Abstract
Accumulation of toxic protein aggregates—amyloid-β (Aβ) plaques and hyperphosphorylated tau tangles—is the pathological hallmark of Alzheimer disease (AD). Aβ accumulation has been hypothesized to result from an imbalance between Aβ production and clearance; indeed, Aβ clearance seems to be impaired in both early and late forms of AD. To develop efficient strategies to slow down or halt AD, it is critical to understand how Aβ is cleared from the brain. Extracellular Aβ deposits can be removed from the brain by various clearance systems, most importantly, transport across the blood–brain barrier. Findings from the past few years suggest that astroglial-mediated interstitial fluid (ISF) bulk flow, known as the glymphatic system, might contribute to a larger portion of extracellular Aβ (eAβ) clearance than previously thought. The meningeal lymphatic vessels, discovered in 2015, might provide another clearance route. Because these clearance systems act together to drive eAβ from the brain, any alteration to their function could contribute to AD. An understanding of Aβ clearance might provide strategies to reduce excess Aβ deposits and delay, or even prevent, disease onset. In this Review, we describe the clearance systems of the brain as they relate to proteins implicated in AD pathology, with the main focus on Aβ.
Introduction
Alzheimer disease (AD) is the most common type of dementia and comprises early-onset AD (EOAD) and sporadic or late-onset AD (LOAD).1–3 EOAD affects a minority of AD patients, whereas LOAD afflicts over 95% of patients with AD.4–6 Both EOAD and LOAD are characterized by excessive accumulation of toxic forms of amyloid-β (Aβ), which has been hypothesized to result from an imbalance between its production and clearance.7–9 Emerging evidence suggests that Aβ clearance is impaired in both early-onset and late-onset forms of AD.10,11 Specifically, carriers of EOAD-associated presenilin mutations show both increased Aβ production10,12 and decreased Aβ clearance,10 whereas individuals with LOAD exhibit decreased Aβ clearance only.11
Failure of Aβ clearance is increasingly recognized in the pathogenesis of AD. It is critical to understand how Aβ is cleared from the brain, and to find new ways of investigating this process in carefully phenotyped patients and healthy controls. Because Aβ deposition can be increased in presymptomatic individuals years or even decades before the hallmark symptoms of AD manifest,20 an understanding of Aβ clearance might eventually provide strategies to reduce excess Aβ deposits and delay, or even prevent, disease onset.
Soluble Aβ can be removed from the brain by various clearance systems, including enzymatic degradation and cellular uptake, transport across the blood–brain barrier (BBB) and blood–cerebrospinal fluid barrier (BCSFB), interstitial fluid (ISF) bulk flow, and cerebrospinal fluid (CSF) absorption into the circulatory and lymphatic systems.
In the early 2000s, mouse studies demonstrated that the majority (75%) of extracellular Aβ (eAβ) is cleared by the BBB, with only a minority (10%) being cleared by ISF bulk flow.14,15 However, two-photon imaging studies from the past few years have suggested that ISF bulk flow—facilitated by astroglial aquaporin-4 (AQP4) channels and named the glymphatic (glial + lymphatic) system—contributes to a larger portion of eAβ clearance than previously thought.16,17 Furthermore, the discovery of meningeal lymphatic vessels suggests yet another potential clearance route.18 Although the relative contributions of each of these systems to overall clearance are unknown, they act together to drive eAβ from the brain, meaning that alterations in any given system can contribute to the altered pathophysiology and accumulation of lesions in AD.
In this Review, we aim to describe the brain’s clearance systems that are related to removal of toxic accumulation of proteins in AD. Here, ‘clearance’ is defined broadly as the removal of any substance, such as Aβ, from the brain. We focus on Aβ, given its ability to form aggregates within the extracellular space, but also briefly cover tau, which needs to be investigated in parallel with Aβ.
Background
Risk factors for AD
When characterized by autosomal dominant inheritance, EOAD is related to mutations in the presenilin 1 (PSEN1), presenilin 2 (PSEN2) or amyloid precursor protein (APP) genes.2–4 However, epidemiological data suggest that only a minority of EOAD cases demonstrate autosomal dominant transmission, leaving the genetic association of the majority of EOAD cases unexplained.2,19
Various factors have been reported to positively and negatively modulate the risk of LOAD. Specifically, the greatest overall risk factor for LOAD is ageing;20 for example, in the USA, over 40% of individuals above the age of 85 years have been diagnosed with AD.21–24 The strongest identified genetic risk factor for LOAD is the apolipoprotein E (APOE) ε4 allele (APOE*ε4),25,26 although genome-wide association studies have linked LOAD to several other genetic variants, such as TREM2 (triggering receptor expressed on myeloid cells 2),27 clusterin (CLU),28 and phosphatidylinositol-binding clathrin assembly protein (PICALM).28,29 Known environmental risk factors for LOAD include cardiovascular disease, and factors conferring a risk of cardiovascular disease, such as diabetes mellitus and hypertension. Head trauma, physical and mental inactivity, and sleep impairment are additional risk factors for LOAD.13,30–35
Pathological changes
AD is characterized by specific neuropathological and biomarker changes. The gross pathological changes consist of brain atrophy, particularly in the hippocampal formation, temporal lobes and parietotemporal cortices, accompanied by cortical thinning, enlarged ventricles and white matter abnormalities, as evident on MRI.36,37 Microscopic changes include accumulation of Aβ into parenchymal senile plaques (also known as neuritic plaques) or in the walls of cerebral capillaries and arteries (known as cerebral amyloid angiopathy, or CAA), as well as aggregation of hyperphosphorylated tau into intracellular neurofibrillary tangles (NFTs) and neuropil threads.36,38 The severity of CAA, the NFT load, and the magnitude of synapse loss—but not the number and extent of amyloid plaques—correlate well with the degree of cognitive decline.39–44 These AD-related neuro-pathological changes presumably occur decades before symptom onset, because they are also found in individuals with mild cognitive impairment (MCI), and in people with no cognitive symptoms.20
In vivo AD biomarkers
Recent advances now enable several AD-related brain changes to be detected in vivo: 18F-FDG-PET detects decreases in glucose metabolism,45,46 and MRI detects brain atrophy, as well as diffusion and perfusion abnormalities, which are most prominent in the vulnerable hippocampal formation and cortical regions.47–49 The pathological accumulation of Aβ and tau proteins in the brain can be inferred by analysing their levels in the CSF, with longitudinal changes having been described and modelled.50–53 Specifically, Aβ accumulation into extracellular plaques is marked by decreased CSF levels of Aβ1–42, and tau accumulation into NFTs is marked by increased CSF levels of total tau and hyperphosphorylated tau.51,54 In addition, PET can be used to assess Aβ brain accumulation directly,50 and PET for tau is currently under investigation.52,55
Clearance systems
The removal of soluble waste from the brain occurs via various overlapping clearance systems, which can be classified according to the compartment from which the waste is directly cleared, and the compartment into which the waste is directly cleared. Protein waste can be cleared from the intracellular compartment, or from the extracellular compartment, which comprises the ISF that surrounds neurons and the CSF that surrounds the brain. These proteins can then be removed by enzymes or cellular uptake, exported into the blood or lymph, or recirculated in the CSF (Table 1). The relative contributions of each of the various clearance systems are currently unknown; the prevailing view is that the BBB clearance predominates, though recent studies involving perivascular CSF circulation challenge this view, making clearance systems of the brain an important area for future research.
Table 1.
Clearance systems in the brain
| Clearance system | Source | Destination | Factors affecting clearance system | Clearance pathways |
|---|---|---|---|---|
| Blood–brain barrier clearance61 | ISF | Blood | Transporter expression and activity Ligand affinity and competition Vascular integrity |
Efflux transporters and mediators Influx transporters and mediators |
| Degradation clearance56 | ||||
| Intracellular | ICS | Degradation | Enzyme expression and activity Ligand affinity and competition Initiation of intracellular degradation pathways |
Ubiquitin–proteasome pathway Autophagy–lysosome pathway Endosome–lysosome pathway Proteases |
| Extracellular | ISF | Degradation or cellular uptake |
Enzyme expression and activity Ligand affinity and competition Activation of cellular uptake |
Proteases Glial phagocytes |
| ISF bulk fow clearance86 | ||||
| CSF sink | ISF | CSF sink (subarachnoid space, ventricles) |
Intrinsic ISF fow rate | ISF efflux into CSF sink |
| Perivascular drainage | ISF | Periarterial space to peripheral lymph |
APOE*ε4 Immune complex deposition Arterial age Arterial pulsation (hypothetical) |
ISF efflux into basement membrane of capillary and arterial walls |
| Perivascular glymphatic | ISF | Perivenous space to peripheral lymph or ventricles |
Molecular size Arterial pulsation AQP4 expression and localization Sleep |
CSF influx into periarterial space CSF–ISF exchange within interstitium CSF–ISF efflux along perivenous space |
| CSF absorption clearance18,98 | ||||
| Circulatory | CSF | Blood | CSF production BCSFB transporters Arachnoid villi resistance |
Arachnoid villi integrity and BCSFB efflux and influx ransporters and mediators |
| Lymphatic | CSF | Peripheral lymph | Lymphatic absorption of CSF | Perivascular space Perineural space |
| Meningeal lymphatic vessels | CSF | Lymph | Unknown | Subarachnoid CSF into meningeal lymphatic vessels |
Abbreviations: APOE*ε4, apolipoprotein E ε4 allele; AQP4, aquaporin-4; BCSFB, blood–CSF barrier; CSF, cerebrospinal fluid; ICS, intracellular space; ISF, interstitial fluid.
Degradation clearance
Degradation clearance is the enzymatic breakdown of proteins in the brain, and entails both extracellular and intracellular degradation. Extracellular degradation of ISF proteins mainly consists of degradation by proteases expressed and secreted by cells such as astrocytes.56,57 ISF proteins can also be taken up from the extracellular space to be degraded intracellularly in neurons or glia, including phagocytic microglia and astrocytes.58–60 Intracellular degradation of proteins occurs via the ubiquitin– proteasome pathway, the autophagy–lysosome pathway, and the endosome–lysosome pathway.56
Blood–brain barrier clearance
Interstitial proteins can be cleared into the blood directly at the BBB through specialized transport systems located in the brain endothelium.61–64 The BBB endothelial cells are connected by tight junctions and have two functionally distinct sides: the luminal side facing the blood circulation, and the abluminal side facing the brain parenchyma.65 In addition to the BBB, the brain is also protected by the so-called ‘glial barrier’ (also known as the glia limitans) that surrounds the BBB and consists of astroglial endfeet processes that cover the majority of the parenchymal vasculature, with the remaining area consisting of intercellular astrocytic endfeet clefts forming gap junctions.66 The BBB and glial barrier are part of the neurovascular unit, which comprises various components, including cerebral microvascular endothelium, basement membrane, contractile pericytes (which share the capillary basement membrane with the endothelium), smooth muscle cells (which invest the endothelium of precapillary arterioles), astroglia, and neurons.67
Transport at the neurovascular unit across the glial barrier and BBB depends on the solubility, molecular weight and diameter of the protein.68,69 The relatively large size of the intercellular clefts (20 nm)70,71 implies that the glial barrier is permeable to nearly all proteins.66 Given the size of AD-related proteins, monomeric Aβ1–40, Aβ1–42 and tau, should be able to pass freely through astrocytic endfeet clefts at the glial barrier.72 However, endothelial tight junctions at the BBB prevent free passage of Aβ and tau into the blood, so they must instead be transported across the endothelium by specialized transporters, which, as descried below, have been identified for Aβ, but not for tau.
Interstitial fluid bulk-flow clearance
ISF proteins can also be cleared directly into the CSF via ISF bulk flow73 that enters the CSF sink (described below) or the perivascular space (sometimes referred to the paravascular space—we use the term ‘perivascular space’ to describe the region surrounding the parenchymal vasculature).
Cerebrospinal fluid sink clearance
In parts of the body other than the CNS, lymphatic vessels run in parallel with the circulatory system to clear waste from the ISF in the form of lymph. Lymphatic vessels have recently been described in the meninges surrounding the mouse brain,18 but the brain parenchyma itself is devoid of such vessels, leading to the long-held assumption that CSF serves as a ‘lymph equivalent’ to clear waste from the CNS.74,75 Apart from transport across the endothelium, the removal of ISF from the brain parenchyma was traditionally believed to occur by diffusion76 or ISF bulk flow into the CSF sink, which comprises the ventricles and subarachnoid space.77 Given that diffusion is dependent on molecular size, diffusion into the CSF sink has been proposed to be too slow for the highly metabolic and large human brain.77 As such, ISF bulk flow, which is independent of molecular size, has been proposed as the predominant pathway for movement of large molecules into the CSF sink.77 ISF bulk flow was initially thought to course through the brain in a diffuse manner, but later evidence (described below) suggests the existence of definite pathways.78
Perivascular clearance
Support for an anatomically specific bulk-flow system came from a study of perivascular circulation.79 Following infusion of horseradish peroxidase into the lateral ventricles or subarachnoid space of anaesthetized cats and dogs, CSF within the subarachnoid space flowed freely through the Virchow–Robin space—a histologically defined space where the subarachnoid space meets the perivascular space. From the Virchow–Robin space, CSF travelled into the periarterial spaces that surround penetrating arteries, moving along specific pathways in the same direction as blood flow.79 CSF was also shown to move from the perivascular space into the interstitial ISF.79 This perivascular circulation hypothesis challenged the traditional model of one-way flow of ISF into the CSF sink.77,80,81
Mouse model82 and human83 studies involving fluorescent soluble tracers and confocal microscopy have demonstrated that following intracerebral injection, ISF solutes diffuse and enter perivascular drainage pathways along the basement membrane of capillary and arterial walls separating smooth muscle cells,84 then move towards the leptomeningeal arteries at the surface of the brain and, ultimately, to cervical lymph nodes (Figure 1).84 This pathway was named the perivascular drainage pathway, and was deemed to be the lymphatic drainage of the brain.44
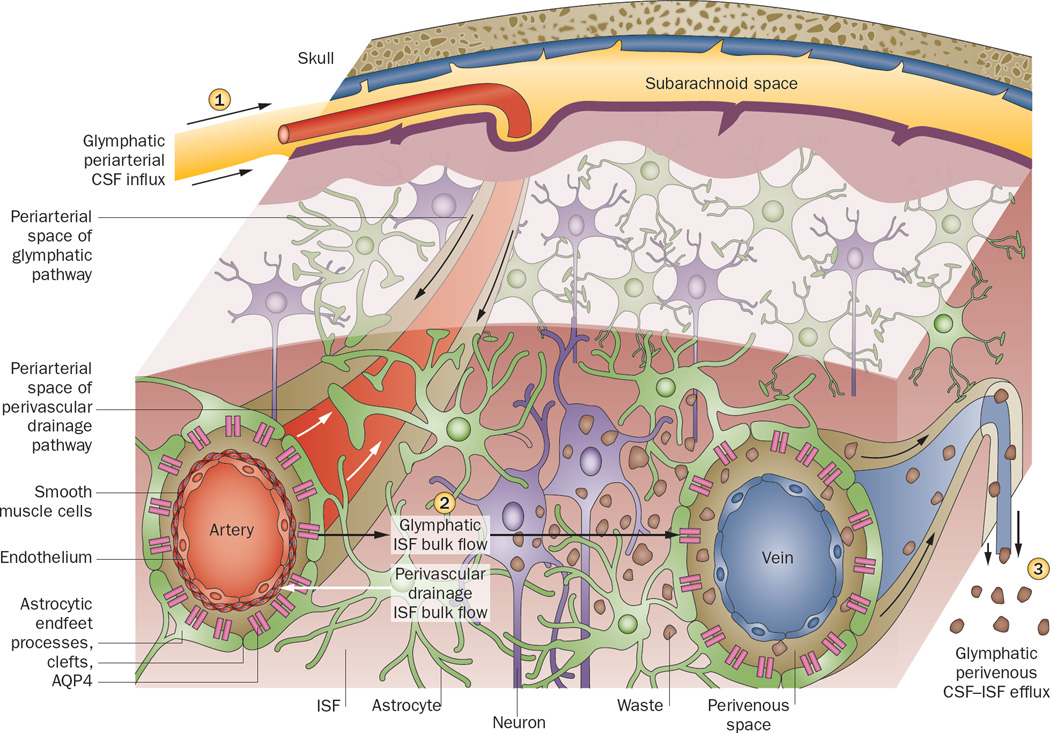
Figure 1.
Perivascular clearance comprises perivascular drainage and glymphatic pathways. The perivascular drainage Nature Reviews | Neurology pathway (white arrows) moves waste into the periarterial space (located along smooth muscle cells and the capillary basement membrane) and towards the subarachnoid space in the direction opposite to blood flow. The glymphatic pathway (black arrows) clears waste from the ISF through the brain parenchyma, and comprises three functional components. (1) CSF influx, unidirectionally with blood flow, into the periarterial space (between the basement membrane of smooth muscle cells and pia mater), where the water component of CSF crosses astrocytic AQP4 channels to enter the brain parenchyma. CSF solutes can be cleared with astroglial transporters or channels, or can pass through the astrocytic endfeet clefts. (2) CSF–ISF exchange within the brain parenchyma. (3) CSF–ISF movement into the perivenous space of deep-draining veins. Effluxed waste can then recirculate with the CSF, or eventually be absorbed into the lymphatic system. Arrows indicate direction of flow. Abbreviations: AQP4, aquaporin-4; CSF, cerebrospinal fluid; ISF, interstitial fluid. Permission obtained from Cell Press © Nedergaard, M. Science 340, 1529–1530 (2013).
The perivascular circulation hypothesis79 was recently confirmed and expanded on by a study in mice.16 Following tracer injection into the CSF at the cisterna magna, two-photon microscopy was used to visualize in real time the flux of CSF in living mice through a closed cranial window.16 As already hypothesized decades ago,74 CSF was found to act like lymph: it flushed out interstitial substances in a process facilitated by glial cells, prompting the authors to name it the glymphatic system (Figure 1).16 This evidence corroborated prior findings that CSF, driven by arterial pulsation, flows into the periarterial space, following the course of the arterial vascular smooth muscle basement membrane to reach the basal lamina of the brain capillary bed, and entering the interstitium at all levels of this perivascular route.79 The work also confirmed that ISF moves by bulk flow.77 Moreover, the study extended these findings to include a role for astroglial AQP4 channels. These channels were found to mediate CSF transport from the periarterial space across the pial–glial membrane into the interstitium, where it mixes with ISF. The pia has been shown to be relatively permeable:16,85 tracers injected into the subarachnoid space rapidly enter the perivascular space and brain parenchyma.16 CSF–ISF movement from the interstitium into the perivenous space of deep draining veins runs ventromedially towards ventricular and deep white matter structures.85 Hydrostatic pressure of periarterial bulk flow has been speculated to drive CSF water through the AQP4 channels, which is followed by astrocytic passage of molecules both through clefts and across astrocytes to maintain osmotic balance, although the mechanism has not been fully elucidated.16 Any remaining CSF components course along the capillary basal lamina.87,79
It is unclear whether the perivascular drainage pathway and the glymphatic pathway are in fact distinct pathways, or whether they simply reflect transport along the same pathway captured under differing physiological or experimental conditions. In another study using two-photon imaging, fluorescent tracers were injected directly into the mouse brain via an open skull. Periarterial tracer accumulation was observed, although the direction of the flow was not discerned.88 Opening of the skull is suggested to be a confounding factor in many experiments involving circulation of CSF, because skull removal can lead to inflammation and mechanical injuries to the cortical surface, or disturb local blood perfusion, BBB permeability and brain homeostasis.89–92 Regardless, as recently suggested, the perivascular drainage and glymphatic pathways are not mutually exclusive: both could be active depending on the conditions, and the pathway in use could even be different between vessels or within the same vessel at different times (Figure 1).86
Cerebrospinal fluid absorption clearance
Following clearance from the ISF into the CSF, proteins must be cleared from the brain. Circulating CSF can be absorbed directly into the circulatory or lymphatic systems.
Circulatory clearance
Although other CSF production sites have also been suggested,93–95 the majority of CSF seems to be produced at the BCSFB by the choroid plexus, a vascular unit of capillaries comprised of fenestrated endothelium and covered by choroid plexus epithelium (modified epen-dymal cells with tight junctions), located in the ventricles. The BCSFB serves not only as a CSF production site, but also as a ventricular CSF solute clearance site.96,97 According to the traditional view, following CSF production by the choroid plexus, CSF circulates within the subarachnoid space, from where it is primarily cleared from the brain at arachnoid villi (also known as arachnoid granulations)— one-way valve structures leading to the dural venous sinuses.98
Lymphatic clearance
As described above, circulating CSF within the perivascular space can be cleared from the brain to cervical lymph nodes.84,16 Another clearance route for circulating CSF to cervical lymph nodes is along perineural spaces, extensions of the subarachnoid space surrounding nerves.99,100 Furthermore, the recent development of a method for mounting of whole meninges, such that mouse meninges can be examined intact on a single slide, led to the discovery of meningeal lymphatic vessels, which might provide another clearance route for circulating CSF proteins.18 These meningeal lymphatic vessels might also provide a more conventional path for immune cells to exit the CNS, and dysfunction of these vessels might have important implications for neurological disorders associated with altered immune responses.18
Clearance of amyloid-β
Amyloid- β aggregation
Aβ is produced during neuronal activity101 from amyloid precursor protein (APP), a membrane protein that acts as a signalling receptor.101,102 In nonpathological conditions, APP is cleaved by α-secretase, which precludes formation of Aβ, and the resulting carboxy-terminal fragment is then cleaved by γ-secretase.103 The resulting products do not aggregate.104
If APP is first cleaved by β-secretase 1 (also known as BACE1) instead of α-secretase, the subsequent γ-secretase cleavage will result in soluble monomeric Aβ. The most common soluble monomeric isoforms of Aβ are Aβ1–40 (<80%), Aβ1–38 (<20%) and Aβ1–42 (10%).105 Aβ1–40 is prone to be deposited in the vasculature, as seen in CAA.15,106 Aβ1–38 is less likely to aggregate in either the vasculature or the brain than the other isoforms.107,108 Aβ1–42 has two additional amino acids, making it more hydrophobic than Aβ1–40;109 thus, it is capable of forming insoluble aggregates.110 The tendency of Aβ1–42 to form hard-to-clear aggregates is particularly increased when the concentration of Aβ1–42 is high, and at a lower pH.111–114
The different forms of Aβ are in a dynamic equilibrium, and dense amyloid plaques can slough off soluble monomeric Aβ1–42, which can then reform into aggregates.115 Reflecting the equilibrium, in a longitudinal study of individuals carrying EOAD-linked presenilin mutation, CSF Aβ levels were initially reduced owing to aggregation of Aβ1–42 into plaques.10 Impaired clearance can, thus, result from Aβ aggregation— especially aggregation of Aβ1–42—rather than from an intrinsic defect in the clearance system. Nonetheless, Aβ clearance systems can also become dysfunctional, as discussed below (Figure 2 and Table 2).
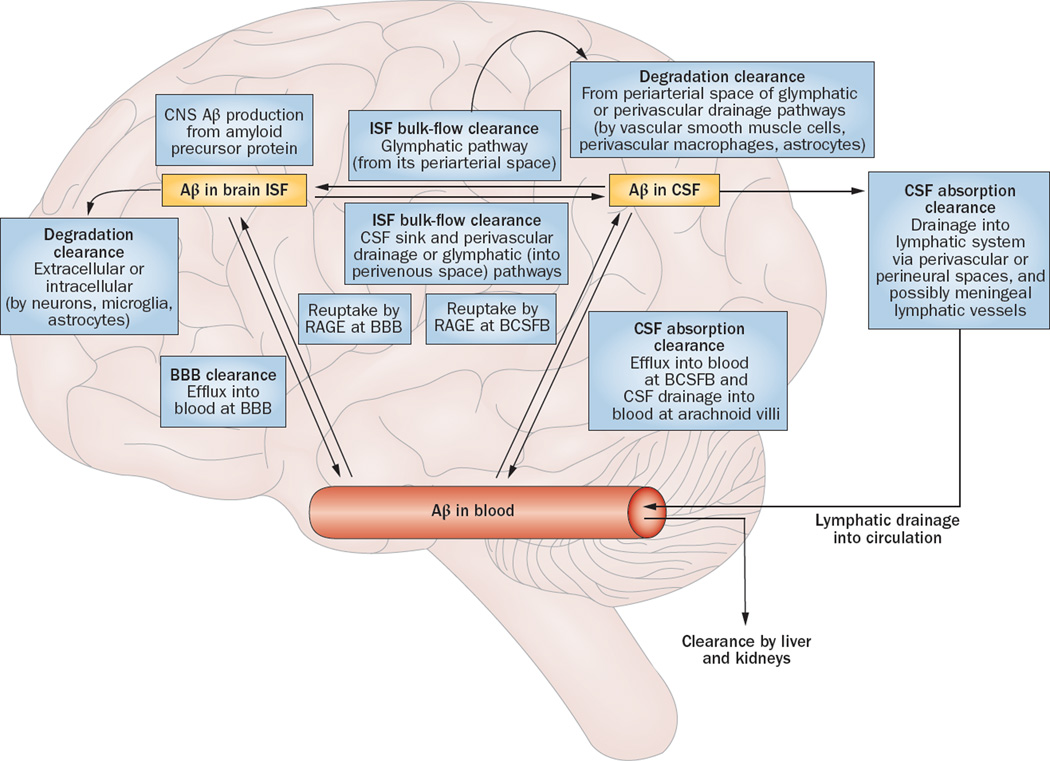
Figure 2.
Aβ clearance systems. Soluble Aβ can be removed from the brain by various clearance systems. Degradation clearance via extracellular and intracellular degradation pathways can involve either cellular uptake from the interstitium by neurons, microglia, and astrocytes, or uptake from the perivascular space by smooth muscle cells, perivascular macrophages, and astrocytes. BBB clearance involves Aβ efflux into the blood. ISF bulk flow clearance can occur into the CSF sink (ventricles and subarachnoid space), via perivascular drainage pathway, or via glymphatic pathway. CSF absorption clearance involves absorption either into the circulatory system from the arachnoid villi and BCSFB, or into the lymphatic system from the perivascular and perineural spaces—and possibly through meningeal lymphatic vessels. Abbreviations: Aβ, amyloid-β; BBB, blood–brain barrier; BCSFB, blood–CSF barrier; CSF, cerebrospinal fluid; ISF, interstitial fluid; RAGE, advanced glycosylation end productspecific receptor. Adapted with permission from Nature Publishing Group © Erickson, M. A. & Banks, W. A. J. Cerebr. Blood Flow & Metabol. 33, 1500–1513 (2013).
Table 2.
Clearance of Aβ and tau from the brain
| Clearance system | Aβ9,16,44,109,125 | Tau135,210 |
|---|---|---|
| Blood–brain barrier clearance | Majority of eAβ clearance LRP1 efflux ABCB1 efflux ApoE-mediated efflux α2M–mediated efflux LRP2-mediated efflux RAGE influx |
Unknown |
| Degradation clearance | ||
| Intracellular | Ubiquitin–proteasome pathway Autophagy–lysosome pathway Endosome–lysosome pathway Proteases |
Ubiquitin–proteasome pathway Autophagy–lysosome pathway Endosome–lysosome pathway Proteases |
| Extracellular | Proteases Glial phagocytosis |
Unknown |
| ISF bulk flow clearance | ||
| CSF sink | Contributes to eAβ clearance | Unknown |
| Perivascular drainage | Contribution % to eAβ clearance unknown |
Unknown |
| Perivascular glymphatic | Contributes to eAβ clearance (55–65%) Likely to facilitate blood–brain barrier clearance |
Might contribute to clearance of non-endocytosed tau |
| CSF absorption clearance | ||
| Circulatory | Arachnoid villi Blood–CSF barrier transporters (e.g. LRP1 efflux) |
Arachnoid villi |
| Lymphatic | CSF lymphatic absorption | Unknown |
Abbreviations: α2M, α2-macroglobulin; Aβ, amyloid- β; ABCB1, multidrug resistance protein 1 (also known as P-glycoprotein 1); ApoE, apolipoprotein E; CSF, cerebrospinal fluid; e-tau, extracellular tau; eAβ, extracellular Aβ; ISF, interstitial fluid; LRP, LDL receptor-related protein; RAGE, advanced glycosylation end product-specific receptor.
Degradation clearance
Intracellular Aβ (iAβ) can be degraded by proteasomes via the ubiquitin–proteasome pathway in neurons,116 lysosomal cathepsin enzymes,117 proteases (such as insulin-degrading enzyme, a thiol metalloendopeptidase that degrades monomeric Aβ) and insulin.118 Extracellular Aβ can also be degraded by proteases, such as neprilysin (a membrane-anchored zinc metalloendopeptidase that degrades the Aβ monomers Aβ1–40 and Aβ1–42, and Aβ oligomers),119 matrix metalloproteinases 2, 3 and 9,120 glutamate carboxypeptidase II,121 endothelin-converting enzyme,122 tissue plasminogen activator,123 plasmin,120 angiotensin-converting enzyme,120 and insulin-degrading enzyme.124 In addition, eAβ can be degraded following glial phagocytosis. Specifically, ISF Aβ can be taken up by microglia and astrocytes, whereas perivascular Aβ can be degraded by vascular smooth muscle cells, perivascular macrophages, and astrocytes (Figure 2).125
Degradation clearance of Aβ is affected by four main factors: enzyme expression and activity, ligand affinity and competition, activation of cellular uptake, and initiation of intracellular degradation pathways (Table 1), all of which become impaired with ageing and in AD. First, expression of neprilysin is decreased in AD,126 especially in regions with high Aβ loads such as the hippocampus and temporal gyrus.127 Although overall matrix metallo-proteinase 2 expression is increased in AD,58 its activity is reduced in astrocytes that surround Aβ plaques.128 Second, both Aβ and insulin are ligands that compete for degradation by insulin-degrading enzyme; thus, hyper-insulinaemia can reduce clearance of Aβ, which might partly explain the link between type 2 diabetes mellitus and AD.13 Third, plaques activate the immune effectors of the CNS—microglia and astrocytes129—inducing both phagocytosis of Aβ, which facilitates clearance from the extracellular space, and production of neurotoxic inflammatory cytokines.130 Aβ that has undergone cellular uptake can then be degraded, for example via the autophagy–lysosome pathway131 or be released back into the extracellular space,130 as found in the brains of patients with AD.132,133 Last, in AD, Aβ degradation via the endosome–lysosome pathway is increased relative to lysosomal degradation:134 endocytic activity is elevated, resulting in accumulation of autophagic vacuoles, presence of lysosomal cathepsin enzymes in Aβ plaques, and abnormally enlarged endosomes containing Aβ, leading to generalized proteasome dysfunction.134,135
Blood-brain barrier clearance
Mechanisms of amyloid-β influx and efflux
Aβ is transported from the interstitial space across the BBB and into blood, and vice versa (Figure 3).123 Specifically, local soluble Aβ is transferred from the interstitium to the brain by LDL receptor (LDLR) family members such as LRP1, and ATP-binding cassette transporters (ABC transporters).14,136 Some evidence suggests that LRP1 is the main transporter for Aβ efflux at the BBB, whereas other studies have demonstrated its role to be quite minor.137–139
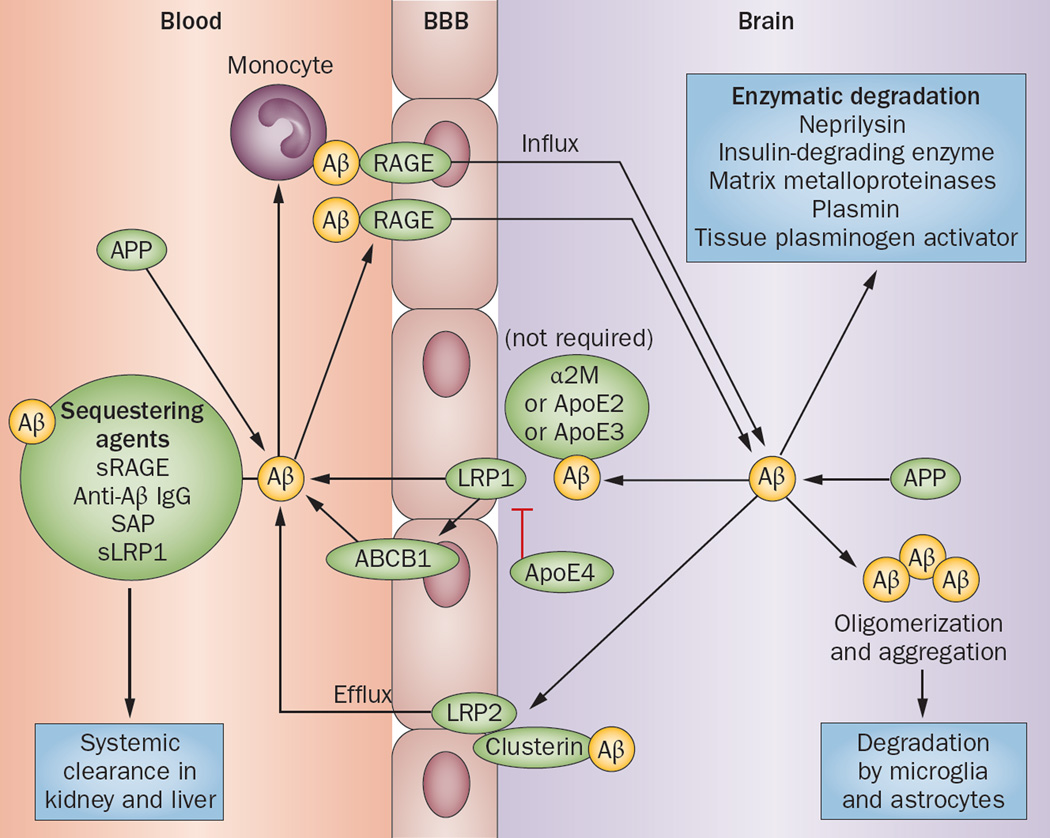
Figure 3.
Aβ efflux and influx through the BBB. Aβ can enter the brain via RAGE as a free plasma-derived peptide, or can be transported by monocytes. Sequestering agents (soluble transporters that chaperone Aβ for systemic degradation) can prevent Aβ entry from the circulation into the brain. Aβ is eliminated from the brain enzymatically or by transportation through the BBB. LRP1 mediates efflux of unbound Aβ and Aβ bound to ApoE2, ApoE3 or α2M from the brain parenchyma into the blood with the help of ABCB1; ApoE4 inhibits this transport process. Aβ bound to clusterin is transported through the BBB by LRP2. Abbreviations: α2M, α2-macroglobulin; Aβ, amyloid-β; ABCB1, multidrug resistance protein 1 (also known as P-glycoprotein 1); ApoE, apolipoprotein E; BBB, blood–brain barrier; LRP, LDL receptor-related protein; RAGE, advanced glycosylation end product-specific receptor; SAP, serum amyloid P; sLRP1, soluble LRP1; sRAGE, soluble form of RAGE. Permission obtained from Nature Publishing Group © Zlokovic, B. V. et al Nat. Rev. Neurosci. 12, 723–738 (2011).
The main ABC transporter responsible for Aβ efflux is ABCB1 (also known as P-glycoprotein 1 or MDR1), which directly exports Aβ into the circulation. ABCA1, which is located on the abluminal side of the brain endothelium,140 does not directly bind and extrude Aβ,141 but mediates Aβ clearance in an ApoE-dependent manner.142 The precise mechanism by which abluminal ABCA1 mediates Aβ clearance is unknown, although this transporter has been proposed to induce ApoE lipidation, which facilitates ApoE-Aβ interaction in the perivascular space, making Aβ more accessible to transport by LRP1 or ABCB1.143 Clearance of Aβ through the BBB is also mediated by α2-macroglobulin (α2M),14 and LDLR-related protein 2 (LRP2, also known as megalin) when LRP2 forms a complex with clusterin (also known as ApoJ).14,136 In addition, insulin-degrading enzyme has been proposed to have a role in Aβ clearance through the BBB, which might explain why BBB clearance is sensitive to insulin.144
Free Aβ can be transported from the circulation into the interstitium via RAGE (advanced glycosylation end product-specific receptor).136,145 Soluble transporters (also known as sequestering agents)—such as the soluble form of RAGE (sRAGE),14 anti-Aβ IgG,14 serum amyloid P component (SAP),14 and the soluble form of LRP (sLRP), which binds 70–90% of plasma Aβ—bind to soluble Aβ and inhibit its binding to RAGE, thereby preventing Aβ from entering the interstitium.146
Factors impairing amyloid-β clearance in AD
Clearance of Aβ through the BBB is affected by transporter expression and activity, ligand affinity and competition, and vascular integrity (Table 1). In AD, these factors are impaired in a number of ways. First, expression of the blood efflux transporters LRP1123 and ABCB1147 is decreased, whereas expression of the blood influx transporter RAGE is upregulated.123
Second, oxidative changes in AD are linked to changes in sLRP that reduce its affinity for Aβ, potentially facilitating Aβ influx into the interstitium by RAGE.123 Inflammation, a common feature of AD, can affect ligand affinity by making the pH more acidic, which promotes hyperphosphorylation of tau and induces conformational changes in Aβ that hinder its clearance.148,149 ApoE is a cholesterol transporter that competes with Aβ for efflux by LRP1 from the interstitium into the circulation;150 competition for shared receptors is the primary mechanism by which ApoE mediates Aβ clearance.151 The strongest genetic risk factor for AD is APOE*ε4152 (APOE*ε4>APOE*ε3>APOE*ε2151), which codes for an ApoE isoform that is less efficient at mediating Aβ clearance than are the other ApoE isoforms.153
Third, ApoE4 is also associated with lower antioxidant activity than other ApoE isoforms,154,155 and it mediates BBB breakdown through a proinflammatory pathway involving cyclophilin A in pericytes.156 These findings are in line with evidence suggesting that increased oxidative stress157 and loss of vascular integrity contribute to ageing158 and AD,159 as demonstrated by accelerated breakdown of the BBB and the neurovascular unit.
Interstitial fluid bulk-flow clearance
ISF bulk-flow clearance removes ISF—which contains eAβ—from the interstitium via ISF bulk flow into the CSF sink and perivascular space.16,44 Here, we will discuss perivascular clearance of Aβ specifically via the perivascular drainage and glymphatic pathways.
Perivascular drainage
Aβ is cleared along perivascular drainage pathways.83 In both AD44,160 and CAA44 (commonly associated with AD84), perivascular drainage of Aβ is impaired. Known factors affecting perivascular drainage of Aβ include APOE*ε4, deposition of immune complexes, arterial age, and—possibly—arterial pulsation (Table 1). The presence of ApoE4 is associated with reduced perivascular drainage of Aβ,161 which in turn is linked to deposition of immune complexes.162 Perivascular drainage of Aβ fails as arteries age;163 this failure is associated not only with loss of homeostasis164 and elevated levels of soluble Aβ in the brain, but also with accumulation of Aβ in arterial walls (as seen in CAA), which increases the risk of intracerebral lobar haemorrhages.165 One of the main complications following immunization against Aβ is the solubilization of Aβ from plaques and entrapment in perivascular drainage pathways, which worsens CAA.162,166 It is possible that arterial pulsation drives perivascular drainage of ISF solutes,88,167 and that morphological changes associated with age-related arteriosclerosis result in failure of perivascular drainage.168 Of note, a high-fat prenatal maternal diet has recently been reported to result in a failure of Aβ clearance along cerebrovascular basement membranes. This failure was exacerbated if the high-fat diet had been lifelong, suggesting a role for epigenetic changes and diet in AD pathogenesis.169,170
Glymphatic clearance
Recent mouse studies suggest that the AQP4-dependent glymphatic pathway is an important clearance system for driving the removal of soluble Aβ from the interstitium. In mice, Aβ is cleared along perivascular pathways, and Aβ clearance was reduced by 55–65% in Aqp4 knockout mice compared with wild-type mice.16,171 Furthermore, glymphatic clearance was reduced by 40% in aged relative to young mice,17 suggesting that the glymphatic pathway is impaired with age, which, as mentioned above, is the primary risk factor for LOAD.
Potential factors affecting glymphatic ISF bulk flow include molecular size, arterial pulsation, AQP4 expression and localization, and sleep (Table 1). Following subarachnoid injection, larger tracer molecules are slower to enter the parenchyma than are smaller tracers, and soluble perivascular Aβ can cross the 20 nm astrocytic endfeet clefts.16
Arterial pulsation is critical for perivascular circulation and transport of CSF into the interstitium.79,172,173 Recirculating Aβ-rich CSF within the periarterial space, might be taken up by vascular smooth muscle cells, particularly in the presence of glymphatic stasis (caused by reduced arterial pulsation) that could facilitate protein misfolding and aggregation.81,174–176 This is one mechanism by which Aβ might accumulate in the periarterial space, as seen in CAA, and the resulting Aβ accumulation might block perivascular pathways, further reducing glymphatic clearance.87
In Aqp4 knockout mice, interstitial clearance is reduced by about 70%, resulting in a 55–65% reduction in Aβ clearance.16,171 In AD, AQP4 expression could be decreased, given that in cultured mouse cortical astrocytes, interstitial Aβ1–42 reduces AQP4 expression,177 which can lead to additional accumulation of plaque-forming Aβ1–42.178 In traumatic brain injury (TBI)—a risk factor for AD— reactive gliosis is increased.178 Initially, AQP4 expression is increased in TBI, but long-lasting AQP4 mislocalization from perivascular endfeet to the astrocytic soma occurs, resulting in reduced perivascular AQP4 availability, which can reduce Aβ clearance.66,171 Both TBI and AD are associated with perivascular inflammation,16,66 and these changes might partly explain the link between these conditions.13
In mice, Aβ clearance during sleep is twice as fast as during awake periods.179 This increase in Aβ elimination is mediated by a 60% increase in the volume of the extracellular space, which might be modulated by a change in astrocyte cell volume in response to change in adrenergic signalling, as would be expected during sleep.179–182 This expansion of the extracellular space was caused by sleep itself rather than circadian rhythms, as it not only occurred during normal sleep, but could also be induced with anaesthesia.17,179 It should be noted, however, that circadian rhythm disturbances have been reported in patients with AD,183 and might affect clearance through a different mechanism involving increased oxidative stress caused by decreased expression of circadian clock genes, which are involved in protection from oxidative damage.184,185
The recent study describing the glymphatic system demonstrated that accelerated ISF-to-CSF bulk flow was partly responsible for the increase in total Aβ clearance during sleep, representing about 40% of total clearance, which can be calculated from the clearance rate constant data.179 The remaining 60% is probably attributable to accelerated BBB transport of Aβ, because during these transport clearance measurements, the degradation of AB was minimal, which is in line with previous reports.178,179 This finding might result from the glymphatic system flushing Aβ toward the BBB during sleep. Thus, sleep could indirectly increase BBB clearance of Aβ through increased glymphatic bulk flow, but it might also directly increase clearance through the BBB via various mechanisms, such as molecular changes (for example, upregulated LRP1), as seen with AD-protective physical and cognitive activity in mice.187–189 These findings might partly explain why sleep impairment increases the risk of AD.33,35
Cerebrospinal fluid absorption clearance
Aβ in the circulating CSF can be absorbed either through the arachnoid villi196 and BCSFB136 into the circulation, or through the perivascular16,44 and perineural spaces191— and possibly the meningeal lymphatics18—into the lymphatic system.
CSF absorption clearance of Aβ by the circulatory and lymphatic systems depends on CSF production, BCSFB integrity and transporters, arachnoid villi resistance, and lymphatic absorption of the CSF (Table 1). In ageing and AD, these factors are impaired in a number of ways. First, in ageing, and particularly in AD, CSF production by the choroid plexus is reduced, as shown by decreased water secretion into the ventricles via AQP1 water channels.192 In AD, the choroid plexus undergoes many structural changes, such as calcification, fibrosis and Aβ deposition, all of which can obstruct CSF production.193
Second, these structural changes affect BCSFB integrity, thereby reducing Aβ clearance. Many of the Aβ transporters expressed at the BBB, including LRP1, LRP2, ABCB1 and RAGE, are also found at the BCSFB.194 LRP1 is likely to have an important role in ventricular Aβ clearance at the BCSFB, given that the overall clearance rate of Aβ from the CSF is fivefold faster than the rate observed via CSF flow through the arachnoid villi.195 However, the age-related change in expression of many BCSFB transporters follows an opposite pattern to that observed at the BBB for Aβ, such that there is increased efflux and decreased influx transporter expression, which is suggested to be a result of the BCSFB compensating for age-dependent BBB transporter defects.136
Third, CSF outflow resistance at the arachnoid villi is increased in AD.196 This increased resistance is mechanistically similar to normal pressure hydrocephalus,196 and has been proposed to result from amyloid deposition and fibrosis at the arachnoid villi,197 resulting in decreased CSF bulk outflow and, thus, decreased CSF Aβ absorption into the blood. Although no evidence has yet been obtained that CSF Aβ levels initially increase in LOAD, reduced CSF turnover would be expected to result in heavily Aβ-laden recirculating CSF, subsequently resulting in reduced concentration as Aβ is deposited in plaques,111 in the vasculature as CAA,175 and in the meninges, thereby increasing outflow resistance at the arachnoid villi.98
Last, lymphatic absorption of CSF decreases with age98—the primary risk factor for LOAD. In EOAD, by contrast, overproduction of Aβ might result in increased absorption of Aβ by the lymphatic system, as demonstrated in a transgenic mouse model of AD, in which increasing Aβ levels in cervical and axillary lymph nodes mirrored increased Aβ levels in the brain.198
Clearance of tau
Tau—a splicing variant of the microtubule- associated protein tau (MAPT)—is an intracellular neuronal protein that stabilizes axons.199 Intracellular tau (i-tau) can undergo two transformations that are relevant to its clearance: modification and release. Tau modification is regulated by phosphorylation. i-Tau can undergo nondegradative cleavage by proteolytic enzymes, such as aminopeptidases, thrombin, HTRA1, calpain and caspases.135 Rather than degrading tau, these enzymes produce proteolytic fragments, which can ultimately be degraded; however, these fragments have an increased propensity to form aggregates, resulting in reduced clearance. In AD, i-tau is hyperphosphorylated, which induces the formation of insoluble NFTs that cannot readily be cleared, and can also be neurotoxic.200 Neuronal activation (namely, presynaptic glutamate release),201 neuronal death and increased i-tau concentration or aggregation202 trigger the release of i-tau into the extracellular space, leading to elevated CSF tau levels.
Tau clearance is less well understood than Aβ clearance, but also seems to be less complex. Transporters that specifically transport tau through the BBB have not been identified, which suggests that tau does not undergo clearance through the BBB, except after brain injury, when BBB permeability is temporarily increased.203 Instead, tau is thought to be cleared from the brain primarily by degradation, ISF bulk flow, and CSF absorption clearance (Table 2). Recent studies using passive immunization with anti-tau oligomer antibodies have shown that like Aβ, pathological tau can be cleared from the brain by a peripheral sink mechanism, indicating that enhancement of tau clearance might be a therapeutic strategy in AD.204
Degradation clearance
Tau is mainly cleared through intracellular degradation by lysosomes via the autophagy–lysosome pathway, and by proteasomes via the ubiquitin– proteasome pathway.202 AD-related dysfunction of these pathways has been suggested to result in the accumulation of soluble i-tau.135 i-Tau can also be degraded by proteases (such as caspases) in response to apoptosis- inducing stressors, and by calpain in response to elevated intracellular calcium concentrations. Phosphorylation of tau by protein kinase A increases its resistance to degradation by calpain; thus, AD-associated hyperphosphorylation of tau has been suggested to impair tau turnover and result in tau accumulation in the form of NFTs.205 Following release of i-tau into the extracellular space—a process that could result from neuronal death or stimulation202—e-tau can be internalized by other neurons via endocytosis, leading to prion-like spreading of tau pathology.206 In addition, soluble e-tau might bind to muscarinic type 1 and type 3 receptors, thereby increasing intracellular calcium levels, which might facilitate further release of i-tau.202 Tau released into the extracellular space is highly stable:207 its CSF half-life is 12–14 h,208 compared with about 2 h for Aβ.209
Interstitial fluid bulk-flow clearance
If e-tau is not cleared by endocytosis, it might be cleared via the glymphatic system.210 Following TBI, glymphatic clearance of ISF solutes was impaired by about 60% in wild-type mice, and to an even greater extent in Aqp4 knockout mice that displayed NFTs, neuroinflammatory reactive gliosis, and neurodegeneration.210 These findings support the link between TBI and tau aggregation, with resulting neurodegeneration similar to that seen in AD and chronic traumatic encephalopathy.210,211 Recirculation of CSF poses an additional challenge to tau clearance: cells closest to the periarterial boundary might internalize tau from the tau-laden recirculating CSF within the periarterial space.175
Cerebrospinal fluid absorption clearance
As for any soluble substance in circulating CSF, tau can be absorbed either into the circulatory system from the arachnoid villi and BCSFB, or from the lymphatic system through the perivascular and perineural spaces. Meningeal lymphatic vessels18 provide another possible route, although their specific contribution to tau elimination has not been tested.
Conclusions
Removal of proteins from the brain occurs via various overlapping clearance systems: enzymatic degradation and cellular uptake, transport across the BBB and BCSFB, ISF bulk flow, and absorption of CSF into the circulatory and lymphatic systems (Table 1). The majority of eAβ is cleared across the BBB, with a minority being cleared by ISF bulk flow (Figure 2 and Table 2).14,15 However, the recently discovered glymphatic pathway (Figure 1) also seems to be an important contributor to eAβ clearance, because it can flush Aβ towards the perivascular space, thereby mediating clearance through the BBB (Figure 3) or re-entry into the capillary basement membranes.16,17 The recently discovered meningeal lymphatic vessels might provide another clearance route,18 but their role in Aβ and tau clearance has not yet been assessed. In contrast with Aβ, tau does not seem to undergo receptor-mediated BBB clearance; thus, the elimination of tau seems less complex than that of Aβ. However, the mechanisms involved in the clearance of tau from the brain are not completely understood, and further research into tau trafficking could help us better understand its role in AD.
Recent evidence for Aβ accumulation in LOAD points to reduced clearance, as opposed to overproduction, as the main culprit, but which specific clearance systems are defective is unclear.10,11 The evidence for clearance failure in AD comes from the use of stable isotope labelling kinetics,212 which enable measurement of both Aβ production and clearance in humans. However, this technique does not provide information on the specific clearance systems themselves but, rather, provides a composite of production and clearance measures for Aβ. This technological progress has introduced an urgent need for detailed information on the specific Aβ clearance defects in patients with AD. Likewise, a technique to measure overall tau clearance and specific tau clearance defects in AD is needed, because such defects might be expected in light of the reduced CSF production and arachnoid villi defects that are observed in ageing individuals and patients with AD.
Because Aβ deposition can be increased in presymptomatic individuals years or even decades before the hallmark symptoms of AD manifest, an understanding of Aβ clearance might eventually provide strategies to restore clearance mechanisms, so as to eliminate excess Aβ deposits and delay or possibly even prevent disease onset. Whether the observed clearance defect in AD is a cause or a consequence of pathology, or merely coincidental, remains unknown. Regardless, Aβ clearance defects are a consistent finding in patients with AD, and might provide a useful biomarker and indicator of reversible clinical pathology.
Key points.
-
▪
Accumulation of neurotoxic forms of amyloid-β (Aβ) and tau proteins is the pathological hallmark of Alzheimer disease (AD)
-
▪
Excess deposition of Aβ results from an imbalance between its production and clearance; in both early-onset and late-onset forms of AD, Aβ clearance seems already impaired at the prodromal stage
-
▪
Aβ is removed from the brain by various overlapping and interacting clearance systems: degradation, blood-brain barrier (BBB) transport, interstitial fluid (ISF) bulk flow, and cerebrospinal fluid (CSF) absorption into the circulatory and peripheral lymphatic systems
-
▪
Although most extracellular Aβ undergoes BBB clearance, the recently discovered glymphatic pathway seems to be important for Aβ clearance
-
▪
Specific BBB transporters for tau have not been identified, suggesting that clearance of tau is less complex than that of Aβ, and mainly relies on degradation, ISF bulk flow, and CSF absorption
-
▪
Precise understanding of the mechanisms of clearance dysfunction in AD is paramount to develop strategies to reduce excess deposition of neuroxic protein and to halt the related pathological changes
Acknowledgements
The authors acknowledge the following grants: NIH/NIA/NHLBI AG022374, AG13616, AG12101 and AG008051 (to M.J.d.L.), HL118624 (to R.S.O), HL111724 (to L.G.), AG20245 and AG008051 (to T.W.), and NIH/NINDS NS028642 (to C.N.). K.B. has received funding from the Torsten Söderberg Foundation at the Royal Swedish Academy of Sciences, and H.Z. has received funding from the Swedish Research Council and the Knut and Alice Wallenberg Foundation.
Footnotes
Competing interests
K.B. and H.Z. are co-founders of Brain Biomarker Solutions.
The other authors declare no competing interests.
Author contributions
M.J.d.L., J.M.T.-C., R.O.C., R.S.O., T.B., H.R., C.N., B.V.Z., K.B., H.Z. and T.W. researched data for article. M.J.d.L., J.M.T.-C., R.O.C., B.V.Z., H.Z. and T.W. wrote the article. M.J.d.L. and J.M.T.C. provided substantial contributions to discussion of the content. All authors participated in reviewing and editing of the manuscript before submission.
References
- 1.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerreiro R, Hardy J. Genetics of Alzheimer’s disease. Neurotherapeutics. 2014;11:732–737. doi: 10.1007/s13311-014-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014;545:185–193. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Bruni AC, Conidi ME, Bernardi L. Genetics in degenerative dementia: current status and applicability. Alzheimer Dis. Ass. Disord. 2014;28:199–205. doi: 10.1097/WAD.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Aging, amyloid, and Alzheimer’s disease: a perspective in honor of Carl Cotman. Neurochem. Res. 2003;28:1705–1713. doi: 10.1023/a:1026065122854. [DOI] [PubMed] [Google Scholar]
- 8.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 9.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid β-peptide from brain: transport or metabolism? Nat. Med. 2000;6:718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 10.Potter R, et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci. Transl. Med. 2013;5:189ra77. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mawuenyega KG, et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiman EM, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case- control study. Lancet Neurol. 2012;11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Zlokovic BV, Frangione B. Transport-clearance hypothesis for Alzheimer’s disease and potential therapeutic implications. Madame Curie Bioscience Database. 2003 [online], http://www.ncbi.nlm.nih.gov/books/NBK5975/.
- 15.Shibata M, et al. Clearance of Alzheimer’s amyloid-β1-40 peptide from brain by LDL receptor- related protein-1 at the blood–brain barrier. J. Clin. Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kress BT, et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louveau AE, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. doi: 10.1038/nature14432. http://dx.doi.org/10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingo TS, Lah JJ, Levey AI, Cutler DJ. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 2012;69:59–64. doi: 10.1001/archneurol.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 23.Hetzel L. 65 Years and Over Population: 2000. Census 2000 Brief. DIANE Publishing; 2008. [Google Scholar]
- 24.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Potter H, Wisniewski T. Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. Int. J. Alzheimers Dis. 2012;2012:489428. doi: 10.1155/2012/489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol. Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Boutajangout A, Wisniewski T. The innate immune system in Alzheimer’s disease. Int. J. Cell Biol. 2013;2013 doi: 10.1155/2013/576383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harold D, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert JC, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Marco LY, et al. Modifiable lifestyle factors in dementia: a systematic review of longitudinal observational cohort studies. J. Alzheimers Dis. 2014 doi: 10.3233/JAD-132225. [DOI] [PubMed] [Google Scholar]
- 31.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picchioni D, Reith RM, Nadel JL, Smith CB. Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain Sci. 2014;4:150–201. doi: 10.3390/brainsci4010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat. Rev. Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spira AP, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Leon MJ, Bobinski M, Convit A, De Santi S. In: Neurobiology of Mental Illness. 1st edn. Charney DS, Nestler EJ, editors. Oxford University Press; 1999. pp. 698–714. Ch. 5. [Google Scholar]
- 38.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thal DR, et al. Pathology of clinical and preclinical Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(Suppl. 2):S137–S145. doi: 10.1007/s00406-013-0449-5. [DOI] [PubMed] [Google Scholar]
- 40.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 41.Blennow K, Bogdanovic N, Alafuzoff I, Ekman R, Davidsson P. Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the APOE4 allele. J. Neural Transm. 1996;103:603–618. doi: 10.1007/BF01273157. [DOI] [PubMed] [Google Scholar]
- 42.Giacobini E, Gold G. Alzheimer disease therapy—moving from amyloid-β to tau. Nat. Rev. Neurol. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 43.Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 44.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosconi L, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 46.Ferris SH, et al. Positron emission tomography in the study of aging and senile dementia. Neurobiol. Aging. 1981;1:127–131. doi: 10.1016/0197-4580(80)90005-6. [DOI] [PubMed] [Google Scholar]
- 47.de Leon MJ. In: Alzheimer: 100 Years and Beyond Illness. 1st edn. Jucker M, et al., editors. Springer; 2006. pp. 385–390. [Google Scholar]
- 48.de Leon MJ, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am. J .Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 49.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen AD, Klunk WE. Early detection of Alzheimer’s disease using PiB and FDG PET. Neurobiol. Disease. 2014 doi: 10.1016/j.nbd.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blennow K. CSF biomarkers for AD: state of the art and new developments. Neurobiol. Aging. 2014;35:S3–S3. [Google Scholar]
- 52.Harada R, et al. [18F]THK-5117 PET for assessing neurofibrillary pathology in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:1052–1061. doi: 10.1007/s00259-015-3035-4. [DOI] [PubMed] [Google Scholar]
- 53.Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79:915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 55.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015;14:114–124. doi: 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]
- 56.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid. Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 57.Yin K-J, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-β peptide catabolism. J. Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcock DM, et al. Microglial activation facilitates Aβ plaque removal following intracranial anti-Aβ antibody administration. Neurobiol. Dis. 2004;15:11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of Β-amyloid in cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guenette SY. Astrocytes: a cellular player in Aβ clearance and degradation. Trends Mol. Med. 2003;9:279–280. doi: 10.1016/s1471-4914(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 61.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 62.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enke phalin,D-alanine2 D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 63.Zloković BV, Lipovac MN, Begley DJ, Davson H, Rakić L. Transport of leucine- enkephalin across the blood-brain barrier in the perfused guinea pig brain. J. Neurochem. 1987;49:310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 64.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm. Res. 1995;12:1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 65.Hermann DM, ElAli A. The abluminal endothelial membrane in neurovascular remodeling in health and disease. Sci. Signal. 2012;5:re4. doi: 10.1126/scisignal.2002886. [DOI] [PubMed] [Google Scholar]
- 66.Thrane AS, Thrane VR, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014;37:620–628. doi: 10.1016/j.tins.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong AD, et al. The blood-brain barrier: an engineering perspective. Front. Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Felgenhauer K. Protein filtration and secretion at human body fluid barriers. Pflügers Arch. 1980;384:9–17. doi: 10.1007/BF00589509. [DOI] [PubMed] [Google Scholar]
- 70.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 71.Garai K, Crick SL, Mustafi SM, Frieden C. Expression and purification of amyloid-β peptides from Escherichia coli. Protein Expr. Purif. 2009;66:107–112. doi: 10.1016/j.pep.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang L, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Milford H. In: Studies in Intracranial Physiology and Surgery. 1st edn. Cushing H, editor. Oxford University Press; 1926. pp. 1–50. Ch. 1. [Google Scholar]
- 75.Loukas M, et al. The lymphatic system: a historical perspective. Clin. Anat. 2011;24:807–816. doi: 10.1002/ca.21194. [DOI] [PubMed] [Google Scholar]
- 76.Fenstermacher J, Patlak C. In: Fluid Environment of the Brain. 1st edn. Cserr H, editor. Academic Press; 1975. pp. 201–214. Ch. 12. [Google Scholar]
- 77.Cserr HF. Physiology of the choroid plexus. Physiol. Rev. 1971;51:273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- 78.Cserr H, Cooper D, Suri P, Patlak C. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 1981;240:F319–F328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 79.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 80.Pullen RG, DePasquale M, Cserr HF. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. Am. J. Physiol. 1987;253:F538–F545. doi: 10.1152/ajprenal.1987.253.3.F538. [DOI] [PubMed] [Google Scholar]
- 81.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 1984;246:F835–F844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 82.Carare R, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 83.Preston S, Steart P, Wilkinson A, Nicoll J, Weller R. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid β from the human brain. Neuropathol. Appl. Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 84.Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014;24:396–403. doi: 10.1111/bpa.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iliff JJ, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem. Res. doi: 10.1007/s11064-015-1581-6. http://dx.doi.org/10.1007/s11064-015-15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arbel-Ornath M, et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta Neuropathol. 2013;126:353–364. doi: 10.1007/s00401-013-1145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nimmerjahn A. Two-photon imaging of microglia in the mouse cortex in vivo. Cold Spring Harb. Protoc. doi: 10.1101/pdb.prot069294. http://dx.doi.org/10.1101/pdb.prot069294. [DOI] [PubMed] [Google Scholar]
- 90.Navari R, Wei E, Kontos H, Patterson J. Comparison of the open skull and cranial window preparations in the study of the cerebral microcirculation. Microvasc. Res. 1978;16:304–315. doi: 10.1016/0026-2862(78)90064-x. [DOI] [PubMed] [Google Scholar]
- 91.Bacskai BJ, et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat. Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 92.Kawamura S, et al. An improved closed cranial window technique for investigation of blood- brain barrier function and cerebral vasomotor control in the rat. Int. J. Microcirc. Clin. Exp. 1990;9:369–383. [PubMed] [Google Scholar]
- 93.Igarashi H, Tsujita M, Kwee IL, Nakada T. Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. Neuroreport. 2014;25:39–43. doi: 10.1097/WNR.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orešković D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 2010;64:241–262. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Bering EA., Jr Water exchange of central nervous system and cerebrospinal fluid. J. Neurosurgery. 1952;9:275–287. doi: 10.3171/jns.1952.9.3.0275. [DOI] [PubMed] [Google Scholar]
- 96.Johanson CE, et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wraith DC, Nicholson LB. The adaptive immune system in diseases of the central nervous system. J. Clin. Invest. 2012;122:1172–1179. doi: 10.1172/JCI58648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bradbury M, Cserr H, Westrop R. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 1981;240:F329–F336. doi: 10.1152/ajprenal.1981.240.4.F329. [DOI] [PubMed] [Google Scholar]
- 100.Bradbury M, Westrop R. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J. Physiol. 1983;339:519–534. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neve RL, McPhie DL. Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis. Biochim. Biophys. Acta. 2007;1772:430–437. doi: 10.1016/j.bbadis.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromol. Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 105.Zheng L, et al. Intracellular distribution of amyloid beta peptide and its relationship to the lysosomal system. Transl. Neurodegener. 2012;1:19. doi: 10.1186/2047-9158-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morris AW, Carare RO, Schreiber S, Hawkes CA. The cerebrovascular basement membrane: role in the clearance of β-amyloid and cerebral amyloid angiopathy. Front. Aging Neurosci. 2014;6:251. doi: 10.3389/fnagi.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mueller-Steiner S, et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 108.Moro ML, et al. APP mutations in the Aβ coding region are associated with abundant cerebral deposition of Aβ38. Acta Neuropathol. 2012;124:809–821. doi: 10.1007/s00401-012-1061-x. [DOI] [PubMed] [Google Scholar]
- 109.Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer’s disease. J. Mol. Neurosci. 2001;17:137–145. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- 110.Dawkins E, Small DH. Insights into the physiological function of the beta-amyloid precursor protein: beyond Alzheimer’s disease. J. Neurochem. 2014;129:756–769. doi: 10.1111/jnc.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 112.Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 2013;126:631–641. doi: 10.1007/s00401-013-1139-0. [DOI] [PubMed] [Google Scholar]
- 113.Grimm MO, et al. Neprilysin and Aβ clearance: impact of the APP intracellular domain in NEP regulation and implications in Alzheimer’s disease. Front. Aging Neurosci. 2013;5:98. doi: 10.3389/fnagi.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lührs T, et al. 3D structure of Alzheimer’s amyloid-β(1-42) fibrils. Proc. Natl. Acad. Sci. USA. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schnabel J. Amyloid: little proteins, big clues. Nature. 2011;475:S12–S14. doi: 10.1038/475S12a. [DOI] [PubMed] [Google Scholar]
- 116.Perez FP, et al. Late-onset Alzheimer’s disease, heating up and foxed by several proteins: pathomolecular effects of the aging process. J. Alzheimers Dis. 2014;40:1–17. doi: 10.3233/JAD-131544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L, Sheng R, Qin Z. The lysosome and neurodegenerative diseases. Acta Biochim. Biophys. Sin. (Shanghai) 2009;41:437–445. doi: 10.1093/abbs/gmp031. [DOI] [PubMed] [Google Scholar]
- 118.Querfurth HW, LaFerla FM. Mechanisms of disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 119.Iwata N, et al. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 120.Miners JS, et al. Aβ-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J. Neurochem. 2012;120:167–185. doi: 10.1111/j.1471-4159.2011.07510.x. [DOI] [PubMed] [Google Scholar]
- 122.Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer’s amyloid β peptide by endothelin-converting enzyme. J. Biol. Chem. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- 123.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qiu WQ, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 125.Yoon SS, Jo SA. Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol. Ther. (Seoul) 2012;20:245–255. doi: 10.4062/biomolther.2012.20.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang DS, Iwata N, Hama E, Saido TC, Dickson DW. Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2003;310:236–241. doi: 10.1016/j.bbrc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Yasojima K, McGeer E, McGeer P. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]
- 128.Lim NK, et al. Investigation of matrix metalloproteinases, MMP-2 and MMP-9, in plasma reveals a decrease of MMP-2 in Alzheimer’s disease. J. Alzheimers Dis. 2011;26:779–786. doi: 10.3233/JAD-2011-101974. [DOI] [PubMed] [Google Scholar]
- 129.Morrone CD, Liu M, Black SE, McLaurin J. Interaction between therapeutic interventions for Alzheimer’s disease and physiological Aβ clearance mechanisms. Front. Aging Neurosci. 2015;7 doi: 10.3389/fnagi.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cho MH, et al. Autophagy in microglia degrades extracellular beta-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy. 2014;10:1761–1775. doi: 10.4161/auto.29647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nagele RG, D’Andrea MR, Lee H, Venkataraman V, Wang HY. Astrocytes accumulate Aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- 133.Lee CD, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 2006;9:277–289. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- 135.Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front. Neurol. 2013;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pascale CL, et al. Amyloid-beta transporter expression at the blood–CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. doi: 10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ito S, Ohtsuki S, Terasaki T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-β peptide (1–40) across the rat blood–brain barrier. Neurosci. Res. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 138.Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-β peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J. Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- 139.Ito S, Ueno T, Ohtsuki S, Terasaki T. Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-β peptide(1–40) in mouse: involvement of an LRP-1-independent pathway. J. Neurochem. 2010;113:1356–1363. doi: 10.1111/j.1471-4159.2010.06708.x. [DOI] [PubMed] [Google Scholar]
- 140.Panzenboeck U, et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood–brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- 141.Akanuma S, et al. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-β peptide (1–40) at the blood–brain barrier. Neurochem. Int. 2008;52:956–961. doi: 10.1016/j.neuint.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 142.Fitz NF, et al. Abca1 deficiency affects Alzheimer’s disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J. Neurosci. 2012;32:13125–13136. doi: 10.1523/JNEUROSCI.1937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.ElAli A, Rivest S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease. Front. Physiol. 2013;4:45. doi: 10.3389/fphys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ito S, et al. Involvement of insulin-degrading enzyme in insulin- and atrial natriuretic peptide-sensitive internalization of amyloid-beta peptide in mouse brain capillary endothelial cells. J. Alzheimers Dis. 2014;38:185–200. doi: 10.3233/JAD-122077. [DOI] [PubMed] [Google Scholar]
- 145.Deane R, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 2012;122:1377. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J. Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pahnke J, Langer O, Krohn M. Alzheimer’s and ABC transporters—new opportunities for diagnostics and treatment. Neurobiol. Dis. 2014;72:A54–A60. doi: 10.1016/j.nbd.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cai ZY, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromol. Med. 2013;15:25–48. doi: 10.1007/s12017-012-8207-9. [DOI] [PubMed] [Google Scholar]
- 149.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 150.Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Verghese PB, et al. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl Acad. Sci. USA. 2013;110:E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for mpaired amyloid beta clearance in Alzheimer’s disease. Alzheimers Res. Ther. 2013;5:33. doi: 10.1186/alzrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miyata M, Smith JD. Apolipoprotein E allele- specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 155.Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochim. Biophys. Acta. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 156.Bell RD, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li M, Chen L, Lee DH, Yu L-C, Zhang Y. The role of intracellular amyloid β in Alzheimer’s disease. Prog. Neurobiol. 2007;83:131–139. doi: 10.1016/j.pneurobio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 158.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer’s disease. J. Alzheimers Dis. 2013;33:S87–S100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weller RO, et al. Cerebral amyloid angiopathy: amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am. J. Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hawkes CA, et al. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLoS ONE. 2012;7:e41636. doi: 10.1371/journal.pone.0041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carare RO, et al. Immune complex formation impairs the elimination of solutes from the brain: implications for immunotherapy in Alzheimer’s disease. Acta Neuropathol. Commun. 2013;1:48. doi: 10.1186/2051-5960-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hawkes CA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 164.Weller RO, Hawkes CA, Carare RO, Hardy J. Does the difference between PART and Alzheimer’s disease lie in the age-related changes in cerebral arteries that trigger the accumulation of Aβ and propagation of tau? Acta Neuropathol. 2015;129:763–766. doi: 10.1007/s00401-015-1416-1. [DOI] [PubMed] [Google Scholar]
- 165.Pezzini A, Padovani A. Cerebral amyloid angiopathy-related hemorrhages. Neurol. Sci. 2008;29:260–263. doi: 10.1007/s10072-008-0957-7. [DOI] [PubMed] [Google Scholar]
- 166.Sakai K, et al. Aβ immunotherapy for Alzheimer’s disease: effects on apoE and cerebral vasculopathy. Acta Neuropathol. 2014;128:777–789. doi: 10.1007/s00401-014-1340-9. [DOI] [PubMed] [Google Scholar]
- 167.Schley D, Carare-Nnadi R, Please C, Perry V, Weller R. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 2006;238:962–974. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 168.Hawkes CA, et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-β from the mouse brain. Aging Cell. 2013;12:224–236. doi: 10.1111/acel.12045. [DOI] [PubMed] [Google Scholar]
- 169.Hawkes CA, Gentleman SM, Nicoll JA, Carare RO. Prenatal high-fat diet alters the cerebrovasculature and clearance of β-amyloid in adult offspring. J. Pathol. 2015;235:619–631. doi: 10.1002/path.4468. [DOI] [PubMed] [Google Scholar]
- 170.Manousopoulou A, et al. Are you also what your mother eats? Distinct proteomic portrait as a result of maternal high-fat diet in the cerebral cortex of the adult mouse. Int. J. Obes. (Lond.) 2015 doi: 10.1038/ijo.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44:S93–S95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stoodley MA, Brown SA, Brown CJ, Jones NR. Arterial pulsation-dependent perivascular cerebrospinal fluid flow into the central canal in the sheep spinal cord. J. Neurosurg. 1997;86:686–693. doi: 10.3171/jns.1997.86.4.0686. [DOI] [PubMed] [Google Scholar]
- 173.Iliff JJ, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maurizi C. Recirculation of cerebrospinal fluid through the tela choroidae is why high levels of melatonin can be found in the lateral ventricles. Med. Hypotheses. 1991;35:154–158. doi: 10.1016/0306-9877(91)90041-v. [DOI] [PubMed] [Google Scholar]
- 175.Nedergaard M. Garbage truck of the brain. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 177.Yang W, et al. Aquaporin-4 mediates astrocyte response to β-amyloid. Mol. Cell. Neurosci. 2012;49:406–414. doi: 10.1016/j.mcn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 178.Ren Z, et al. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013;33:834–845. doi: 10.1038/jcbfm.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McKinley J, McCarthy A, Lynch T. Don’t lose sleep over neurodegeneration-it helps clear amyloid beta. Front. Neurol. 2013;4:206. doi: 10.3389/fneur.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mendelsohn AR, Larrick JW. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 2013;16:518–523. doi: 10.1089/rej.2013.1530. [DOI] [PubMed] [Google Scholar]
- 182.O’Donnell J, Ding F, Nedergaard M. Distinct functional states of astrocytes during sleep and wakefulness: is norepinephrine the master regulator? Curr. Sleep Med. Rep. 2015;1:1–8. doi: 10.1007/s40675-014-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu Y-H, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 184.Ju Y-E, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Musiek ES. Circadian clock disruption in neurodegenerative diseases: cause and effect? Front. Pharmacol. 2015;6:29. doi: 10.3389/fphar.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deane R, et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 187.Lin TW, et al. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015;118:189–197. doi: 10.1016/j.nlm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 188.Herring A, et al. Environmental enrichment counteracts Alzheimer’s neurovascular dysfunction in TgCRND8 mice. Brain Pathol. 2008;18:32–39. doi: 10.1111/j.1750-3639.2007.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Richter H, et al. Wheel-running in a transgenic mouse model of Alzheimer’s disease: protection or symptom? Behav. Brain Res. 2008;190:74–84. doi: 10.1016/j.bbr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 190.Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer’s. Mol. Neurodegener. 2013;8:38. doi: 10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Picken MM. The changing concepts of amyloid. Arch. Pathol. Lab. Med. 2001;125:38–43. doi: 10.5858/2001-125-0038-TCCOA. [DOI] [PubMed] [Google Scholar]
- 192.Nakada T, Igarashi H, Suzuki Y, Kwee I. Alzheimer patients show significant disturbance in water influx into CSF space strongly supporting β-amyloid clearance hypothesis [abstract S58.0001] Neurology. 2014;82(Suppl. 1) S58.001. [Google Scholar]
- 193.Serot JM, Zmudka J, Jouanny P. A possible role for CSF turnover and choroid plexus in the pathogenesis of late onset Alzheimer’s disease. J. Alzheimers Dis. 2012;30:17–26. doi: 10.3233/JAD-2012-111964. [DOI] [PubMed] [Google Scholar]
- 194.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fujiyoshi M, et al. Amyloid-β peptide(1–40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood–cerebrospinal fluid barrier. J. Neurochem. 2011;118:407–415. doi: 10.1111/j.1471-4159.2011.07311.x. [DOI] [PubMed] [Google Scholar]
- 196.Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2:506–511. doi: 10.1016/s1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 197.Silverberg G, Mayo M, Saul T, Fellmann J, McGuire D. Elevated cerebrospinal fluid pressure in patients with Alzheimer’s. Cerebrospinal Fluid Res. 2006;3:7. doi: 10.1186/1743-8454-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pappolla M, et al. Evidence for lymphatic Aβ clearance in Alzheimer’s transgenic mice. Neurobiol. Dis. 2014;71:215–219. doi: 10.1016/j.nbd.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shea T, Beermann M. Respective roles of neurofilaments, microtubules, MAP1B, and tau in neurite outgrowth and stabilization. Mol. Biol. Cell. 1994;5:863–875. doi: 10.1091/mbc.5.8.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Binder LI, Guillozet-Bongaarts AL, Garcia- Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 201.Yamada K, et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Avila J, Simon D, Diaz-Hernandez M, Pintor J, Hernandez F. Sources of extracellular tau and its signaling. J. Alzheimers Dis. 2014;40(Suppl. 1):S7–S15. doi: 10.3233/JAD-131832. [DOI] [PubMed] [Google Scholar]
- 203.Irazuzta JE, de Courten-Myers G, Zemlan FP, Bekkedal MY, Rossi J., 3rd Serum cleaved tau protein and neurobehavioral battery of tests as markers of brain injury in experimental bacterial meningitis. Brain Res. 2001;913:95–105. doi: 10.1016/s0006-8993(01)02764-0. [DOI] [PubMed] [Google Scholar]
- 204.Castillo-Carranza DL, et al. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J. Neurosci. 2014;34:4260–4272. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Litersky JM, Johnson G. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J. Biol. Chem. 1992;267:1563–1568. [PubMed] [Google Scholar]
- 206.Medina M, Avila J. The role of extracellular tau in the spreading of neurofibrillary pathology. Front. Cell. Neurosci. 2014;8:13. doi: 10.3389/fncel.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gómez-Ramos A, et al. Characteristics and consequences of muscarinic receptor activation by tau protein. Europ. Neuropsychopharmacol. 2009;19:708–717. doi: 10.1016/j.euroneuro.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 208.David DC, et al. Proteasomal degradation of tau protein. J. Neurochemist. 2002;83:176–185. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 209.Cirrito JR, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Iliff JJ, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Plog BA, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bateman RJ, et al. Quantifying CNS protein production and clearance rates in humans using in vivo stable isotope labeling, immunoprecipitation, and tandem mass spectrometry. Nat. Med. 2006;12:856. [Google Scholar]