Abstract
Anemia is associated with poor prognosis in patients hospitalized with acute decompensated heart failure (ADHF). Whether the impact of anemia differs by heart failure with preserved (HFpEF) or reduced (HFrEF) ejection fraction is uncertain. We examined hospital surveillance data captured by the Atherosclerosis Risk in Communities Study from January 1, 2005 – December 31, 2010. Diagnoses of ADHF were validated by standardized physician review of the medical record. Anemia was classified using WHO criteria (<12 g/dL for women, < 13 g/dL for men), and heart failure type was determined by the ejection fraction (<40% for HFrEF, ≥ 40% for HFpEF). Hospital length of stay and 1-year mortality outcomes were analyzed by multivariable regression, weighted to account for the sampling design, and adjusted for demographics and clinical covariates. Over 6 years, 15,461 (weighted) hospitalized events for ADHF (59% HFrEF) occurred in the ARIC catchment, based on 3,309 sampled events. Anemia was associated with a mortality hazard ratio of 2.1 (95% CI: 1.6 – 2.7) in patients classified with HFpEF, and 1.4 (95% CI: 1.1 – 1.7) among those with HFrEF; p for interaction = 0.05. The mean increase in length of hospital stay associated with anemia was 3.5 days (95% CI: 3.4 – 3.6) for patients with HFpEF, compared with 1.8 days (95% CI: 1.7 – 1.9) for those with HFrEF; p for interaction <0.0001. In conclusion, the incremental risks of death and lengthened hospital stay associated with anemia are more pronounced in ADHF patients classified with HFpEF than HFrEF.
Keywords: Heart failure, Anemia, Epidemiology
Introduction
Anemia is a common comorbidity of heart failure (HF), and has been associated with poor functional status1, longer hospitalizations2, re-hospitalizations3, and death4. In clinical trials, the benefit of anemia treatment to HF patients has been questionable5-7. However, most research has focused on HF with reduced ejection fraction (HFrEF), and there is some evidence to suggest the impact of anemia may differ by HF type8. This trend has not been consistently reported though9, 10, and few studies have examined outcomes in patients hospitalized with acute decompensated heart failure (ADHF). We investigated whether the mortality risk and length of hospital stay (LOS) associated with anemia would differ by HF type, by analyzing a population-based sample of ADHF hospitalizations captured by the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Since 2005, the ARIC study has conducted population-based retrospective surveillance of hospitalized events in Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and 8 northwest suburbs of Minneapolis, Minnesota. Surveillance eligibility is restricted to residents 55 years of age or older, with a hospitalization spanning at least one day and, for the purposes of our analysis, a discharge date between January 1, 2005 – December 31, 2010. Hospitalizations with any discharge codes for congestive HF, rheumatic heart disease, hypertensive heart disease, acute cor pulmonale, chronic pulmonary heart disease, cardiomyopathies, acute edema of lung, or dyspnea were randomly sampled, using pre-specified sampling fractions within strata of ARIC communities, ICD-9 code (428.x or all other eligible codes), age (55-74, 75-84, or ≥85), sex, and race (black or white).
Hospitalized medical records indicating signs or symptoms of HF were fully abstracted and reviewed by ARIC physicians, as previously described11. Using standardized criteria, hospitalizations were classified as definite ADHF, probable ADHF, stable chronic HF, not HF, or unclassifiable; based on diagnostic reports from the hospital record, physician notes, and discharge summaries. ADHF was differentiated from stable, chronic HF by evidence of new onset or worsening signs or symptoms.
Hemoglobin and serum creatinine were abstracted from the medical record, by recording the lowest and last values over the course of the hospitalization. To minimize potential confounding by hemodilution12, the last values were used to determine anemia and glomerular filtration rate. For the purposes of our analysis, anemia was defined by World Health Organization criteria (hemoglobin < 12 g/dL for women and < 13 g/dL for men). Polycythemia was considered a hemoglobin value ≥ 17 g/dL. Glomerular filtration rate was estimated by the Chronic Kidney Disease Epidemiology Collaboration formula13.
HF type was determined by the abstracted ejection fraction, from either inpatient diagnostic tests, or when absent, pre-admission imaging studies. The majority of abstracted ejection fractions (71%) were in-hospital, and of these, 96% were based on transthoracic echocardiograms. Consistent with previous analyses of hospitalized ADHF and anemia8, 14-16, HFpEF was classified by a normal or mildly decreased systolic function (ejection fraction ≥ 40%) whereas HFrEF was considered an ejection fraction < 40%.
Length of stay (LOS) was calculated by subtracting the admission date from the discharge date, excluding transfers to and from another hospital. Mortality outcomes were ascertained for up to one year post-admission, by linking hospital records with death files.
Over six years, 6,291 hospitalizations were sampled and abstracted. Of these, 4,271 were classified as definite or probable ADHF. We omitted hospitalizations with no abstracted hemoglobin values (n = 33) or ejection fraction (n = 576), and patients transferred to or from another hospital (n=77). In addition, we excluded patients receiving dialysis (n = 337), as this represents a distinct subgroup of anemic HF, and patients identified with polycythemia (n = 19), due to the known U-shaped relation between hemoglobin and mortality risk17-19. After exclusions, 3,309 unweighted events remained in analysis, corresponding to 15,461 weighted hospitalizations for definite (79%) or probable (21%) ADHF.
All analyses were performed using SAS 9.3 (SAS Institute; Cary, NC) and weighted by the inverse of the sampling probability. Proportions were compared using Rao-Scott χ2, and means were compared using analysis of variance. Correlations of hemoglobin with ejection fraction, age and GFR were assessed by Pearson regression. The crude incidence of death was examined by Kaplan-Meier regression. Adjusted mortality hazard ratios were examined using Cox regression, and associations with length of hospital stay were analyzed by linear regression. All multivariable regression models were adjusted for age, race, sex, and clinical covariates associated with anemia (glomerular filtration rate, smoking, systolic blood pressure, diastolic blood pressure, diabetes, coronary heart disease, beta blockers, and diuretics). Interactions were analyzed by entering the cross product of anemia and HF type, or the cross product of hemoglobin and HF type, into the fully adjusted models. Significance of interactions was assessed by Wald χ2 tests, using a threshold of α=0.10, due to reduced power to detect interaction20.
Results
All results are weighted to account for the sampling design, unless otherwise indicated. Of 15,461 hospitalizations with verified ADHF, 6,414 (41%) were classified as HFpEF, and 9,047 (59%) as HFrEF. The mean age at discharge was 76 years; approximately half (52%) were women, and nearly one third (29%) were black. The overall anemia prevalence was 70%, and did not differ by HF type (p=0.4). In unadjusted analyses, hemoglobin was positively correlated with glomerular filtration rate (r = 0.21; p<0.0001) and negatively correlated with age (r = −0.11; p<0.0001) and ejection fraction (r = −.15; p<0.001).
Patients classified with HFpEF were more often women (61% vs. 46%, p<0.0001), white (74% vs. 69%, p=0.003), and older (76 vs. 75 years, p=0.02) than those with HFrEF. Hypertension was more prevalent with HFpEF (86% vs. 83%, p=0.02), however myocardial infarction was less common (20% vs. 33%, p<0.0001). As shown in Table 1, gender-specific mean hemoglobin levels were similar among patients with HFpEF and HFrEF. In univariate analyses, anemia was strongly associated with race, kidney function, blood pressure, smoking, and history of coronary heart disease for both HF types (p<0.05 for each). However, diabetes was associated with anemia only in HFpEF patients, while use of diuretics correlated with anemia only in those with HFrEF.
Table 1.
Weighted demographics and characteristics of hospitalized acute decompensated heart failure patients sampled by the ARIC Study community surveillance (2005 - 2010), stratified by heart failure type and anemia status
| Characteristic |
HFpEF
|
HFrEF
|
||
|---|---|---|---|---|
| Anemia (n=4,566) | No Anemia (n=1,848) | Anemia (n=6,289) | No Anemia (n=2,758) | |
| No. (%) or Mean ± SEM | No. (%) or Mean ± SEM | |||
| Female* | 2,675 (59%) | 1,253 (68%) | 2,844 (45%) | 1,341 (49%) |
| Black*† | 1,297 (28%) | 356 (19%) | 1,826 (29%) | 944 (34%) |
| Age (years)† | 77 ± 0.3 | 76 ± 0.4 | 76 ± 0.2 | 73 ± 0.3 |
| Hemoglobin (g/dL) | ||||
| Women | 10.3 ± 0.05 | 13.1 ± 0.06 | 10.4 ± 0.04 | 13.2 ± 0.06 |
| Men | 10.6 ± 0.08 | 14.2 ± 0.07 | 10.9 ± 0.05 | 14.1 ± 0.05 |
| Creatinine (mg/dL)*† | 1.6 ± 0.03 | 1.2 ± 0.02 | 1.6 ± 0.02 | 1.3 ± 0.02 |
| GFR (mL/min/1.73 m2)*† | 48 ± 0.8 | 58 ± 0.9 | 48 ± 0.6 | 57 ± 0.8 |
| BMI (kg/m2) | 31 ± 0.3 | 31 ± 0.7 | 28 ± 0.2 | 29 ± 0.3 |
| Systolic BP (mmHg)*† | 146 ± 1.1 | 148 ± 1.4 | 135 ± 0.9 | 142 ± 1.2 |
| Diastolic BP (mmHg)*† | 75 ± 0.7 | 80 ± 0.8 | 75 ± 0.6 | 83 ± 0.8 |
| Ejection Fraction (%)† | 54 ± 0.3 | 55 ± 0.4 | 34 ± 0.4 | 29 ± 0.5 |
| Coronary Heart Disease*† | 2,078 (46%) | 690 (37%) | 3,189 (51%) | 1,272 (46%) |
| Myocardial Infarction | 975 (21%) | 337 (18%) | 2,109 (34%) | 878 (32%) |
| Hypertension* | 4,414 (88%) | 1,525 (83%) | 5,240 (83%) | 2,260 (82%) |
| Current Smoker*† | 487 (11%) | 317 (17%) | 799 (13%) | 540 (20%) |
| COPD | 1,406 (31%) | 636 (34%) | 2,215 (35%) | 890 (32%) |
| Diabetes* | 2,350 (51%) | 561 (30%) | 2,953 (47%) | 1,183 (43%) |
| ACE Inhibitors | 1,727 (38%) | 667 (36%) | 2,555 (41%) | 1,235 (45%) |
| Beta blockers* | 2,914 (64%) | 1,050 (57%) | 4,362 (69%) | 1,790 (65%) |
| ARB | 648 (14%) | 333 (18%) | 851 (14%) | 360 (13%) |
| Diuretics† | 3,142 (68%) | 1,208 (65%) | 4,820 (77%) | 1,991 (72%) |
| Statins | 1,964 (43%) | 745 (40%) | 3,023 (48%) | 1,187 (43%) |
| Mean stay (days)*† | 8.9 ± 0.3 | 5.6 ± 0.2 | 7.5 ± 0.2 | 5.7 ± 0.2 |
| In-hospital Mortality*† | 348 (8%) | 50 (3%) | 517 (8%) | 83 (3%) |
| 28 day Mortality*† | 563 (12%) | 122 (7%) | 824 (13%) | 250 (9%) |
| 365 day Mortality*† | 1,652 (36%) | 379 (21%) | 2,724 (43%) | 800 (29%) |
significantly associated with anemia in patients with HFpEF (p<0.05)
significantly associated with anemia in patients with HFrEF (p<0.05)
HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, GFR = glomerular filtration rate, BMI = body mass index, BP = blood pressure, COPD = chronic obstructive pulmonary disease, ACE = angiotensin converting enzyme, ARB = angiotensin receptor blocker
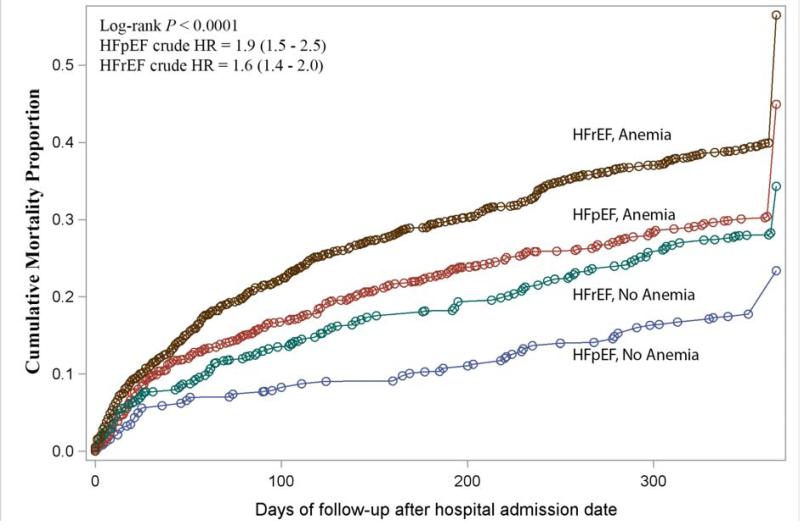
Overall, HFrEF was associated with greater mortality. In both types of HF, anemia was related to a higher incidence of death, and those with HFrEF and anemia fared worst (Figure 1). However, in relative analyses comparing outcomes of anemic with non-anemic HFpEF patients, and anemic with non-anemic HFrEF patients, the incremental risk of death was higher with HFpEF. After adjustments, anemia was associated with more than twice the risk of death in patients with HFpEF (HR = 2.1, 95% CI: 1.6 – 2.7), but only a 40% higher risk in those with HFrEF (HR = 1.4, 95% CI: 1.1 – 1.7); p for interaction = 0.05. As shown in Table 2, a slightly higher relative risk of death was also observed for HFpEF (HR = 1.2, 95% CI: 1.1 – 1.3) compared with HFrEF (HR = 1.1, 95% CI: 1.0 – 1.2), when examining mortality risk per 1 g/dL decrement of hemoglobin.
Figure 1.
Unadjusted cumulative mortality one year following hospital admission date, stratified by heart failure type and anemia status
HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio of death associated with anemia
Table 2.
Adjusted risks associated with anemia status and hemoglobin (per 1 g/dL decrement) in patients hospitalized with acute decompensated heart failure, stratified by heart failure of preserved and reduced ejection fraction
| Model* | Estimate | P-value for interaction† |
|---|---|---|
| 1-Year mortality (Hazard ratio, 95% CI) | ||
| Anemia vs. No Anemia | 0.05 | |
| HFpEF | 2.1 (1.6 – 2.7) | |
| HFrEF | 1.4 (1.1 – 1.7) | |
| Hemoglobin (per 1 g/dL decrement) | 0.08 | |
| HFpEF | 1.2 (1.1 – 1.3) | |
| HFrEF | 1.1 (1.0 – 1.2) | |
| Length of stay increase (Days, 95% CI) | ||
| Anemia vs. No Anemia | < 0.0001 | |
| HFpEF | 3.5 (3.3 – 3.6) | |
| HFrEF | 1.8 (1.7 – 1.9) | |
| Hemoglobin (per 1 g/dL decrement) | < 0.0001 | |
| HFpEF | 1.2 (1.1 – 1.2) | |
| HFrEF | 0.7 (0.6 – 0.7) | |
Models adjusted for age, sex, race, glomerular filtration rate, smoking, systolic blood pressure, diastolic blood pressure, diabetes, coronary heart disease, beta blockers use, and diuretics use
Interaction terms = (anemia × heart failure type) in anemia models and (hemoglobin × heart failure type) in hemoglobin models
On average, patients with HFpEF were hospitalized 1 day longer than those with HFrEF (8.0 vs. 7.0 days, p=0.0009). Anemic patients had longer stays regardless of HF type, but those with HFpEF had the lengthiest time to discharge (8.9 vs. 7.5 days, p<0.0001). After adjustments, the absolute increase in mean LOS associated with anemia was 3.5 (3.3 – 3.6) days for patients with HFpEF, compared to 1.8 (1.7 – 1.9) days for those with HFrEF; p for interaction < 0.0001. The association remained robust when examining hemoglobin as a continuous measure. For each 1 g/dL decrement of hemoglobin, hospital stay was lengthened by 1.2 (1.1 – 1.2) days for patients with HFpEF, and 0.7 (0.6 – 0.7) days for those with HFrEF. When the analysis was restricted to patients discharged alive, the estimates were unchanged (data not shown).
Discussion
In this population-based sample of patients hospitalized with ADHF, anemia was associated with both greater mortality risk and longer hospital stays. We observed significantly stronger associations in patients with HFpEF, which remained robust when examining hemoglobin as a continuous measure.
Many studies have established anemia as risk factor for death in HF patients; however, comparisons of risk by HF type have been inconsistent for hospitalized ADHF. In a study from the National Heart Care Project involving 50,405 patients hospitalized with HF, slightly higher adjusted mortality risks were observed in patients with lower hematocrit levels, independent of left ventricular ejection fraction15. In contrast, the OPTIMIZE-HF registry, which prospectively followed 5,117 ADHF hospitalizations for mortality outcomes, reported significant interaction between HF type and hemoglobin level8. As with our study, the overall worst mortality outcomes were noted in anemic patients with HFrEF. Although the OPTIMIZE-HF registry did not report relative risks within HFpEF and HFrEF groups, a crude analysis of the published data contrasting the lowest with highest hemoglobin quartiles yields a lower relative risk for patients with HFpEF (RR = 1.2) than HFrEF (RR = 2.2). The lower mortality risk associated with anemia in HFpEF patients is contrary to our observations from the ARIC study. Consistent with our analysis, the adjusted hazard ratio of death associated with anemia was also greater with HFpEF (HR = 2.78) than HFrEF (HR = 1.93) in a Scottish registry of 528 consecutive HF hospitalizations16.
We are aware of only two previous studies examining the association between anemia and hospital LOS in ADHF patients, and a single study which examined interaction by HF type. In an analysis of 8,569 patients hospitalized with HF, those with the lowest hemoglobin quartile were hospitalized an average 1.8 days longer than patients in the highest quartile, and incurred an additional $8,406 in mean charges2. In the OPTIMIZE-HF registry8, which reported crude LOS stratified by HF type, contrasts of the lowest with highest hemoglobin quartiles yield a similar increase in LOS for patients with HFpEF (1.30 days) and HFrEF (1.38 days), by our calculations. However, our analyses from the ARIC study revealed a different pattern. Contrasts of anemia with no anemia yielded a hospital stay lengthened by 3.5 days for patients with HFpEF, and 1.8 days for those with HFrEF.
The anemia prevalence in our study, 70%, is consistent with previous reports of anemia and advanced HF. In ambulatory HF patients, anemia prevalence has been shown to increase with HF severity, from 9%, 19%, 53%, and 79% for NYHA class I-IV, respectively 21. However, national registries of ADHF hospitalizations with demographics similar to our study population have reported lower estimates of anemia prevalence. The ADHERE registry, based on 105,388 ADHF hospitalizations, reported an anemia prevalence of 53% 22. Similarly, the reported anemia prevalence in the OPTIMIZE-HF registry was 51% 8. The reason for the greater anemia prevalence in our study population is uncertain, but may be related to differing inclusion criteria. In the ARIC study, potential ADHF hospitalizations were identified for medical review using a broader range of ICD-9 codes than the OPTIMIZE-HF or ADHERE registries, and consequently, the ARIC study may have captured a higher prevalence of ADHF cases with comorbid conditions causing anemia.
We observed that anemic patients with HFpEF were less often treated with diuretics, and that anemia was only associated with diuretics in patients with HFrEF. This raises the possibility that hemodilutional anemia prevalence may differ by HF type. In a small study involving 46 ambulatory HF patients identified with anemia, examination of plasma volume by I-131 labeled albumin revealed 12% with HFpEF to be hemodilutional, compared to 41% with HFrEF23. To counter the possibility of hemodilution in our analysis, we defined anemia using the last hemoglobin value recorded in the hospital record. However, the specific time point of hemoglobin measurement was not abstracted, and hemodilution cannot be entirely ruled out. In the PROTECT trial, involving 1,969 patients hospitalized with HFrEF, hemoglobin levels were closely monitored by measurements on days 1-4 and day 7 of the hospitalization24. Short term improvement in hemoglobin levels was attributed to diuresis and abatement of hemodilution, and associated with better mortality outcomes. Though not possible to confirm from the abstracted data, the disparity in relative outcomes that we observed among anemic HFpEF and HFrEF patients may relate to differing anemia etiologies and duration of anemia.
Our study has some limitations. Anemia therapies and red cell indices were not abstracted from the medical record, and we were unable to consider NYHA class as a potential modifier of associations between hemoglobin and mortality or LOS outcomes. We were also limited to hospitalizations with ejection fractions documented in the medical record. However, the ARIC study community surveillance provides a real-world glimpse into hospitalized ADHF, mitigating the selection bias by hospital catchment, medical insurance status, or trial inclusion criteria that may be encountered with other study designs. All hospitalizations for ADHF were validated by physician review of the medical record, minimizing misclassification of events, and sampled within pre-specified strata, to ensure an adequately representative study population.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HSN268201100006C, HHSN268201100007C, HHSN268201100008C, HSN268201100009C, HHSN268201100010C, HHSN268201100011C, and SN268201100012C). M.C.C. was supported by grant R00-HL098458. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD, Scheffold T, Zugck C, Maisch B, Regitz-Zagrosek V, Hasenfuss G, Pieske BM, Wachter R. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–764. doi: 10.1007/s00392-011-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordyke RJ, Kim JJ, Goldberg GA, Vendiola R, Batra D, McCamish M, Thomasson JW. Impact of anemia on hospitalization time, charges, and mortality in patients with heart failure. Value Health. 2004;7:464–471. doi: 10.1111/j.1524-4733.2004.74009.x. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, O'Connor CM. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92:625–628. doi: 10.1016/s0002-9149(03)00740-9. [DOI] [PubMed] [Google Scholar]
- 4.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 5.Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113:2454–2461. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- 6.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O'Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ, RED-HF Committees, RED-HF Investigators Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 7.Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159:746–757. doi: 10.7326/0003-4819-159-11-201312030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Young JB, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O'Connor CM, She L, Sun JL, Yancy CW, Fonarow GC, OPTIMIZE-HF Investigators and Coordinators Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry) Am J Cardiol. 2008;101:223–230. doi: 10.1016/j.amjcard.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 9.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 10.O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, Young JB, Solomon SD, Granger CB, Ostergren J, Olofsson B, Michelson EL, Pocock S, Yusuf S, Swedberg K, Pfeffer MA, CHARM Committees and Investigators Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, Mancini DM. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–229. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerzner R, Gage BF, Freedland KE, Rich MW. Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction. Am Heart J. 2003;146:286–290. doi: 10.1016/S0002-8703(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 15.Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, Havranek EP, Krumholz HM. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. 2005;165:2237–2244. doi: 10.1001/archinte.165.19.2237. [DOI] [PubMed] [Google Scholar]
- 16.Berry C, Norrie J, Hogg K, Brett M, Stevenson K, McMurray JJ. The prevalence, nature, and importance of hematologic abnormalities in heart failure. Am Heart J. 2006;151:1313–1321. doi: 10.1016/j.ahj.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R, Francis DP, Pitt B, Poole-Wilson PA, Coats AJ, Anker SD. Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE II trial. Eur Heart J. 2004;25:1021–1028. doi: 10.1016/j.ehj.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 19.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121:726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Stat Med. 1983;2:243–251. doi: 10.1002/sim.4780020219. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail. 2002;4:681–686. doi: 10.1016/s1388-9842(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 22.Galvao M, Kalman J, DeMarco T, Fonarow GC, Galvin C, Ghali JK, Moskowitz RM. Gender differences in in-hospital management and outcomes in patients with decompensated heart failure: analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Card Fail. 2006;12:100–107. doi: 10.1016/j.cardfail.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol. 2008;102:1069–1072. doi: 10.1016/j.amjcard.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meer P, Postmus D, Ponikowski P, Cleland JG, O'Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013;61:1973–1981. doi: 10.1016/j.jacc.2012.12.050. [DOI] [PubMed] [Google Scholar]