Abstract
Stroke is the third leading cause of death in developed nations. Up to 88% of strokes are ischemic in nature. Extracranial carotid artery atherosclerotic disease is the third leading cause of ischemic stroke in the general population and the second most common non-traumatic cause among adults <45 years of age. The aim of this paper is to provide comprehensive, evidence-based recommendations for the management of extracranial atherosclerotic disease, including imaging for screening and diagnosis, medical management and interventional management.
Keywords: Carotid disease, Carotid stenosis, Atherosclerotic disease, Stroke, Carotid endarterectomy, Carotid angioplasty and stenting, Antiplatelet therapy
Introduction
Epidemiology
When considered as an independent diagnosis separate from other cardiovascular diseases, stroke is the third leading cause of death in developed nations and a leading cause of long-term disability.1 Approximately 87% of all strokes are ischemic, 10% are hemorrhagic, and 3% are subarachnoid hemorrhages.2–10
Based on the Framingham Heart Study and Cardiovascular Health Study populations, the prevalence of >50% carotid stenosis is approximately 9% in men and 6–7% in women.11,12 Carotid stenosis or occlusion as a cause of stroke has been more difficult to determine from population studies. Approximately 7–18% of all first strokes were associated with carotid stenosis.13,14 The risk for recurrent strokes among survivors is 4–15% within a year after the initial stroke and 25% by 5 years.8
Extracranial atherosclerotic disease accounts for up to 15–20% of all ischemic strokes.15,16 While intracranial atherosclerotic disease has shown to be consistently more common among Blacks, Hispanics and Asians compared to Whites,15,17 the racial differences for extracranial atherosclerotic disease is less apparent. The Northern Manhattan Stroke study reported equal incidence of extracranial atherosclerotic disease among patients of all races presenting with an acute ischemic stroke.15 However, a smaller study reported that Whites were more likely than Blacks to have extracranial carotid artery lesions (33% versus 15%, p=0.001).16 While the male gender appears to be an independent predictor for intracranial atherosclerotic disease, no gender differences were reported for extracranial disease.16
Natural History
Stroke associated with extracranial carotid atherosclerotic disease could occur via several mechanisms:18
Atheroembolism of cholesterol crystals or other debris
Artery to artery embolism of thrombus
Structural disintegration of the wall (dissection)
Acute thrombotic occlusion
Reduced cerebral perfusion with plaque growth
In symptomatic patients, there is a clear correlation between the degree of stenosis and the risk of stroke.19 In the North America Symptomatic Carotid Endarterectomy Trial (NASCET), the stroke rate after 18 months of medical therapy without revascularization was 19% in patients with 70–79% stenosis, 28% in patients with 80–89% stenosis, and 33% in patients with 90–99% stenosis.19
This correlation is less apparent in asymptomatic patients. In the Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST), asymptomatic patients with 60–80% stenosis had higher strokes rates compared to those with more severe stenosis.20,21 The presence of a carotid bruit also does not appear to be a reliable predictor of stroke risk in asymptomatic patients. Despite the Framingham Heart Study population showing that asymptomatic patients with carotid bruit had a 2.6 fold increased incidence of strokes compared to those without carotid bruit, less than half of these stroke events involved the ipsilateral cerebral hemisphere.3
While the degree of carotid stenosis remains the main determinant of disease severity, additional imaging markers of plaque vulnerability are also important in determining the risk for transient ischemic attack (TIA) and strokes.22–24 Imaging markers for plaque vulnerability on ultrasonography include:22,23
Ulceration
Echolucency
Intraplaque hemorrhage
High lipid content
Thin or ruptured fibrous caps, intraplaque hemorrhage and large lipid-rich or necrotic plaque cores, and overall plaque thickness seen on magnetic resonance imaging (MRI) have also been associated with subsequent ischemic events.25
Recently, the utility of biomarkers and imaging makers for inflammation in predicting plaque vulnerability and risk for stroke has also been investigated. Carotid plaques from patients with ipsilateral stroke demonstrated infiltration of the fibrous cap by inflammatory cells.26,27 F-fluorodeoxyglucose measured by positron emission tomography (PET) is believed to reflect inflammation.28,29 Macrophage activity quantified by PET has been observed in experimental models. In addition, biomarkers such as C-reactive protein and different matrix metalloproteinase are currently being studied for their predictive value of plaque instability.30–32 However, the reliability of these markers remains uncertain.
Evaluation of Carotid Atherosclerotic Disease
Carotid Ultrasound
When performed by well-trained, experienced technologist, carotid ultrasound (US) is accurate and relatively inexpensive.33–38 Carotid US is also noninvasive, does not require a venipuncture, or exposure to contrast material or radiation. As such, carotid US is recommended for the initial evaluation of symptomatic and asymptomatic patients with suspicion for carotid atherosclerotic disease.39
Carotid US should be performed in asymptomatic patients with two or more of the following risk factors:
Hypertension
Hyperlipidemia
Family history of atherosclerosis or ischemic stroke before 60 years of age
Tobacco smoking
US remains an appropriate screening tool for high-risk, asymptomatic patients irrespective of auscultation findings because the sensitivity and positive predictive value of a carotid bruit for a hemodynamically significant carotid stenosis are relatively low.
Carotid US, however, is not recommended for routine screening of asymptomatic patients without risk factors for atherosclerotic disease due to the lack of data from health economic studies to support mass screening of the general population.40,41
Carotid US should also be performed annually to assess the progression or regression of disease and response to therapeutic measures in patients with >50% stenosis. Once stability has been established or a patient’s candidacy for further intervention has changed, longer intervals may be appropriate.39
Carotid US does not directly measure the luminal diameter of the artery or stenotic section. Instead, it relies on blood flow velocity as an indicator for the degree of stenosis. Several schemes have been developed for assessment of carotid stenosis.42–44 Measuring the internal carotid artery (ICA) peak systolic velocity and ratio of ICA peak systolic velocity over the ipsilateral common carotid artery velocity correlate best with angiographic stenosis. Potential pitfalls of velocity-based estimation of stenosis are the higher velocities in women than in men, and elevated velocities in the presence of a contralateral occlusion.45,46 Subtotal arterial occlusion may also sometimes be mistaken for total occlusion, which is crucial to differentiate in determining management strategies. Other factors that may further reduce the accuracy of carotid US include highly operator-dependent reliability, obesity, high carotid bifurcation, severe arterial tortuosity, extensive calcifications, and presence of a carotid stent.33–35,39,47
Despite varying results between imaging centers and operators, the overall sensitivity and specificity for detection of occlusion or stenosis >70% have been reported to be 85–90% when compared to catheter angiography.48–50
Computed Tomography Angiography (CTA) and Magnetic Resonance Angiography (MRA)
Both MRA and CTA are able to generate high-resolution images of the cervical arteries.51–57 When compared to catheter angiography, MRA has a sensitivity range of 97–100% and a specificity range of 82–96%,58–62 while CTA has 100% sensitivity and 63% specificity (95% CI: 25 – 88%).63 Both are indicated in symptomatic patients when carotid US cannot be obtained, yield equivocal results or show complete occlusion.39 In patients with high pretest probability for disease, MRA and CTA may be used as the initial test. MRA and CTA of the intracranial vessels should be done when an extracranial source cannot be identified in symptomatic patients or in patients with risk factors for intracranial atherosclerotic disease. MRA and CTA are helpful in determining the exact severity of stenosis and anatomical details that will influence treatment decisions.
MRA has the benefit of its relative insensitivity to arterial calcification. Contrast-enhanced MRA allows for more detailed evaluation of the cervical arteries, especially in lesions with a slow blood flow when compared to non-contrasted studies.58–61,64,65 However, if contrast is contraindicated, non-contrast-enhanced MRA may be used.51
Potential pitfalls for MRA include a tendency to overestimate the degree of stenosis, and an inability to discriminate between total occlusion and subtotal occlusion. This effect is reduced with the use of contrast-enhanced MRA. Additional barriers of MRA include patients who are claustrophobic, extreme obesity, or incompatible implanted devices, such as pacemakers or defibrillators. For these patients, CTA is a good alternative.39
Unlike both MRA and carotid US, CTA provides direct imaging of the arterial lumen, making it suitable for evaluation of stenosis. It is an accurate test to determine severity of stenosis and is highly accurate for detection or exclusion of complete occlusions as well.55 However, CTA exposes patients to radiation, and the relatively high volume of iodinated contrast needed for the study precludes patients with impaired renal function. The presence of heavily calcified plaques may affect the accuracy of CTA in determining the degree of stenosis.66 In addition, foreign metal objects, such as dental implants and surgical clips in the neck, can generate artifacts, which may obscure the targeted vessels.
Catheter Angiography
While non-invasive imaging can provide the information needed in guiding the choice of medical, endovascular or surgical treatment in most cases,39 catheter angiography remains the gold standard for diagnosing and grading of carotid atherosclerotic disease.
Due to its inherent cost and risk for complications, such as ischemic strokes, catheter angiography should be reserved for patients in whom noninvasive imaging is contraindicated, inconclusive, or yields discordant results. The risks of catheter angiography include allergic reactions to contrast, kidney dysfunction due to contrast toxicity, femoral artery injuries, infections or hematomas of the puncture site, strokes, or death, typically at a rate lower than 1/1000 for the most serious complications and <5% for the minor events in specialized centers with high volumes.67,68
Catheter angiography is useful in patients with renal insufficiency. Selective angiography of a single suspected vascular territory could provide definitive imaging with limited exposure to contrast material and is unlikely to exacerbate renal insufficiency.39
Several methods to measure stenosis have been described, producing marked variability in measurements of vessels with the same degree of actual anatomic narrowing. Measurement methods based on NASCET have been used in most modern clinical trials, taking into account the luminal diameter at the section with highest degree of stenosis (A), and the luminal diameter of a normal section just distal to the stenosis (B).20
Medical Management
Pharmacologic therapy for patients with carotid atherosclerotic disease consists mainly of antiplatelet therapy and medical management of the risk factors for atherosclerotic disease.
Antithrombotic Therapy
The use of antiplatelet agents has been shown to reduce the risk of stroke in patients with TIA or a previous stroke.40,69–71 Single-agent antiplatelet therapies are recommended for all symptomatic patients, independent of whether or not they are candidates for revascularization. Aspirin 75–325 mg daily should be the first line of therapy. Clopidogrel 75 mg daily or ticlopidine 250 mg daily are reasonable alternatives when aspirin is contraindicated by factors other than active hemorrhage.39,69,70,72
Several randomized, controlled, double-blinded studies have shown that dual antiplatelet combination therapy is not superior to single agents. The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial and Management of Atherothrombosis With Clopidogrel in High-Risk Patients (MATCH), both showed that combination therapy of aspirin plus clopidogrel did not reduce stroke risk significantly compared to either drug alone.73,74 The Second European Stroke Prevention Study (ESPS-2), which included 6,602 patients, showed that the combination of aspirin and extended release dipyridamole was superior to aspirin alone in patients with prior TIA or stroke.75 However, a much larger study, with over 20,000 patients, The Prevention Regimen for Effectively Avoiding Second Strokes (PROFESS) trial, showed that combination therapy of aspirin and extended release dipyridamole was not superior to clopidogrel alone in recurrent stroke prevention.76 Furthermore, there was an increased risk for major hemorrhagic events, including intracranial hemorrhage, in the combination therapy group.76 Despite clopidogrel monotherapy showing equal efficacy and lower hemorrhage risk than aspirin plus extended release dipyridamole, and equal efficacy with aspirin plus clopidogrel the variations in response to clopidogrel due to genetic factors and drug interactions makes it crucial for individualized treatment selection for optimum stroke prevention.
Variability in response to clopidogrel is a result of both clinical and genetic factors. Conversion of clopidogrel to its active form by the cytochrome p450 system depends highly on CYP enzyme, which has significant genetic variability. CYP2C19*2 is the most common genetic variant associated with impaired response to clopidogrel.39 However, other genetic polymorphisms may also contribute to poor response. Aspirin resistance has also been described and was more frequent in patients taking low-dose aspirin (81 mg daily) and the enteric-coated preparations.77 Clopidogrel or aspirin resistance due to the inability of these agents to inhibit platelet function is a potential cause for failure in stroke prevention. However, whether or not variations in response to antiplatelet therapy are associated with greater stroke risk and whether or not treatment of resistance improves outcomes have not been established. There also a lack in consensus on which platelet function test should be used to determine such resistance.39
The efficacy of antiplatelet therapy in stroke prevention for asymptomatic patients is less apparent.40,69,70,78 In the Asymptomatic Cervical Bruit Study, a randomized, double-blinded study, the annual rate of ischemic events and death due to any cause in patients with >50% carotid stenosis was 11.0% in the aspirin group compared to 12.3% in the placebo group during a 2-year follow up. However, the sample size of 372 patients may have been insufficient to detect a clinically meaningful difference.79
Anticoagulation with warfarin along with its potential risk for increased hemorrhagic complications has not been shown to be superior to antiplatelet agents. Antiplatelet therapy is recommended over anticoagulation for both symptomatic and asymptomatic patients where antithrombotic therapy is indicated.39 The Warfarin-Aspirin Recurrent Stroke Study (WARSS), a randomized, double-blinded trial with 2206 patients, compared warfarin to aspirin for stroke prevention or recurrent ischemic stroke in patients with a recent stroke.80 No significant benefit of warfarin over aspirin was found after 2 years. Parental anticoagulation with unfractionated heparin or low molecular weight heparin is also not recommended for patients with extracranial carotid atherosclerosis with acute ischemic stroke or TIA.81–83 In patients who have other indications for anticoagulation, such as a mechanical prosthetic valve or atrial fibrillation, a vitamin K antagonist such as warfarin may be used over antiplatelet therapy. The goal international normalized ratio (INR) should be 2.0–3.0.84
Treatment of Hypertension
Antihypertensive therapy has shown to reduce the risk of stroke, with a 33% reduction in stroke risk for every 10 mmHg decrease in systolic blood pressure up to 115/75 mmHg.85,86 Antihypertensive therapy also reduces the risk for recurrent strokes by 24%.87 These effects appear to be consistent between Whites and Blacks across a wide age range88 and between sexes, regions, and stroke subtypes.85 As such, antihypertensive treatment is recommended for all patients with concurrent hypertension and asymptomatic extracranial carotid atherosclerotic disease, with a goal blood pressure below 140/90 mmHg.85–87,89,90 The protective value of antihypertensive therapy also appears to extend to patients without concurrent hypertension, as demonstrated by the Heart Outcomes Protection Evaluation trial (HOPE).91
The exact benefits of antihypertensive treatment in symptomatic patients with severe carotid stenosis remain unclear due to concerns for reduction in cerebral perfusion and exacerbation of cerebral ischemia. Patients with severe carotid stenosis may have impaired cerebrovascular reactivity due to chronic hypoperfusion, thereby increasing the risk for ipsilateral ischemic events.92 Antihypertensive treatment is likely indicated in patients with hypertension and symptomatic extracranial atherosclerosis after the hyperacute period.39 However, a specific blood pressure goal has yet to be established.
Treatment of Hyperlipidemia
According to the 2011 American Heart Association guidelines on the management of extracranial carotid and vertebral artery disease, statins are recommended for all patients with extracranial carotid stenosis to reduce low-density lipoprotein (LDL) levels below 100mg/dL.39,70,89,93 A target LDL level of 70 mg/dL is reasonable in patients who have sustained an ischemic stroke. Niacin and bile acid sequestrants are reasonable alternatives in patients who do not tolerate statins.94–96 They can also be used in combination with a statin if treatment with a statin does not achieve targeted LDL levels.94,95,97,98
Epidemiological studies have consistently shown a positive association between cholesterol levels and carotid artery atherosclerosis.99–101 Lipid-lowering therapy with statins has been shown to reduce the risk of ischemic stroke in patients with atherosclerosis.102,103 A meta-analysis of 26 trials involving approximately 90,000 patients showed that statins reduced the risk for all stroke by 21% [odds ratio (OR): 0.79, 95% confidence interval (CI): 0.73−0.85], with a 15.6% reduction in stroke risk for every 10% decrease in serum LDL levels (95% CI: 6.7−23.6).103 Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL), a randomized, prospective trial, showed that 80 mg daily of atorvastatin reduced the absolute risk for stroke at 5 years by 2.2%, the relative risk (RR) of all stroke by 16% and RR of ischemic stroke by 22%.93 Statins also reduce the progression and induce regression of carotid atherosclerosis.104 A meta-analysis of 9 randomized trials showed that statins reduced stroke risk by 15.6% and intima-media thickness (IMT) by 0.73% per year for every 10% reduction in LDL levels.103 The Atorvastatin versus Simvastatin on Atherosclerosis Progression (ASAP) trial involving patients with familial hypercholesterolemia, 80 mg daily of atorvastatin decreased carotid IMT after 2 years of treatment, but carotid IMT increased in patients randomized to 40 mg daily of simvastatin.105 Atorvastatin’s effects on IMT were further supported by the Arterial Biology for Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) trial which showed that carotid IMT regressed after 12 months of treatment with 80 mg daily of atorvastatin but remained unchanged with 40 mg daily of pravastatin.106 The Measuring Effects of Intima-Media Thickness: An Evaluation of Rosuvastatin (METEOR) trial showed that in patients with elevated LDL levels and a low Framingham risk score, rosuvastatin reduced the progression of carotid IMT over 2 years when compared to placebo.107
The effects of non-statin lipid-modifying therapies on stroke risk reduction are less apparent.39 Niacin only showed a small benefit in reduction of risk of death caused by cerebrovascular disease in patients participating in the Coronary Drug Project.108 Fenofibrate did not reduce stroke rates in patients with diabetes mellitus in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study.109 Genfibrozil reduced the risk of total strokes and ischemic strokes in patients with coronary artery disease and low HDL levels in the Veteran Affairs HDL Intervention trial.110
The ARBITER-2 and Effect of Combination Ezetimibe and High-Dose vs. Simvastatin Alone on the Atherosclerosis Process in Patients with Heterozygous Familial Hypercholesterolemia (ENHANCE) studies showed that the addition of extended-release niacin and ezetimibe, respectively, to statin therapy did not affect progression of carotid IMT more than statin therapy alone.106,111 The Cholesterol Lowering Atherosclerosis (CLAS) trial however showed that niacin and colestipol combination therapy reduced progression of carotid IMT.112
Diabetes Mellitus Management
Elevated fasting and post challenge glucose levels were associated with an increased risk of stroke.113 The risk of ischemic stroke in diabetic patients is increased 2 to 5 fold compared with non-diabetic patients.114–116 The Cardiovascular Health Study showed that diabetes was associated with carotid IMT and severity of carotid stenosis.12 Both the Atherosclerosis Risk in Communities (ARIC) study and Insulin Resistance Atherosclerosis Study and Epidemiology of Diabetes Interventions and Complications (EDIC) showed that diabetes was associated with progression of carotid IMT.116–122 Several randomized, controlled, double-blinded studies have shown that the use of pioglitazone has lead to substantial regression of carotid IMT.123,124 The affect of pioglitazone appears to be independent of improved glycemic control.123
Smoking Cessation
Cigarette smoking increases the relative risk for ischemic stroke by 25–50%.125–131 This risk decreases substantially within 5 years among those who quit smoking.126,128 The Framingham Heart Study showed that the degree of extracranial carotid stenosis correlated with the quantity of cigarettes smoked over time.132 These findings were corroborated by the Cardiovascular Health Study, in which the severity of carotid stenosis was greater among current smokers compared to former smokers and there was a significant association between pack-years of tobacco exposure to the severity of carotid stenosis.133 The ARIC study revealed that current and past cigarette smoking were associated with a 50% and 25% increase, respectively, in risk of progression of IMT over a 3-year period when compared to nonsmokers.130 Smoking cessation counseling and interventions should be offered to patients with extracranial carotid atherosclerosis to reduce the risk for disease progression and stroke.125–128,134
Obesity and Physical Inactivity
Abdominal adiposity has a strong positive association with the risk of stroke or TIA.135 Adjusted odds ratio for the waist-to-hip ratio showed successive increases in stroke/TIA risk for every successive tertile. There was also significant association with waist circumference and waist-to-stature ration with the risk of stroke/TIA.
Physical inactivity is a significant modifiable risk factor for stroke, with a 25% prevalence, a 30% attributable risk, and a RR of 2.7.40,136 However, the risk reduction associated with intervention remains unclear. Several observational studies and meta-analyses have suggested a lower risk for stroke among individuals engaging in regular moderate to high levels of physical activity.137 However, it is unclear whether exercise alone has a significant stroke risk reduction in the absence of effects on other risk factors, such as reduction in obesity and improvement in glycemic control and serum lipid levels.
Interventional Management
Atherosclerotic disease of the extracranial carotid arteries carries significant morbidity and mortality risk despite maximal medical therapy. NASCET demonstrated a stroke rate of 19–33% after 18 months of medical therapy without intervention among symptomatic patients, depending on the degree of stenosis.19 Interventional management consisting mainly of carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAS) has been shown to decrease the stroke rate among these patients.8,19,138–146
In general, intervention when indicated should be done within 6 months of original presentation.8,19,147,148 However, intervention within 2 weeks of the index event is reasonable for patients with no contraindications for early revascularization.149
The indications for intervention will be discussed in detail in the sections below. The general contraindications for interventions include:
Severe, disabling stroke [modified Rankin Scale (mRS) score ≤3]
Chronic total carotid artery occlusion
Carotid stenosis <50%
Extreme high-risk for peri-procedural complications
Carotid revascularization is not recommended for patients with near–complete occlusion or stenosis <50% since the risk for stroke is low in these patients.19 Revascularization has also not shown to have any benefit in these patients.19 Carotid revascularization is also not recommended for patients with cerebral infarction causing severe disability that precludes preservation of useful function.
Carotid Endarterectomy
Carotid Endarterectomy in Symptomatic Patients
CEA has been shown to significantly reduce the risk for ipsilateral stroke beyond the 30-day perioperative period in symptomatic patients. However, the inherent risk for periprocedural complications, such as stroke and myocardial infarction (MI), must be considered in the overall assessment for safety and efficacy.
Patients with a non-disabling ischemic stroke (mRS >3) or TIA and >70% stenosis of the ipsilateral internal carotid artery by noninvasive imaging, or >50% stenosis by catheter angiography should undergo CEA.8,147
In NASCET, a randomized trial comparing stroke risk in symptomatic patients receiving CEA and medical management versus medical management alone, patients were stratified according to severity of stenosis.19 The trial for high-grade stenosis group (70–99%) was stopped after 18 months, after randomizing 328 patients because a significant benefit for CEA was evident. There was 17% absolute stroke risk reduction with CEA at 2 years.19 At the end of NASCET, the investigators also reported a benefit for CEA in patients with 50–69% stenosis. The rate of ipsilateral stroke including perioperative events was 15.7% at 5 years compared to 22% in the medical management only group. The rate of operative mortality or perioperative stroke at 30 days was 6.7%. CEA had no benefit in patients with carotid stenosis <50%.
The European Carotid Surgery Trial (ECST), which randomized 2518 patients over a 10- year period, showed similar results to that of NASCET in symptomatic patients with 70–99% stenosis, showing a highly significant benefit for CEA, but did not show any benefit in patients with milder stenosis.150,151 The lack of benefit of CEA in symptomatic patients with 50–69% stenosis based on ECST was attributed to the difference in angiographic measurement of stenosis.
The Veterans Affairs Cooperative Study (VASC) was stopped before completion, after only randomizing 189 symptomatic patients with a mean follow-up of 11.9 months, due to significant benefit of CEA over medical therapy alone. The primary endpoint of death, stroke or TIA occurred in 7.7% of CEA patients versus 19.4% of patients receiving medical therapy alone.152
A meta-analysis of these three trials showed that CEA was most effective in patients with >70% stenosis without complete or near occlusion.150 Benefits of CEA in patients with 50–69% stenosis were only modest, but increased with time. Surgery offered little to no long-term benefits in patients with complete or near occlusion. When the combined outcome of perioperative stroke or death and fatal or disabling ipsilateral ischemic stroke was considered, the clinical benefits of CEA were only evident in patients with 80–99% stenosis.
Carotid Endarterectomy in Asymptomatic Patients
The benefits of CEA for stroke risk reduction in asymptomatic patients is less profound compared to symptomatic patients. CEA is reasonable in asymptomatic patients who have >70% internal carotid artery stenosis if the risk of perioperative MI, stroke and death is low.138,153–156 While CEA in symptomatic patients showed an increased benefit of surgery with increased degree of stenosis, CEA in asymptomatic patients did not show a similar trend. Equal benefits were seen in all patients within the 60–99% stenosis range.156
The Veterans Affair Cooperative Study (VACS) group was the first major trial of CEA in asymptomatic patients.153 A total of 444 patients with ≥50% stenosis were randomized over a 54-month period into either the CEA group or the medically therapy group. The 30-day mortality rate among patients undergoing CEA was 1.9% and incidence of stroke was 2.4%. The study showed a statistically significant reduction in TIA, stroke and death 5 years post-CEA, with a 10% overall rate of adverse events in the surgical group compared with 20% in the group given medical therapy alone. However, the inclusion of TIA in the primary composite endpoint remains controversial given that the study was underpowered to detect a difference in a composite endpoint of death and stroke without TIA.153,157,158
ACAS also sought to determine whether the addition of CEA to medical management reduced the incidence of cerebral infarction in asymptomatic patients, but excluded TIA in their primary endpoint.138 The trial was stopped before completion after randomizing 1662 patients, due to the apparent advantage of CEA among patients with >60% carotid stenosis. After a mean follow up of 2.7 years, the projected 5-year risk for ipsilateral stroke and any perioperative stroke or death was estimated to be 5.1% for surgical patients and 11.0% for patients treated medically. The aggregate risk reduction was 53% (95% CI: 22%−72%).
These findings were further corroborated by the ACST, which randomized 3120 asymptomatic patients with >60% stenosis to immediate CEA versus delayed surgery with initial medical management.21 The 30-day stroke risk was 3.1% in both groups, but the 5-year rates were 6.4% in the early surgery group compared to 11.8% in the group initially managed medically.139
The benefits of CEA for asymptomatic patients are even less apparent in women. This is due to the higher operative risk and lower stroke risk without intervention among asymptomatic women compared to men.156 Such benefits remain unclear despite a meta-analysis done combining the data from both ACST and ACAS.156
Interpretation of CEA Trials
The interpretation of CEA trials for both symptomatic and asymptomatic patients should be done in the context of the evolution of medical therapy for atherosclerotic disease over the years. Although pharmacotherapy was included in most trials, guidelines and strategies for medical management have changed over the years. Best medical therapy during the period of older trials such as NASCET was scant by modern standards. In NASCET, only approximately 70% of patients were placed on antihypertensive drugs and an even smaller proportion of patients were given lipid-lowering agents.8 Medical therapy was not described in ACAS. The ACST investigators reported a change in medical therapy over the 10-year trial period.139 Towards the end of the trial in 2003, 70% of patients were on lipid-lowering agents and 81% were on antihypertensive drugs. However, the outcomes for CEA were only reported for the first 5 years of the trial, ending in 1998, during which such medical therapy was considerably less frequent. Additionally, 60% of patients had systolic blood pressure (SBP) >160 mmHg, while 33% had total serum cholesterol >250 mg/dL.
Concurrently, surgical outcomes of CEA have improved over time with advances in training, increased hospital and surgeon volumes, and improved peri-operative medical management.159–162
Therefore, with advances in both medical management and operative/peri-operative management and outcomes over time, which has lead to decline in rates of adverse events, the comparative outcomes of CEA over medical therapy must be interpreted with caution.
Demographic and Clinical Considerations
Advanced age does not preclude CEA in appropriately selected patients. Despite several reports showing a higher risk for complications among older patients,163,164 patients ≥75 years of age with few cardiovascular risk factors have been shown to have comparable risk for perioperative stroke and death when compared to younger patients.165 However, in ACST, no benefit from CEA was observed in patients ≥80 years of age.21 In NASCET, the greatest benefit of CEA was observed in older patients up to 80 years of age.19 Patients older than 80 years of age were excluded from NASCET (prior to 1991) and ACAS.19,138
Women undergoing CEA have a higher risk for complications than men.147,166–168 Both in ACAS and NASCET, women had a higher risk for surgical mortality, neurologic morbidity, recurrent stenosis or gaining little to no benefit form surgery.19,138
There are insufficient data to determine the effects of ethnicity on outcomes.39
Anatomical Considerations
Several factors that affect patient anatomy must be taken into account when considering the safety and technical challenges associated with CEA. Unfavorable factors include:
High carotid bifurcation or arterial stenosis above the level of the second cervical vertebra
Arterial stenosis below the clavicle (intrathoracic)
Contralateral carotid occlusion
Contralateral vocal cord paralysis
Previous ipsilateral CEA
Prior radical neck surgery or radiation
Prior tracheostomy
A high carotid bifurcation or arterial stenosis above the level of the second vertebra may require high cervical exposure, which increases the risk for cranial nerve injury.169,170 The risk for cranial nerve injury is also higher in patients with prior radical neck surgery or tracheostomy. In these cases, there usually is added difficulty in exposing the artery and increased risk for perioperative infection. Contralateral laryngeal nerve palsy is a relative contraindication for CEA because bilateral laryngeal nerve palsy can lead to significant compromise of the airway.171 Prior radiation can make CEA technically challenging, but several series have shown that CEA can still be performed safely.172 Although in this situation, CAS may be a safer option, but the rate of restenosis is high, ranging from 18–80% over 3 years.173–175
Technical Consideration
There have been considerable variations in surgical technique with CEA over the past 50 years. Local anesthesia was initially recommended to permit observation of patient’s level of consciousness during temporary carotid artery clamping. Several authors also advocated local anesthesia due to the possibility of less perioperative adverse cardiac events.39 However, there has been no significant data demonstrating an advantage of local anesthesia over general anesthesia.
Patients undergoing general anesthesia for CEA should undergo intraoperative monitoring of cerebral function to determine the need for shunting during arterial clamping.176–178 Selective shunting of patients is preferable due to potential complications associated with shunting, such as mechanical injury to distal ICA, air embolism or thromboembolism through the shunt, and obscuring of distal arterial anatomy during endarterectomy.39 Intraoperative monitoring include:
Electroencephalography (EEG)
Somasosentory evoked potential (SSEP)
Transcranial Doppler ultrasonography
Computerized topographic brain mapping, measurement of residual collateral perfusion pressure or internal carotid artery (ICA) back pressure
Shunting was generally indicated when EEG abnormalities associated with ischemia appeared.190 In our institution, shunts are used when a depression of ≥50% of EEG amplitude or SSEP P25 amplitude is observed. Shunts are used in all patients with contralateral carotid occlusion.
Patch closure of the arteriotomy may reduce the incidence of residual or recurrent stenosis. However, there is increased operative time and increased carotid clamp time. Multiple studies have failed to demonstrate a consistent difference in outcomes between patch closure and primary closure.179–189 A Cochrane meta-analysis, analyzing the combined results of 10 trials showed that patch closure reduces the risk of peri-operative arterial occlusion and ipsilateral stroke. There was also reduction in the subsequent risk of restenosis, death or stroke.190 As such, most surgeons now advocate for patch closure. Several different patch materials have been described in the literature, including the use of bovine pericardium, vein, polyethylene terephthalate, and polytertrafluoroethyelene.191–194 However, the outcomes have appeared to be similar independent of the patch material used.
The use of peri-operative antiplatelet therapy such aspirin or clopidogrel reduces the risk for adverse cardiac and neurologic events without a significant increase in risk for postoperative bleeding.195,196 However, peri-operative combination therapy consisting of aspirin and clopidogrel was associated with increased risk for postoperative bleeding or incisional hematoma.197,198
Peri-operative Management
Antiplatelet therapy with aspirin 81–325 mg daily is recommended before CEA, and should be continued indefinitely post-operatively.71,199 In the Acetylsalicylic Acid and Carotid Endarterectomy (ACE) study, where 2849 patients were randomized into 4 different daily doses of aspirin, the risk of stroke, MI and death within 30 days and 3 months after CEA was higher in patients taking higher doses of aspirin (650 or 1300 mg daily) when compared to those taking lower doses (81 mg or 325 mg daily). (At 30 days, 7.0% versus 5.4%, RR 1.31 [95% CI 0.98−1.75]; and at 3 months, 8.4% versus 6.2%, RR 1.34 [95% CI 1.03−1.75])199 Clopidogrel 75 mg daily or a combination of low-dose aspirin plus extended-release dipyridamole 25–200 mg twice daily are reasonable alternatives as well.72,74,80
The use of peri-operative lipid-lowering drugs such as statins for prevention of ischemic events regardless of serum lipid levels after CEA is reasonable.200 However, the optimum agents and doses for prevention of restenosis have not been established. A retrospective review by 13 surgeons of 1566 patients undergoing CEA at a single, large academic center, revealed that receiving statin medication at least 1 week before surgery (42% of total patients reviewed) was associated with lower rates of:
Perioperative stroke (1.2% versus 4.5%; p <0.01)
TIA (1.5% versus 3.6%; p <0.01)
All causes of mortality (0.3% versus 2.1%; p <0.01)
Median (interquartile range) length of hospitalization (2 days [2–5 days] versus 3 days [2–7 days]; p <.05)
Antihypertensive medication is recommended before CEA and should be resumed post-operatively.39
A summary of peri-operative management pearls based on our institution’s experience:
General anesthesia
Continues EEG and SSEP monitoring
Discuss with anesthesia the potential need for barbiturates in the reduction of cerebral metabolic demand
Intravenous antibiotics: ancef or vancomycin
Patient is kept normocapnic (35–45 mmHg)
Patient is kept normotensive with permissive hypertension to 20% above baseline during carotid clamping
Strict control of blood pressure to avoid hypertension is initiated immediately after removal of carotid clamps
Patient is kept nomothermic
Goal hematocrite of ≥30%
Shunt is used with any reduction in 50% in EEG amplitude or 50% in the P25 median nerve SSEP activity or in cases of contralateral occlusion
A single dose of intravenous heparin is given before cross clamping, usually 5,000 units. In smaller patients or more heavyset patients, an alternative dosing of 85 units/kg can be used. If small patient or too large can use 85U/Kg (?)
During dissection of the carotid bulb, arrhythmias may occur. Atropine or glycopyrrolate should be ready
Complications
Complications associated with CEA are listed below, and include neurological and non-neurological complications:201
Cranial nerve palsy
Infection
Hemorrhage
Stroke
Venous thromboembolism
Acute arterial occlusion
Arterial restenosis
Myocardial infarction
Hemodynamic Instability (hypertension or hypotension)
Death
Risk factors associated with increased perioperative stroke and death include:201–203
Symptomatic before CEA (OR 1.62, p <0.0001)
Hemispheric symptoms (OR 2.31, p <0.001 versus retinal symptoms)
Urgent operations (OR 4.9, p <0.001)
Reoperation (OR 1.95, p <0.018)
Contralateral carotid arterial occlusion (RR 2.2, CI 1.1−4.5)
A large, retrospective, cohort study reviewing CEAs performed at 6 different hospitals by 64 different surgeons in a 2-year period revealed a 30-day post-operative stroke or death rate of 2.28% in asymptomatic patients, 2.93% in patients with TIA, and 7.11% among patients presenting with stroke.204 These results were similar to those of NASCET, which had a 30-day post-operative stroke or mortality rate of 6.7% among symptomatic patients.19 The pooled analysis of NASCET, ECST and VACS revealed a 30-day stroke and death rate after CEA of 7.1%.150 The results for asymptomatic patients were also similar to prospective trials such as ACAS and ACST, which had 30-day stroke and mortality rates of 2.3% and 3.1%, respectively.21,138 High-risk anatomic criteria, such as restenosis after CEA and contralateral carotid occlusion, further increase this risk as seen in NASCET and ACAS.138,201 The perioperative stroke and death rate have been reported to be as high as 19.9% in patients undergoing re-operative CEA and 14.3% among patients with contralateral carotid occlusion.205
However, more recent reports suggest a much lower risk than was previously reported. Case volume and surgical training are important factors in determining the clinical outcomes after a CEA. A population-based study in the state of Virginia investigating all CEAs performed from 1997 to 2001, with approximately 14,000 procedures, reported a cumulative stroke rate of 1.0% and mortality rate of 0.5%.206 There was a progressive decline in these rates each successive year. Similar results were found in Maryland from 1994 to 2003, which included 23,237 CEA procedures. The cumulative stroke rate was 0.73%; 2.12% in 1994, 1.47% in 1995, and 0.29–0.65% from 1996 to 2003.207 The cumulative stroke rate in California from 1999 to 2003 was similar at 0.54%. During this time, 51,231 CEA procedures were performed.207 Mortality rates in both states were relatively stable over the reported years.
Intracerebral hemorrhage can also occur as a result of hyperperfusion syndrome despite adequate control of blood pressure, which occurs in <1% of patients with a stable pre-operative blood pressure and blood pressure well managed peri-operatively.208–211
Cranial nerve injury occurs in up to 7% of patients undergoing CEA; however, permanent injury remained in <1% of patients.150,171,212 Cranial neuropathy typically appeared early in the post-operative period, with most patients showing complete resolution over time.171 Only 3.7% had residual cranial nerve deficits. Listed below in decreasing order of frequency, the involvement of cranial nerves or their branches:171,201,213–215
Hypoglossal
Marginal mandibular
Recurrent laryngeal
Spinal accessory
Cervical sympathetic chain (Horner syndrome)
Cardiovascular events have been reported in up 20% of patients undergoing CEA, with hypotension occurring in 5%, hypertension in 20%, and peri-operative MI in 1%.39 Local anesthesia and cervical block may lessen cardiovascular instability in selected patient groups..216 Myocardial ischemia including non-fatal MI is a major cause of morbidity in patients undergoing CEA due to carotid bifurcation atherosclerosis commonly being associated with coronary atherosclerosis.39 In NASCET and ECST, respectively, the incidence of MI was 0.3% and 0.2%..19,147 The risk for cardiopulmonary complications is associated with:217–219
Advanced age
Active angina pectoris
New York Heart Association class III or IV heart failure
Left ventricular ejection fraction ≤30%
MI within 30 days
Urgent cardiac surgery 30 days prior
Severe chronic lung disease
Severe renal insufficiency
Wound infections occur in ≤1% of patients.220,221 Wound hematoma occurred in ≤5% of patients and was associated with perioperative antiplatelet therapy,222 duration of surgery, perioperative use of heparin and protamine, and other factors.39
Carotid Angioplasty and Stenting
CAS has shown varying outcome differences when compared to CEA based on different patient factors. CAS appears to be a good alternative to CEA in certain patient groups, such as those with unfavorable surgical anatomy (noted previously). When performed with an embolic protection device (EPD), the risk associated with CAS may be lower compared to that of CEA in patients with increased risk for surgical complications.
Carotid Angioplasty and Stenting in Asymptomatic Patients
CAS has been reported to have superior outcomes when compared to CEA in high surgical risk patients. In a selected group of asymptomatic patients with unfavorable surgical anatomy and significant co-morbidities, it is reasonable to recommend CAS over CEA when intervention is indicated. High surgical risk patients were defined as having one or more of following criteria:223,224
New York Heart Association class III or IV heart failure
Chronic obstructive pulmonary disease
>50% contralateral carotid artery stenosis
Prior CEA or CAS
Prior coronary artery bypass graft surgery
The Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy (SAPHIRE) trial randomized high-risk patients into CEA and CAS with EPD groups, with an inclusion criteria of symptomatic stenosis >50% or asymptomatic stenosis >80%. The primary endpoint was defined as death, stroke or MI within 30 days plus death due to neurological causes or ipsilateral stroke between 31 days and 1 year. The secondary endpoint was defined as the primary endpoint events plus death or ipsilateral stroke between 1 and 3 years. Technical success was achieved in 95.6% of patients who underwent CAS. However, the study incurred a selection bias by excluding patients from the CEA arm who were considered a priori to have exceedingly high risk for complication. The trial was stopped before completion, after randomizing 334 patients, due to a sharp decline in enrolment rate. Three-year follow up data were available in only 85.6% of patients.143,144 In asymptomatic patients, the occurrence of the primary endpoint was greater after CEA (21.5%) versus after CAS (9.9%). The peri-procedural death, MI or stroke rate was also greater after CEA (10.2%) versus after CAS (5.4%). The 3-year stroke rates were comparable between CEA and CAS, at 9.2% and 10.3%, respectively.
CAS does not appear to be superior to CEA in asymptomatic patients with conventional surgical risk for intervention. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) was a multicenter, randomized trial comparing CAS to CEA in both symptomatic (carotid stenosis >50%) and asymptomatic patients (carotid stenosis >60%).141,225,226 Among 2502 patients followed for 2 years, the estimated 4-year rate of stroke, death of MI was similar in both CAS and CEA (7.2% and 6.8%, respectively; stenting HR: 1.11, 95% CI: 0.81−1.51, p=0.51). However, peri-procedural stroke alone was more frequent after CAS (4.1% versus 2.3%, p=0.01), while peri-procedural MI alone was more frequent after CEA (2.3% versus 1.1%, p=0.03). In the subgroup of asymptomatic patients, the 4-year stroke and death rates were higher after CAS (4.5% and 2.7%, respectively; HR: 1.86, p=0.07). In addition, CREST also showed that quality of life was significantly impacted by major and minor stroke but not by MI, based on quality of life studies done at 1 year. The outcomes with CEA and CAS also appeared to be affected by age, with a crossover occurring at approximately 70 years. CEA showed greater efficacy at older ages and CAS at younger ages.141 The comparative primary results did not vary by sex or symptom status. As seen in previous randomized trials, cranial nerve palsy was more common after CEA.
The Asymptomatic Carotid Surgery Trial-2 (ACST-2) is an ongoing, large, multicenter, randomized trial comparing CAS to CEA in asymptomatic patients with severe carotid stenosis. The trial aims to randomize 5000 patients. After randomizing 986 patients, interim safety results show that the combined CAS and CEA outcome is on par with other recent trials; however, comparison results between CAS and CEA are not currently available.227 CREST-2 is another study that will evaluate intensive medical management versus CEA or CAS in asymptomatic patients. The study is designed as two independent, multicenter, randomized controlled trials evaluating medical management versus CEA in one and CAS in the other.228
Carotid Angioplasty and Stenting in Symptomatic Patients
In symptomatic patients, CEA has been reported to have superior outcomes over CAS in both conventional- and high surgical risk patients. In high surgical risk symptomatic patients, SAPHIRE showed that despite a similar occurrence of the primary endpoint at 1 year (CAS 16.8% versus CEA 16.5%), the secondary endpoint at 3 years was higher after CAS (32% versus 21.7%). Of note, only a smaller portion of symptomatic patients underwent 3-year follow-up compared to asymptomatic patients.143,144
Several studies have compared the outcomes of CEA and CAS in conventional surgical risk symptomatic patients. One of the most comprehensive and better designed is CREST, a multicenter, randomized trial comparing CAS to CEA in both symptomatic (carotid stenosis >50%) and asymptomatic patients (carotid stenosis >60%).141,225,226 The 4-year stroke and death rate was higher after CAS in symptomatic patients (8.0% versus 6.4%, HR: 1.37, p=0.14). As mentioned above, although peri-procedural MI was more frequent after CEA, the study showed that quality of life was significantly impacted by major and minor stroke but not by MI.
Other studies comparing CAS to CEA in symptomatic patients with conventional surgical risk for intervention include the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), which was a multicenter, randomized trial comparing CAS with CEA. A total of 504 patients were randomized, 90% of whom were symptomatic.229–231 Of note, EPDs were not used and only 22% of CAS patients were stented. The combined stroke and death rate at 30 days was similar in both groups (10%). However, cranial neuropathy occurred more frequently in CEA patients (8.7% versus 0%, p <0.0001). Major incisional hematoma after CEA occurred more frequently than access site hematoma after CAS (6.7% versus 1.2%, p <0.0015). The rate of ipsilateral stroke after 3 years of follow up was similar in both groups (adjusted hazard ratio=1.04, 95% CI 0.63–1.70, p=0.9). However, the 8-year incidence and HR for ipsilateral non-perioperative stroke was 11.3% versus 8.6% (HR 1.22, 95% CI 0.59–2.54). There was also a higher rate of restenosis associated with CAS, with an estimated 5-year incidence of 30.7% compared to 10.5% after CEA. The investigators found that several factors were associated with the higher incidence of restenosis, including longer segments of stenosis at baseline and performing a balloon angioplasty alone without stenting.231,232
The Endarterectomy Versus Angioplasty in Symptomatic Severe Carotid Stenosis (EVA-3S) trial randomized patients with a completed stroke or TIA within the past 120 days and an ipsilateral carotid stenosis >60%.233 Patients with disabling stroke were excluded from the trial [mRS score >3]. After randomizing 520 patients, the trial was stopped before completion due to both safety and futility reasons. The 30-day incidence of stroke or death was 9.6% after CAS versus 3.9% after CEA, with a RR of 2.5 (95% CI 0.5−4.2). However, there were several factors in the EVA-3S trial, which may have confounded its results, including inadequate training requirements for operators performing CAS and no uniform requirement for the use of EPDs.234 In addition, 5 different carotid stent devices and 7 EPDs were used. While experts have agreed that the EVA-3S trial results should not affect management guidelines, the trial has highlighted the importance of rigorous and standardized training criteria required for interventionalists performing carotid stent placement.234
The Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study was a randomized, non-inferiority trial comparing CAS to CEA in symptomatic patients with a stroke or TIA within the past 180 days and ipsilateral carotid stenosis >70%.142,235 Patients with severe disabling stroke (mRS score >3) were excluded. The initial planned sample size of 1900 was not met; only 1214 patients were successfully randomized due to the inability to further enroll patients. Surgeons were required to have at least 25 CEAs done with acceptable rates of mortality and morbidity in the past year; and CAS operators were required to have performed at least 25 successful angioplasties or stenting, although not necessarily in the carotid artery. The rate of ipsilateral stroke and death were similar in both groups within 30 days (6.8% CAS versus 6.3% CEA) and also within 2 years (9.5% versus 8.8%, HR: 1.10, 95%, CI 0.75−1.61). Recurrent stenosis of ≥70% was more frequent in CAS patients compared to CEA (10.7% versus 4.6%, p=0.0009).
The International Carotid Stenting Study (ICSS) is a multicenter, randomized trial comparing the safety and efficacy of CAS to CEA in symptomatic patients with ipsilateral carotid stenosis ≥50%.236 The clinical phase of the trial is complete. In ICSS, participating centers were classified into ‘experienced’ or ‘supervised’. Experienced centers were defined as having at least 1 surgeon and 1 interventionalist who have performed 50 CEA (minimum of 10 per year) or 50 CAS (at least 10 involving the carotid), respectively. Supervised centers were designated as experienced after randomization and treatment of 20 cases of CEA or CAS, if the results were acceptable to a proctor and credentialing committee. In total, 88% of patients were treated at an experienced center. Interim safety analysis reported that the risk for stroke and death by all causes was higher in the CAS group (stroke: 7.6% after CAS versus 4.1% after CEA, HR 1.92, 95% CI: 1.27–2.89; death: 2.2% versus 0.8%, HR 2.76, 95% CI: 1.16–6.56). In the MRI sub-study, CAS was associated with more acute and persisting ischemic brain lesions.237 Peri-procedural hemodynamic instability, including bradycardia, asystole, or hypotension requiring treatment, were more likely to cause ischemic brain lesions in CAS patients versus CEA patients (RR, 3.36; 95% CI: 1.73–6.50).238
Anatomical Considerations
Several patient anatomic factors are considered to be unfavorable for endovascular intervention, including:239
Type II or III aortic arch
Arch vessel origin stenosis >50%
Common and internal carotid artery tortuosity >30°
Significant plaque calcifications
Long segment stenosis
These factors increase the technical difficulty of CAS, and also increase the risk for perioperative stroke. They are more prevalent in the elderly (>80 years of age), but may also be found in patients of all ages.
Cerebral Embolism Prevention
The outcomes associated with the use of EPDs have not been studied in randomized trials. Several observational studies have suggested that EPDs when used by experienced operators lead to reduced rates of adverse events, including major and minor strokes.240,241 An international survey involving 53 sites with a total of 11392 CAS procedures performed by experienced operators reported a combined stroke and death rate of 2.8% when EPDs were used and 6.2% when they were not.240 Several other studies have also shown an improvement in outcome with the use of EPDs.143,144,242–244
However, when used by operators who are not experienced with the device, EPDs have been associated with worse clinical outcome229,233,235 and increased incidence of ischemic abnormalities seen in post-procedural brain imaging.245 The ACCULINK for Revascularization of Carotids in High-Risk Patients (ARCHeR) trial, a non-randomized, multiphase trial that included experienced operators, did not show an improvement in outcome with the use of EPDs.
Peri-procedural Management
Dual antiplatelet therapy consisting of aspirin 81–325 mg daily and clopidogrel 75 mg daily is recommended before CAS and for a minimum of 30 days after. After which at least 1 antiplatelet agent should be continued long term. Ticlopidine 250 mg twice daily is an acceptable alternative for patients intolerant of clopidogrel. Adequate intra-procedural anticoagulation can be achieved with unfractionated heparin with a goal activated clotting time of 250–300 seconds. Alternatively, bivalirudin may be used, which has an added advantage over heparin, in that there is no need to monitor activated clotting time.246,247
CAS is associated with hemodynamic instability, including hypotension and vasovagal responses. Several intra-procedural steps can be taken to minimize the associated risk:39
Continuous electrocardiogram and blood pressure monitoring
Adequate hydration and adjustment of antihypertensive medication immediately prior to CAS to avoid persistent intra-procedural hypotension
Prophylactic administration of atropine 0.5 to 1 mg intravenously prior to angioplasty and stenting
Temporary transvenous pacemaker for persistent bradycardia
Phenylephrine 1–10mcg/kg/min or dopamine 5–15mcg/kg/min for persistent hypotension
To minimize risk of intracerebral hemorrhage or hyperperfusion syndrome, the SBP should be maintained below 180 mmHg before and during the procedure. In our experience, strict control of SBP below 140 mmHg immediately after revascularization has consistently prevented hemorrhages.
Complications
Complications associated with CAS include:
Cardiovascular: baroreflex responses, MI, arterial dissection, target vessel perforation, vasospasm, restenosis
Neurologic: TIA, stroke, hemorrhage, seizure
Device failure
Access-site injury
Baroreflex responses such as hypotension, bradycardia and vasovagal reactions occur in 5–10% of cases, but have been reported to be as high as 33%.248–250 Most are transient and do not require additional treatment after the procedure. With the introduction of appropriate pre-procedural management, rates can be kept in the lower range.249,251–256
The risk for MI is approximately 1%, with rates as low as 0.9% as reported in the CAPTURE registry of 3500 patients. However, this may be higher among high-risk patients, with up to 2.4% reported in the ARCHeR trial.154,250,257–266
The risk for arterial dissection or thrombosis was <1%. The risk for target vessel perforation was also <1% in what study.39 External carotid stenosis or occlusion occurred in 5–10% of cases, but were usually benign with no further intervention required.154,250,257–264,267 Transient vasospasm occurred in 10–15% of cases and was associated with vessel manipulation by guide wires, catheters and capture devices. This is also more common among smokers and patients with hypertension.268–271 Restenosis occurs in 3–5% of cases and can be minimized by avoiding multiple or high-pressure balloon angioplasties, particularly in heavily calcified vessels.174,272–289
The CAPTURE registry reported an overall stroke rate of 4.9%, with disabling strokes occurring in 2% of patients.267,290–298 The ARCHeR trial reported similar results with an overall stroke rate of 5.5% and disabling strokes occurring in 1.5% of patients.154,258–260,262,263,265,266 TIA occurs in up to 1–2% of patients undergoing CAS. Subclinical ischemic injury detected by magnetic resonance imaging (MRI) has also been reported.146,299,300
Intracranial hemorrhage associated with hyperperfusion, hypertension and anticoagulation occurs in <1% of cases.301–304 Seizures, which are predominantly associated with hyperperfusion occur in <1% of cases.305
Device malfunction occurs in <1% of procedures and include:268,269,306,307
Stent malformation
Stent migration
Failure of deployment of device
EPD failure (inability to deliver EPD to target zone, reduced steerability, and ischemia due to EPD overloaded by embolic material)
EPDs can reduce the stroke risk associated with CAS, but the device itself is also associated with failures.244,266,267,306,308–314 The use of appropriately sized EPDs is crucial, because undersized EPDs may allow passage of debris into distal circulation, while oversized EPDs may cause endothelial injury or cause vasospasm.
Access-site injuries occur in up to 5% of cases and mostly consist of local pain and hematoma, which are largely self-limited requiring no further intervention.315–318 Other access site injuries include:
Groin infection (<1%)
Pseudoaneurysm (1–2%)
Puncture site bleeding or retroperitoneal hematoma requiring blood transfusion (2–3%)
Contrast nephropathy is rare and has been reported in <1% of cases, largely because CAS is generally avoided in patients with severe renal dysfunction.319
Evaluation for Recurrence and Recurrence Management
Noninvasive imaging at the 1-month interval, followed by the 6-month interval, and then annually after revascularization is recommended for both CAS and CEA patients. Regular imaging allows for adequate assessment of ipsilateral carotid patency and to exclude development of contralateral lesions. Once stability has been established, surveillance at longer intervals may be appropriate. Surveillance may not be indicated when the patient is no longer a candidate for intervention.
The mechanism responsible for arterial restenosis after CEA is related to the postoperative interval. Early stenosis within 2 years is largely attributed to intimal hyperplasia, whereas later restenosis is usually due to progression of the atherosclerotic disease. Very early stenosis, detected on the first postoperative duplex US, usually represents an unsatisfactory or incomplete CEA. This usually occurs in <1% of cases and can be minimized by using intraoperative duplex US or a completion angiography.39
The CAVATAS investigators reported that long segment carotid stenosis (>0.65 times common carotid artery diameter) was associated with an increased risk for long-term restenosis. The risk for restenosis in long segment carotid stenosis was significantly greater in CAS patients compared to CEA patients.231,232 In CAS patients, performing an angioplasty alone without stenting was also associated with increased rates of restenosis.231,232
The reported incidence of recurrent stenosis depends on the methods used for detection. When assessed by ultrasonography, the rate of restenosis has been reported to be 5–10%. However, in more recent series in which patch closures were used, the restenosis rate has consistently been below 5%.191,192,203,215,320–323 When duplex US is used, hemodynamically significant restenosis occurred in 5–7% of cases.180,187,188,203,321,324–339
Comparison data on restenosis after CAS and CEA should be interpreted with caution.39 Most studies utilize ultrasonography as the follow-up imaging, which introduces potential bias. While stent placement has been shown to be associated with decreased rates of restenosis,231,232 the role of stent-generated artifacts in US velocity measurements have yet to be resolved with angiographic comparison. In our experience, this effect may be partially overcome by performing an intra-procedural carotid ultrasonography immediately after CAS. This allows for a direct comparison of carotid US results with post-procedural catheter angiography results for future reference. In the CAVATAS study, a carotid US at 1 year detected 70–99% stenosis in 4% of CEA patients and in 14% of CAS patients (p <0.001). Of note, only 22% of CAS patients had stent placement.229–231 In the SAPHIRE trial, where all CAS patients had stent placement, carotid US at 1 year was available in 218 patients (96 CEA, 122 CAS), and the rate of restenosis >70% was 4.2% in CEA patients and 0.8% in CAS patients (p=0.17).143,144 In the SPACE trial, carotid US at 1 year showed recurrent stenosis >70% in 4.6% of CEA patients and in 10.7% of CAS patients (p=0.0009).142,235
In patients with recurrent symptomatic carotid stenosis, a repeat CEA or CAS can be considered, using the same criteria as recommended for initial revascularization (discussed previously). Repeat intervention is also recommended when duplex ultrasonography and an additional confirmatory imaging (MRA, CTA or catheter angiography) shows rapidly progressive restenosis, indicating risk of complete occlusion.39 A repeat CEA can be considered under the hands of an experienced surgeon. CAS is an alternative to repeat CEA in patients with recurrent stenosis after CEA, and may be appropriate in asymptomatic patients with restenosis >80% or symptomatic restenosis >50%. Repeat intervention can also be considered in patients with asymptomatic recurrent stenosis, using the same criteria for initial intervention, but should not be performed in patients with <70% stenosis.
Summary
In summary, there are several imaging modalities that are available for the screening and diagnosis of carotid atherosclerotic disease, and treatment consists mainly of medical and interventional management.
Carotid US has a relatively low cost, minimal side effects and discomfort, and is widely available. It should be utilized as the initial screening tool for both symptomatic and asymptomatic patients with suspected carotid disease. Other more advanced non-invasive imaging, such as MRA and CTA, can be employed when US yields equivocal results, or is not available. MRA and CTA are helpful in determining the exact severity of stenosis and anatomical details in patients undergoing interventional management. Catheter angiography remains the gold standard for diagnosing carotid atherosclerotic disease and for grading stenosis degree. However, due to its inherent cost and risk for complications such as ischemic strokes, it should be reserved for patients in whom noninvasive imaging is contraindicated, inconclusive, does not provide adequate delineation of the disease, or yields discordant results.
Medical therapy consists mainly of antithrombotic therapy and risk factor modification. Dual antiplatelet combination therapy has not been shown to be superior to single agents. Anticoagulation with warfarin along with its potential risk for increased hemorrhagic complications also has not been shown to be superior to antiplatelet agents. Comprehensive risk factor management should be employed in these patients, including blood pressure control, cholesterol management, diabetes management, weight loss, cessation of smoking and other lifestyle modifications.
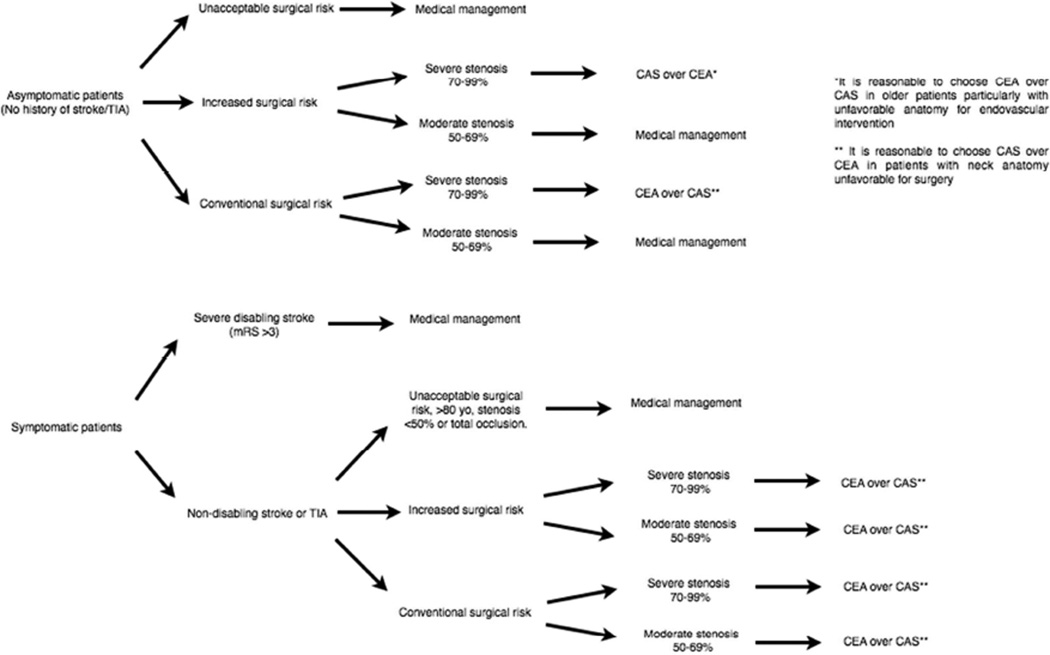
Randomized trials such as NASCET, ECST, ACAS, ACST, SPACE, EVA-3S, SAPHIRE and CREST (Table 1) have shown that revascularization decreases the long-term risk for adverse ischemic events in both asymptomatic patients with non-occlusive severe stenosis (>70%) and symptomatic patients without a devastating stroke (mRS >3), and moderate to severe stenosis (>50%). However, patient comorbidities, overall life expectancy and risk for peri-procedural complications, such as ischemic stroke, MI and death, must be taken into account (Table 2). The decision-making algorithm for medical treatment and types of revascularization are summarized in Figure 1.
Table – 1.
Summary of key randomized clinical trials
| Trial, Year (Reference) |
Study population, degree of stenosis |
Intervention | Comparison | No. of patients | Event | Events (%) | ||
|---|---|---|---|---|---|---|---|---|
| Treatment group |
Comparison group |
Treatment group |
Control Group |
|||||
| NASCET 1991140 | S (70–90% by angio) | CEA | Med | 328 | 321 | Ipsilateral stroke at 2 yr | 9.00 | 26.00 |
| NASCET 19988 | S (50–69% by angio) | CEA | Med | 320 | 428 | Ipsilateral stroke at 5 yr | 15.70 | 22.20 |
| ECST 2003151 | S (70–99% by angio) | CEA | Med | 429 | 850 | Stroke or surgical death | 6.80 | NA |
| S (50–69% by angio) | CEA | Med | 646 | 850 | Stroke or surgical death | 10.00 | NA | |
| ACAS 1995138 | AS (>60% by angio) | CEA | Med | 825 | 834 | Ipsilateral stroke, peri-procedural stroke or death | 5.10 | 11.0 |
| ACST 2004139 | AS (>60% by angio) | Immediate CEA | Delayed CEA | 1560 | 1560 | 5-yr stroke risk | 3.8 | 11.0 |
| SPACE 2008142 | S (≥70% by US) | CEA | CAS | 589 | 607 | All stroke at 2 yr | 10.10 | 10.90 |
| All peri-procedural strokes or deaths and ipsilateral strokes up to 2 yr | 8.80 | 9.50 | ||||||
| Ipsilateral stroke between 31d and 2 yr | 1.90 | 2.20 | ||||||
| EVA-3S 2008145 | S (≥60%) | CEA | CAS | 262 | 265 | All stroke at 4 yr | 3.40 | 9.10 |
| Ipsilateral stroke at 4 yr | 1.50 | 1.50 | ||||||
| All peri-procedural stroke, death and non-procedural ipsilateral stroke at 4 yr | 6.20 | 11.10 | ||||||
| SAPHIRE 2004 and 2008143,144 | S (≥50% by US) + AS (≥80% by US) | CEA | CAS | 167 | 167 | All strokes a 1 yr | 7.90 | 6.20 |
| Ipsilateral stroke at 1 yr | 4.80 | 4.20 | ||||||
| All stroke, death or MI within 30d of procedure, ipsilateral stroke between 31d and 1 yr | 20.10 | 12.20 | ||||||
| All strokes at 3 yr | 9.00 | 9.00 | ||||||
| Ipsilateral stroke at 3 yr | 5.40 | 6.60 | ||||||
| All stroke, death or MI within 30d of procedure, ipsilateral stroke between 31d and 1080 d | 26.90 | 24.60 | ||||||
| ICSS 2010146 | S (≥50% by angio or 2 non-invasive imaging) | CEA | CAS | 858 | 855 | All strokes within 30 d of randomization | 3.30 | 7.00 |
| All strokes within 120 d of randomization | 4.10 | 7.70 | ||||||
| CREST 2010141 | S (≥50% by angio, ≥70% by US) | CEA | CAS | 653 | 688 | All peri-procedural strokes, MI, death and post-procedural ipsilateral strokes up to 4 yr | 8.40 | 8.60 |
| All peri-procedural strokes, death and post-procedural ipsilateral strokes up to 4 yr | 6.40 | 8.00 | ||||||
| All peri-procedural strokes and post-procedural ipsilateral strokes up to 4 yr | 6.40 | 7.60 | ||||||
| AS (≥60% by angio, ≥70% by US) | CEA | CAS | 587 | 594 | All peri-procedural strokes, MI, death and post-procedural ipsilateral strokes up to 4 yr | 4.90 | 5.60 | |
| All peri-procedural strokes, death and post-procedural ipsilateral strokes up to 4 yr | 2.70 | 4.50 | ||||||
| All peri-procedural strokes and post-procedural ipsilateral strokes up to 4 yr | 2.70 | 4.50 | ||||||
| S + AS | CEA | CAS | 1240 | 1262 | All stroke up to 4 yr | 7.90 | 10.20 | |
US = ultrasonography, angio = catheter angiography, yr = year(s), d = day(s), S = symptomatic, AS = asymptomatic, NNT = number needed to treat; CEA = carotid endarterectomy; CAS = carotid angioplasty and stenting.
Table 2.
Factors Influencing the Decision of CEA versus CAS
| CEA | CAS |
|---|---|
Anatomic factors
|
Anatomic factors
|
Plaque factors
|
Plaque factors
|
Patient factors
|
Patient factors
|
As long as distal segment can be surgically reached below the angle of the
Figure 1.
Carotid disease management flow chart. Medical management should be started on all patients with carotid atherosclerotic disease independent of intervention. Carotid endarterectomy (CEA) should be considered in all patients who require intervention. CAS may be a better alternative to CEA in asymptomatic patients with severe stenosis and increased risk for surgery.
Key Points.
Asymptomatic patients without risk factors should not be screened for carotid atherosclerotic disease.
Carotid ultrasound should be the initial screening tool for symptomatic patients.
Medical management, including antiplatelet therapy, is indicated in all symptomatic patients with carotid atherosclerotic disease, independent of degree of stenosis.
In general, carotid revascularization is indicated in symptomatic patients with non-occlusive moderate to severe stenosis (>50%) and asymptomatic patients with severe stenosis (>70%).
When revascularization is indicated, patient anatomy, risk factors and plaque factors should be considered in the decision for carotid endarterectomy versus angioplasty and stenting.
Acknowledgments
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (award number K23NS079477). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yinn Cher Ooi, Email: Yooi@mednent.ucla.edu, Department of Neurosurgery, University of California, Los Angeles.
Nestor R. Gonzalez, Email: NGonzalez@mednet.ucla.edu, Department of Neurosurgery and Radiology, University of California, Los Angeles, 100 UCLA Med Plaza Suite# 219, Los Angeles, CA 90095, +1(310)825-5154.
References
- 1.States U, Bureau TUS, Participation P, August--november D, Disability A, Module T. Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(7):120–125. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11393491. [PubMed] [Google Scholar]
- 2.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. [Accessed July 15, 2014];Stroke. 1998 29(2):415–421. doi: 10.1161/01.str.29.2.415. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9472883. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Kannel WB, Sorlie P, McNamara P. Asymptomatic carotid bruit and risk of stroke. The Framingham study. [Accessed July 23, 2014];JAMA. 1981 245(14):1442–1445. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7206146. [PubMed] [Google Scholar]
- 4.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. [Accessed July 23, 2014];Am J Epidemiol. 2000 151(5):478–487. doi: 10.1093/oxfordjournals.aje.a010233. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10707916. [DOI] [PubMed] [Google Scholar]
- 5.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. [Accessed July 23, 2014];Am J Epidemiol. 1991 134(3):250–256. doi: 10.1093/oxfordjournals.aje.a116078. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1877584. [DOI] [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Garrett E, Klag MJ, Coresh J. Trends in stroke prevalence between 1973 and 1991 in the US population 25 to 74 years of age. [Accessed July 9, 2014];Stroke. 2002 33(5):1209–1213. doi: 10.1161/01.str.0000015031.57955.d1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11988592. [DOI] [PubMed] [Google Scholar]
- 8.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339(20):1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 9.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. [Accessed July 15, 2014];Stroke. 1996 27(9):1459–1466. doi: 10.1161/01.str.27.9.1459. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8784113. [DOI] [PubMed] [Google Scholar]
- 10.Wolf PA, Clagett GP, Easton JD, et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack : a statement for healthcare professionals from the Stroke Council of the American Heart Association. [Accessed July 22, 2014];Stroke. 1999 30(9):1991–1994. doi: 10.1161/01.str.30.9.1991. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10471455. [DOI] [PubMed] [Google Scholar]
- 11.Fine-Edelstein JS, Wolf PA, O’Leary DH, et al. Precursors of extracranial carotid atherosclerosis in the Framingham Study. [Accessed July 22, 2014];Neurology. 1994 44(6):1046–1050. doi: 10.1212/wnl.44.6.1046. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8208397. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. [Accessed July 22, 2014];Stroke. 1992 23(12):1752–1760. doi: 10.1161/01.str.23.12.1752. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1448826. [DOI] [PubMed] [Google Scholar]
- 13.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 14.Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. [Accessed July 23, 2014];Stroke. 1999 30(12):2513–2516. doi: 10.1161/01.str.30.12.2513. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10582970. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. [Accessed July 22, 2014];Stroke. 1995 26(1):14–20. doi: 10.1161/01.str.26.1.14. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7839388. [DOI] [PubMed] [Google Scholar]
- 16.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. [Accessed July 22, 2014];Stroke. 1996 27(11):1974–1980. doi: 10.1161/01.str.27.11.1974. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8898801. [DOI] [PubMed] [Google Scholar]
- 17.Rincon F, Sacco RL, Kranwinkel G, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis. 2009;28(1):65–71. doi: 10.1159/000219299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez NR, Liebeskind DS, Dusick JR, Mayor F, Saver J. Intracranial arterial stenoses: current viewpoints, novel approaches, and surgical perspectives. Neurosurg Rev. 2013;36(2):175–1784. doi: 10.1007/s10143-012-0432-z. discussion 184–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy T. [Accessed July 22, 2014];Stroke. 1991 22(6):816–817. doi: 10.1161/01.str.22.6.816. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2057984. [DOI] [PubMed] [Google Scholar]
- 20.Young B, Moore WS, Robertson JT, et al. An analysis of perioperative surgical mortality and morbidity in the asymptomatic carotid atherosclerosis study. ACAS Investigators. Asymptomatic Carotid Atherosclerosis Study. [Accessed July 22, 2014];Stroke. 1996 27(12):2216–2224. doi: 10.1161/01.str.27.12.2216. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8969784. [DOI] [PubMed] [Google Scholar]
- 21.Halliday AW, Thomas D, Mansfield A. The Asymptomatic Carotid Surgery Trial (ACST). Rationale and design. Steering Committee. [Accessed July 22, 2014];Eur J Vasc Surg. 1994 8(6):703–710. doi: 10.1016/s0950-821x(05)80650-4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7828747. [DOI] [PubMed] [Google Scholar]
- 22.Fisher M, Paganini-Hill A, Martin A, et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke. 2005;36(2):253–257. doi: 10.1161/01.STR.0000152336.71224.21. [DOI] [PubMed] [Google Scholar]
- 23.Lal BK, Hobson RW, Pappas PJ, et al. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. [Accessed July 21, 2014];J Vasc Surg. 2002 35(6):1210–1217. doi: 10.1067/mva.2002.122888. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12042733. [DOI] [PubMed] [Google Scholar]
- 24.Redgrave JNE, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke. 2007;38(5):1482–1488. doi: 10.1161/STROKEAHA.106.477380. [DOI] [PubMed] [Google Scholar]
- 25.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke. 2006;37(3):818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 26.Spagnoli LG, Mauriello A, Sangiorgi G, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA. 2004;292(15):1845–1852. doi: 10.1001/jama.292.15.1845. [DOI] [PubMed] [Google Scholar]
- 27.Redgrave JNE, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113(19):2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 28.Rudd JHF, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. [Accessed July 22, 2014];Circulation. 2002 105(23):2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12057982. [DOI] [PubMed] [Google Scholar]
- 29.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez B, Ruiz C, Chacón P, Alvarez-Sabin J, Matas M. Serum values of metalloproteinase-2 and metalloproteinase-9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J Vasc Surg. 2004;40(3):469–475. doi: 10.1016/j.jvs.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Arthurs ZM, Andersen C, Starnes BW, Sohn VY, Mullenix PS, Perry J. A prospective evaluation of C-reactive protein in the progression of carotid artery stenosis. J Vasc Surg. 2008;47(4):744–750. doi: 10.1016/j.jvs.2007.11.066. discussion 751. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez Garcia B, Ruiz C, Chacon P, Sabin JA, Matas M. High-sensitivity C-reactive protein in high-grade carotid stenosis: risk marker for unstable carotid plaque. J Vasc Surg. 2003;38(5):1018–1024. doi: 10.1016/s0741-5214(03)00709-2. [DOI] [PubMed] [Google Scholar]
- 33.Howard G, Baker WH, Chambless LE, Howard VJ, Jones AM, Toole JF. An approach for the use of Doppler ultrasound as a screening tool for hemodynamically significant stenosis (despite heterogeneity of Doppler performance). A multicenter experience. Asymptomatic Carotid Atherosclerosis Study Investigators. [Accessed July 22, 2014];Stroke. 1996 27(11):1951–1957. doi: 10.1161/01.str.27.11.1951. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8898797. [DOI] [PubMed] [Google Scholar]
- 34.Kuntz KM, Polak JF, Whittemore AD, Skillman JJ, Kent KC. Duplex ultrasound criteria for the identification of carotid stenosis should be laboratory specific. [Accessed July 22, 2014];Stroke. 1997 28(3):597–602. doi: 10.1161/01.str.28.3.597. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9056618. [DOI] [PubMed] [Google Scholar]
- 35.Alexandrov AV. Ultrasound and angiography in the selection of patients for carotid endarterectomy. [Accessed July 23, 2014];Curr Cardiol Rep. 2003 5(2):141–147. doi: 10.1007/s11886-003-0082-4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12583859. [DOI] [PubMed] [Google Scholar]
- 36.Paciaroni M, Caso V, Cardaioli G, et al. Is ultrasound examination sufficient in the evaluation of patients with internal carotid artery severe stenosis or occlusion? Cerebrovasc Dis. 2003;15(3):173–176. doi: 10.1159/000068832. doi:68832. [DOI] [PubMed] [Google Scholar]
- 37.Filis KA, Arko FR, Johnson BL, et al. Duplex ultrasound criteria for defining the severity of carotid stenosis. Ann Vasc Surg. 2002;16(4):413–421. doi: 10.1007/s10016-001-0175-8. [DOI] [PubMed] [Google Scholar]
- 38.Mattos MA, Hodgson KJ, Faught WE, et al. Carotid endarterectomy without angiography: is color-flow duplex scanning sufficient? [Accessed July 22, 2014];Surgery. 1994 116(4):776–782. discussion 782–3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7940178. [PubMed] [Google Scholar]
- 39.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task F. Circulation. 2011;124(4):e54–e130. doi: 10.1161/CIR.0b013e31820d8c98. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Counc. Circulation. 2006;113(24):e873–e923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 41.Whitty CJ, Sudlow CL, Warlow CP. Investigating individual subjects and screening populations for asymptomatic carotid stenosis can be harmful. [Accessed July 22, 2014];J Neurol Neurosurg Psychiatry. 1998 64(5):619–623. doi: 10.1136/jnnp.64.5.619. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2170073&tool=pmcent rez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229(2):340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 43.Blakeley DD, Oddone EZ, Hasselblad V, Simel DL, Matchar DB. Noninvasive carotid artery testing. A meta-analytic review. [Accessed July 22, 2014];Ann Intern Med. 1995 122(5):360–367. doi: 10.7326/0003-4819-122-5-199503010-00007. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7847648. [DOI] [PubMed] [Google Scholar]
- 44.AbuRahma AF, Robinson PA, Strickler DL, Alberts S, Young L. Proposed new duplex classification for threshold stenoses used in various symptomatic and asymptomatic carotid endarterectomy trials. Ann Vasc Surg. 1998;12(4):349–358. doi: 10.1007/s100169900166. [DOI] [PubMed] [Google Scholar]
- 45.Busuttil SJ, Franklin DP, Youkey JR, Elmore JR. Carotid duplex overestimation of stenosis due to severe contralateral disease. Am J Surg. 1996;172(2):144–147. doi: 10.1016/S0002-9610(96)00137-7. discussion 147–8. [DOI] [PubMed] [Google Scholar]
- 46.Comerota AJ, Salles-Cunha SX, Daoud Y, Jones L, Beebe HG. Gender differences in blood velocities across carotid stenoses. J Vasc Surg. 2004;40(5):939–944. doi: 10.1016/j.jvs.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Chi Y-W, White CJ, Woods TC, Goldman CK. Ultrasound velocity criteria for carotid in-stent restenosis. Catheter Cardiovasc Interv. 2007;69(3):349–354. doi: 10.1002/ccd.21032. [DOI] [PubMed] [Google Scholar]
- 48.Utter GH, Hollingworth W, Hallam DK, Jarvik JG, Jurkovich GJ. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203(6):838–848. doi: 10.1016/j.jamcollsurg.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Jahromi AS, Cinà CS, Liu Y, Clase CM. Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg. 2005;41(6):962–972. doi: 10.1016/j.jvs.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 50.Nederkoorn PJ, van der Graaf Y, Hunink MGM. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review. Stroke. 2003;34(5):1324–1332. doi: 10.1161/01.STR.0000068367.08991.A2. [DOI] [PubMed] [Google Scholar]
- 51.DeMarco JK, Huston J, Bernstein MA. Evaluation of classic 2D time-of-flight MR angiography in the depiction of severe carotid stenosis. AJR Am J Roentgenol. 2004;183(3):787–793. doi: 10.2214/ajr.183.3.1830787. [DOI] [PubMed] [Google Scholar]
- 52.Belsky M, Gaitini D, Goldsher D, Hoffman A, Daitzchman M. Color-coded duplex ultrasound compared to CT angiography for detection and quantification of carotid artery stenosis. [Accessed July 22, 2014];Eur J Ultrasound. 2000 12(1):49–60. doi: 10.1016/s0929-8266(00)00101-4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10996770. [DOI] [PubMed] [Google Scholar]
- 53.Grønholdt M-LM. B-mode ultrasound and spiral CT for the assessment of carotid atherosclerosis. [Accessed July 22, 2014];Neuroimaging Clin N Am. 2002 12(3):421–435. doi: 10.1016/s1052-5149(02)00015-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12486830. [DOI] [PubMed] [Google Scholar]
- 54.Hollingworth W, Nathens AB, Kanne JP, et al. The diagnostic accuracy of computed tomography angiography for traumatic or atherosclerotic lesions of the carotid and vertebral arteries: a systematic review. [Accessed July 22, 2014];Eur J Radiol. 2003 48(1):88–102. doi: 10.1016/s0720-048x(03)00200-6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14511863. [DOI] [PubMed] [Google Scholar]
- 55.Koelemay MJW, Nederkoorn PJ, Reitsma JB, Majoie CB. Systematic review of computed tomographic angiography for assessment of carotid artery disease. Stroke. 2004;35(10):2306–2312. doi: 10.1161/01.STR.0000141426.63959.cc. [DOI] [PubMed] [Google Scholar]
- 56.Enterline DS, Kapoor G. A practical approach to CT angiography of the neck and brain. Tech Vasc Interv Radiol. 2006;9(4):192–204. doi: 10.1053/j.tvir.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Clevert D-A, Johnson T, Jung EM, et al. Color Doppler, power Doppler and B-flow ultrasound in the assessment of ICA stenosis: Comparison with 64-MD-CT angiography. Eur Radiol. 2007;17(8):2149–2159. doi: 10.1007/s00330-006-0488-7. [DOI] [PubMed] [Google Scholar]
- 58.Wutke R, Lang W, Fellner C, et al. High-resolution, contrast-enhanced magnetic resonance angiography with elliptical centric k-space ordering of supra-aortic arteries compared with selective X-ray angiography. [Accessed July 22, 2014];Stroke. 2002 33(6):1522–1529. doi: 10.1161/01.str.0000016972.70366.d6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12052985. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez-Linera J, Benito-Leon J, Escribano J, Campollo J, Gesto R. Prospective evaluation of carotid artery stenosis: elliptic centric contrast-enhanced MR angiography and spiral CT angiography compared with digital subtraction angiography. [Accessed July 22, 2014];AJNR Am J Neuroradiol. 2003 24(5):1012–1019. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12748115. [PMC free article] [PubMed] [Google Scholar]
- 60.Remonda L, Senn P, Barth A, Arnold M, Lovblad K-O, Schroth G. Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. [Accessed July 22, 2014];AJNR Am J Neuroradiol. 2002 23(2):213–219. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11847044. [PMC free article] [PubMed] [Google Scholar]
- 61.Cosottini M, Pingitore A, Puglioli M, et al. Contrast-enhanced three-dimensional magnetic resonance angiography of atherosclerotic internal carotid stenosis as the noninvasive imaging modality in revascularization decision making. Stroke. 2003;34(3):660–664. doi: 10.1161/01.STR.0000057462.02141.6F. [DOI] [PubMed] [Google Scholar]
- 62.Yucel EK, Anderson CM, Edelman RR, et al. AHA scientific statement. Magnetic resonance angiography : update on applications for extracranial arteries. [Accessed July 22, 2014];Circulation. 1999 100(22):2284–2301. doi: 10.1161/01.cir.100.22.2284. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10578005. [DOI] [PubMed] [Google Scholar]
- 63.Josephson SA, Bryant SO, Mak HK, Johnston SC, Dillon WP, Smith WS. Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. [Accessed July 22, 2014];Neurology. 2004 63(3):457–460. doi: 10.1212/01.wnl.0000135154.53953.2c. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15304575. [DOI] [PubMed] [Google Scholar]
- 64.Glor FP, Ariff B, Crowe LA, et al. Carotid geometry reconstruction: a comparison between MRI and ultrasound. [Accessed July 22, 2014];Med Phys. 2003 30(12):3251–3261. doi: 10.1118/1.1628412. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14713092. [DOI] [PubMed] [Google Scholar]
- 65.Teng MMH, Tsai F, Liou AJ-K, et al. Three-dimensional contrast-enhanced magnetic resonance angiography of carotid artery after stenting. J Neuroimaging. 2004;14(4):336–341. doi: 10.1177/1051228404267620. [DOI] [PubMed] [Google Scholar]
- 66.Chen C-J, Lee T-H, Hsu H-L, et al. Multi-Slice CT angiography in diagnosing total versus near occlusions of the internal carotid artery: comparison with catheter angiography. Stroke. 2004;35(1):83–85. doi: 10.1161/01.STR.0000106139.38566.B2. [DOI] [PubMed] [Google Scholar]
- 67.Kaufmann TJ, Huston J, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243(3):812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 68.Thiex R, Norbash AM, Frerichs KU. The safety of dedicated-team catheter-based diagnostic cerebral angiography in the era of advanced noninvasive imaging. AJNR Am J Neuroradiol. 2010;31(2):230–234. doi: 10.3174/ajnr.A1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. [Accessed July 14, 2014];BMJ. 2002 324(7329):71–86. doi: 10.1136/bmj.324.7329.71. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=64503&tool=pmcentre z&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39(5):1647–1652. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.A randomized trial of aspirin and sulfinpyrazone in threatened stroke. The Canadian Cooperative Study Group. N Engl J Med. 1978;299(2):53–59. doi: 10.1056/NEJM197807132990201. [DOI] [PubMed] [Google Scholar]
- 72.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. [Accessed July 22, 2014];Lancet. 1996 348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8918275. [DOI] [PubMed] [Google Scholar]
- 73.Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 74.Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 75.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. [Accessed July 22, 2014];J Neurol Sci. 1996 143(1–2):1–13. doi: 10.1016/s0022-510x(96)00308-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8981292. [DOI] [PubMed] [Google Scholar]
- 76.Sacco RL, Diener H-C, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alberts MJ, Bergman DL, Molner E, Jovanovic BD, Ushiwata I, Teruya J. Antiplatelet effect of aspirin in patients with cerebrovascular disease. Stroke. 2004;35(1):175–178. doi: 10.1161/01.STR.0000106763.46123.F6. [DOI] [PubMed] [Google Scholar]
- 78.Chen L-C, Ashcroft DM. Do selective COX-2 inhibitors increase the risk of cerebrovascular events? A meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2006;31(6):565–576. doi: 10.1111/j.1365-2710.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 79.Côté R, Battista RN, Abrahamowicz M, Langlois Y, Bourque F, Mackey A. Lack of effect of aspirin in asymptomatic patients with carotid bruits and substantial carotid narrowing. The Asymptomatic Cervical Bruit Study Group. [Accessed July 22, 2014];Ann Intern Med. 1995 123(9):649–655. doi: 10.7326/0003-4819-123-9-199511010-00002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7574219. [DOI] [PubMed] [Google Scholar]
- 80.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 81.Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atheros. Stroke. 2007;38(5):1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 82.Woessner R, Grauer M, Bianchi O, et al. Treatment with anticoagulants in cerebral events (TRACE) Thromb Haemost. 2004;91(4):690–693. doi: 10.1160/TH03-11-0680. [DOI] [PubMed] [Google Scholar]
- 83.Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. [Accessed July 22, 2014];JAMA. 279(16):1265–1272. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9565006. [PubMed] [Google Scholar]
- 84.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. [Accessed July 20, 2014];Arch Intern Med. 1994 154(13):1449–1457. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8018000. [PubMed] [Google Scholar]
- 85.Lawes CMM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35(3):776–785. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- 86.Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. [Accessed July 22, 2014];Lancet. 2000 356(9246):1955–1964. doi: 10.1016/s0140-6736(00)03307-9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11130523. [DOI] [PubMed] [Google Scholar]
- 87.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34(11):2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 88.MacMahon S, Rodgers A. Blood pressure, antihypertensive treatment and stroke risk. [Accessed July 22, 2014];J Hypertens Suppl. 1994 12(10):S5–S14. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7769492. [PubMed] [Google Scholar]
- 89.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on C. Stroke. 2006;37(2):577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 90.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 91.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 92.Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. [Accessed July 22, 2014];JAMA. 2000 283(16):2122–2127. doi: 10.1001/jama.283.16.2122. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10791504. [DOI] [PubMed] [Google Scholar]
- 93.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 94.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. [Accessed July 10, 2014];Circulation. 2002 106(25):3143–3421. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12485966. [PubMed] [Google Scholar]
- 95.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. [Accessed July 22, 2014];Circulation. 2002 106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12438303. [DOI] [PubMed] [Google Scholar]
- 96.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 97.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 98.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341(6):410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 99.Sacco RL, Roberts JK, Boden-Albala B, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. [Accessed July 22, 2014];Stroke. 1997 28(5):929–935. doi: 10.1161/01.str.28.5.929. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9158627. [DOI] [PubMed] [Google Scholar]
- 100.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. [Accessed July 22, 2014];Stroke. 1996 27(2):224–231. doi: 10.1161/01.str.27.2.224. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8571414. [DOI] [PubMed] [Google Scholar]
- 101.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. [Accessed July 22, 2014];Arterioscler Thromb. 1994 14(7):1098–1104. doi: 10.1161/01.atv.14.7.1098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8018665. [DOI] [PubMed] [Google Scholar]
- 102.Briel M, Studer M, Glass TR, Bucher HC. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2004;117(8):596–606. doi: 10.1016/j.amjmed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 103.Amarenco P, Labreuche J, Lavallee P, Touboul P-J. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35(12):2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 104.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 105.Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. [Accessed July 11, 2014];Lancet. 2001 357(9256):577–581. doi: 10.1016/s0140-6736(00)04053-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11558482. [DOI] [PubMed] [Google Scholar]
- 106.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 107.Crouse JR, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297(12):1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 108.Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. [Accessed July 22, 2014];J Am Coll Cardiol. 1986 8(6):1245–1255. doi: 10.1016/s0735-1097(86)80293-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3782631. [DOI] [PubMed] [Google Scholar]
- 109.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 110.Bloomfield Rubins H, Davenport J, Babikian V, et al. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT) [Accessed July 22, 2014];Circulation. 2001 103(23):2828–2833. doi: 10.1161/01.cir.103.23.2828. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11401940. [DOI] [PubMed] [Google Scholar]
- 111.Kastelein JJP, Akdim F, Stroes ESG, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 112.Blankenhorn DH, Selzer RH, Crawford DW, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery. Two- and four-year reduction of intima-media thickness measured by ultrasound. [Accessed July 22, 2014];Circulation. 1993 88(1):20–28. doi: 10.1161/01.cir.88.1.20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8319334. [DOI] [PubMed] [Google Scholar]
- 113.Smith NL, Barzilay JI, Shaffer D, et al. Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. [Accessed July 22, 2014];Arch Intern Med. 2002 162(2):209–216. doi: 10.1001/archinte.162.2.209. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11802755. [DOI] [PubMed] [Google Scholar]
- 114.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. [Accessed July 22, 2014];Arch Intern Med. 1991 151(6):1141–1147. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2043016. [PubMed] [Google Scholar]
- 115.Karapanayiotides T, Piechowski-Jozwiak B, van Melle G, Bogousslavsky J, Devuyst G. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. [Accessed July 22, 2014];Neurology. 2004 62(9):1558–1562. doi: 10.1212/01.wnl.0000123252.55688.05. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15136681. [DOI] [PubMed] [Google Scholar]
- 116.Folsom AR, Rasmussen ML, Chambless LE, et al. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. [Accessed July 22, 2014];Diabetes Care. 1999 22(7):1077–1083. doi: 10.2337/diacare.22.7.1077. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10388971. [DOI] [PubMed] [Google Scholar]
- 117.Dobs AS, Nieto FJ, Szklo M, Barnes R, Sharrett AR, Ko WJ. Risk factors for popliteal and carotid wall thicknesses in the Atherosclerosis Risk in Communities (ARIC) Study. [Accessed July 24, 2014];Am J Epidemiol. 1999 150(10):1055–1067. doi: 10.1093/oxfordjournals.aje.a009929. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10568620. [DOI] [PubMed] [Google Scholar]
- 118.Wagenknecht LE, D’Agostino R, Savage PJ, O’Leary DH, Saad MF, Haffner SM. Duration of diabetes and carotid wall thickness. The Insulin Resistance Atherosclerosis Study (IRAS) [Accessed July 22, 2014];Stroke. 1997 28(5):999–1005. doi: 10.1161/01.str.28.5.999. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9158641. [DOI] [PubMed] [Google Scholar]
- 119.Haffner SM, Agostino RD, Saad MF, et al. Carotid artery atherosclerosis in type-2 diabetic and nondiabetic subjects with and without symptomatic coronary artery disease (The Insulin Resistance Atherosclerosis Study) [Accessed July 22, 2014];Am J Cardiol. 2000 85(12):1395–1400. doi: 10.1016/s0002-9149(00)00784-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10856382. [DOI] [PubMed] [Google Scholar]
- 120.Wagenknecht LE, Zaccaro D, Espeland MA, Karter AJ, O’Leary DH, Haffner SM. Diabetes and progression of carotid atherosclerosis: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 2003;23(6):1035–1041. doi: 10.1161/01.ATV.0000072273.67342.6D. [DOI] [PubMed] [Google Scholar]
- 121.Chambless LE, Folsom AR, Davis V, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. [Accessed July 22, 2014];Am J Epidemiol. 2002 155(1):38–47. doi: 10.1093/aje/155.1.38. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11772783. [DOI] [PubMed] [Google Scholar]
- 122.Nathan DM, Lachin J, Cleary P, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Langenfeld MR, Forst T, Hohberg C, et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation. 2005;111(19):2525–2531. doi: 10.1161/01.CIR.0000165072.01672.21. [DOI] [PubMed] [Google Scholar]
- 124.Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296(21):2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 125.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. [Accessed July 24, 2014];BMJ. 1989 298(6676):789–794. doi: 10.1136/bmj.298.6676.789. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1836102&tool=pmcent rez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and decreased risk of stroke in women. [Accessed July 22, 2014];JAMA. 1993 269(2):232–236. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8417241. [PubMed] [Google Scholar]
- 127.Robbins AS, Manson JE, Lee IM, Satterfield S, Hennekens CH. Cigarette smoking and stroke in a cohort of U.S. male physicians. [Accessed July 13, 2014];Ann Intern Med. 1994 120(6):458–462. doi: 10.7326/0003-4819-120-6-199403150-00002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8311368. [DOI] [PubMed] [Google Scholar]
- 128.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Smoking cessation and the risk of stroke in middle-aged men. [Accessed July 22, 2014];JAMA. 1995 274(2):155–160. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7596004. [PubMed] [Google Scholar]
- 129.Rohr J, Kittner S, Feeser B, et al. Traditional risk factors and ischemic stroke in young adults: the Baltimore-Washington Cooperative Young Stroke Study. [Accessed July 22, 2014];Arch Neurol. 1996 53(7):603–607. doi: 10.1001/archneur.1996.00550070041010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8929167. [DOI] [PubMed] [Google Scholar]
- 130.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. [Accessed July 22, 2014];Stroke. 1998 29(5):913–917. doi: 10.1161/01.str.29.5.913. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9596234. [DOI] [PubMed] [Google Scholar]
- 131.Lu M, Ye W, Adami H-O, Weiderpass E. Stroke incidence in women under 60 years of age related to alcohol intake and smoking habit. Cerebrovasc Dis. 2008;25(6):517–525. doi: 10.1159/000131669. [DOI] [PubMed] [Google Scholar]
- 132.Wilson PW, Hoeg JM, D’Agostino RB, et al. Cumulative effects of high cholesterol levels, high blood pressure, and cigarette smoking on carotid stenosis. N Engl J Med. 1997;337(8):516–522. doi: 10.1056/NEJM199708213370802. [DOI] [PubMed] [Google Scholar]
- 133.Tell GS, Rutan GH, Kronmal RA, et al. Correlates of blood pressure in community-dwelling older adults. The Cardiovascular Health Study. Cardiovascular Health Study (CHS) Collaborative Research Group. [Accessed July 22, 2014];Hypertension. 1994 23(1):59–67. doi: 10.1161/01.hyp.23.1.59. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8282331. [DOI] [PubMed] [Google Scholar]
- 134.Wolf PA, D’Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The Framingham Study. [Accessed July 22, 2014];JAMA. 1988 259(7):1025–1029. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3339799. [PubMed] [Google Scholar]
- 135.Winter Y, Rohrmann S, Linseisen J, et al. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39(12):3145–3151. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 136.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. [Accessed July 22, 2014];Stroke. 1998 29(2):380–387. doi: 10.1161/01.str.29.2.380. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9472878. [DOI] [PubMed] [Google Scholar]
- 137.Hankey GJ. Potential new risk factors for ischemic stroke: what is their potential? Stroke. 2006;37(8):2181–2188. doi: 10.1161/01.STR.0000229883.72010.e4. [DOI] [PubMed] [Google Scholar]
- 138.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. [Accessed July 22, 2014];JAMA. 1995 273(18):1421–1428. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7723155. [PubMed] [Google Scholar]
- 139.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 140.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 141.Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7(10):893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 143.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358(15):1572–1579. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 144.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 145.Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7(10):885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 146.Bonati LH, Jongen LM, Haller S, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS) Lancet Neurol. 2010;9(4):353–362. doi: 10.1016/S1474-4422(10)70057-0. [DOI] [PubMed] [Google Scholar]
- 147.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) [Accessed July 22, 2014];Lancet. 1998 351(9113):1379–1387. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9593407. [PubMed] [Google Scholar]
- 148.Rothwell PM, Slattery J, Warlow CP. A systematic review of the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis. [Accessed July 22, 2014];Stroke. 1996 27(2):260–265. doi: 10.1161/01.str.27.2.260. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8571420. [DOI] [PubMed] [Google Scholar]
- 149.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363(9413):915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 150.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. [Accessed July 22, 2014];Lancet. 2003 361(9352):107–116. doi: 10.1016/s0140-6736(03)12228-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12531577. [DOI] [PubMed] [Google Scholar]
- 151.Rothwell PM, Gutnikov Sa, Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34(2):514–523. doi: 10.1161/01.str.0000054671.71777.c7. [DOI] [PubMed] [Google Scholar]
- 152.Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. [Accessed July 22, 2014];JAMA. 1991 266(23):3289–3294. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1960828. [PubMed] [Google Scholar]
- 153.Hobson RW, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328(4):221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 154.Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44(2):258–268. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 155.Katzen BT, Criado FJ, Ramee SR, et al. Carotid artery stenting with emboli protection surveillance study: thirty-day results of the CASES-PMS study. Catheter Cardiovasc Interv. 2007;70(2):316–323. doi: 10.1002/ccd.21222. [DOI] [PubMed] [Google Scholar]
- 156.Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 2004;35(10):2425–2427. doi: 10.1161/01.STR.0000141706.50170.a7. [DOI] [PubMed] [Google Scholar]
- 157.Role of carotid endarterectomy in asymptomatic carotid stenosis. A Veterans Administration Cooperative Study. [Accessed July 22, 2014];Stroke. 17(3):534–539. doi: 10.1161/01.str.17.3.534. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2872740. [DOI] [PubMed] [Google Scholar]
- 158.Towne JB, Weiss DG, Hobson RW. First phase report of cooperative Veterans Administration asymptomatic carotid stenosis study--operative morbidity and mortality. [Accessed July 22, 2014];J Vasc Surg. 1990 11(2):252–258. discussion 258–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2405197. [PubMed] [Google Scholar]
- 159.Cowan JA, Dimick JB, Thompson BG, Stanley JC, Upchurch GR. Surgeon volume as an indicator of outcomes after carotid endarterectomy: an effect independent of specialty practice and hospital volume. [Accessed July 23, 2014];J Am Coll Surg. 2002 195(6):814–821. doi: 10.1016/s1072-7515(02)01345-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12495314. [DOI] [PubMed] [Google Scholar]
- 160.Killeen SD, Andrews EJ, Redmond HP, Fulton GJ. Provider volume and outcomes for abdominal aortic aneurysm repair, carotid endarterectomy, and lower extremity revascularization procedures. J Vasc Surg. 2007;45(3):615–626. doi: 10.1016/j.jvs.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 161.Holt PJE, Poloniecki JD, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between hospital volume and outcome following carotid endarterectomy. Eur J Vasc Endovasc Surg. 2007;33(6):645–651. doi: 10.1016/j.ejvs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 162.Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke. 2005;36(10):2072–2076. doi: 10.1161/01.STR.0000183623.28144.32. [DOI] [PubMed] [Google Scholar]
- 163.Kazmers A, Perkins AJ, Huber TS, Jacobs LA. Carotid surgery in octogenarians in Veterans Affairs medical centers. J Surg Res. 1999;81(1):87–90. doi: 10.1006/jsre.1998.5459. [DOI] [PubMed] [Google Scholar]
- 164.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. [Accessed July 22, 2014];JAMA. 279(16):1278–1281. doi: 10.1001/jama.279.16.1278. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9565008. [DOI] [PubMed] [Google Scholar]
- 165.Debing E, Van den Brande P. Carotid endarterectomy in the elderly: are the patient characteristics, the early outcome, and the predictors the same as those in younger patients? Surg Neurol. 2007;67(5):467–471. doi: 10.1016/j.surneu.2006.08.084. discussion 471. [DOI] [PubMed] [Google Scholar]
- 166.Hellings WE, Pasterkamp G, Verhoeven BAN, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg. 2007;45(2):289–296. doi: 10.1016/j.jvs.2006.09.051. discussion 296–7. [DOI] [PubMed] [Google Scholar]
- 167.Debing E, Von Kemp K, Van den Brande P. Gender differences in cardiovascular risk factors in a carotid endarterectomy population. [Accessed July 22, 2014];Int Angiol. 2006 25(1):18–25. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16520720. [PubMed] [Google Scholar]
- 168.Alamowitch S, Eliasziw M, Barnett HJM. The risk and benefit of endarterectomy in women with symptomatic internal carotid artery disease. Stroke. 2005;36(1):27–31. doi: 10.1161/01.STR.0000149622.12636.1f. [DOI] [PubMed] [Google Scholar]
- 169.Bryant MF. Anatomic considerations in carotid endarterectomy. [Accessed July 22, 2014];Surg Clin North Am. 1974 54(6):1291–1296. doi: 10.1016/s0039-6109(16)40484-6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4432208. [DOI] [PubMed] [Google Scholar]
- 170.Hans SS, Shah S, Hans B. Carotid endarterectomy for high plaques. [Accessed July 22, 2014];Am J Surg. 1989 157(4):431–434. doi: 10.1016/0002-9610(89)90593-x. discussion 434–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2929868. [DOI] [PubMed] [Google Scholar]
- 171.Bond R, Warlow CP, Naylor AR, Rothwell PM. Variation in surgical and anaesthetic technique and associations with operative risk in the European carotid surgery trial: implications for trials of ancillary techniques. Eur J Vasc Endovasc Surg. 2002;23(2):117–126. doi: 10.1053/ejvs.2001.1566. [DOI] [PubMed] [Google Scholar]
- 172.Kashyap VS, Moore WS, Quinones-Baldrich WJ. Carotid artery repair for radiation-associated atherosclerosis is a safe and durable procedure. [Accessed July 22, 2014];J Vasc Surg. 1999 29(1):90–96. doi: 10.1016/s0741-5214(99)70351-4. discussion 97–9. Available at http://www.ncbi.nlm.nih.gov/pubmed/9882793. [DOI] [PubMed] [Google Scholar]
- 173.Harrod-Kim P, Kadkhodayan Y, Derdeyn CP, Cross DT, Moran CJ. Outcomes of carotid angioplasty and stenting for radiation-associated stenosis. [Accessed July 22, 2014];AJNR Am J Neuroradiol. 2005 26(7):1781–1788. Available at http://www.ncbi.nlm.nih.gov/pubmed/16091530. [PMC free article] [PubMed] [Google Scholar]
- 174.Protack CD, Bakken AM, Saad WE, Illig KA, Waldman DL, Davies MG. Radiation arteritis: a contraindication to carotid stenting? J Vasc Surg. 2007;45(1):110–117. doi: 10.1016/j.jvs.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 175.Favre J-P, Nourissat A, Duprey A, Nourissat G, Albertini JN, Becquemin J-P. Endovascular treatment for carotid artery stenosis after neck irradiation. J Vasc Surg. 2008;48(4):852–858. doi: 10.1016/j.jvs.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 176.Baker JD, Gluecklich B, Watson CW, Marcus E, Kamat V, Callow AD. An evaluation of electroencephalographic monitoring for carotid study. [Accessed July 23, 2014];Surgery. 1975 78(6):787–794. Available at http://www.ncbi.nlm.nih.gov/pubmed/1188621. [PubMed] [Google Scholar]
- 177.Elmore JR, Eldrup-Jorgensen J, Leschey WH, Herbert WE, Dillihunt RC, Ray FS. Computerized topographic brain mapping during carotid endarterectomy. [Accessed July 23, 2014];Arch Surg. 1990 125(6):734–737. doi: 10.1001/archsurg.1990.01410180058011. discussion 738. Available at http://www.ncbi.nlm.nih.gov/pubmed/2088317. [DOI] [PubMed] [Google Scholar]
- 178.Moore WS, Yee JM, Hall AD. Collateral cerebral blood pressure. An index of tolerance to temporary carotid occlusion. [Accessed July 23, 2014];Arch Surg. 1973 106(4):521–523. doi: 10.1001/archsurg.1973.01350160134022. Available at http://www.ncbi.nlm.nih.gov/pubmed/4696724. [DOI] [PubMed] [Google Scholar]
- 179.Golledge J, Cuming R, Davies AH, Greenhalgh RM. Outcome of selective patching following carotid endarterectomy. [Accessed July 23, 2014];Eur J Vasc Endovasc Surg. 1996 11(4):458–463. doi: 10.1016/s1078-5884(96)80182-1. Available at http://www.ncbi.nlm.nih.gov/pubmed/8846183. [DOI] [PubMed] [Google Scholar]
- 180.Gelabert HA, el-Massry S, Moore WS. Carotid endarterectomy with primary closure does not adversely affect the rate of recurrent stenosis. [Accessed July 23, 2014];Arch Surg. 1994 129(6):648–654. doi: 10.1001/archsurg.1994.01420300092016. Available at http://www.ncbi.nlm.nih.gov/pubmed/8204041. [DOI] [PubMed] [Google Scholar]
- 181.Myers SI, Valentine RJ, Chervu A, Bowers BL, Clagett GP. Saphenous vein patch versus primary closure for carotid endarterectomy: long-term assessment of a randomized prospective study. [Accessed July 23, 2014];J Vasc Surg. 1994 19(1):15–22. doi: 10.1016/s0741-5214(94)70116-4. Available at http://www.ncbi.nlm.nih.gov/pubmed/8301727. [DOI] [PubMed] [Google Scholar]
- 182.De Letter JA, Moll FL, Welten RJ, et al. Benefits of carotid patching: a prospective randomized study with long-term follow-up. Ann Vasc Surg. 1994;8(1):54–58. doi: 10.1007/BF02133406. [DOI] [PubMed] [Google Scholar]
- 183.Fietsam R, Ranval T, Cohn S, Brown OW, Bendick P, Glover JL. Hemodynamic effects of primary closure versus patch angioplasty of the carotid artery. Ann Vasc Surg. 1992;6(5):443–449. doi: 10.1007/BF02007000. [DOI] [PubMed] [Google Scholar]
- 184.Rosenthal D, Archie JP, Garcia-Rinaldi R, et al. Carotid patch angioplasty: immediate and long-term results. [Accessed July 23, 2014];J Vasc Surg. 1990 12(3):326–333. Available at http://www.ncbi.nlm.nih.gov/pubmed/2144599. [PubMed] [Google Scholar]
- 185.Vanmaele R, Van Schil P, De Maeseneer M. Closure of the internal carotid artery after endarterectomy: the advantages of patch angioplasty without its disadvantages. Ann Vasc Surg. 1990;4(1):81–84. doi: 10.1007/BF02042696. [DOI] [PubMed] [Google Scholar]
- 186.Clagett GP, Patterson CB, Fisher DF, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. [Accessed July 23, 2014];J Vasc Surg. 1989 9(2):213–223. doi: 10.1067/mva.1989.vs0090213. Available at http://www.ncbi.nlm.nih.gov/pubmed/2645441. [DOI] [PubMed] [Google Scholar]
- 187.Eikelboom BC, Ackerstaff RG, Hoeneveld H, et al. Benefits of carotid patching: a randomized study. [Accessed July 23, 2014];J Vasc Surg. 1988 7(2):240–247. doi: 10.1067/mva.1988.avs0070240. Available at http://www.ncbi.nlm.nih.gov/pubmed/3276933. [DOI] [PubMed] [Google Scholar]
- 188.Hertzer NR, Beven EG, O’Hara PJ, Krajewski LP. A prospective study of vein patch angioplasty during carotid endarterectomy. Three-year results for 801 patients and 917 operations. [Accessed July 23, 2014];Ann Surg. 1987 206(5):628–635. doi: 10.1097/00000658-198711000-00013. Available at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1493295&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Katz MM, Jones GT, Degenhardt J, Gunn B, Wilson J, Katz S. The use of patch angioplasty to alter the incidence of carotid restenosis following thromboendarterectomy. [Accessed July 23, 2014];J Cardiovasc Surg (Torino) 28(1):2–8. Available at http://www.ncbi.nlm.nih.gov/pubmed/3805106. [PubMed] [Google Scholar]
- 190.Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane database Syst Rev. 2009;(4):CD000160. doi: 10.1002/14651858.CD000160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bond R, Rerkasem K, Naylor R, Rothwell PM. Patches of different types for carotid patch angioplasty. Cochrane database Syst Rev. 2004;(2):CD000071. doi: 10.1002/14651858.CD000071.pub2. [DOI] [PubMed] [Google Scholar]
- 192.Matsagas MI, Bali C, Arnaoutoglou E, et al. Carotid endarterectomy with bovine pericardium patch angioplasty: mid-term results. Ann Vasc Surg. 2006;20(5):614–619. doi: 10.1007/s10016-006-9102-3. [DOI] [PubMed] [Google Scholar]
- 193.Mannheim D, Weller B, Vahadim E, Karmeli R. Carotid endarterectomy with a polyurethane patch versus primary closure: a prospective randomized study. J Vasc Surg. 2005;41(3):403–407. doi: 10.1016/j.jvs.2004.11.036. discussion 407–8. [DOI] [PubMed] [Google Scholar]
- 194.Krishnan S, Clowes AW. Dacron patch infection after carotid endarterectomy: case report and review of the literature. Ann Vasc Surg. 2006;20(5):672–677. doi: 10.1007/s10016-006-9064-5. [DOI] [PubMed] [Google Scholar]
- 195.Schoenefeld E, Donas K, Radicke A, Osada N, Austermann M, Torsello G. Perioperative use of aspirin for patients undergoing carotid endarterectomy. Vasa. 2012;41(4):282–287. doi: 10.1024/0301-1526/a000204. [DOI] [PubMed] [Google Scholar]
- 196.Stone DH, Goodney PP, Schanzer A, et al. Clopidogrel is not associated with major bleeding complications during peripheral arterial surgery. J Vasc Surg. 2011;54(3):779–784. doi: 10.1016/j.jvs.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rosenbaum A, Rizvi AZ, Alden PB, et al. Outcomes related to antiplatelet or anticoagulation use in patients undergoing carotid endarterectomy. Ann Vasc Surg. 2011;25(1):25–31. doi: 10.1016/j.avsg.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 198.Oldag A, Schreiber S, Schreiber S, et al. Risk of wound hematoma at carotid endarterectomy under dual antiplatelet therapy. Langenbecks Arch Surg. 2012;397(8):1275–1282. doi: 10.1007/s00423-012-0967-z. [DOI] [PubMed] [Google Scholar]
- 199.Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. [Accessed July 23, 2014];Lancet. 1999 353(9171):2179–2184. doi: 10.1016/s0140-6736(99)05388-x. Available at http://www.ncbi.nlm.nih.gov/pubmed/10392981. [DOI] [PubMed] [Google Scholar]
- 200.LaMuraglia GM, Stoner MC, Brewster DC, et al. Determinants of carotid endarterectomy anatomic durability: effects of serum lipids and lipid-lowering drugs. J Vasc Surg. 2005;41(5):762–768. doi: 10.1016/j.jvs.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 201.Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy Trial : surgical results in 1415 patients. [Accessed July 23, 2014];Stroke. 1999 30(9):1751–1758. doi: 10.1161/01.str.30.9.1751. Available at http://www.ncbi.nlm.nih.gov/pubmed/10471419. [DOI] [PubMed] [Google Scholar]
- 202.Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20(2):69–77. doi: 10.1159/000086509. [DOI] [PubMed] [Google Scholar]
- 203.Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg. 2004;40(6):1126–1135. doi: 10.1016/j.jvs.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 204.Halm EA, Hannan EL, Rojas M, et al. Clinical and operative predictors of outcomes of carotid endarterectomy. J Vasc Surg. 2005;42(3):420–428. doi: 10.1016/j.jvs.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 205.Mericle RA, Kim SH, Lanzino G, et al. Carotid artery angioplasty and use of stents in high-risk patients with contralateral occlusions. J Neurosurg. 1999;90(6):1031–1036. doi: 10.3171/jns.1999.90.6.1031. [DOI] [PubMed] [Google Scholar]
- 206.Harthun NL, Baglioni AJ, Kongable GL, Meakem TD, Cherry KJ. Carotid endarterectomy: update on the gold standard treatment for carotid stenosis. [Accessed July 23, 2014];Am Surg. 2005 71(8):647–651. discussion 651–2. Available at http://www.ncbi.nlm.nih.gov/pubmed/16217946. [PubMed] [Google Scholar]
- 207.Matsen SL, Chang DC, Perler BA, Roseborough GS, Williams GM. Trends in the in-hospital stroke rate following carotid endarterectomy in California and Maryland. J Vasc Surg. 2006;44(3):488–495. doi: 10.1016/j.jvs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 208.Gupta AK, Purkayastha S, Unnikrishnan M, Vattoth S, Krishnamoorthy T, Kesavadas C. Hyperperfusion syndrome after supraaortic vessel interventions and bypass surgery. [Accessed July 23, 2014];J Neuroradiol. 2005 32(5):352–358. doi: 10.1016/s0150-9861(05)83170-0. Available at http://www.ncbi.nlm.nih.gov/pubmed/16424839. [DOI] [PubMed] [Google Scholar]
- 209.Van Mook WNKA, Rennenberg RJMW, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4(12):877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- 210.Nouraei SAR, Al-Rawi PG, Sigaudo-Roussel D, Giussani DA, Gaunt ME. Carotid endarterectomy impairs blood pressure homeostasis by reducing the physiologic baroreflex reserve. J Vasc Surg. 2005;41(4):631–637. doi: 10.1016/j.jvs.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 211.Posner SR, Boxer L, Proctor M, Upchurch GR, Stanley JC, Henke PK. Uncomplicated carotid endarterectomy: factors contributing to blood pressure instability precluding safe early discharge. [Accessed July 23, 2014];Vascular. 12(5):278–284. doi: 10.1258/rsmvasc.12.5.278. Available at http://www.ncbi.nlm.nih.gov/pubmed/15765908. [DOI] [PubMed] [Google Scholar]
- 212.Maroulis J, Karkanevatos A, Papakostas K, Gilling-Smith GL, McCormick MS, Harris PL. Cranial nerve dysfunction following carotid endarterectomy. [Accessed July 23, 2014];Int Angiol. 2000 19(3):237–241. Available at http://www.ncbi.nlm.nih.gov/pubmed/11201592. [PubMed] [Google Scholar]
- 213.Stoner MC, Cambria RP, Brewster DC, et al. Safety and efficacy of reoperative carotid endarterectomy: a 14-year experience. J Vasc Surg. 2005;41(6):942–949. doi: 10.1016/j.jvs.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 214.Sajid MS, Vijaynagar B, Singh P, Hamilton G. Literature review of cranial nerve injuries during carotid endarterectomy. [Accessed July 23, 2014];Acta Chir Belg. 107(1):25–28. doi: 10.1080/00015458.2007.11680006. Available at http://www.ncbi.nlm.nih.gov/pubmed/17405594. [DOI] [PubMed] [Google Scholar]
- 215.Cunningham EJ, Bond R, Mayberg MR, Warlow CP, Rothwell PM. Risk of persistent cranial nerve injury after carotid endarterectomy. J Neurosurg. 2004;101(3):445–448. doi: 10.3171/jns.2004.101.3.0445. [DOI] [PubMed] [Google Scholar]
- 216.Sternbach Y, Illig KA, Zhang R, et al. Hemodynamic benefits of regional anesthesia for carotid endarterectomy. [Accessed July 23, 2014];J Vasc Surg. 2002 35(2):333–339. doi: 10.1067/mva.2002.121579. Available at http://www.ncbi.nlm.nih.gov/pubmed/11854732. [DOI] [PubMed] [Google Scholar]
- 217.Cywinski JB, Koch CG, Krajewski LP, Smedira N, Li L, Starr NJ. Increased risk associated with combined carotid endarterectomy and coronary artery bypass graft surgery: a propensity-matched comparison with isolated coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2006;20(6):796–802. doi: 10.1053/j.jvca.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 218.Stoner MC, Abbott WM, Wong DR, et al. Defining the high-risk patient for carotid endarterectomy: an analysis of the prospective National Surgical Quality Improvement Program database. J Vasc Surg. 2006;43(2):285–295. doi: 10.1016/j.jvs.2005.10.069. discussion 295–6. [DOI] [PubMed] [Google Scholar]
- 219.Debing E, Van den Brande P. Does the type, number or combinations of traditional cardiovascular risk factors affect early outcome after carotid endarterectomy? Eur J Vasc Endovasc Surg. 2006;31(6):622–626. doi: 10.1016/j.ejvs.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 220.Asciutto G, Geier B, Marpe B, Hummel T, Mumme A. Dacron patch infection after carotid angioplasty. A report of 6 cases. Eur J Vasc Endovasc Surg. 2007;33(1):55–57. doi: 10.1016/j.ejvs.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 221.Borazjani BH, Wilson SE, Fujitani RM, Gordon I, Mueller M, Williams RA. Postoperative complications of carotid patching: pseudoaneurysm and infection. Ann Vasc Surg. 2003;17(2):156–161. doi: 10.1007/s10016-001-0400-5. [DOI] [PubMed] [Google Scholar]
- 222.Moore M, Power M. Perioperative hemorrhage and combined clopidogrel and aspirin therapy. [Accessed July 23, 2014];Anesthesiology. 2004 101(3):792–794. doi: 10.1097/00000542-200409000-00030. Available at http://www.ncbi.nlm.nih.gov/pubmed/15329606. [DOI] [PubMed] [Google Scholar]
- 223.Carotid revascularization using endarterectomy or stenting systems (CARESS): phase I clinical trial. J Endovasc Ther. 2003;10(6):1021–1030. doi: 10.1177/152660280301000601. [DOI] [PubMed] [Google Scholar]
- 224.Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg. 2005;42(2):213–219. doi: 10.1016/j.jvs.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 225.Hobson RW. CREST (Carotid Revascularization Endarterectomy versus Stent Trial): background, design, and current status. [Accessed July 23, 2014];Semin Vasc Surg. 2000 13(2):139–143. Available at http://www.ncbi.nlm.nih.gov/pubmed/10879554. [PubMed] [Google Scholar]
- 226.Hobson RW, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40(6):1106–1111. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 227.Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2) Eur J Vasc Endovasc Surg. 2013;46(5):510–518. doi: 10.1016/j.ejvs.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 228.Carotid Revascularization for Primary Prevention of Stroke - Full Text View - ClinicalTrials.gov. [Accessed August 13, 2014]; Available at http://clinicaltrials.gov/ct2/show/study/NCT02089217?term=CREST+2&rank=1.
- 229.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. [Accessed July 23, 2014];Lancet. 2001 357(9270):1729–1737. Available at http://www.ncbi.nlm.nih.gov/pubmed/11403808. [PubMed] [Google Scholar]
- 230.Ederle J, Bonati LH, Dobson J, et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8(10):898–907. doi: 10.1016/S1474-4422(09)70228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bonati LH, Ederle J, McCabe DJH, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009;8(10):908–917. doi: 10.1016/S1474-4422(09)70227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bonati LH, Ederle J, Dobson J, et al. Length of carotid stenosis predicts peri-procedural stroke or death and restenosis in patients randomized to endovascular treatment or endarterectomy. Int J Stroke. 2014;9(3):297–305. doi: 10.1111/ijs.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mas J-L, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 234.Qureshi AI. Carotid angioplasty and stent placement after EVA-3S trial. Stroke. 2007;38(6):1993–1996. doi: 10.1161/STROKEAHA.107.484352. [DOI] [PubMed] [Google Scholar]
- 235.Ringleb PA, Allenberg J, Brückmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368(9543):1239–1247. [Google Scholar]
- 236.Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375(9719):985–997. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rostamzadeh A, Zumbrunn T, Jongen LM, et al. Predictors of acute and persisting ischemic brain lesions in patients randomized to carotid stenting or endarterectomy. Stroke. 2014;45(2):591–594. doi: 10.1161/STROKEAHA.113.003605. [DOI] [PubMed] [Google Scholar]
- 238.Altinbas A, Algra A, Bonati LH, et al. Periprocedural hemodynamic depression is associated with a higher number of new ischemic brain lesions after stenting in the international carotid stenting study-MRI substudy. Stroke. 2014;45(1):146–151. doi: 10.1161/STROKEAHA.113.003397. [DOI] [PubMed] [Google Scholar]
- 239.Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007;45(5):875–880. doi: 10.1016/j.jvs.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 240.Wholey MH, Al-Mubarek N, Wholey MH. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60(2):259–266. doi: 10.1002/ccd.10645. [DOI] [PubMed] [Google Scholar]
- 241.Kastrup A, Gröschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34(3):813–819. doi: 10.1161/01.STR.0000058160.53040.5F. [DOI] [PubMed] [Google Scholar]
- 242.Roubin GS, New G, Iyer SS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. [Accessed July 23, 2014];Circulation. 2001 103(4):532–537. doi: 10.1161/01.cir.103.4.532. Available at http://www.ncbi.nlm.nih.gov/pubmed/11157718. [DOI] [PubMed] [Google Scholar]
- 243.White CJ, Iyer SS, Hopkins LN, Katzen BT, Russell ME. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day results. Catheter Cardiovasc Interv. 2006;67(4):503–512. doi: 10.1002/ccd.20689. [DOI] [PubMed] [Google Scholar]
- 244.Safian RD, Bresnahan JF, Jaff MR, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol. 2006;47(12):2384–2389. doi: 10.1016/j.jacc.2005.12.076. [DOI] [PubMed] [Google Scholar]
- 245.Barbato JE, Dillavou E, Horowitz MB, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg. 2008;47(4):760–765. doi: 10.1016/j.jvs.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 246.Katzen BT, Ardid MI, MacLean AA, et al. Bivalirudin as an anticoagulation agent: safety and efficacy in peripheral interventions. J Vasc Interv Radiol. 2005;16(9):1183–1187. doi: 10.1097/01.RVI.0000171694.01237.26. quiz 1187. [DOI] [PubMed] [Google Scholar]
- 247.Schneider LM, Polena S, Roubin G, et al. Carotid stenting and bivalirudin with and without vascular closure: 3-year analysis of procedural outcomes. Catheter Cardiovasc Interv. 2010;75(3):420–426. doi: 10.1002/ccd.22322. [DOI] [PubMed] [Google Scholar]
- 248.Cayne NS, Faries PL, Trocciola SM, et al. Carotid angioplasty and stent-induced bradycardia and hypotension: Impact of prophylactic atropine administration and prior carotid endarterectomy. J Vasc Surg. 2005;41(6):956–961. doi: 10.1016/j.jvs.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 249.Leisch F, Kerschner K, Hofmann R, et al. Carotid sinus reactions during carotid artery stenting: predictors, incidence, and influence on clinical outcome. Catheter Cardiovasc Interv. 2003;58(4):516–523. doi: 10.1002/ccd.10483. [DOI] [PubMed] [Google Scholar]
- 250.Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane database Syst Rev. 2004;(2):CD000515. doi: 10.1002/14651858.CD000515.pub2. [DOI] [PubMed] [Google Scholar]
- 251.Bak S, Andersen M, Tsiropoulos I, et al. Risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nested case-control study. [Accessed July 23, 2014];Stroke. 2003 34(2):379–386. Available at http://www.ncbi.nlm.nih.gov/pubmed/12574546. [PubMed] [Google Scholar]
- 252.Criado E, Doblas M, Fontcuberta J, et al. Carotid angioplasty with internal carotid artery flow reversal is well tolerated in the awake patient. J Vasc Surg. 2004;40(1):92–97. doi: 10.1016/j.jvs.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 253.Sganzerla P, Bocciarelli M, Savasta C, et al. The treatment of carotid artery bifurcation stenoses with systematic stenting: experience of first 100 consecutive cardiological procedures. [Accessed July 23, 2014];J Invasive Cardiol. 2004 16(10):592–595. Available at http://www.ncbi.nlm.nih.gov/pubmed/15505359. [PubMed] [Google Scholar]
- 254.Tan KT, Cleveland TJ, Berczi V, McKevitt FM, Venables GS, Gaines PA. Timing and frequency of complications after carotid artery stenting: what is the optimal period of observation? [Accessed July 23, 2014];J Vasc Surg. 2003 38(2):236–243. doi: 10.1016/s0741-5214(03)00316-1. Available at http://www.ncbi.nlm.nih.gov/pubmed/12891103. [DOI] [PubMed] [Google Scholar]
- 255.Srimahachota S, Singhatanadgige S, Boonyaratavej S, Chayanont D. Bilateral carotid stenting prior to coronary artery bypass graft: a case report. [Accessed July 23, 2014];J Med Assoc Thai. 2002 85(11):1232–1235. Available at http://www.ncbi.nlm.nih.gov/pubmed/12546322. [PubMed] [Google Scholar]
- 256.Leisch F, Kerschner K, Hofman R, Bibl D, Engleder C, Bergmann H. Carotid stenting: acute results and complications. [Accessed July 23, 2014];Z Kardiol. 1999 88(9):661–668. doi: 10.1007/s003920050342. Available at http://www.ncbi.nlm.nih.gov/pubmed/10525928. [DOI] [PubMed] [Google Scholar]
- 257.Illig KA, Zhang R, Tanski W, Benesch C, Sternbach Y, Green RM. Is the rationale for carotid angioplasty and stenting in patients excluded from NASCET/ACAS or eligible for ARCHeR justified? J Vasc Surg. 2003;37(3):575–581. doi: 10.1067/mva.2003.79. [DOI] [PubMed] [Google Scholar]
- 258.Coward LJ, Featherstone RL, Brown MM. Safety and efficacy of endovascular treatment of carotid artery stenosis compared with carotid endarterectomy: a Cochrane systematic review of the randomized evidence. Stroke. 2005;36(4):905–911. doi: 10.1161/01.STR.0000158921.51037.64. [DOI] [PubMed] [Google Scholar]
- 259.Back MR. Commentary. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. [Accessed July 23, 2014];Perspect Vasc Surg Endovasc Ther. 2006 18(4):349–351. Available at http://www.ncbi.nlm.nih.gov/pubmed/17396364. [PubMed] [Google Scholar]
- 260.Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane database Syst Rev. 2005;(2):CD000516. doi: 10.1002/14651858.CD000516.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Crawley F, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane database Syst Rev. 2000;(2):CD000515. doi: 10.1002/14651858.CD000515. [DOI] [PubMed] [Google Scholar]
- 262.Gray WA. Endovascular treatment of extra-cranial carotid artery bifurcation disease. [Accessed July 23, 2014];Minerva Cardioangiol. 2005 53(1):69–77. Available at http://www.ncbi.nlm.nih.gov/pubmed/15788981. [PubMed] [Google Scholar]
- 263.Gray WA. A cardiologist in the carotids. J Am Coll Cardiol. 2004;43(9):1602–1605. doi: 10.1016/j.jacc.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 264.Kasirajan K. What is the latest in inventory for carotid stenting and cerebral protection? [Accessed July 23, 2014];Perspect Vasc Surg Endovasc Ther. 2005 17(2):135–141. doi: 10.1177/153100350501700217. Available at http://www.ncbi.nlm.nih.gov/pubmed/16110380. [DOI] [PubMed] [Google Scholar]
- 265.Naylor AR. Regarding “Protected carotid stenting in high-surgical-risk patients: the ARCHeR results”. J Vasc Surg. 2007;45(1):222–223. doi: 10.1016/j.jvs.2006.08.089. author reply 223–4. [DOI] [PubMed] [Google Scholar]
- 266.Schonholz CJ, Uflacker R, Parodi JC, Hannegan C, Selby B. Is there evidence that cerebral protection is beneficial? Clinical data. [Accessed July 23, 2014];J Cardiovasc Surg (Torino) 2006 47(2):137–141. Available at http://www.ncbi.nlm.nih.gov/pubmed/16572087. [PubMed] [Google Scholar]
- 267.Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69(3):341–348. doi: 10.1002/ccd.21050. [DOI] [PubMed] [Google Scholar]
- 268.Kwon BJ, Han MH, Kang H-S, Jung C. Protection filter-related events in extracranial carotid artery stenting: a single-center experience. J Endovasc Ther. 2006;13(6):711–722. doi: 10.1583/06-1900.1. [DOI] [PubMed] [Google Scholar]
- 269.Cardaioli P, Giordan M, Panfili M, Chioin R. Complication with an embolic protection device during carotid angioplasty. Catheter Cardiovasc Interv. 2004;62(2):234–236. doi: 10.1002/ccd.20061. [DOI] [PubMed] [Google Scholar]
- 270.Van den Berg JC. The nature and management of complications in carotid artery stenting. [Accessed July 23, 2014];Acta Chir Belg. 2004 104(1):60–64. Available at http://www.ncbi.nlm.nih.gov/pubmed/15053467. [PubMed] [Google Scholar]
- 271.Griewing B, Brassel F, von Smekal U, Al Ahmar MT, Kessler C. Carotid artery stenting in patients at surgical high risk: clinical and ultrasound findings. Cerebrovasc Dis. 10(1):44–48. doi: 10.1159/000016024. doi:16024. [DOI] [PubMed] [Google Scholar]
- 272.Kadkhodayan Y, Moran CJ, Derdeyn CP, Cross DT. Carotid angioplasty and stent placement for restenosis after endarterectomy. Neuroradiology. 2007;49(4):357–364. doi: 10.1007/s00234-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 273.Kitta Y, Obata J, Takano H, et al. Echolucent carotid plaques predict in-stent restenosis after bare metal stenting in native coronary arteries. Atherosclerosis. 2008;197(1):177–182. doi: 10.1016/j.atherosclerosis.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 274.Geary GG. The vascular therapist. Heart Lung Circ. 2007;16(3):193–199. doi: 10.1016/j.hlc.2007.02.103. [DOI] [PubMed] [Google Scholar]
- 275.Teng Z, Ji G, Chu H, et al. Does PGA external stenting reduce compliance mismatch in venous grafts? Biomed Eng Online. 2007;6:12. doi: 10.1186/1475-925X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bosiers M, De Donato G, Deloose K, et al. Are there predictive risk factors for complications after carotid artery stenting? [Accessed July 23, 2014];J Cardiovasc Surg (Torino) 2007 48(2):125–130. Available at http://www.ncbi.nlm.nih.gov/pubmed/17410060. [PubMed] [Google Scholar]
- 277.Parodi JC, Schönholz C, Parodi FE, Sicard G, Ferreira LM. Initial 200 cases of carotid artery stenting using a reversal-of-flow cerebral protection device. [Accessed July 23, 2014];J Cardiovasc Surg (Torino) 2007 48(2):117–124. Available at http://www.ncbi.nlm.nih.gov/pubmed/17410059. [PubMed] [Google Scholar]
- 278.Peynircioglu B, Geyik S, Yavuz K, Cil BE, Saatci I, Cekirge S. Exclusion of atherosclerotic plaque from the circulation using stent-grafts: alternative to carotid stenting with a protection device? Cardiovasc Intervent Radiol. 30(5):854–860. doi: 10.1007/s00270-007-9010-0. [DOI] [PubMed] [Google Scholar]
- 279.Younis GA, Gupta K, Mortazavi A, et al. Predictors of carotid stent restenosis. Catheter Cardiovasc Interv. 2007;69(5):673–682. doi: 10.1002/ccd.20809. [DOI] [PubMed] [Google Scholar]
- 280.De Souza JM, Espinosa G, Santos Machado M, Soares PJ. Bilateral occlusion associated to steal phenomenon of internal carotid and left subclavian arteries: treatment by angioplasty and stenting. Surg Neurol. 2007;67(3):298–302. doi: 10.1016/j.surneu.2006.04.013. discussion 302. [DOI] [PubMed] [Google Scholar]
- 281.Chahwan S, Miller MT, Pigott JP, Whalen RC, Jones L, Comerota AJ. Carotid artery velocity characteristics after carotid artery angioplasty and stenting. J Vasc Surg. 2007;45(3):523–526. doi: 10.1016/j.jvs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 282.De Borst GJ, Ackerstaff RGA, de Vries J-PPM, et al. Carotid angioplasty and stenting for postendarterectomy stenosis: long-term follow-up. J Vasc Surg. 2007;45(1):118–123. doi: 10.1016/j.jvs.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 283.Ali ZA, Alp NJ, Lupton H, et al. Increased in-stent stenosis in ApoE knockout mice: insights from a novel mouse model of balloon angioplasty and stenting. Arterioscler Thromb Vasc Biol. 2007;27(4):833–840. doi: 10.1161/01.ATV.0000257135.39571.5b. [DOI] [PubMed] [Google Scholar]
- 284.Park B, Aiello F, Dahn M, Menzoian JO, Mavanur A. Follow-up results of carotid angioplasty with stenting as assessed by duplex ultrasound surveillance. Am J Surg. 2006;192(5):583–588. doi: 10.1016/j.amjsurg.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 285.Gupta R, Al-Ali F, Thomas AJ, et al. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 2006;37(10):2562–2566. doi: 10.1161/01.STR.0000242481.38262.7b. [DOI] [PubMed] [Google Scholar]
- 286.Hauth EAM, Drescher R, Jansen C, et al. Complications and follow-up after unprotected carotid artery stenting. Cardiovasc Intervent Radiol. 29(4):511–518. doi: 10.1007/s00270-005-0050-z. [DOI] [PubMed] [Google Scholar]
- 287.Cao P, De Rango P, Verzini F, Maselli A, Norgiolini L, Giordano G. Outcome of carotid stenting versus endarterectomy: a case-control study. Stroke. 2006;37(5):1221–1226. doi: 10.1161/01.STR.0000217435.21051.60. [DOI] [PubMed] [Google Scholar]
- 288.Lal BK, Hobson RW. Management of carotid restenosis. [Accessed July 23, 2014];J Cardiovasc Surg (Torino) 2006 47(2):153–160. Available at http://www.ncbi.nlm.nih.gov/pubmed/16572089. [PubMed] [Google Scholar]
- 289.Halabi M, Gruberg L, Pitchersky S, et al. Carotid artery stenting in surgical high-risk patients. Catheter Cardiovasc Interv. 2006;67(4):513–558. doi: 10.1002/ccd.20640. [DOI] [PubMed] [Google Scholar]
- 290.Maleux G, Demaerel P, Verbeken E, et al. Cerebral ischemia after filter-protected carotid artery stenting is common and cannot be predicted by the presence of substantial amount of debris captured by the filter device. [Accessed July 23, 2014];AJNR Am J Neuroradiol. 2006 27(9):1830–1833. Available at http://www.ncbi.nlm.nih.gov/pubmed/17032852. [PMC free article] [PubMed] [Google Scholar]
- 291.Reimers B, Tübler T, de Donato G, et al. Endovascular treatment of in-stent restenosis after carotid artery stenting: immediate and midterm results. J Endovasc Ther. 2006;13(4):429–435. doi: 10.1583/06-1811.1. [DOI] [PubMed] [Google Scholar]
- 292.Imai K, Mori T, Izumoto H, et al. Successful stenting seven days after atherothrombotic occlusion of the intracranial internal carotid artery. J Endovasc Ther. 2006;13(2):254–259. doi: 10.1583/05-1742R.1. [DOI] [PubMed] [Google Scholar]
- 293.Macdonald S. Is there any evidence that cerebral protection is beneficial? Experimental data. [Accessed July 23, 2014];J Cardiovasc Surg (Torino) 2006 47(2):127–136. Available at http://www.ncbi.nlm.nih.gov/pubmed/16572086. [PubMed] [Google Scholar]
- 294.Quan V-H, Huynh R, Seifert PA, et al. Morphometric analysis of particulate debris extracted by four different embolic protection devices from coronary arteries, aortocoronary saphenous vein conduits, and carotid arteries. Am J Cardiol. 2005;95(12):1415–1419. doi: 10.1016/j.amjcard.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 295.Sprouse LR, Peeters P, Bosiers M. The capture of visible debris by distal cerebral protection filters during carotid artery stenting: Is it predictable? J Vasc Surg. 2005;41(6):950–955. doi: 10.1016/j.jvs.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 296.Ohki T, Veith FJ. Critical analysis of distal protection devices. [Accessed July 23, 2014];Semin Vasc Surg. 2003 16(4):317–325. doi: 10.1053/j.semvascsurg.2003.08.010. Available at http://www.ncbi.nlm.nih.gov/pubmed/14691774. [DOI] [PubMed] [Google Scholar]
- 297.Grube E, Colombo A, Hauptmann E, et al. Initial multicenter experience with a novel distal protection filter during carotid artery stent implantation. Catheter Cardiovasc Interv. 2003;58(2):139–146. doi: 10.1002/ccd.10348. [DOI] [PubMed] [Google Scholar]
- 298.Sievert H, Rabe K. Role of distal protection during carotid stenting. [Accessed July 23, 2014];J Interv Cardiol. 2002 15(6):499–504. doi: 10.1111/j.1540-8183.2002.tb01095.x. Available at http://www.ncbi.nlm.nih.gov/pubmed/12476654. [DOI] [PubMed] [Google Scholar]
- 299.Capoccia L, Speziale F, Gazzetti M, et al. Comparative study on carotid revascularization (endarterectomy vs stenting) using markers of cellular brain injury, neuropsychometric tests, and diffusion-weighted magnetic resonance imaging. J Vasc Surg. 2010;51(3):584–591. 591.e1–591.e3. doi: 10.1016/j.jvs.2009.10.079. discussion 592. [DOI] [PubMed] [Google Scholar]
- 300.Tedesco MM, Lee JT, Dalman RL, et al. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46(2):244–250. doi: 10.1016/j.jvs.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 301.Morrish W, Grahovac S, Douen A, et al. Intracranial hemorrhage after stenting and angioplasty of extracranial carotid stenosis. [Accessed July 23, 2014];AJNR Am J Neuroradiol. 21(10):1911–1916. Available at http://www.ncbi.nlm.nih.gov/pubmed/11110546. [PMC free article] [PubMed] [Google Scholar]
- 302.Buhk J-H, Cepek L, Knauth M. Hyperacute intracerebral hemorrhage complicating carotid stenting should be distinguished from hyperperfusion syndrome. [Accessed July 23, 2014];AJNR Am J Neuroradiol. 2006 27(7):1508–1513. Available at http://www.ncbi.nlm.nih.gov/pubmed/16908570. [PMC free article] [PubMed] [Google Scholar]
- 303.Hartmann M, Weber R, Zoubaa S, Schranz C, Knauth M. Fatal subarachnoid hemorrhage after carotid stenting. [Accessed July 24, 2014];J Neuroradiol. 2004 31(1):63–66. doi: 10.1016/s0150-9861(04)96880-0. Available at http://www.ncbi.nlm.nih.gov/pubmed/15026733. [DOI] [PubMed] [Google Scholar]
- 304.Chuang YM, Wu HM. Early recognition of cerebral hyperperfusion syndrome after carotid stenting--a case report. [Accessed July 23, 2014];Kaohsiung J Med Sci. 2001 17(9):489–494. Available at http://www.ncbi.nlm.nih.gov/pubmed/11842653. [PubMed] [Google Scholar]
- 305.Ho DS, Wang Y, Chui M, Ho SL, Cheung RT. Epileptic seizures attributed to cerebral hyperperfusion after percutaneous transluminal angioplasty and stenting of the internal carotid artery. Cerebrovasc Dis. 10(5):374–379. doi: 10.1159/000016093. doi:16093. [DOI] [PubMed] [Google Scholar]
- 306.Eskandari MK, Najjar SF, Matsumura JS, Kibbe MR, Morasch MD. Technical limitations of carotid filter embolic protection devices. Ann Vasc Surg. 2007;21(4):403–407. doi: 10.1016/j.avsg.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 307.Schillinger M, Exner M, Sabeti S, et al. Excessive carotid in-stent neointimal formation predicts late cardiovascular events. J Endovasc Ther. 2004;11(3):229–239. doi: 10.1583/04-1214.1. [DOI] [PubMed] [Google Scholar]
- 308.DeRubertis BG, Chaer RA, Gordon R, et al. Determining the quantity and character of carotid artery embolic debris by electron microscopy and energy dispersive spectroscopy. J Vasc Surg. 2007;45(4):716–724. doi: 10.1016/j.jvs.2006.12.015. discussion 724–5. [DOI] [PubMed] [Google Scholar]
- 309.Rapp JH, Wakil L, Sawhney R, et al. Subclinical embolization after carotid artery stenting: new lesions on diffusion-weighted magnetic resonance imaging occur postprocedure. J Vasc Surg. 2007;45(5):867–872. doi: 10.1016/j.jvs.2006.12.058. discussion 872–4. [DOI] [PubMed] [Google Scholar]
- 310.Hart JP, Peeters P, Verbist J, Deloose K, Bosiers M. Do device characteristics impact outcome in carotid artery stenting? J Vasc Surg. 2006;44(4):725–730. doi: 10.1016/j.jvs.2006.06.029. discussion 730–1. [DOI] [PubMed] [Google Scholar]
- 311.Powell RJ, Alessi C, Nolan B, et al. Comparison of embolization protection device-specific technical difficulties during carotid artery stenting. J Vasc Surg. 2006;44(1):56–61. doi: 10.1016/j.jvs.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 312.Hamood H, Makhoul N, Hassan A, Shefer A, Rosenschein U. Embolic protection: limitations of current technology and novel concepts. Int J Cardiovasc Intervent. 2005;7(4):176–182. doi: 10.1080/14628840500285038. [DOI] [PubMed] [Google Scholar]
- 313.Gruberg L, Beyar R. Cerebral embolic protection devices and percutaneous carotid artery stenting. Int J Cardiovasc Intervent. 2005;7(3):117–121. doi: 10.1080/14628840500280542. [DOI] [PubMed] [Google Scholar]
- 314.Yadav JS. Embolic protection devices: methods, techniques, and data. Tech Vasc Interv Radiol. 2004;7(4):190–193. doi: 10.1053/j.tvir.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 315.Cil BE, Türkbey B, Canyiğit M, Geyik S, Yavuz K. An unusual complication of carotid stenting: spontaneous rectus sheath hematoma and its endovascular management. [Accessed July 23, 2014];Diagn Interv Radiol. 2007 13(1):46–48. Available at http://www.ncbi.nlm.nih.gov/pubmed/17354196. [PubMed] [Google Scholar]
- 316.Pipinos II, Johanning JM, Pham CN, Soundararajan K, Lynch TG. Transcervical approach with protective flow reversal for carotid angioplasty and stenting. J Endovasc Ther. 2005;12(4):446–453. doi: 10.1583/05-1561.1. [DOI] [PubMed] [Google Scholar]
- 317.Zorger N, Finkenzeller T, Lenhart M, et al. Safety and efficacy of the Perclose suture-mediated closure device following carotid artery stenting under clopidogrel platelet blockade. Eur Radiol. 2004;14(4):719–722. doi: 10.1007/s00330-003-2143-x. [DOI] [PubMed] [Google Scholar]
- 318.Gupta A, Bhatia A, Ahuja A, Shalev Y, Bajwa T. Carotid stenting in patients older than 65 years with inoperable carotid artery disease: a single-center experience. [Accessed July 23, 2014];Catheter Cardiovasc Interv. 2000 50(1):1–8. doi: 10.1002/(sici)1522-726x(200005)50:1<1::aid-ccd1>3.0.co;2-r. discussion 9. Available at http://www.ncbi.nlm.nih.gov/pubmed/10816271. [DOI] [PubMed] [Google Scholar]
- 319.Schneider LM, Roubin GS. Minimal contrast use in carotid stenting: avoiding contrast pitfalls. [Accessed July 23, 2014];J Invasive Cardiol. 2007 19(1):37–38. Available at http://www.ncbi.nlm.nih.gov/pubmed/17297184. [PubMed] [Google Scholar]
- 320.Bond R, Rerkasem K, AbuRahma AF, Naylor AR, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane database Syst Rev. 2004;(2):CD000160. doi: 10.1002/14651858.CD000160.pub2. [DOI] [PubMed] [Google Scholar]
- 321.Moore WS, Kempczinski RF, Nelson JJ, Toole JF. Recurrent carotid stenosis : results of the asymptomatic carotid atherosclerosis study. [Accessed July 23, 2014];Stroke. 1998 29(10):2018–2025. doi: 10.1161/01.str.29.10.2018. Available at http://www.ncbi.nlm.nih.gov/pubmed/9756575. [DOI] [PubMed] [Google Scholar]
- 322.Cunningham EJ, Bond R, Mehta Z, Mayberg MR, Warlow CP, Rothwell PM. Long-term durability of carotid endarterectomy for symptomatic stenosis and risk factors for late postoperative stroke. [Accessed July 23, 2014];Stroke. 2002 33(11):2658–2663. doi: 10.1161/01.str.0000034397.72390.d3. Available at http://www.ncbi.nlm.nih.gov/pubmed/12411657. [DOI] [PubMed] [Google Scholar]
- 323.Cikrit DF, Larson DM, Sawchuk AP, et al. Discretionary carotid patch angioplasty leads to good results. Am J Surg. 2006;192(5):e46–e50. doi: 10.1016/j.amjsurg.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 324.AbuRahma AF, Robinson PA, Saiedy S, Richmond BK, Khan J. Prospective randomized trial of bilateral carotid endarterectomies: primary closure versus patching. [Accessed July 23, 2014];Stroke. 1999 30(6):1185–1189. doi: 10.1161/01.str.30.6.1185. Available at http://www.ncbi.nlm.nih.gov/pubmed/10356097. [DOI] [PubMed] [Google Scholar]
- 325.Rockman CB, Halm EA, Wang JJ, et al. Primary closure of the carotid artery is associated with poorer outcomes during carotid endarterectomy. J Vasc Surg. 2005;42(5):870–877. doi: 10.1016/j.jvs.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 326.Hansen F, Lindblad B, Persson NH, Bergqvist D. Can recurrent stenosis after carotid endarterectomy be prevented by low-dose acetylsalicylic acid? A double-blind, randomised and placebo-controlled study. [Accessed July 23, 2014];Eur J Vasc Surg. 1993 7(4):380–385. doi: 10.1016/s0950-821x(05)80253-1. Available at http://www.ncbi.nlm.nih.gov/pubmed/8359292. [DOI] [PubMed] [Google Scholar]
- 327.Petrik PV, Gelabert HA, Moore WS, Quinones-Baldrich W, Law MM. Cigarette smoking accelerates carotid artery intimal hyperplasia in a dose-dependent manner. [Accessed July 23, 2014];Stroke. 1995 26(8):1409–1414. doi: 10.1161/01.str.26.8.1409. Available at http://www.ncbi.nlm.nih.gov/pubmed/7631346. [DOI] [PubMed] [Google Scholar]
- 328.Salvian A, Baker JD, Machleder HI, Busuttil RW, Barker WF, Moore WS. Cause and noninvasive detection of restenosis after carotid endarterectomy. [Accessed July 23, 2014];Am J Surg. 1983 146(1):29–34. doi: 10.1016/0002-9610(83)90254-4. Available at http://www.ncbi.nlm.nih.gov/pubmed/6869676. [DOI] [PubMed] [Google Scholar]
- 329.AbuRahma AF, Robinson PA, Saiedy S, Kahn JH, Boland JP. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. [Accessed July 23, 2014];J Vasc Surg. 1998 27(2):222–232. doi: 10.1016/s0741-5214(98)70353-2. discussion 233–4. Available at http://www.ncbi.nlm.nih.gov/pubmed/9510277. [DOI] [PubMed] [Google Scholar]
- 330.Lord RS, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. Part I. Perioperative results. [Accessed July 23, 2014];J Vasc Surg. 1989 9(4):521–529. Available at http://www.ncbi.nlm.nih.gov/pubmed/2709521. [PubMed] [Google Scholar]
- 331.Curley S, Edwards WS, Jacob TP. Recurrent carotid stenosis after autologous tissue patching. [Accessed July 23, 2014];J Vasc Surg. 1987 6(4):350–354. doi: 10.1067/mva.1987.avs0060350. Available at http://www.ncbi.nlm.nih.gov/pubmed/3309379. [DOI] [PubMed] [Google Scholar]
- 332.Awad IA, Little JR. Patch angioplasty in carotid endarterectomy. Advantages, concerns, and controversies. [Accessed July 23, 2014];Stroke. 1989 20(3):417–422. doi: 10.1161/01.str.20.3.417. Available at http://www.ncbi.nlm.nih.gov/pubmed/2604762. [DOI] [PubMed] [Google Scholar]
- 333.Bernstein EF, Torem S, Dilley RB. Does carotid restenosis predict an increased risk of late symptoms, stroke, or death? [Accessed July 23, 2014];Ann Surg. 1990 212(5):629–636. doi: 10.1097/00000658-199011000-00011. Available at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1358192&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nicholls SC, Phillips DJ, Bergelin RO, Beach KW, Primozich JF, Strandness DE. Carotid endarterectomy. Relationship of outcome to early restenosis. [Accessed July 24, 2014];J Vasc Surg. 1985 2(3):375–381. doi: 10.1067/mva.1985.avs0020375. Available at http://www.ncbi.nlm.nih.gov/pubmed/3889378. [DOI] [PubMed] [Google Scholar]
- 335.O’Donnell TF, Callow AD, Scott G, Shepard AD, Heggerick P, Mackey WC. Ultrasound characteristics of recurrent carotid disease: hypothesis explaining the low incidence of symptomatic recurrence. [Accessed July 23, 2014];J Vasc Surg. 1985 2(1):26–41. Available at http://www.ncbi.nlm.nih.gov/pubmed/3880832. [PubMed] [Google Scholar]
- 336.Zierler RE, Bandyk DF, Thiele BL, Strandness DE. Carotid artery stenosis following endarterectomy. [Accessed July 23, 2014];Arch Surg. 1982 117(11):1408–1415. doi: 10.1001/archsurg.1982.01380350016003. Available at http://www.ncbi.nlm.nih.gov/pubmed/7138302. [DOI] [PubMed] [Google Scholar]
- 337.Stoney RJ, String ST. Recurrent carotid stenosis. [Accessed July 23, 2014];Surgery. 1976 80(6):705–710. Available at http://www.ncbi.nlm.nih.gov/pubmed/1006517. [PubMed] [Google Scholar]
- 338.Hertzer NR, Martinez BD, Benjamin SP, Beven EG. Recurrent stenosis after carotid endarterectomy. [Accessed July 23, 2014];Surg Gynecol Obstet. 1979 149(3):360–364. Available at http://www.ncbi.nlm.nih.gov/pubmed/472995. [PubMed] [Google Scholar]
- 339.DeGroote RD, Lynch TG, Jamil Z, Hobson RW. Carotid restenosis: long-term noninvasive follow-up after carotid endarterectomy. [Accessed July 23, 2014];Stroke. 18(6):1031–1036. doi: 10.1161/01.str.18.6.1031. Available at http://www.ncbi.nlm.nih.gov/pubmed/3318001. [DOI] [PubMed] [Google Scholar]