Abstract
Background
Randomized trials of radiation after breast-conserving surgery (BCS) for DCIS found substantial rates of recurrence, with half of recurrences invasive. Decreasing local recurrence rates for invasive breast carcinoma have been observed, and are largely attributed to systemic therapy improvements. Here we examine recurrence rates after BCS for DCIS over 3 decades at one institution.
Methods
We retrospectively reviewed a prospectively maintained database of DCIS patients undergoing BCS from 1978–2010. Cox proportional hazard models were used to investigate the association between treatment period and recurrence, controlling for other variables.
Results
363 (12%) recurrences among 2996 cases were observed. Median follow-up for patients without recurrence was 75 months (range 0–30 years); 732 were followed for ≥10 years. The 5-year recurrence rate for 1978–1998 was 13.6% versus 6.6% for 1999–2010 (hazard ratio [HR] 0.62, p<0.0001). Controlling for age, family history, presentation, nuclear grade, necrosis, number of excisions, margin status, radiation, and endocrine therapy, treatment period remained significantly associated with recurrence, with later years associated with a lower HR (0.74, p=0.02) compared to earlier. After stratification by radiation use, association of recurrence with treatment period persisted in those treated without radiation (HR 0.62, p=0.003).
Conclusions
Recurrence rates for DCIS have fallen over time. Increases in screen-detection, negative margins, and use of adjuvant therapies only partially explain the decrease. The unexplained decline persists in women not receiving radiation, suggesting it is not due to changes in radiation efficacy, but may be due to improvements in radiologic detection and pathologic assessment.
INTRODUCTION
Ductal carcinoma in situ (DCIS) accounts for over 20% of all breast cancer diagnosed in the United States annually.1 A 500% increase in the incidence of DCIS between 1983 and 2003 was observed for women 50 years of age and older, likely due to screening mammography.2
Reported recurrence rates for DCIS treated with breast-conserving surgery (BCS) from 4 prospective randomized trials of radiation range from 26–36% for those treated without radiation therapy, and 9–23% for those treated with radiation at 13–20 years of follow-up.3–6 These rates are higher than the 12-year ipsilateral breast tumor recurrence rates of 5–8% for node-negative invasive breast cancer treated with radiation and systemic therapy.7
Local recurrence rates in early invasive cancer have declined over time.8–11 This decline has been attributed, at least in part, to advances in systemic therapy for invasive cancer.12
Temporal trends in DCIS recurrence are less well studied. Here we sought to examine changes in recurrence rates over time among women treated with BCS for DCIS, and to explore the reasons for the changes found.
MATERIALS AND METHODS
After obtaining Institutional Review Board approval, we analyzed outcomes from a prospectively maintained database of DCIS patients undergoing BCS from 1978–2010 at Memorial Sloan Kettering Cancer Center. Follow-up is routinely obtained by yearly contact with the patient, at follow-up clinician visits or by mail, phone, or e-mail. Synchronous (n = 30) or metachronous bilateral lesions (n = 29) were entered as separate cases of DCIS.
Variables examined included age at diagnosis, menopausal status (pre- or peri- versus postmenopausal), family history (at least 1 first- or second-degree family member with breast cancer), radiologic versus clinical presentation of DCIS, nuclear grade (non-high grade versus high grade), necrosis, number of excisions (≤ 2 versus ≥ 3), margin status (positive or close [≤ 2 mm] versus negative [> 2 mm]), radiation, endocrine therapy, and year of definitive surgery.
An event was defined as ipsilateral breast recurrence of DCIS or invasive cancer, ipsilateral regional recurrence in the absence of breast recurrence, or distant recurrence in the absence of locoregional recurrence or diagnosis of other malignancy. Time to recurrence was defined as the length of time between the last surgical excision and first event. Kaplan-Meier curves were created to compare recurrence rates by treatment period; logrank tests were used to determine significance. Patient, pathological, and treatment variables were compared between treatment periods using chi-square. The Cochran-Armitage test was used to test for trend in the proportion of women undergoing BCS versus mastectomy over time. Multivariable Cox models were built to examine differences in recurrence rates over time, controlling for other variables. Proportionality of hazards was checked for all Cox models and found to be appropriate. Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
From 1978–2010, 2996 cases of DCIS treated with BCS were identified. Population characteristics are shown in Table 1. Patients without recurrence were followed for a median of 75 months (range 0–30 years); 732 were followed for at least 10 years. The median age of the entire population, and for the cohorts from both the early and late treatment periods, was 57 years (range 20–92 years).
Table 1.
Characteristics of entire population (n = 2996) and patients treated between 1978–1998 and 1999–2010.
| Entire population (n = 2996)
|
1978–1998 (n = 785)
|
1999–2010 (n = 2211)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p-value* | |
| Age | ≤ 50 years | 845 | 28.2% | 237 | 30.2% | 608 | 27.5% | 0.1 |
| > 50 years | 2151 | 71.8% | 548 | 69.8% | 1603 | 72.5% | ||
|
| ||||||||
| Menopausal status | Pre/peri | 1038 | 34.6% | 264 | 33.6% | 774 | 35.0% | 0.6 |
| Post | 1946 | 64.9% | 514 | 65.5% | 1432 | 64.8% | ||
| Unknown | 12 | 0.4% | 7 | 0.9% | 5 | 0.2% | ||
|
| ||||||||
| Family history | No | 1816 | 60.6% | 501 | 63.8% | 1315 | 59.5% | 0.005 |
| Yes | 1136 | 37.9% | 261 | 33.2% | 875 | 39.6% | ||
| Unknown | 44 | 1.4% | 23 | 2.9% | 21 | 0.9% | ||
|
| ||||||||
| Presentation | Clinical | 386 | 12.9% | 193 | 24.6% | 193 | 8.7% | < 0.0001 |
| Radiologic | 2606 | 86.9% | 588 | 74.9% | 2018 | 91.3% | ||
| Unknown | 4 | 0.1% | 4 | 0.5% | 0 | 0% | ||
|
| ||||||||
| Nuclear grade | Low/intermediate | 1787 | 59.6% | 305 | 38.9% | 1482 | 67.0% | < 0.0001 |
| High | 994 | 33.2% | 294 | 37.5% | 700 | 31.7% | ||
| Unknown | 215 | 7.2% | 186 | 23.7% | 29 | 1.3% | ||
|
| ||||||||
| Necrosis | Absent | 1029 | 34.3% | 239 | 30.4% | 790 | 35.7% | 0.7 |
| Present | 1802 | 60.1% | 431 | 54.9% | 1371 | 62.0% | ||
| Unknown | 165 | 5.5% | 115 | 14.6% | 50 | 2.3% | ||
|
| ||||||||
| Number of excisions | ≤ 2 | 2775 | 92.6% | 738 | 94.0% | 2037 | 92.1% | 0.04 |
| ≥ 3 | 217 | 7.2% | 44 | 5.6% | 173 | 7.8% | ||
| Unknown | 4 | 0.1% | 3 | 0.4% | 1 | 0.04% | ||
|
| ||||||||
| Margin status | Positive/close (≤ 2 mm) | 553 | 18.5% | 185 | 23.6% | 368 | 16.6% | < 0.0001 |
| Negative (> 2 mm) | 2235 | 74.6% | 440 | 56.1% | 1795 | 81.2% | ||
| Unknown | 208 | 6.9% | 160 | 20.4% | 48 | 2.2% | ||
|
| ||||||||
| Radiation therapy | No | 1374 | 45.9% | 458 | 58.3% | 916 | 41.4% | < 0.0001 |
| Yes | 1588 | 53.0% | 310 | 39.5% | 1278 | 57.8% | ||
| Unknown | 34 | 1.1% | 17 | 2.2% | 17 | 0.8% | ||
|
| ||||||||
| Endocrine therapy | No | 2321 | 77.4% | 642 | 81.8% | 1679 | 76.0% | < 0.0001 |
| Yes | 628 | 20.9% | 121 | 15.4% | 507 | 22.9% | ||
| Unknown | 47 | 1.7% | 22 | 2.8% | 25 | 1.1% | ||
|
| ||||||||
| Treatment period | 1978–1998 | 785 | 26.2% | 785 | 100% | -- | ||
| 1998–2010 | 2211 | 73.8% | -- | 2211 | 100% | |||
Chi-square test of difference between early and later treatment periods
Among the 1374 who underwent BCS alone, there were 223 recurrences; 117 were ipsilateral breast recurrences of DCIS, 98 were invasive (91 ipsilateral breast recurrences, 2 ipsilateral regional nodal recurrences, 5 ipsilateral breast and nodal recurrences), and 8 were ipsilateral breast recurrence of unknown type.
Among the 1588 who underwent BCS and radiation, there were 140 recurrences; 75 were ipsilateral breast recurrences of DCIS, 61 were invasive (56 ipsilateral breast recurrences, 5 ipsilateral breast and nodal recurrences), 3 were ipsilateral breast recurrence of unknown type, and there was a single case of distant metastasis without ipsilateral locoregional recurrence or diagnosis of other malignancy.
Recurrence rates over time
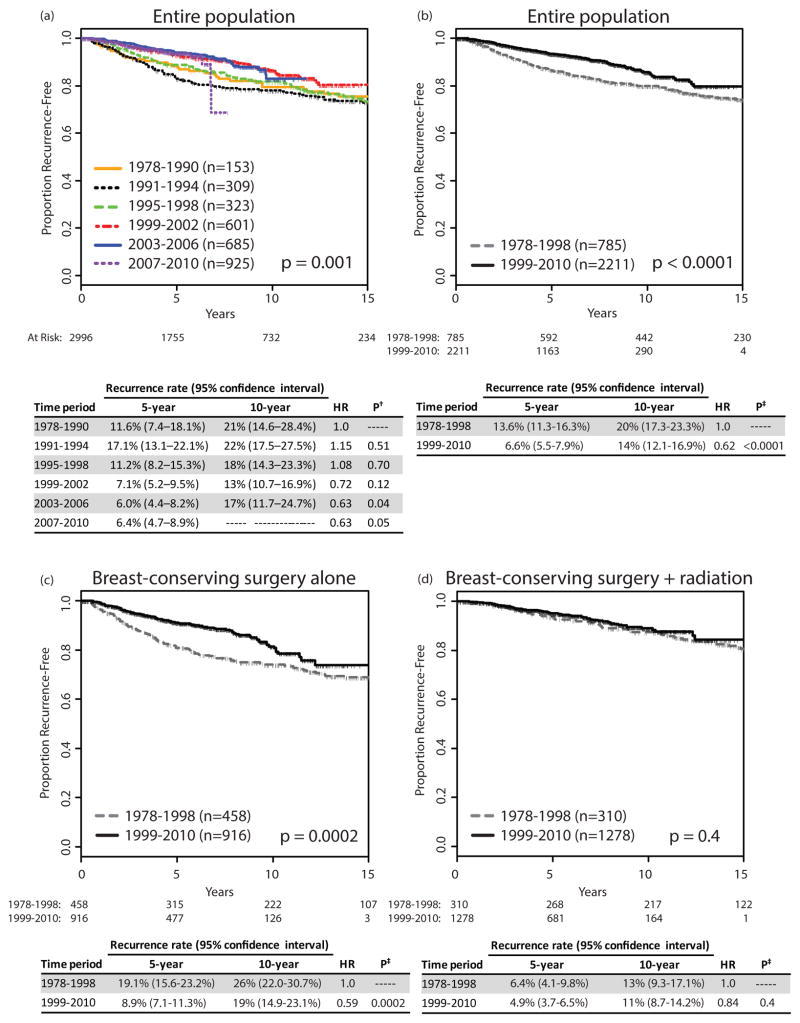
Fig 1a shows recurrence rates by treatment period, dividing the study interval into 6 treatment periods. A significant decrease in recurrence rates over time was observed (p = 0.001). The change over time appeared non-linear, with an apparent break between the 3 earlier intervals and the 3 later intervals; we therefore dichotomized the treatment period into intervals of 1978–1998 and 1999–2010 for further analysis. Fig 1b shows a decline in 5-year recurrence rates from 13.6% (95% confidence interval [CI] 11.3–16.3%) in 1978–1998 to 6.6% (95% CI 5.5–7.9%) in 1999–2010 (p < 0.0001), with a hazard ratio (HR) of 0.62 (p < 0.0001) in the later as compared to earlier period.
Fig 1.
Proportion recurrence-free, by year of surgery, (a) for 6 treatment periods, (b) for 2 treatment periods, (c) for breast-conserving surgery alone over 2 treatment periods, (d) for breast-conserving surgery with radiation over 2 treatment periods.
†p-value for difference compared to 1978–1990
‡p-value for difference compared to 1978–1998
HR, hazard ratio
Change in patient, tumor, and treatment variables over time
Patient, tumor, and treatment variables were compared between the dichotomized treatment periods to identify factors potentially contributing to the reduction in recurrence (Table 1).
For nearly all variables, there were more missing data from the earlier time period. In the more recent time period, family history was more frequently recorded as positive, patients more frequently presented as a result of radiologic screening, nuclear grade was less frequently rated as high, more women underwent at least 3 excisions to enable breast conservation, close margins were less frequent, and adjuvant radiation and endocrine therapies were more commonly used. Age at diagnosis, menopausal status, and the presence of necrosis did not change over time.
Multivariable analysis
Recurrence rates by treatment period were compared using a multivariable model to control for known risk factors and the factors that changed over time (Table 2). Even after controlling for 9 variables from Table 1, the later time period was associated with a lower risk of recurrence, with an HR of 0.74 compared to the earlier period (p = 0.02). The persistent association of time period with recurrence, even after controlling for other variables, indicates that increases in screen detection, negative margins, and use of radiation and endocrine therapies do not fully explain the decrease in recurrence rates observed over time.
Table 2.
Multivariable Cox regression analysis of the association of treatment period with recurrence in 2558 women* with DCIS treated with BCS, controlling for other factors.
| Variable | N | Events | HR | p-value | |
|---|---|---|---|---|---|
| Age | Continuous, per year | 2558 | 297 | 0.977 | < 0.0001 |
|
| |||||
| Family history | No | 1566 | 170 | 1.00 | |
| Yes | 992 | 127 | 1.28 | 0.04 | |
|
| |||||
| Presentation | Radiologic | 2278 | 245 | 1.00 | |
| Clinical | 280 | 52 | 1.40 | 0.03 | |
|
| |||||
| Nuclear grade | Non-high | 1630 | 183 | 1.00 | |
| High | 928 | 114 | 1.03 | 0.8 | |
|
| |||||
| Necrosis | Absent | 879 | 90 | 1.00 | |
| Present | 1679 | 207 | 1.43 | 0.01 | |
|
| |||||
| Number of excisions | ≤ 2 | 2362 | 267 | 1.00 | |
| ≥ 3 | 196 | 30 | 1.56 | 0.03 | |
|
| |||||
| Margin status | Positive/close (≤ 2mm) | 507 | 76 | 1.00 | |
| Negative (> 2 mm) | 2051 | 221 | 0.72 | 0.02 | |
|
| |||||
| Radiation therapy | No | 1111 | 181 | 1.00 | |
| Yes | 1447 | 116 | 0.40 | < 0.0001 | |
|
| |||||
| Endocrine therapy | No | 1989 | 259 | 1.00 | |
| Yes | 569 | 38 | 0.52 | 0.0002 | |
|
| |||||
| Treatment period | 1978–1998 | 514 | 129 | 1.00 | |
| 1999–2010 | 2044 | 168 | 0.74 | 0.02 | |
N=2558 due to missing data in 438 patients
DCIS, ductal carcinoma in situ; BCS, breast-conserving surgery; HR, hazard ratio
Change in recurrence rates over time, stratified by use of radiation
To determine whether the unexplained decline in recurrence occurred in patients treated with and without radiotherapy, we fit multivariable models stratified by radiation use (Table 3). This analysis demonstrated that the decrease in recurrence rates over time, not accounted for by change in other variables, was limited to the group not receiving radiation (HR for treatment period 0.62, p = 0.003), suggesting that improvement in radiation efficacy is not the primary cause of the observed decrease in recurrence rates.
Table 3.
Multivariable Cox regression analysis of the association of treatment period with recurrence, stratified by use of radiation in 2558 women with DCIS treated with BCS, controlling for other factors.
| No radiation (N = 1111*)
|
Radiation (N = 1447*)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Events | HR | p-value | N | Events | HR | p-value | |
| Age | Continuous, per year | 1111 | 181 | 0.984 | 0.007 | 1447 | 116 | 0.960 | < 0.0001 |
|
| |||||||||
| Family history | No | 679 | 102 | 1.00 | 887 | 68 | 1.00 | ||
| Yes | 432 | 79 | 1.31 | 0.08 | 560 | 48 | 1.28 | 0.2 | |
|
| |||||||||
| Presentation | Radiologic | 980 | 148 | 1.00 | 1298 | 97 | 1.00 | ||
| Clinical | 131 | 33 | 1.50 | 0.04 | 149 | 19 | 1.21 | 0.5 | |
|
| |||||||||
| Nuclear grade | Non-high | 868 | 129 | 1.00 | 762 | 54 | 1.00 | ||
| High | 243 | 52 | 1.04 | 0.8 | 685 | 62 | 1.02 | 0.9 | |
|
| |||||||||
| Necrosis | Absent | 550 | 68 | 1.00 | 329 | 22 | 1.00 | ||
| Present | 561 | 113 | 1.54 | 0.01 | 1118 | 94 | 1.10 | 0.7 | |
|
| |||||||||
| Number of excisions | ≤ 2 | 1072 | 167 | 1.00 | 1290 | 100 | 1.00 | ||
| ≥ 3 | 39 | 14 | 2.11 | 0.009 | 157 | 16 | 1.18 | 0.5 | |
|
| |||||||||
| Margin status | Positive/close (≤ 2mm) | 194 | 46 | 1.00 | 313 | 30 | 1.00 | ||
| Negative (> 2mm) | 917 | 135 | 0.58 | 0.001 | 1134 | 86 | 1.00 | 0.9 | |
|
| |||||||||
| Endocrine therapy | No | 932 | 161 | 1.00 | 1057 | 98 | 1.00 | ||
| Yes | 179 | 20 | 0.57 | 0.02 | 390 | 18 | 0.45 | 0.002 | |
|
| |||||||||
| Treatment period | 1978–1998 | 294 | 89 | 1.00 | 220 | 40 | 1.00 | ||
| 1999–2010 | 817 | 92 | 0.62 | 0.003 | 1227 | 76 | 1.13 | 0.6 | |
Numbers do not sum to 2996 due to missing data in 438 patients
DCIS, ductal carcinoma in situ; BCS, breast-conserving surgery; HR, hazard ratio
Rates of total mastectomy versus BCS for DCIS
We compared annual rates of BCS versus mastectomy for DCIS at our institution from 1995–2010 to evaluate the possibility that decreased recurrence rates were due to selection bias. There was no significant change over time in the percentage of patients undergoing mastectomy for DCIS (40.1% in 1995–1998 versus 40.4% in 1999–2010, p = 0.85) (Fig 2).
Fig 2.
Proportion and number of DCIS cases undergoing breast-conserving surgery versus therapeutic mastectomy, by year. Proportion of each bar shaded black or gray represents the proportion of all cases of DCIS that were treated with breast-conserving surgery or mastectomy, respectively, each year. The number treated by each type of surgery is shown overlying the appropriate portion of the bar.
DCIS, ductal carcinoma in situ
DISCUSSION
Several groups have reported decreasing locoregional recurrence rates for invasive cancer.8–11 These improvements are largely attributed to improved systemic treatments, including chemotherapy, targeted anti-HER2 therapies, and endocrine therapies. In contrast, in DCIS, the only proven systemic therapy is endocrine therapy, and use of endocrine therapy in DCIS is relatively low,13 suggesting that a similar decline in rates of locoregional recurrence might not have occurred.
We undertook this study to examine recurrence rates for DCIS treated with BCS over 30 years at a single institution. Examination of outcomes from 2996 cases revealed that recurrence rates did significantly decline over time. We identified a number of temporal trends in our population which help to explain this outcome.
Patients treated in later years more frequently presented with radiologically detected DCIS, and screen-detected DCIS is associated with lower rates of recurrence than cases presenting clinically—likely due to a lower volume of disease.4,5,14 While it is likely that the pathologically measured size of DCIS would have been smaller in later years, measured pathologic size was not available for the majority of our cases. This is due to the difficulty of accurate measurement of DCIS, which is generally not grossly visible. Size measurements were also missing in the majority of cases in the four prospective randomized trials.15 In the later treatment period, an increased proportion of patients underwent at least 3 surgical excisions. It may reflect a greater effort to achieve margins > 2 mm in the later treatment period, as demonstrated by the decrease in the proportion of women with close or positive margins. This likely contributed to the observed decline in recurrence rates, as margin status is a known risk factor for local recurrence.4–6 Alternatively, it may reflect greater comfort with BCS for larger areas of DCIS in women who might have been advised to undergo mastectomy in prior years.
Over the study period, the proportion of cases with high nuclear grade fell from 49% of those with known nuclear grade to 32%. A similar decrease in the proportion of patients with high-grade DCIS was reported by Habel et al.16 Although studies have shown that local recurrence at 5 years is more common in patients with high-grade DCIS, these differences do not persist with longer follow-up.17,18 In our multivariable analysis, high nuclear grade was not associated with local recurrence, similar to findings in other studies with longer follow-up.17
We also found that patients treated in later years were significantly more likely to receive adjuvant radiation. Patients from the early years of this series were treated prior to publication of the first randomized trial of radiation for DCIS, National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17, in 1993.19 The increasing use of radiation over time is well documented.13,16,20 Baxter et al and Zujewski et al have reported increased use of radiation for DCIS in the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute (SEER) database.13,20 In the community setting, Habel et al also found an increase in use of radiation for the treatment of DCIS, from 25.8% in 1990–1991 to 61.3% in 2000–2001.16 Four prospective randomized studies have been published with over 12 years of follow-up, proving that radiation provides a durable reduction in local recurrence rates of approximately 50%.3–6 Therefore, one clear reason for the observed decrease in recurrence rate in our series is the increased use of radiation.
Use of endocrine therapy also increased significantly over time, likely contributing to the decrease in recurrence rates. NSABP B-24, first published in 1999, was the first randomized study of tamoxifen use in women with DCIS treated with radiation.21 As a result of that study, and the UK/ANZ trial, the use of tamoxifen for DCIS increased.3,13,21 Habel et al noted an increase in tamoxifen use from 2% among those diagnosed in 1990–91, to 34% diagnosed in 2000–2001.16 Hiramatsu et al and Halasz et al reported that none of 76 patients treated prior to 1990 received endocrine therapy as compared to 126 of 246 patients treated from 2001–2007.22,23
After adjustment for all factors that changed over time, including radiologic detection, negative margins, and use of adjuvant radiation and endocrine therapies, all of which clearly influence rates of recurrence, we found that the later treatment period remained significantly associated with a lower risk of recurrence. This suggests that factors not included in our model contributed to the reduction in recurrence risk. One possibility is improved efficacy of adjuvant radiotherapy. However, after stratifying for use of radiation, the unexplained decline in recurrence rate was limited to those not receiving radiation, suggesting that improvement in radiation efficacy is not responsible for the observed decline in recurrence rates.
Others have also reported a decline in local recurrence rates for DCIS treated with BCS. Halasz et al compared the results of a series of 246 women treated with BCS and radiation from 2001–2007, with a median follow-up of 58 months (5-year recurrence rate, 0%) to a series of 76 patients treated from 1976–1990, with a median follow-up of 74 months (10-year recurrence rate, 15%) and concluded that recurrence rates had significantly improved in the modern era of mammographic detection and careful attention to margins.22,23 Similarly, Habel et al reported a reduction in the 5-year local recurrence rate from 14.3% to 7.7% in patients diagnosed in 1990–1991 compared to those diagnosed in 1998–1999.16 Similar to our observation, they found that even after adjustment for adjuvant radiation and endocrine therapy use, there remained a reduced risk of recurrence in the later years. They also noted an increase in the frequency of negative margins and non-high grade DCIS. These factors were not included in their multivariable model, and they hypothesized that the increase in negative margins and non-high grade DCIS contributed to the decreased recurrence rates. However, in our analysis, even after inclusion of these factors in a multivariable model, treatment period remained significantly associated with a reduced recurrence risk.
Factors potentially responsible for the improved outcomes we observed and which we were unable to study include improvements in breast imaging and pathologic evaluation. Digital mammography, as compared to film screen, is better able to detect faint microcalcifications,24–27 which may lead to earlier detection and to more complete excision of DCIS lesions, thereby improving recurrence rates.
Pathologic assessment of DCIS has changed over time, with an increased number of slides examined per case as well as standardized reporting with regard to extent of disease and margin status.28–30 The report of a negative margin in more recent years, after a more intensive pathologic examination, may indicate in a lower burden of residual disease and result in lower recurrence rates.
Our findings have important implications for patients being treated in the modern era. The metaanalysis of the 4 randomized trials of BCS with and without radiation for DCIS reported a 5-year ipsilateral recurrence rate of 18% in patients undergoing BCS alone.15 Those trials began between 1985 and 1990. Our results suggest that current rates of local recurrence are substantially lower. Newer prospective studies support this contention, albeit in favorable subsets of patients.
McCormick et al reported 5-year recurrence rates of 0.4% among those randomized to radiation and 3.5% among those randomized to no radiation in selected low-risk women with DCIS treated from 1999–2006.31 Two single-arm prospective studies have evaluated BCS without radiation for selected women treated in a more recent time period. Both required ≤2.5cm of DCIS and widely negative inked margins. Wong et al accrued patients between1995–2002 and reported 5-year recurrence rates of 9.8%.32 Hughes et al accrued patients from 1997–2002 and reported 5-year recurrence rates of 6.1% for low/intermediate-grade DCIS, and 15.3% for high-grade DCIS.18
Although these represent selected populations, these rates are reassuringly lower than those from the first 4 randomized trials, and are consistent with our finding that recurrence rates have declined. The lower recurrence risk estimate for patients treated in recent years can be critical when counseling patients, especially in this era of increased use of uni- and bilateral mastectomy.33,34 An online risk estimation tool, which incorporates various factors, including year of treatment, has been validated in independent populations and may be helpful for patients and clinicians in weighing various treatment options and in obtaining more current and individualized risk estimates (http://nomograms.mskcc.org/Breast/DuctalCarcinomaInSituRecurrencePage.aspx). 35–39
Conclusion
Recurrence rates after BCS for DCIS have declined over time. The increased proportion of patients with screen-detected DCIS, negative margins, and the increased use of radiation and endocrine therapies, only partly explains decreased recurrence rates. Advances in digital mammography and improvements in pathological assessment likely result in earlier detection and more complete resection, and thereby contribute to the reduction in recurrences seen in recent years. The expected recurrence rate for a woman treated today may be lower than that seen in the prospective randomized trials that began decades ago. This has implications for patient decision-making, especially in view of the marked increase in recent years of the number of women choosing unilateral or bilateral mastectomy for treatment of their DCIS.
Synopsis.
Analysis of 2996 women with DCIS treated with breast-conserving surgery over 30 years showed decreasing recurrence rates. Increases in mammographic detection, negative margins and use of endocrine and radiation therapies only partly explain the observed reduction in recurrences.
Footnotes
Disclosures: This study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015 Jan;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Kerlikowske K. Epidemiology of ductal carcinoma in situ. Journal of the National Cancer Institute. Monographs. 2010;2010(41):139–141. doi: 10.1093/jncimonographs/lgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. The Lancet. Oncology. 2011 Jan;12(1):21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013 Nov 10;31(32):4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 5.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. Journal of the National Cancer Institute. 2011 Mar 16;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014 Nov 10;32(32):3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 May 20;27(15):2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabioglu N, Hunt KK, Buchholz TA, et al. Improving local control with breast-conserving therapy: a 27-year single-institution experience. Cancer. 2005 Jul 1;104(1):20–29. doi: 10.1002/cncr.21121. [DOI] [PubMed] [Google Scholar]
- 9.Canavan J, Truong PT, Smith SL, Lu L, Lesperance M, Olivotto IA. Local recurrence in women with stage I breast cancer: declining rates over time in a large, population-based cohort. International journal of radiation oncology, biology, physics. 2014 Jan 1;88(1):80–86. doi: 10.1016/j.ijrobp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009 Jan;90(1):14–22. doi: 10.1016/j.radonc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Ernst MF, Voogd AC, Coebergh JW, Poortmans PM, Roukema JA. Using loco-regional recurrence as an indicator of the quality of breast cancer treatment. European journal of cancer (Oxford, England: 1990) 2004 Mar;40(4):487–493. doi: 10.1016/j.ejca.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer--bigger is not better. The New England journal of medicine. 2012 Jul 5;367(1):79–82. doi: 10.1056/NEJMsb1202521. [DOI] [PubMed] [Google Scholar]
- 13.Zujewski JA, Harlan LC, Morrell DM, Stevens JL. Ductal carcinoma in situ: trends in treatment over time in the US. Breast cancer research and treatment. 2011 May;127(1):251–257. doi: 10.1007/s10549-010-1198-z. [DOI] [PubMed] [Google Scholar]
- 14.Collins LC, Achacoso N, Haque R, et al. Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast cancer research and treatment. 2013 Jun;139(2):453–460. doi: 10.1007/s10549-013-2539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists’ Collaborative G. Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. Journal of the National Cancer Institute. Monographs. 2010;2010(41):162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habel LA, Achacoso NS, Haque R, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast cancer research: BCR. 2009;11(6):R85. doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solin LJ, Kurtz J, Fourquet A, et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1996 Mar;14(3):754–763. doi: 10.1200/JCO.1996.14.3.754. [DOI] [PubMed] [Google Scholar]
- 18.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 Nov 10;27(32):5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. The New England journal of medicine. 1993 Jun 3;328(22):1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 20.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. Journal of the National Cancer Institute. 2004 Mar 17;96(6):443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999 Jun 12;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 22.Hiramatsu H, Bornstein BA, Recht A, et al. Local recurrence after conservative surgery and radiation therapy for ductal carcinoma in situ: Possible importance of family history. The cancer journal from Scientific American. 1995 May-Jun;1(1):55–61. [PubMed] [Google Scholar]
- 23.Halasz LM, Sreedhara M, Chen YH, et al. Improved outcomes of breast-conserving therapy for patients with ductal carcinoma in situ. International journal of radiation oncology, biology, physics. 2012 Mar 15;82(4):e581–586. doi: 10.1016/j.ijrobp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Feeley L, Kiernan D, Mooney T, et al. Digital mammography in a screening programme and its implications for pathology: a comparative study. Journal of clinical pathology. 2011 Mar;64(3):215–219. doi: 10.1136/jcp.2010.085860. [DOI] [PubMed] [Google Scholar]
- 25.Karssemeijer N, Bluekens AM, Beijerinck D, et al. Breast cancer screening results 5 years after introduction of digital mammography in a population-based screening program. Radiology. 2009 Nov;253(2):353–358. doi: 10.1148/radiol.2532090225. [DOI] [PubMed] [Google Scholar]
- 26.Hambly NM, McNicholas MM, Phelan N, Hargaden GC, O’Doherty A, Flanagan FL. Comparison of digital mammography and screen-film mammography in breast cancer screening: a review in the Irish breast screening program. AJR. American journal of roentgenology. 2009 Oct;193(4):1010–1018. doi: 10.2214/AJR.08.2157. [DOI] [PubMed] [Google Scholar]
- 27.Del Turco MR, Mantellini P, Ciatto S, et al. Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR. American journal of roentgenology. 2007 Oct;189(4):860–866. doi: 10.2214/AJR.07.2303. [DOI] [PubMed] [Google Scholar]
- 28.Miller KL, Marks LB, Barrier RC, Jr, et al. Increased sectioning of pathologic specimens with ductal carcinoma in situ of the breast: are there clinical consequences? Clinical breast cancer. 2003 Aug;4(3):198–202. doi: 10.3816/cbc.2003.n.025. [DOI] [PubMed] [Google Scholar]
- 29.Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Archives of pathology & laboratory medicine. 2009 Jan;133(1):15–25. doi: 10.5858/133.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Siziopikou KP. Ductal carcinoma in situ of the breast: current concepts and future directions. Archives of pathology & laboratory medicine. 2013 Apr;137(4):462–466. doi: 10.5858/arpa.2012-0078-RA. [DOI] [PubMed] [Google Scholar]
- 31.McCormick B, Winter K, Hudis C, et al. RTOG 9804: A Prospective Randomized Trial for Good-Risk Ductal Carcinoma In Situ Comparing Radiotherapy With Observation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015 Mar 1;33(7):709–715. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS) Breast cancer research and treatment. 2014 Jan;143(2):343–350. doi: 10.1007/s10549-013-2813-6. [DOI] [PubMed] [Google Scholar]
- 33.Rutter CE, Park HS, Killelea BK, Evans SB. Growing Use of Mastectomy for Ductal Carcinoma-In Situ of the Breast Among Young Women in the United States. Annals of surgical oncology. 2015 Jan 7; doi: 10.1245/s10434-014-4334-x. [DOI] [PubMed] [Google Scholar]
- 34.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 Mar 20;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 35.Memorial Sloan Kettering Cancer Center. [Accessed April 8, 2015];Breast Cancer Nomogram: Ductal Carcinoma In Situ Recurrence. 2014 Available at: http://nomograms.mskcc.org/Breast/DuctalCarcinomaInSituRecurrencePage.aspx.
- 36.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Aug 10;28(23):3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 37.Sweldens C, Peeters S, van Limbergen E, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer journal. 2014 Jan-Feb;20(1):1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Li H, Tan PH, et al. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clinical oncology. 2014 Nov;26(11):684–691. doi: 10.1016/j.clon.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Collins LC, Achacoso N, Haque R, et al. Risk prediction for local breast cancer recurrence among women with DCIS treated in a community practice: A nested case-control study. Annals of surgical oncology. 2015 doi: 10.1245/s10434-015-4641-x. in press. [DOI] [PubMed] [Google Scholar]