Abstract
Circulating Tumor Cell (CTC) enumeration provides prognostic but not predictive information for chemotherapy in metastatic breast cancer. Because CTC measurement is reproducible and allows molecular profiling, an assay to assess endocrine resistance was developed. Whether this CTC-based assay can be used to reliably select endocrine therapy must be tested.
In this issue of Clinical Cancer Research, Paoletti and colleagues describe the development of Circulating Tumor Cell–Endocrine Therapy Index (CTC-ETI), an assay based on both enumeration of circulating tumor cells (CTC) and measurement of protein expression using immunofluorescence staining of CTC (1).
Circulating tumor cells (CTC) are malignant cells found in peripheral blood that originate from primary or metastatic cancer tissue (Fig. 1) (2). Since circulating cancer cells are extremely rare, their detection requires enrichment techniques based on biological properties (e.g., tumor-specific cell surface antigens or protein secretion) or physical characteristics (e.g., size, density, deformability or electric charges) of tumor cells (2). Paoletti and colleagues used the CellSearch™ system, the only FDA-approved CTC enumeration technology for metastatic breast cancer, as the basis for their work. This technology uses epithelial cell-adhesion molecule-based (EpCAM) immuno-magnetic beads for enrichment of CTC, followed by positive immunofluorescence staining for a cytokeratin (CK) epithelial marker (CK-8, 18 and 19) and a nuclear dye (DAPI) and negative staining for common leukocyte antigen (CD45) (3).
Figure 1.
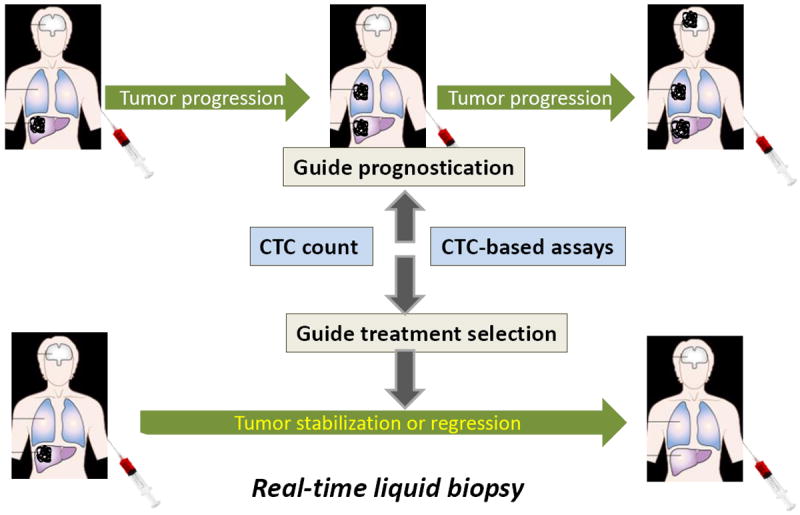
Circulating tumor cells (CTC) are malignant cells found in peripheral blood and their analysis could represent a real-time liquid biopsy. CTC count is prognostic for poor overall survival in metastatic breast cancer. The desired goal of CTC-based assays like CTC-ETI is to guide the selection of treatment of patients with metastatic breast cancer, so that breast cancer-related outcomes are improved.
Over the last decade, numerous clinical trials have investigated the prognostic role of CTC detection in MBC (3-5). A recent individual patient data meta-analysis, using 1944 MBC patients from 20 studies, confirmed the independent poor prognosis of an elevated CTC count of ≥5/7.5ml of whole blood in terms of progression-free survival and overall survival compared with a baseline CTC count <5/7.5ml (6). When compared to imaging studies, CTC assessment was more reproducible (inter-observer variability - 0.7%) and was an earlier marker of disease progression (4). In contrast to tumor markers such as CA15-3 and CA27.29, CTC does not reflect tumor bulk (4). The SWOG S0500 trial, investigating the strategy of changing chemotherapy in patients with MBC based on persistently elevated CTC, failed to show improved overall survival for an early CTC-driven switch in therapy compared with continuation of initial therapy (7).
Despite the results of SWOG 0500, CTC technology has the potential to be a significant part of our arsenal against MBC if it can be adapted to serve as a predictive assay to individualize therapy. However, intratumoral heterogeneity and clonal evolution of cancers, especially under the selective pressure of therapy, pose considerable challenges to attaining this goal (8, 9). Tissue biopsy at various stages of progression would be desirable but remains challenging. Investigators are now focused on assessment of CTC and circulating tumor-specific DNA (ctDNA) in blood as a means of performing real time “liquid biopsies” (2,10), in the hope that these tests will identify critical tumor characteristics along the disease continuum that will help predict the most effective therapy.
Several well-defined predictive markers, including estrogen receptor alpha (ER) and progesterone receptor expression, are routinely assessed in primary or metastatic tumor tissues to predict response to hormone agents. In an attempt to identify endocrine-resistant patients earlier and without tissue biopsy, Paoletti et al. developed a new CTC-based assay, CTC-ETI (1). They empirically chose four endocrine therapy-specific markers, Estrogen Receptor alpha (ER), B-Cell Lymphoma-2 (BCL-2), Human Epidermal Growth Factor Receptor 2 (HER2) and Ki67, assessable by immunofluorescence on CTC, to provide a numerical Bio-Score. They hypothesized that low ER and BCL-2 expression and high HER2 and Ki67 expression would predict resistance to endocrine therapy. Missing from this panel (for unstated reasons) was another well-established predictive marker, progesterone receptor (PR).
Using CTC enumeration and analysis of protein expression in human breast cancer cell lines, the group developed a CTC-ETI assay, which is a composite of CTC enumeration and Bio-Score. A CTC-Enumeration point reflected the average number of CTC from 4 aliquots of 7.5 ml of whole blood, whereas the Bio-Score is based on the percentage of CTC that were positive for that specific biomarker. Multiple logical but unproven assumptions allowed definition of CTC-ETI categories. The CTC-ETI was arbitrarily considered low if CTC count was less than 5/7.5ml of whole blood or if CTC-ETI is 0-3; intermediate if CTC-ETI was 4-6; and high if the score was 7-14. A high CTC-ETI was hypothesized to be suggestive of ET-resistant disease.
Paoletti and colleagues then conducted a two stage prospective study of 50 patients with MBC to test analytical validity of the CTC-ETI. A CTC-ETI was determined in 88% of patients; and the analytical failure rate was acceptable (12%). The inter-observer agreement for CTC-ETI was high with a concordance of >90% for all four biomarkers. Thus the test was highly reproducible and reliable, especially when the CTC count was higher. Since CTC yield is reduced with prior administration of systemic therapy, and the inter-observer variability is higher in patients with low CTC counts, the utility and reliability of CTC-ETI may be suspect in heavily pre-treated patients (11). Interestingly, 39% of the study population had a high CTC-ETI, suggesting that the test could identify a significant subset of patients who may have endocrine therapy-resistant disease. Since the ultimate goal of CTC-ETI is to guide personalized management of hormone receptor positive metastatic cancer, an even distribution of patients among the three pre-specified categories (low, intermediate and high) potentially enhances the clinical relevance of this assay.
Not surprisingly, the CTC-ETI assay identified significant intra-patient cell-to-cell heterogeneity in the intensity of staining as well as discordance with biomarker expression previously determined in primary or metastatic biopsy. Limitations of this study are absence of information about treatment and the lack of analysis of paired samples, i.e. a CTC-ETI assay performed at the same time as a metastatic biopsy. It is simply not known whether these discordant results reflect differences in tumor cells evaluated at two different points in time, or in the subset of metastatic cells that circulate versus those found in the metastasis, or in the methodologies used to assess biomarker expression by conventional immunohistochemistry of metastatic lesion versus CTC.
Nonetheless, Paoletti and colleagues successfully established the analytical validity of the CTC-ETI assay in a small cohort of patients and have now embarked on the necessary clinical validation through two randomized clinical trials (COMETI P2 and SWOG S1222). These clinical trials will help determine the correlation of CTC-ETI with clinical outcomes in patients with hormone receptor-positive MBC whose disease has progressed through several lines of endocrine therapy. Once the clinical validity of the assay is confirmed, clinical trials to assess capacity for early prediction of endocrine therapy resistance will be required to move the assay to the clinic.
It should be noted that development of CTC-ETI, a putative cell-based assay for endocrine resistance, occurred in parallel with the observation that ESR1 mutations can be detected in up 30% of metastatic breast cancers that recur in the presence of endocrine therapy, and that these mutations appear to confer hormone resistance (12). This finding has galvanized work to detect ESR1 mutations in ctDNA as a potential marker of endocrine resistance mediated by another mechanism. Efforts to move this finding into the clinic will require the same attention to rigorous methods and standards that are exemplified by this report of Paoletti et al. on the analytical validity of CTC-ETI. Given the heterogeneity of potential mechanisms of endocrine resistance, it is conceivable that the optimal test for endocrine resistance in the future will combine CTC- and ctDNA-based assays. This paper is a first step in the search for the Holy Grail of personalized medicine - a non-invasive test, which is reproducible, reliable, and predictive for response or resistance to cancer treatment.
Acknowledgments
Grant Support
N.E. Davidson was supported by the Breast Cancer Research Foundation and the NIH under award number P30CA047904.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Paoletti C, Muniz MC, Thomas DG, Griffith KA, Kidwell KM, Tokudome N, et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor positive breast cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-1913. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–8. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 3.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 4.Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 5.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 6.Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 7.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–9. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–13. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignatiadis M, Dawson SJ. Circulating tumor cells and circulating tumor DNA for precision medicine: dream or reality? Ann Oncol. 2014;25:2304–1. doi: 10.1093/annonc/mdu480. [DOI] [PubMed] [Google Scholar]
- 11.Ignatiadis M, Riethdorf S, Bidard FC, Vaucher I, Khazour M, Rothe F, et al. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res. 2014;16:R43. doi: 10.1186/bcr3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oesterreich S, Davidson NE. The search for ESR1 mutations in breast cancer. Nat Genet. 2013;45:1415–6. doi: 10.1038/ng.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]


