patients diagnosed with OPC who failed azole therapy. Previous studies have demonstrated that these azole-resistant isolates overexpress either MDR1 (isolates F5, G5, 6692) or CDR1 and CDR2 (isolates Gu5, C56, 5674) [11, 13, 20–22]. As overexpression of azole resistance genes in these resistant isolates is due to gain-of-function mutations in their respective transcription factors, we measured changes in protein expression between the azole-susceptible and azole-resistant isolates in each of these matched isolate pairs using a comparative proteomic approach to reveal the differentially expressed proteins co-regulated with either Mdr1p or Cdr1p and Cdr2p. In order to determine which differentially expressed proteins were influenced specifically by activation of Mrr1p or Tac1p, we examined the protein expression profiles of an MRR1 disruption mutant derived from clinical isolate F5 and a TAC1 disruption mutant derived from clinical isolate 5674. We also examined the protein expression profiles of two additional mutant strains, each containing a gain-of-function allele of either MRR1 or TAC1 introduced into a wild-type background. This investigation expands on previous proteomic studies in C. albicans and identifies proteins that are specifically co-regulated with known azole resistance genes and those that are influenced by Mrr1p or Tac1p. Our findings also provide unique insight into the correlation between gene and protein expression profiling as all of the isolate pairs examined here have previously been subjected to microarray analysis [17, 19]. It is possible that some of these differentially expressed proteins contribute directly to azole antifungal resistance.
2. Materials and Methods
2.1 Organisms and culture conditions
All strains used in this study are detailed in Table 1. The clinical C. albicans matched isolate pairs used in this study were originally obtained from AIDS patients diagnosed with OPC who failed azole therapy. Briefly, the matched isolate pairs (susceptible/resistant) used in this study overexpress MDR1 (F1/F5, G1/G5, and 5833/6692) or CDR1/CDR2 (Gu2/Gu5, C43/C56, and 5457/5674). Strains SC∆zcf36MK3A and SZY91 are derivatives of strain SC5314 in which the endogenous MRR1 and TAC1 alleles, respectively, were disrupted and gain-of-function alleles were introduced [17, 19]. MRR1 and TAC1 disruption mutants F5MRR1M4B and SZY31 were derived from clinical isolates F5 and 5674, respectively [17, 19]. In three independent experiments, C. albicans isolates were cultured overnight at 30°C with agitation in 10 mL of Yeast Peptone Dextrose (YPD, Sigma, St. Louis, MO) broth consisting of 1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) dextrose. For each isolate, cells were diluted into 500 mL of YPD broth to an OD600 of 0.2 and subsequently grown for 4.5 hours at 30°C to early logarithmic phase in a shaking incubator rotating at 250 rpm.
Table 1.
Description of C. albicans clinical isolates and mutant strains used in this study.
| C. albicans strain | Relevant Genotype or Description | Description of Mutations
|
Referenceor source | ||
|---|---|---|---|---|---|
| Protein | Mutation | Reference | |||
| F1 | Clinical isolate from patient F, fluconazole-susceptible | – | – | – | [10] |
| F5 | Clinical isolate from patient F, fluconazole-resistant | Mrr1p | P683S | [17] | [10] |
| G1 | Clinical isolate from patient G, fluconazole-susceptible | – | – | – | [10] |
| G5 | Clinical isolate from patient G, fluconazole-resistant | Mrr1p | G997V | [17] | [10] |
| 5833 | Clinical isolate, fluconazole-susceptible | – | – | – | [20] |
| 6692 | Clinical isolate, fluconazole-resistant | Mrr1p | T360I, K335N | [22] | [20] |
| Gu2 | Clinical isolate, fluconazole-susceptible | – | – | – | [11] |
| Gu5 | Clinical isolate, fluconazole-resistant | Tac1p | L979E | [Unpub. data] | [11] |
| C43 | Clinical isolate, fluconazole-susceptible | – | – | – | [12] |
| C56 | Clinical isolate, fluconazole-resistant | Tac1p | N977D | [18] | [12] |
| 5457 | Clinical isolate, fluconazole-susceptible | – | – | – | [20] |
| 5674 | Clinical isolate, fluconazole-resistant | Tac1p | N972D | [44] | [20] |
| SC5314 | Wild-type C. albicans model strain | – | – | – | [43] |
| SCΔzcf36MK3A | mrr1Δ::FRT/MRR1(F5)-caSAT1 | Mrr1p | P683S | [17] | [17] |
| SZY91 | tac1Δ::FRT/TAC1-2(5674)-MPAR-FLP | Tac1p | N972D | [44] | [44] |
| F5MRR1M4B | mrr1Δ::FRT/mrr1Δ::FRT | – | – | – | [17] |
| SZY31 | tac1Δ::FRT/tac1Δ::FRT | – | – | – | [44] |
2.2 Preparation of protein extracts
Early logarithmic C. albicans cells were collected by centrifugation at 3000 × g for 10 minutes. The cell pellets were washed three times with ultrapure water and broken with 0.5 mm diameter glass beads using a Mini-Bead Beater 8 (BioSpec Products, Inc., Bartlesville, OK) in cold 50 mM Tris buffer (pH 7.4) containing 1 mM EDTA and Complete protease inhibitor cocktail (Roche, Penzberg, Germany). Briefly, equal volumes of glass beads were added to cell pellets in 2 mL conical bottom tubes and beaten for six 20-second bursts. Between bursts, the samples were incubated on ice for one minute. After disruption, the samples were centrifuged at 20,000 × g for 15 minutes at 4°C to remove the glass beads, unbroken cells, and particulate debris from the homogenate. The supernatant was collected, and the protein content was quantitated using the bicinchoninic acid (BCA) method using BSA as a standard [23]. The protein samples were stored at −80°C for further studies.
2.3 2-DE
For 2-DE, samples containing 300 μg of total protein were processed using the 2-D cleanup kit according to the manufacturer’s protocol (Bio-Rad Laboratories, Inc, Hercules, CA). The resulting acetone-washed protein pellets were subsequently added to 300 μL of rehydration buffer consisting of 7 M urea, 2 M thiourea, 50 mM DTT, 4% (w/v) CHAPS, 0.6% (v/v) Bio-Lyte 3–10 ampholytes, and 0.002% (w/v) bromophenol blue. The samples were then subjected to IEF on a PROTEAN IEF Cell (Bio-Rad) over a pI range from four to seven using 17 cm ReadyStrip IPG strips (Bio-Rad) at 20°C. The IPG strips were subjected to passive rehydration for 12 hours and run at 250V for the first 15 minutes, followed by a linear increase in voltage to a maximum of 10,000V, where they ran for an additional five hours. After IEF, the IPG strips were reduced in equilibration solution consisting of 1.5 M Tris-HCl (pH 8.8), 7 M urea, 2% (w/v) SDS, 20% (v/v) glycerol, and 1% (w/v) DTT for 10 minutes followed by an alkylation step in the same equilibration buffer containing 1.5% (w/v) iodoacetamide instead of the DTT for 10 minutes. Proteins were subsequently separated in the second dimension based on their relative molecular mass using homogenous 12% acrylamide gels (20cm × 20cm 1mm) containing 2.6% N, N’-methylene-bis-acrylamide and run at a constant 200V for six to seven hours at 15°C in a PROTEAN® plus Dodeca™ Cell (Bio-Rad). After electrophoresis, the gels were fixed, washed, and stained with Sypro® Ruby.
2.4 Gel imaging and analysis software
2-D gels were imaged on an FX imager (BioRad) at the medium Sypro® Ruby intensity setting at a resolution of 300 dpi. Spot comparison of 2-D gel images was performed using PDQuest software version 7.1 (BioRad) to measure the differences in spot intensity. Protein spots were compared between gel images using an automated matching program, and any mismatched spots were corrected using a manual matching feature in the software. After background removal, the individual spot volume for each resolved protein spot was normalized against the total spot volume and area in the gel. Protein spots that were consistently differentially expressed in mean normalized spot volume by at least 1.5 fold (50%) in three independent experiments were selected for excision and subsequent identification. Statistical analysis was performed using a Student’s t-test.
2.5 Protein trypsinization and extraction
Protein spots were excised from gels and transferred to 1.5 mL microfuge tubes pre-washed three times with 50% methanol and 10% glacial acetic acid. Proteins were then destained twice in 200 μL of 50% acetonitrile at 23°C and vortexed for 30 minutes. The samples were subsequently placed in a 96-well Millipore PTFE 0.45 μm filter plate with the wells pretreated three times with 20% methanol and 0.1% TFA. Trypsinization of the proteins was carried out by adding 200 μL of neat acetonitrile to the wells. The solution was removed, and the samples dried under vacuum for one minute. Another 200 μL of acetonitrile was applied to the samples and removed by vacuum. The washed proteins were subsequently digested in a 100 μL aliquot of fresh digestion buffer containing 12.5 ng/μL ultrapure sequencing grade trypsin (Promega, Madison, WI), 25 mM ammonium bicarbonate, and 10% acetonitrile and incubated at 37°C overnight. The tryptic polypeptides were extracted in a 25 μL aliquot of fresh extraction solution containing 50% acetonitrile and 5% TFA.
2.6 Protein identification and database search
Protein identification was carried out according to the methods described by Cummings et al. 2007 [24]. Briefly, MS spectra were recorded on an Ultraflex matrix assisted laser desorption ionization reflecting time-of-flight (MALDI-ToF/ToF) mass spectrometer (Bruker Daltonics, Bremen, Germany). The autolytic trypsin fragment peaks of 842.509 m/z and 2211.104 m/z were used for internal calibration of peptide mass spectra with mass resolution between 2500 and 8000 m/z. External calibration of this instrument was carried out using a 2:1 mixture of des-R-bradykinin and ACTH (Sigma). Mass spectra parameter settings were 20 kV for the extraction voltage and 11.8 kV for the reflector voltage. Both FlexControl version 2.0 and FlexAnalysis version 2.0 software were used for recording the mass spectra data and sample analysis, respectively. For each sample, the S/N was calculated using the SNAP algorithm using the default settings within the FlexAnalysis software. For each protein identified, MALDI-TOF mass fingerprint data were blasted against a custom database (http://genolist.pasteur.fr/CandidaDB/) containing known C. albicans ORF DNA sequences using PROWL software. For each protein identified, there was a Z-score value calculated from the software that measured the significance of the identification. A Z-score of 1.65 ranked the mass data search to a ≥95% level of confidence of nonrandom matches to the specific ORF.
3. Results
A description of the C. albicans isolates used in this study is provided in Table 1. In three independent experiments, the combination of 2-DE and PMF revealed the identities of 31 differentially expressed proteins that were found to be coregulated with the overexpression of either MDR1 or CDR1 and CDR2 by at least 1.5-fold (Tables 2 and 3). The overexpression of proteins corresponding to these efflux pump genes was not detected by this approach as we analyzed only the soluble proteins. We report here the proteins that were found to be differentially expressed in at least two of the three pairs of matched isolate sets that overexpress either MDR1 or CDR1 and CDR2. A summary of all differentially expressed proteins in each of these matched sets can be found in the supplementary data section tables S1–S3 for proteins coregulated with Mdr1p and tables S4–S6 for those coregulated with Cdr1p and Cdr2p.
Table 2.
A summary of the differentially expressed proteins co-regulated with Mdr1p identified by 2-DE and MALDI-TOF MS
| Protein | Systematica Name |
Function | pI |
Mr (kDa) |
Z-scoreb | Protein coverage (%) |
Matched peptides |
Fold change |
Fold change |
Fold change |
t-test p-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| F5 | G5 | 6692 | F5 | G5 | 6692 | F5 | G5 | 6692 | F5/F1 | G5/G1 | 6692/5833 | F5 | G5 | 6692 | |||||
|
|
|||||||||||||||||||
| Eno1p | orf19.395 | Enolase (2-Phosphoglycerate dehydratase) | 5.5 | 47.2 | 2.4 | 2.4 | 2.4 | 21 | 61 | 53 | 7 | 20 | 16 | 2.1 ± 0.5 | 2.2 ± 0.7 | 2.4 ± 0.7 | 0.04 | 0.04 | 0.00 |
| Tdh3p | orf19.6814 | Glyceraldedyde-3-phosphate dehydrogenasef | 6.6 | 35.8 | 2.4 | 2.4 | 1.8 | 45 | 25 | 38 | 9 | 9 | 10 | 2.0 ± 1.9 | 1.9 ± 0.3 | 1.8 ± 0.4 | 0.21 | 0.05 | 0.09 |
| Gpx1p | orf19.86 | Glutathione peroxidasec | 6.6 | 18.1 | 2.4 | 2.4 | 2.4 | 35 | 41 | 39 | 7 | 8 | 8 | 3.7 ± 1.0 | 3.8 ± 0.3 | 3.1 ± 0.6 | 0.00 | 0.04 | 0.00 |
| Grp2p | orf19.4309 | Reductasec, d, f | 6.0 | 37.6 | 2.4 | 2.4 | 2.4 | 31 | 22 | 18 | 9 | 9 | 5 | 4.1 ± 0.9 | 4.4 ± 1.1 | 11.6 ± 0.8 | 0.00 | 0.05 | 0.01 |
| Grp2p | orf19.4309 | Reductasec, d, f | 6.0 | 37.6 | 2.3 | 2.4 | 2.4 | 28 | 35 | 55 | 8 | 13 | 22 | 5.5 ± 2.3 | 5.3 ± 0.9 | 13.4 ± 3.9 | 0.07 | 0.02 | 0.04 |
| Ifd1p | orf19.1048 | Putative aryl-alcohol dehydrogenasec, d, e | 5.6 | 39.1 | 2.4 | 1.8 | 2.2 | 42 | 28 | 21 | 15 | 8 | 9 | 10 ± 6.2 | 3.9 ± 0.4 | 6.4 ± 1.3 | 0.11 | 0.00 | 0.01 |
| Ifd4p | orf19.4477 | Putative aryl-alcohol dehydrogenasec, d, e | 6.0 | 38.3 | 2.4 | 2.4 | 2.3 | 37 | 46 | 31 | 15 | 19 | 8 | 10.1 ± 4.7 | 27.6 ± 16.2 | 2.1 ± 1.1 | 0.08 | 0.19 | 0.06 |
| Ifd5p | orf19.1048 | Putative aryl-alcohol dehydrogenasec, d, e | 5.4 | 39.2 | 2.2 | 2.4 | 2.4 | 31 | 33 | 33 | 9 | 12 | 8 | 4.4 ± 1.9 | 13.9 ± 6.2 | 6.9 ± 1.1 | 0.11 | 0.20 | 0.01 |
| Ipf5987p | orf19.7306 | Aldo-keto reductasec, e | 5.5 | 39.0 | 2.3 | 2.4 | 2.4 | 43 | 60 | 44 | 9 | 17 | 11 | 4.7 ± 0.8 | 201 ± 46 | 3.2 ± 0.3 | 0.02 | 0.00 | 0.00 |
| Oye32p | orf19.3131 | Putative NADPH-dependent flavin oxidoreductasec | 5.9 | 47.5 | 2.4 | 1.8 | 1.3 | 30 | 21 | 16 | 10 | 6 | 4 | 7.3 ± 2.5 | 5.4 ± 3.2 | 1.7 ± 0.3 | 0.00 | 0.04 | 0.01 |
| Adh4p | orf19.271 | 2,4-dienoyl-coenzyme A reductasec | 7.0 | 28.1 | 2.4 | 2.4 | 2.4 | 28 | 22 | 25 | 8 | 6 | 7 | 25.2 ± 5.3 | 4.8 ± 0.4 | 21.8 ± 7.5 | 0.02 | 0.00 | 0.00 |
| Ipf17186p | orf19.251 | Heat shock protein 31 of DJ-1/Pfpl familyc | 4.7 | 25.8 | 2.3 | 2.4 | 2.4 | 20 | 23 | 48 | 5 | 7 | 9 | 6.1 ± 0.6 | 4.5 ± 0.5 | 5.2 ± 0.6 | 0.00 | 0.00 | 0.00 |
| Ipf17186p | orf19.251 | Heat shock protein 31 of DJ-1/Pfpl familyc | 4.7 | 25.8 | 2.1 | 2.3 | 2.4 | 25 | 35 | 36 | 9 | 7 | 7 | 7.9 ± 0.2 | 10.8 ± 2.2 | 7.7 ± 1.2 | 0.04 | 0.00 | 0.01 |
| Adh1p | orf19.3997 | Alcohol dehydrogenasef | 8.6 | 46.1 | N/A | 2.4 | 2.3 | N/A | 39 | 42 | N/A | 14 | 13 | ND | 2 ± 0.6 | 1.7 ± 0.1 | N/A | 0.08 | 0.01 |
| Pdc11p | orf19.2877 | Pyruvate decarboxylase | 5.4 | 62.4 | N/A | 2.4 | 2.3 | N/A | 36 | 17 | N/A | 15 | 7 | ND | 1.7 ± 0.7 | 2.5 ± 1.7 | N/A | 0.19 | 0.16 |
| Ifd6p | orf19.4476 | Putative aryl-alcohol dehydrogenasec, d, e | 5.9 | 39.1 | 2.2 | 1.2 | N/A | 26 | 20 | N/A | 8 | 5 | N/A | 5.1 ± 4.9 | 6.0 ± 0.4 | ND | 0.15 | 0.00 | N/A |
| Gnd1p | orf19.5024 | 6-Phosphogluconate dehydrogenased | 6.1 | 56.9 | 2.4 | N/A | 2.4 | 18 | N/A | 33 | 8 | N/A | 18 | 0.4 ± 0.1 | ND | 0.4 ± 0.1 | 0.00 | N/A | 0.05 |
Systematic names are according to http://www.candidagenome.org.
Z-score values of 1.20 and 1.65 rank the mass data search to a ≥90% and ≥95% level of confidence of nonrandom matches to the specific open reading frame, respectively.
Genes that were found to be differentially expressed by microarray analysis [17].
Proteins previously shown to be differentially expressed in clinical isolates of C. albicans by Hooshdaran et al. 2004 [25].
Proteins differentially expressed in clinical isolates of C. albicans by Kusch et al. 2004 [21]
Proteins found differentially expressed between C. albicans strain SC5314S and SC5314R by Yan et al. 2007 [42] .
Proteins in bold were observed to be differentially expressed in C. albicans mutant SCΔzcf36MK3A.
ND-Not detected
N/A-Not applicable
Table 3.
A summary of the differentially expressed proteins co-regulated with Cdr1p and Cdr2p identified by 2-DE and MALDI-TOF MS.
| Protein | Systematica Name |
Function | pI |
Mr (kDa) |
Z-scoreb | Protein coverage (%) |
Matched peptides |
Fold change |
Fold change |
Fold change |
t-test p-value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| Gu5 | C56 | 5674 | Gu5 | C56 | 5674 | Gu5 | C56 | 5674 | Gu5/Gu2 | C56/C43 | 5674/5457 | Gu5 | C56 | 5674 | |||||
|
|
|||||||||||||||||||
| Eno1p | orf19.395 | Enolase (2-Phosphoglycerate dehydratase) | 5.5 | 47.2 | 2.4 | 2.0 | 2.3 | 49 | 21 | 40 | 18 | 7 | 15 | 2.0 ± 0.3 | 1.6 ± 0.6 | 2.2 ± 0.4 | 0.05 | 0.11 | 0.00 |
| Tdh3p | orf19.6814 | Glyceraldedyde-3-phosphate dehydrogenased, e | 6.6 | 35.8 | 2.4 | 2.3 | 2.1 | 29 | 18 | 46 | 8 | 6 | 10 | 1.7 ± 0.3 | 2.1 ± 1.1 | 2 ± 0.5 | 0.00 | 0.08 | 0.03 |
| Gpx1p | orf19.87 | Glutathione peroxidasec | 6.6 | 18.1 | 2.4 | 2.0 | 2.4 | 37 | 23 | 26 | 7 | 5 | 6 | 4.2 ± 1.3 | 12 ± 8 | 8.5 ± 1.5 | 0.00 | 0.05 | 0.01 |
| Ipf4065p | orf19.1862 | Unknown functionc | 5.2 | 13.1 | 2.3 | 1.7 | 2.2 | 56 | 47 | 50 | 6 | 4 | 4 | 2.0 ± 0.3 | 3.9 ± 1.5 | 2.2 ± 0.2 | 0.01 | 0.01 | 0.01 |
| Ipf15297p | orf19.3053 | Unknown functionc | 4.3 | 20.3 | 1.8 | 1.9 | 2.3 | 25 | 39 | 59 | 5 | 5 | 8 | 1.8 ± 0.2 | 6.1 ± 2.5 | 1.6 ± 0.1 | 0.00 | 0.01 | 0.01 |
| Pdc11p | orf19.2877 | Pyruvate decarboxylase | 5.4 | 62.4 | 2.4 | 2.4 | 2.4 | 31 | 34 | 35 | 14 | 14 | 11 | 2.2 ± 0.8 | 1.6 ± 0.2 | 2.3 ± 0.6 | 0.00 | 0.04 | 0.00 |
| Ssb1p | orf19.6367 | Heat shock protein 70 | 5.3 | 66.4 | 2.4 | 2.3 | 2.4 | 29 | 39 | 40 | 15 | 14 | 24 | 1.7 ± 0.4 | 2.2 ± 0.1 | 1.6 ± 0.3 | 0.00 | 0.03 | 0.06 |
| Adh1p | orf19.271 | Alcohol dehydrogenasee | 8.6 | 46.1 | 2.3 | 2.4 | 2.4 | 20 | 50 | 53 | 7 | 16 | 20 | 2.0 ± 0.4 | 510 ± 456 | 1.5 ± 0 | 0.01 | 0.01 | 0.00 |
| Atp2p | orf19.5653 | F1F0 ATPase complex, beta subunit | 4.9 | 55.7 | 2.4 | 2.4 | N/A | 51 | 52 | N/A | 18 | 16 | N/A | 1.8 ± 0.4 | 1.8 ± 0.5 | ND | 0.05 | 0.00 | ND |
| Ino1p | orf19.7585 | Myoinositol-1-phosphate synthasec | 5.3 | 57.8 | 2.3 | N/A | 2.4 | 28 | N/A | 25 | 9 | N/A | 11 | 2.7 ± 0.5 | ND | 2.6 ± 0.9 | 0.01 | ND | 0.02 |
| Ssb1p | orf19.6367 | Heat shock protein 70 | 5.3 | 66.4 | 2.4 | 1.7 | 1.6 | 56 | 19 | 14 | 28 | 7 | 8 | 0.5 ± 0.3 | 0.5 ± 0 | 0.5 ± 0 | 0.08 | 0.03 | 0.00 |
| Snz1p | orf19.2947 | Stationary phase proteinc | 5.8 | 31.8 | 2.3 | 2.4 | 1.7 | 24 | 24 | 15 | 7 | 7 | 5 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0 | 0.00 | 0.02 | 0.00 |
| Ado1p | orf19.5591 | Adenosine kinase | 5.0 | 38.2 | N/A | 2.4 | 2.3 | N/A | 43 | 35 | N/A | 11 | 10 | ND | 0.4 ± 0.1 | 0.5 ± 0.1 | ND | 0.04 | 0.01 |
| Apt1p | orf19.1448 | Adenine phosphoribosyltransferase | 5.2 | 20.9 | 2.1 | N/A | 2.3 | 49 | N/A | 52 | 6 | N/A | 7 | 0.2 ± 0.3 | ND | 0.5 ± 0.1 | 0.02 | ND | 0.02 |
Systematic names are according to http://www.candidagenome.org.
Z-score values of 1.65 or greater ranked the mass data search to a ≥95% level of confidence of nonrandom matches to the specific open reading frame.
Genes that were found to be differentially expressed in microarray analysis [19].
Proteins found to be differentially expressed in clinical isolates of C. albicans by Hooshdaran et al. 2004 [25].
Proteins previously found to be differentially expressed in C. albicans strain SC5314 by Yan et al. 2007 [42].
Proteins in bold were observed to be differentially expressed in C. albicans mutant SZY91.
ND-Not detected
N/A-Not applicable
3.1 Identification of differentially expressed proteins modulated by Mrr1p activating mutations
A total of 17 differentially protein spots were identified in the MDR1-overexpressing azole-resistant isolates F5, G5, and 6692 when compared to their azole-susceptible counterparts F1, G1, and 5833, respectively. Among them, 16 proteins were found to be up-regulated in the azole-resistant isolates, while only one protein (Gnd1p) was shown to be down-regulated. The proteins that were consistently up-regulated in all MDR1-overexpressing resistant isolates were Ipf5987p, Adh4p, Oye32p, Ipf17186p, Gpx1p, Tdh3p, Grp2p, Eno1p, Ifd1p, Ifd4p, and Ifd5p. Selected differentially expressed proteins are visualized in Figure 1A, which shows detailed regions of 2-D gels displaying Mrr1p-associated changes in expression of selected soluble proteins from the azole-susceptible isolate F1 carrying wild-type alleles of MRR1, azole-resistant isolate F5 harboring gain-of-function mutations in both alleles of MRR1, and the homozygous mrr1Δ strain F5MRR1M4B, a derivative of isolate F5. The changes in the expression of these proteins depicted in this figure are also representative of the alterations we observed in azole-resistant isolates G5 and 6692. The putative biological function, molecular properties, and relative fold changes of these proteins are summarized in Table 2. The protein expression profiles of mutant strain SCΔzcf36MK3A, containing a gain-of-function allele of MRR1 from isolate F5 showed similar patterns of differential protein expression to those observed in azole-resistant isolates F5, G5, and 6692 when compared to its wild-type strain SC5314 (Figure 1B). The proteins that were differentially expressed as a result of the MRR1 gain-of-function allele were Ipf5987p, Adh4p, Ipf17186p, Gpx1p, Grp2p, Ifd1p, Ifd4p, Ifd5p, and Ifd6p. Two proteins, Grp2p and Ipf17186p, were found to be represented by multiple spots in all three MDR1-overexpressing resistant clinical isolates as well as in strain SCΔzcf36MK3A (Figure 1B). Disruption of MRR1 in fluconazole-resistant isolate F5 (F5MRR1M4B) returned the expression of all 10 of these proteins to the same levels as observed in fluconazole susceptible isolate F1 (Figure 1A). These data confirm that these 10 proteins are indeed regulated by Mrr1p and suggest that at least Eno1p and Tdh3p, while increased in expression in resistant clinical isolates F5, G5, and 6692, are not regulated by Mrr1p.
Figure 1.
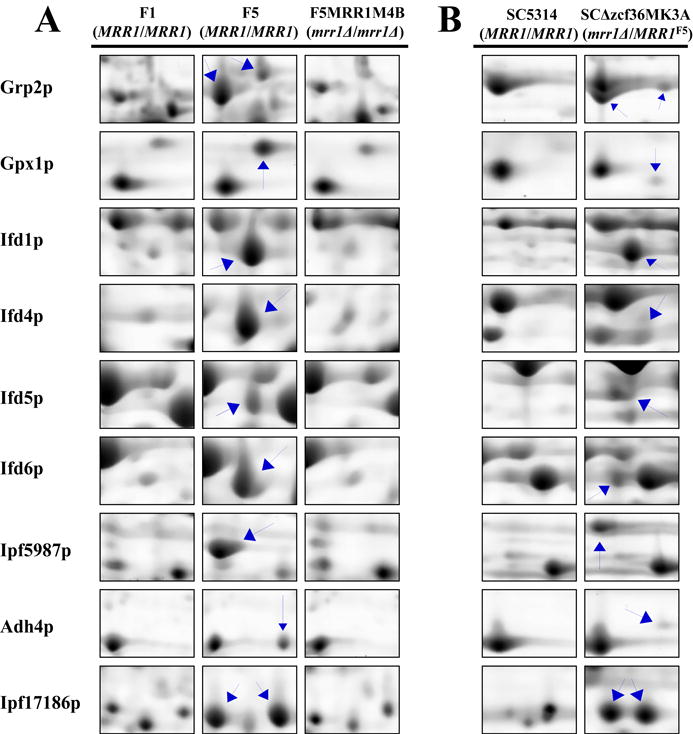
Detailed regions of SYPRO® Ruby –stained 2-D gels displaying MRR1-associated changes in protein expression of selected soluble proteins extracted from (A) azole-susceptible isolate F1 with wild-type alleles of MRR1, azole-resistant isolate F5 carrying gain-of-function mutations in both alleles of MRR1, F5-derivative mutant strain F5MRR1M4B lacking both alleles of MRR1, (B) wild-type strain SC5314 with endogenous MRR1 alleles, and mutant strain SCΔzcf36MK3A with an introduced gain-of-function MRR1 allele from azole-resistant isolate F5.
3.2 Identification of differentially expressed proteins modulated by Tac1p activating mutations
In three independent experiments, 14 differentially expressed proteins were identified in the CDR1- and CDR2-overexpressing azole-resistant isolates Gu5, C56, and 5674 compared to the azole-susceptible isolates Gu2, C43, and 5457, respectively (Table 3). The expression of 10 proteins was found to be up-regulated in the azole-resistant strains while the expression of four proteins was shown to be down-regulated (Table 3). Selected differentially expressed proteins are visualized in Figure 2A, which shows detailed regions of 2-D gels displaying Tac1p-associated changes in expression of selected soluble proteins from the azole-susceptible isolate 5457 carrying wild-type alleles of TAC1, azole-resistant isolate 5674 with gain-of-function mutations in both alleles of TAC1, and the homozygous tac1Δ strain SZY31, which is a derivative of isolate 5674. The alterations in expression of these proteins shown in this figure are also representative of those changes observed in azole-resistant isolates Gu5 and C56. The proteins determined to be differentially expressed in all three azole-resistant isolates were Eno1p, Tdh3p, Gpx1p, Ipf4065p, Ipf15297p, Pdc11p, Snz1p and Ssb1p. Interestingly, the differential expression of a heat shock protein 70 (Ssb1p) was found to be down-regulated in one spot and up-regulated in another adjacent spot on the gel (data not shown). This horizontal shift in position of Ssb1p was observed in the protein expression profiles of all CDR-overexpressing azole-resistant isolates. Similarly, alcohol dehydrogenase (Adh1p) also displayed a relative horizontal shift in position, but this shift was only observed in the 2-D expression profile of SZY91 when compared to that of SC5314 (Figure 2B). The protein expression profiles of SZY91, containing a gain-of-function allele of TAC1, revealed similar patterns of differential protein expression to those observed in azole-resistant isolates Gu5, C56, and 5674. The differentially expressed proteins identified after the introduction of a TAC1 allele were Adh1p, Ipf4065p, Ipf15297p, Gpx1p, and Snz1p (Figure 2B). Disruption of TAC1 in fluconazole-resistant isolate 5674 returned the expression of all five of these proteins to the same levels as observed in fluconazole susceptible isolate 5457 (Figure 2A). This confirms that these five proteins are indeed regulated by Tac1p, and demonstrates that Eno1p, Tdh3p, Pdc11p, and Ssb1p, while all increased in expression in resistant clinical isolates Gu5, C56, and 5674, are not regulated by Tac1p.
Figure 2.
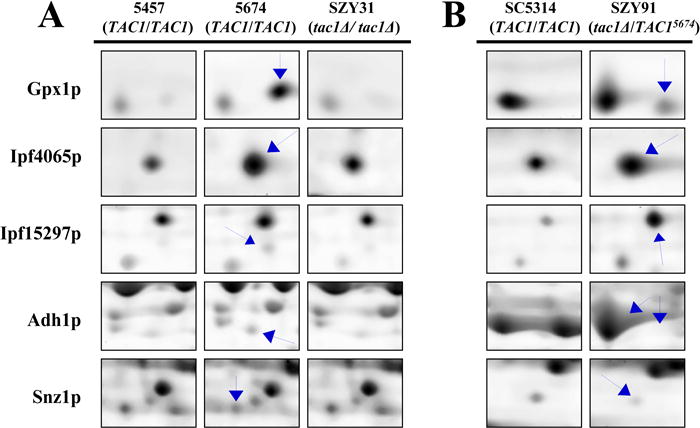
Detailed regions of SYPRO® Ruby –stained 2-D gels displaying TAC1-associated changes in protein expression of selected soluble proteins extracted from (A) azole-susceptible isolate 5457 with wild-type alleles of TAC1, azole-resistant isolate 5674 carrying gain-of-function mutations in both alleles of TAC1, 5674-derivative mutant strain SZY31 lacking both alleles of TAC1, (B) wild-type strain SC5314 with endogenous TAC1 alleles, and mutant strain SZY91 with an introduced gain-of-function allele of TAC1 from azole-resistant isolate 5674.
4. Discussion
The proteomic methods used in this study have proven to be an effective approach to analyze the alterations in protein expression between azole-susceptible and –resistant clinical isolates of C. albicans [21, 25]. It was recently reported that specific mutations in the transcription factor genes MRR1 and TAC1 result in constitutive overexpression of MDR1, CDR1, and CDR2 as well as other Mrr1p and Tac1p target genes and multi-drug resistance [17]. Sequencing of the MRR1 alleles in the matched C. albicans azole-resistant isolates used in the present study revealed single nucleotide substitutions, resulting in a proline to serine (P683S) exchange in isolate F5 and a glycine to valine (G997V) substitution in isolate G5 [17]. When these mutated MRR1 alleles were introduced into a drug-susceptible strain of C. albicans, the resulting mutant exhibited constitutive MDR1 overexpression and multi-drug resistance to fluconazole, diamide, brefeldin A, and cerulenin. Likewise, studies of matched clinical isolates identified multiple mutations in Tac1p, including the amino acid change from asparagine to aspartic acid at position 977 (N977D) that induced increased CDR1 and CDR2 expression and associated drug resistance [18]. Introduction of that mutated TAC1 allele into a tac1/tac1 background, followed by loss of heterozygosity, conferred CDR1 and CDR2 overexpression and drug resistance.
In the present study, we examined six matched clinical isolate sets and mutants disrupted for MRR1 or TAC1 carrying gain-of-function mutations in these genes in an effort to identify proteins whose expression is influenced by these transcription factors. With the exception of the proteins associated with carbohydrate metabolism, our findings confirm that each of these transcription factors regulates a different set of unique proteins associated with specific mechanisms of azole resistance.
The azole-resistant clinical isolates F5, G5, and 6692 belong to a collection of well-characterized strains of C. albicans that have been shown to overexpress MDR1. Several proteins identified in this study were previously observed to be differentially expressed in association with MDR1 overexpression, including, Ifd1p, Ifd4p, Ifd5p, Ifd6p, and Ipf5987p [21]. Proteins encoded by the IFD gene family are putative aldo-keto reductases and are classified as homologues of the putative aryl-alcohol dehydrogenases in the S. cerevisiae family of proteins, YPL088W. Ifd4p is also known as Csh1p and has been shown in C. albicans to function in cell-surface hydrophobicity and to meditate the binding to host target proteins [26]. Another aldo-keto reductase found co-regulated with Mdr1p was Ipf5987p, which is a homologue of the YPR127W gene product in S. cerevisiae and is also regulated by Yrr1p and Yrm1p [27]. It has previously been demonstrated that neither overexpression nor disruption of this gene affected drug resistance or oxidative stress resistance in C. albicans clinical isolates G5 and F5 [21]. Among the differentially expressed proteins identified in the isolates that overexpress MDR1, the aldo-keto reductases were the most abundantly overrepresented. It is tempting to speculate that their overexpression may contribute to azole resistance through detoxification of reactive substrates produced from azole stress.
Another protein found to be consistently differentially expressed in the MDR1-overexpressing isolates was Grp2p, which was shown to be up-regulated in two different spots in azole-resistant isolates F5, G5, and 6692 (Figure 1A). This protein was also previously found to be differentially expressed in C. albicans clinical isolate 12–99, an azole-resistant isolate that overexpresses MDR1, CDR1, CDR2, and ERG11 [25]. Grp2p putatively functions as a stress-induced reductase and is homologous to the NADPH-dependent methylglyoxal reductase or Gre2p in S. cerevisiae. It is believed that Gre2p functions in the detoxification of methylglyoxal-derivatives, and its expression is induced by a variety of cellular stress responses, including those in response to osmotic and oxidative stress, heat shock, heavy metals [28, 29]. The involvement of Gre2p in ergosterol metabolism has also been reported [30]. Mutant strains lacking GRE2 displayed both impaired growth and a striking induction of ergosterol biosynthesis enzymes, including, Erg10p, Erg19p, and Erg6p after membrane stress. Furthermore, these gre2∆ mutants also exhibited impaired tolerance to ergosterol biosynthesis inhibitors.
Another protein up-regulated with MDR1 is Ipf17186p, a protein that belongs to the ThiJ/PfpI protein family [31]. Its ortholog in S. cerevisiae is Hsp31p, and expression of HSP31 is regulated by the transcription factor Yap1p during oxidative stress [32]. Additionally, it has been shown that a mutant lacking this gene is hypersensitive to ROS generators, including hydrogen peroxide, diamide, tert-butyl hydroperoxide, and menadione, which suggests that Hsp31p also functions in protecting the cell against oxidative stressors [32]. Similarly, Oye32p is a putative NAD(P)H oxidoreductase, whose expression is also induced during oxidative stress [33, 34]. Mutants lacking this gene display increased levels of ROS compared to the wild-type strain; therefore, this enzyme may be involved in intracellular redox homeostasis in C. albicans [35].
It was previously shown that while disruption of MDR1 in two fluconazole resistant isolates (including isolate F5) diminished fluconazole resistance, disruption of MRR1 in these isolates had an even stronger effect than inactivation of MDR1, suggesting that MRR1 controls expression of factors in addition to MDR1 that contribute to azole resistance in strains F5 and G5. In an effort to determine which differentially expressed proteins identified in this study were influenced by Mrr1p, we examined the protein expression profiles of a wild-type strain of C. albicans containing the gain-of-function MRR1 allele from isolate F5. We observed changes in the expression levels of many proteins previously identified in the clinical isolates (Table 2). We also examined a MRR1 disruption mutant derived from isolate F5 and found increased expression of these proteins to be dependent upon Mrr1p (Table 2 and Figure 1A). It is possible that at least one of these proteins under the influence of Mrr1p directly contributes to azole resistance in these clinical isolates.
The azole-resistant clinical isolates Gu5, C56, and 5674 are among a variety of well-known clinical isolates previously shown to overexpress the ATP-binding cassette transporter genes CDR1 and CDR2. We identified 14 proteins that were differentially expressed in these azole-resistant isolates. Those proteins identified whose genes have previously been shown to be differentially expressed in association with CDR1 and CDR2, include, Ipf4065p, Gpx1p, and Snz1p [19, 36]. Gpx1p or glutathione peroxidase is an enzyme whose up-regulation was observed in all azole-resistant isolates. This enzyme is involved in the glutathione redox cycle and generally functions in the cell to reduce harmful oxidants using glutathione as a source of reducing power. Studies have implicated Gpx1p to function in quinone resistance in tumor cells [37, 38], and in human drug-resistant cancer cells, overexpression of this enzyme is commonly coregulated with increased activities of the ABC transporter P glycoprotein [39].
Another protein found to be differentially expressed in these azole-resistant isolates was a member of the heat shock protein 70 family, Ssb1p. This protein was found to be down-regulated in one spot and up-regulated in another adjacent spot, displaying a slight horizontal shift in location in the gel. This shift may be due to a change in overall net charge of the protein, possibly as a result of a phosphorylation event. Ssb1p is a cytosolic ATPase that functions as a ribosome-associated molecular chaperone that is involved in the folding of nascent polypeptide chains. These proteins are stress-induced and are considered the major class of Hsp70 chaperones. It has been shown that the Hsp70 protein Ssz1p (formerly Pdr13p) in S. cerevisiae functions to increase the activity of the pleiotropic drug resistance (PDR) transcription factor Pdr1p, resulting in increased expression of the ABC transporter genes YOR1 and PDR5 with associated drug resistance to cyclohexamide and oligomycin [40]. More recently, it has been shown that another cytosolic Hsp70p chaperone Ssa1p in S. cerevisiae is a negative regulator of the PDR transcription factor Pdr3p [41]. The overexpression of Ssa1p repressed expression of PDR5 and attenuated drug resistance to cyclohexamide. It is tempting to speculate that the Hsp70 protein Ssb1p plays a similar role in regulating the transcription factor Tac1p and influencing azole resistance.
As gain-of-function mutations in Tac1p result in increased transcription of its target genes and associated drug resistance, our aim was to identify differentially expressed proteins whose expression is influenced by Tac1p. We studied the protein expression profiles of a wild-type strain of C. albicans containing a TAC1 allele that possesses a gain-of-function mutation (N972D) from azole-resistant isolate 5674. We detected changes in the expression levels of proteins identified in isolates Gu5, C56, and 5674, including, Adh1p, Ipf4065p, Ipf15297p, Gpx1p, and Snz1p (Table 3). We also examined a TAC1 deletion mutant derived from isolate 5674, and found increased expression of these proteins to be dependent upon Tac1p.
Interestingly the up-regulation of enzymes involved in carbohydrate metabolism such as enolase (Eno1p), glyceraldehyde-3-phosphate dehydrogenase (Tdh3p), and pyruvate decarboxylase (Pdc11p) was observed in the azole-resistant isolates studied. The up-regulation of Gap1p and 6-phosphogluconate dehydrogenase (Gnd1p) was reported in a clinical azole-resistant isolate of C. albicans [25], and another study revealed a variety of up-regulated glycolytic enzymes, including Gap1p (Tdh3p) and Pdc11p, in a laboratory-derived fluconazole-resistant strain of C. albicans [42]. Perhaps, glycolysis is up-regulated to produce ATP and reducing equivalents such as NADH and NADPH to complement the azole resistance proteins, particularly the ABC transporters CDR1 and CDR2, ATP-dependent chaperones, and the aldo-keto reductases. However, it is important to note that while introduction of gain-of-function mutant alleles of MRR1 or TAC1 imparts fluconazole resistance, this did not result in increased expression of these metabolic enzymes. Likewise, disruption of MRR1 in isolate F5 or TAC1 in isolate 5674 did not influence the expression of these proteins. Each isolate pair used in this study consists of a pre-treatment isolate and a post-treatment failure isolate. Each treatment failure isolate shares two things in common relative to its respective matched pre-treatment isolate: the development of azole resistance and an increased duration of exposure to the host. It is therefore possible that up-regulation of these proteins reflects adaptation to the host environment.
Our data also provide a unique opportunity to closely examine the overlap between gene and protein expression as all of the isolates studied here have previously been examined by microarray analysis [17, 19, 36]. Overall, there was a significant degree of overlap when comparing our DNA microarray data to the data obtained from our proteomic analysis in these matched isolates, especially for the MDR1-overexpressing isolates F5, G5, and 6692 (Tables 2 and 3). The proteins that were found to be differentially expressed in these isolates whose genes were previously shown to be differentially expressed by DNA microarray analysis, include, Gpx1p, Grp2p, Ifd1p, Ifd4p, Ifd5p, Ifd6p, Ino1p, Ipf4065p, Ipf5987p, Oye32p, Adh4p, Ipf15297p, Ipf17186p, and Snz1p. The correlation between gene and protein expression in this study confirms that a majority of these differentially expressed proteins identified are regulated at the transcriptional level. Furthermore, this integrated approach of studying mRNA and protein expression in combination provides a more global understanding of the regulation of targets of Tac1p and Mrr1p.
Supplementary Material
Acknowledgments
This research was funded by NIH NIAID grant R01A1058145. We would like to thank Qing Zhang for her technical assistance. We also thank our colleagues Martine Raymond and Dominique Sanglard for kindly providing the strains and isolates used in this study, and Martine Raymond for providing helpful comments in the preparation of this manuscript.
Abbreviations
- ABC
ATP binding cassette transporter
- AIDS
Acquired immune deficiency syndrome
- OPC
oropharyngeal candidiasis
Footnotes
Conflict of Interest Statement:
We have no financial or commercial conflicts of interest to declare.
References
- 1.Klein RS, Harris CA, Small CB, Moll B, et al. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 2.Feigal DW, Katz MH, Greenspan D, Westenhouse J, et al. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS. 1991;5:519–525. doi: 10.1097/00002030-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kelly SL, Lamb DC, Kelly DE, Loeffler J, Einsele H. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet. 1996;348:1523–1524. doi: 10.1016/S0140-6736(05)65949-1. [DOI] [PubMed] [Google Scholar]
- 4.Ruhnke M, Eigler A, Tennagen I, Geiseler B, et al. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J Clin Microbiol. 1994;32:2092–2098. doi: 10.1128/jcm.32.9.2092-2098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morschhauser J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta. 2002;1587:240–248. doi: 10.1016/s0925-4439(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 6.Kelly SL, Arnoldi A, Kelly DE. Molecular genetic analysis of azole antifungal mode of action. Biochem Soc Trans. 1993;21:1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- 7.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White TC. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Ribot JL, McAtee RK, Lee LN, Kirkpatrick WR, et al. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franz R, Kelly SL, Lamb DC, Kelly DE, et al. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz R, Ruhnke M, Morschhauser J. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses. 1999;42:453–458. doi: 10.1046/j.1439-0507.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanglard D, Kuchler K, Ischer F, Pagani JL, et al. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perea S, Lopez-Ribot JL, Kirkpatrick WR, McAtee RK, et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2001;45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coste AT, Karababa M, Ischer F, Bille J, Sanglard D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell. 2004;3:1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morschhauser J, Barker KS, Liu TT, Bla BWJ, et al. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007;3:e164. doi: 10.1371/journal.ppat.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coste A, Turner V, Ischer F, Morschhauser J, et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2006;172:2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu TT, Znaidi S, Barker KS, Xu L, et al. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot Cell. 2007 doi: 10.1128/EC.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saidane S, Weber S, De Deken X, St-Germain G, Raymond M. PDR16-mediated azole resistance in Candida albicans. Mol Microbiol. 2006;60:1546–1562. doi: 10.1111/j.1365-2958.2006.05196.x. [DOI] [PubMed] [Google Scholar]
- 21.Kusch H, Biswas K, Schwanfelder S, Engelmann S, et al. A proteomic approach to understanding the development of multidrug-resistant Candida albicans strains. Mol Genet Genomics. 2004;271:554–565. doi: 10.1007/s00438-004-0984-x. [DOI] [PubMed] [Google Scholar]
- 22.Dunkel N, Blass J, Rogers PD, Morschhauser J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PK, Krohn RI, Hermanson GT, Mallia AK, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Cummings ED, Brown JM, Sarva ST, Waldo RH, Hilliard GM. High-throughput proteomics processing of proteins in polyacrylamide in a multiwell format. J Proteome Res. 2007;6:1603–1608. doi: 10.1021/pr060472y. [DOI] [PubMed] [Google Scholar]
- 25.Hooshdaran MZ, Barker KS, Hilliard GM, Kusch H, et al. Proteomic analysis of azole resistance in Candida albicans clinical isolates. Antimicrob Agents Chemother. 2004;48:2733–2735. doi: 10.1128/AAC.48.7.2733-2735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singleton DR, Masuoka J, Hazen KC. Cloning and analysis of a Candida albicans gene that affects cell surface hydrophobicity. J Bacteriol. 2001;183:3582–3588. doi: 10.1128/JB.183.12.3582-3588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucau-Danila A, Delaveau T, Lelandais G, Devaux F, Jacq C. Competitive promoter occupancy by two yeast paralogous transcription factors controlling the multidrug resistance phenomenon. J Biol Chem. 2003;278:52641–52650. doi: 10.1074/jbc.M309580200. [DOI] [PubMed] [Google Scholar]
- 28.Van Wuytswinkel O, Reiser V, Siderius M, Kelders MC, et al. Response of Saccharomyces cerevisiae to severe osmotic stress: evidence for a novel activation mechanism of the HOG MAP kinase pathway. Mol Microbiol. 2000;37:382–397. doi: 10.1046/j.1365-2958.2000.02002.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen CN, Porubleva L, Shearer G, Svrakic M, et al. Associating protein activities with their genes: rapid identification of a gene encoding a methylglyoxal reductase in the yeast Saccharomyces cerevisiae. Yeast. 2003;20:545–554. doi: 10.1002/yea.979. [DOI] [PubMed] [Google Scholar]
- 30.Warringer J, Blomberg A. Involvement of yeast YOL151W/GRE2 in ergosterol metabolism. Yeast. 2006;23:389–398. doi: 10.1002/yea.1363. [DOI] [PubMed] [Google Scholar]
- 31.Braun BR, van Het Hoog M, d’Enfert C, Martchenko M, et al. A human-curated annotation of the Candida albicans genome. PLoS Genet. 2005;1:36–57. doi: 10.1371/journal.pgen.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoneczna A, Micialkiewicz A, Skoneczny M. Saccharomyces cerevisiae Hsp31p, a stress response protein conferring protection against reactive oxygen species. Free Radic Biol Med. 2007;42:1409–1420. doi: 10.1016/j.freeradbiomed.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Cao YY, Jia XM, Cao YB, et al. Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic Biol Med. 2006;40:1201–1209. doi: 10.1016/j.freeradbiomed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Kusch H, Engelmann S, Albrecht D, Morschhauser J, Hecker M. Proteomic analysis of the oxidative stress response in Candida albicans. Proteomics. 2007;7:686–697. doi: 10.1002/pmic.200600575. [DOI] [PubMed] [Google Scholar]
- 35.Jia JH, Wang Y, Cao YB, Gao PH, et al. CaIPF7817 is involved in the regulation of redox homeostasis in Candida albicans. Biochem Biophys Res Commun. 2007;359:163–167. doi: 10.1016/j.bbrc.2007.05.081. [DOI] [PubMed] [Google Scholar]
- 36.Karababa M, Coste AT, Rognon B, Bille J, Sanglard D. Comparison of gene expression profiles of Candida albicans azole-resistant clinical isolates and laboratory strains exposed to drugs inducing multidrug transporters. Antimicrob Agents Chemother. 2004;48:3064–3079. doi: 10.1128/AAC.48.8.3064-3079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black SM, Wolf CR. The role of glutathione-dependent enzymes in drug resistance. Pharmacol Ther. 1991;51:139–154. doi: 10.1016/0163-7258(91)90044-m. [DOI] [PubMed] [Google Scholar]
- 38.Doroshow JH, Akman S, Chu FF, Esworthy S. Role of the glutathione-glutathione peroxidase cycle in the cytotoxicity of the anticancer quinones. Pharmacol Ther. 1990;47:359–370. doi: 10.1016/0163-7258(90)90062-7. [DOI] [PubMed] [Google Scholar]
- 39.Buser K, Joncourt F, Altermatt HJ, Bacchi M, et al. Breast cancer: pretreatment drug resistance parameters (GSH-system, ATase, P-glycoprotein) in tumor tissue and their correlation with clinical and prognostic characteristics. Ann Oncol. 1997;8:335–341. doi: 10.1023/a:1008202723066. [DOI] [PubMed] [Google Scholar]
- 40.Hallstrom TC, Katzmann DJ, Torres RJ, Sharp WJ, Moye-Rowley WS. Regulation of transcription factor Pdr1p function by an Hsp70 protein in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:1147–1155. doi: 10.1128/mcb.18.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahi P, Gulshan K, Moye-Rowley WS. Negative transcriptional regulation of multidrug resistance gene expression by an Hsp70 protein. J Biol Chem. 2007;282:26822–26831. doi: 10.1074/jbc.M704772200. [DOI] [PubMed] [Google Scholar]
- 42.Yan L, Zhang JD, Cao YB, Gao PH, Jiang YY. Proteomic analysis reveals a metabolism shift in a laboratory fluconazole-resistant Candida albicans strain. J Proteome Res. 2007;6:2248–2256. doi: 10.1021/pr060656c. [DOI] [PubMed] [Google Scholar]
- 43.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 44.Znaidi S, De Deken X, Weber S, Rigby T, et al. The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol Microbiol. 2007;66:440–452. doi: 10.1111/j.1365-2958.2007.05931.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


