Abstract
Background
Although accumulating evidence suggests peripheral blood lymphocyte-to-monocyte ratio (LMR) could act as a prognosis predictor in various tumors, the prognostic value of LMR still remains controversial. We carried out this meta-analysis to evaluate the association of pretreatment LMR with survival outcomes in patients with solid tumors.
Methods
Eligible studies were collected and extracted by searching PubMed and Embase databases up to June 3, 2015. The pooled hazard ratios (HRs) and their 95% confidence intervals (CIs) were computed to assess the prognostic value of LMR quantitatively.
Results
Eighteen studies with a total of 8,377 participants were enrolled in this meta-analysis. Our findings indicated that elevated pre-treatment LMR predicted a significantly favorable overall survival (HR=0.59, 95% CI: 0.53–0.67) and disease-free survival (HR=0.74, 95% CI: 0.68–0.80) in solid tumor patients. Subgroup analyses revealed that enhanced LMR was significantly associated with favorable overall survival in patients with digestive system cancers (HR=0.63, 95% CI: 0.49–0.81), urinary tract tumors (HR=0.66, 95% CI: 0.52–0.84), lung cancer (HR=0.62, 95% CI: 0.54–0.72), and nasopharyngeal carcinoma (HR=0.50, 95% CI: 0.43–0.57).
Conclusion
This meta-analysis showed that enhanced LMR may indicate a favorable prognosis in patients with solid tumors.
Keywords: LMR, solid tumors, prognosis, meta-analysis
Introduction
Cancer has been an enormous burden in our society and it is one of the leading causes of death in both developing and developed countries. There were approximately 14.1 million new cancer cases and 8.2 million cancer-related deaths in 2012 worldwide.1 Although the diagnostic and therapeutic approaches have improved in the past decades, cancer patients still have an unsatisfying prognosis mainly due to local recurrence and distal metastasis.2 Therefore, it is of great significance for us to identify a practical biomarker to determine the optimal therapeutic strategies and predict the prognosis of cancers.
It is widely accepted that systemic inflammatory response plays a crucial role in the pathogenesis and progression of cancers.3,4 Several systemic inflammatory indicators, such as platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and neutrophil-to-lymphocyte ratio (NLR), have been explored to predict the prognosis in a wide variety of tumors.5–8 Among these predictors, lymphocytes are considered to play the crucial roles in the antitumor immunological reaction. An elevated pre-treatment lymphocyte count has been reported to be connected with the good prognosis of patients with Hodgkin lymphoma and non-small cell lung cancer.9,10 Monocytes can secrete a variety of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6), which are associated with poor prognosis in cancer patients.11 Therefore, LMR, calculated as a simple ratio between lymphocyte and monocyte, may be a better prognostic factor for hematologic malignancies and solid tumors.9,10,12–17 However, the prognostic value of LMR for solid tumors patients remains controversial. Some studies revealed that the elevated pre-treatment LMR level was associated significantly with favorable prognosis for patients with solid tumors, such as gastric cancer,6,16 pancreatic cancer,8 lung cancer,10,12 nasopharyngeal carcinoma,14,15 and so on.13,18 Other studies had different views on this conclusion.5,6,19,20 Hutterer et al’s19 research showed that LMR was not an independent prognostic factor for renal cell carcinoma patients’ overall survival (OS) in multivariate analysis. A similar result was found for patients with gastric cancer in Deng et al’s6 study. Therefore, we embarked on this meta-analysis to shed light on the association between LMR and the survival of patients with solid tumors.
Materials and methods
Search strategy and eligible criteria
A systematic literature search was carefully performed by using PubMed and Embase databases (updated on June 3, 2015). The search keywords were shown as follows: “lymphocyte monocyte ratio”, “lymphocyte to monocyte ratio”, “lymphocyte-monocyte ratio”, “lymphocyte-to-monocyte ratio”, “LMR” and “cancer”, “carcinoma”, “neoplasm”, and “tumor”. Searches were supplemented by scanning the references of selected articles. If there were overlapping and duplicate datasets detected on the same patient cohort, only the most informative or most recent report was involved in the analysis.
Studies selected in this meta-analysis need to satisfy the following criteria: (1) it researched on the patients with solid tumors; (2) it investigated the association between LMR and OS or disease-free survival (DFS); (3) LMR was calculated from peripheral blood cell count before any treatment was administered. Studies were excluded if they had any of the following items: (1) letters, case reports, conference abstracts, laboratory studies, or reviews; (2) study without sufficient data to extract the HR and its 95% CI; (3) LMR measured after anticancer treatment; and (4) duplicate publications.
Data extraction and qualitative assessment
The following information was independently extracted from each eligible study by two investigators (JJT and TYZ): first authors’ surname, publication year, sample size, country of origin, median age, types of diseases, disease stage, tumor grade, treatment strategy, follow-up, cut-off values, HR, and 95% CI of LMR for survival outcomes. The authors discussed to reach a consensus when there was disagreement. If the results were reported by both univariate and multivariate analysis, we choose the latter because it was more precise due to consideration of the confounding factors.
The quality of selected studies was independently evaluated by two reviewers (JJT and TYZ) according to the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses.21 Each study was judged from three broad perspectives, namely selection, comparability, and outcome. The score was obtained by using a “star system”, and studies with scores of 6 or more were considered as high quality.
Data analysis
The aggregated HRs and 95% CIs were used to evaluate the impact of LMR on OS or DFS. The statistical variables were directly extracted from the study, or calculated from available numerical data based on the method suggested by Tierney et al.22 An observed HR less than 1.0 implied a better prognosis in patients with high LMR. We performed the chi-square-based Q-statistic test and calculated the I-squared (I2) statistic to assess the interstudy heterogeneity. A fixed-effects model was selected if the heterogeneity among the enrolled studies was not significant (P>0.05 for the Q-test and I2<50%). Otherwise, a random-effects model was applied. Publishing bias of literatures was assessed by using the Begg’s funnel plot and Egger’s linear regression test. All P-values were two-tailed, and P<0.05 was considered to be statistically significant. Statistical analyses were conducted using Stata package version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Study selection and quality
According to the literature search methods as described earlier, a total of 1,283 studies were initially selected. After removing the duplicates, 743 studies were identified. However, 702 irrelevant studies were excluded by screening the title and abstract. Because this approach was not informative, 41 full-text articles were reviewed for further evaluation. In this process, we selected 18 studies and excluded 23 studies. Among these excluded studies, 15 were excluded because they reported the prognostic value of other inflammation index but not LMR-specific data; three were excluded, because the data of HR and 95% CI were not available; two were excluded, because the survival outcomes did not focus on OS or DFS. Three other studies were also ineligible, because the LMR involved was measured after treatment. Finally, 18 studies6–8,10,12–16,18–20,23–28 were enrolled in our meta-analysis. The flowchart of the studies selection process is shown in Figure 1. The quality score evaluated by the NOS ranged from 6 to 8 with a mean value of 6.7.
Figure 1.
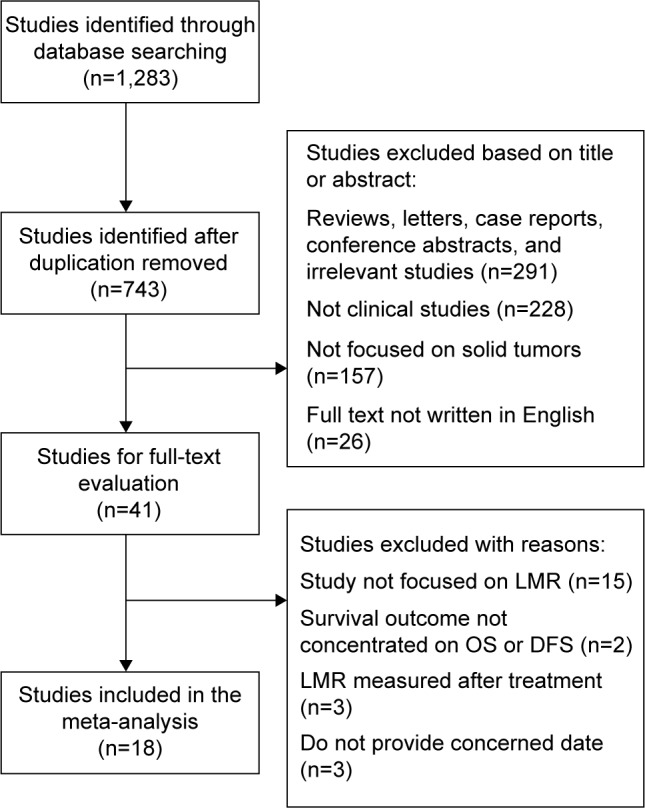
Flow diagram of the study search and selection process.
Abbreviations: DFS, disease-free survival; LMR, Lymphocyte-to-monocyte ratio; OS, overall survival.
Characteristics of the included studies
The main characteristics of the 18 enrolled studies are shown in Table 1. All studies were retrospective. Sixteen studies reported OS, and eight studies investigated DFS. This meta-analysis enrolled a total of 8,377 participants, who were diagnosed with a variety of solid tumors, including pancreatic carcinoma,8 esophageal squamous cell carcinoma,7 gastric cancer,6,16 lung cancer,10,12,23 colorectal cancer,18,24 nasopharyngeal carcinoma,14,15,25 and others.26–28 Among the 18 enrolled studies, 13 studies evaluated Asian populations, and five evaluated Caucasian patients. The LMR cutoff values ranged from 2.14 to 5.22 and were identified with different methods among studies: 13 studies using receiver operating characteristic (ROC) curve analysis, four using the median LMR of the patient group, and one using log-rank test. Nine studies enrolled more than 300 patients, and nine studies enrolled less than 300 patients. All studies used the white blood cell counts based on pre-treatment laboratory data to calculate LMR. For all patients who underwent surgical treatment, LMR was measured preoperative. The effect of pre-treatment LMR on survival outcomes in all studies was assessed by multivariate analysis.
Table 1.
Main characteristics of the eligible studies
| First author publication year | Country of origin | Ethnicity | Number | Age (years) (median and range) | Grade | Cancer type | Stage | Treatment received | Cut-off value | Follow-up (months) | Survival outcome | Hazard ratios | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qi et al, 20158 | People’s Republic of China | Asian | 211 | <60 (101) >60 (110) |
– | PC | Mixed* | Chemotherapy | 3.30 median | NR | OS | Reported | 6 |
| Han et al, 20157 | People’s Republic of China | Asian | 218 | 60.5 (32–84) | G1–3 | ESCC | Non-metastatic | Surgery ± chemotherapy/radiotherapy | 2.57 ROC | 38.6 (3.0–71.0) | OS/DFS | DE | 6 |
| Deng et al, 20156 | People’s Republic of China | Asian | 389 | 65 (29–92) | G1–4 | GC | Mixed* | Surgery | 4.95 ROC | 24.0 (3.0–60.0) | OS/DFS | Reported | 7 |
| Stotz et al, 201418 | Austria | Caucasian | 372 | 64 (27–95) | G1–3 | CC | Non-metastatic | Surgery ± chemotherapy | 2.14 ROC | 68.0 (1.0–190.0) | OS | Reported | 7 |
| Zhou et al, 201416 | People’s Republic of China | Asian | 426 | >60 | G1–3 | GC | Non-metastatic | Surgery ± chemotherapy | 4.32 ROC | 39.6 (2.6–85.6) | OS | Reported | 7 |
| Kozak et al, 201524 | USA | Caucasian | 129 | 67 (30–95) | G1–3 | CRC | Non-metastatic | Surgery ± chemotherapy | 2.60 median | 24.7 (4.2–101.7) | OS/DFS | Reported | 8 |
| Li et al, 201314 | People’s Republic of China | Asian | 1,547 | 51 (6–87) | – | NPC | Non-metastatic | Radiotherapy ± chemotherapy | 5.22 ROC | 67.1 (1.4–99.0) | OS/DFS | Reported | 8 |
| Lin et al, 201415 | People’s Republic of China | Asian | 256 | 53.6 (35–69) | – | NPC | Metastatic | Chemotherapy | 5.07 ROC | 22.6 (5.1–42.3) | OS | Reported | 8 |
| Lin et al, 201410 | People’s Republic of China | Asian | 370 | 63.6 (36–72) | – | NSCLC | Metastatic | Chemotherapy | 4.56 ROC | NR | OS | Reported | 6 |
| Hutterer et al, 201419 | Austria | Caucasian | 678 | 65 (20–88) | G1–4 | ccRCC | Non-metastatic | Surgery | 3.00 ROC | 44.0 (0.0–130.0) | OS | DE | 7 |
| Hutterer et al, 201513 | Austria | Caucasian | 182 | 70 | G1–4 | UTUC | Non-metastatic | Surgery | 2.00 ROC | NR | OS | Reported | 6 |
| Szkandera et al, 201420 (a) | Austria | Caucasian | 170(T) | 64 | G1–3 | STS | Non-metastatic | Surgery + adjuvant treatments | 2.85 LRT | Over120 | OS/DFS | Reported | 6 |
| Szkandera et al, 201420 (b) | Austria | Caucasian | 170(V) | 61 | G1–3 | STS | Non-metastatic | Surgery + adjuvant treatments | 2.85 LRT | Over120 | OS/DFS | Reported | 6 |
| Hu et al, 201412 | People’s Republic of China | Asian | 1,453 | 59 (20–84) | G1–4 | LC | Non-metastatic | Surgery | 3.68 ROC | NR | OS/DFS | DE | 6 |
| Go et al, 201423 | Korea | Asian | 188 | >60 (43–82) | – | SCLC | Mixed* | Chemotherapy ± radiotherapy | 4.19 ROC | 41.9 (2.1–92.5) | OS | DE | 7 |
| Zhang et al, 201526 | People’s Republic of China | Asian | 124 | 65 (30–78) <65 (61) >65 (63) |
G1–G3 | BCa | Mixed* | Surgery ± chemotherapy/radiotherapy | 2.80 ROC | NR | OS | Reported | 6 |
| Jiang et al, 201525 | People’s Republic of China | Asian | 672 | 46 (13–79) | – | NPC | Metastatic | Chemotherapy/radiotherapy | 2.14 ROC | NR | OS | Reported | 6 |
| Ni et al, 201428 | People’s Republic of China | Asian | 542 | 49 | G1–3 | BC | Non-metastatic | Surgery + NCT | 4.25 ROC | 45.9 mean | DFS | Reported | 7 |
| Xiao et al, 201527 | People’s Republic of China | Asian | 280 | <61 (133) >61 (147) |
G1–3 | RC | Non-metastatic | Surgery ± chemotherapy | 3.78 median | 52.0 (0.5–106.4) | DFS | Reported | 7 |
Note:
Represents a mixed group including both no-metastatic and metastatic diseases.
Abbreviations: BCa, bladder cancer; BC, breast cancer; CC, colon cancer; ccRCC, clear cell renal cell carcinoma; CRC, colorectal cancer; DE, data-extrapolated; DFS, disease-free survival; ESCC, esophageal squamous cell carcinoma; GC, gastric cancer; LC, lung cancer; LRT, log-rank test; NOS, Newcastle–Ottawa Scale; NPC, nasopharyngeal carcinoma; NR, not reference; NSCLC, non-small cell lung cancer; NCT, neoadjuvant chemotherapy; OS, overall survival; PC, pancreatic cancer; RC, rectal cancer; ROC, receiver operating characteristic; SCLC, small cell lung cancer; STS, soft tissue sarcoma; T, training set; UTUC, upper urinary tract urothelial carcinoma; V, validation set.
Meta-analysis results
Relationship between LMR and OS
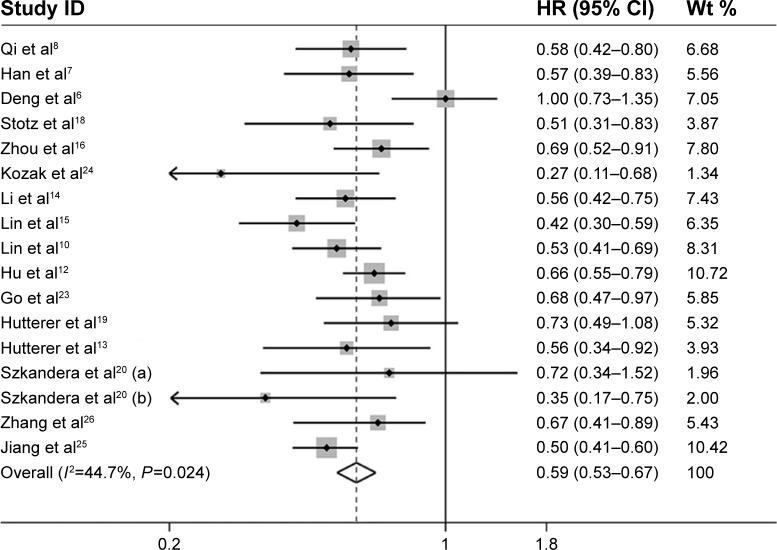
For 16 studies evaluating the correlation between LMR and OS, there appeared to have an overall moderate but not statistically significant level of heterogeneity (I2=44.7%, P=0.024). Hence, a random-effects model was applied to pool the HR and its 95% CI (Figure 2). We found that an elevated pre-treatment LMR predicted a significantly favorable OS with combined HR of 0.59 (95% CI: 0.53–0.67, P<0.001) (Table 2).
Figure 2.
Forest plots of studies evaluating the association between LMR and OS in solid tumor patients.
Note: Weights are from random-effects analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; OS, overall survival; wt, weight.
Table 2.
Overall and subgroup analyses results of OS or DFS stratified by ethnicity, sample size, cut-off value, median age, cancer type, and tumor stage/grade
| Subgroup | OS
|
DFS
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study number | Patients number | HR (95% CI) | P-value | Model | Pheterogeneity | I2 (%) | Study number | Patients number | HR (95% CI) | P-value | Model | Pheterogeneity | I2 (%) | |
| Total | 16 | 7,556 | 0.59 (0.53–0.67) | <0.001 | Random | 0.024 | 44.7 | 8 | 4,898 | 0.74 (0.68–0.80) | <0.001 | Fixed | 0.073 | 44.2 |
| Ethnicity | ||||||||||||||
| Asian | 11 | 5,854 | 0.61 (0.53–0.69) | <0.001 | Random | 0.013 | 55.5 | 6 | 4,429 | 0.75 (0.69–0.82) | <0.001 | Fixed | 0.276 | 20.9 |
| Caucasian | 5 | 1,702 | 0.56 (0.45–0.71) | <0.001 | Fixed | 0.287 | 19.4 | 2 | 469 | 0.36 (0.21–0.60) | <0.001 | Fixed | 0.950 | 0.0 |
| Sample size | ||||||||||||||
| >300 | 8 | 5,907 | 0.63 (0.54–0.73) | <0.001 | Random | 0.011 | 61.8 | 4 | 3,931 | 0.75 (0.67–0.83) | <0.001 | Fixed | 0.201 | 33.9 |
| <300 | 8 | 1,649 | 0.55 (0.48–0.64) | <0.001 | Fixed | 0.332 | 12.3 | 4 | 967 | 0.56 (0.39–0.80) | <0.001 | Random | 0.048 | 58.3 |
| Median age | ||||||||||||||
| >60 | 12 | 3,628 | 0.67 (0.41–0.89) | <0.001 | Fixed | 0.094 | 36.1 | 5 | 1,374 | 0.66 (0.49–0.87) | <0.001 | Random | 0.023 | 61.6 |
| <60 | 4 | 3,928 | 0.54 (0.45–0.65) | <0.001 | Random | 0.063 | 58.8 | 3 | 3,524 | 0.72 (0.64–0.80) | <0.001 | Fixed | 0.653 | 0.0 |
| Tumor stage | ||||||||||||||
| Non-metastatic | 9 | 2,598 | 0.62 (0.55–0.69) | <0.001 | Fixed | 0.476 | 0.0 | 0 | – | – | – | – | – | – |
| Metastatic | 3 | 837 | 0.49 (0.43–0.57) | <0.001 | Fixed | 0.554 | 0.0 | 1 | 389 | – | – | – | – | – |
| Mixed* | 4 | 912 | 0.72 (0.56–0.93) | 0.011 | Random | 0.094 | 53.1 | 7 | 4,509 | 0.72 (0.66–0.79) | <0.001 | Fixed | 0.164 | 33.0 |
| Tumor grade | ||||||||||||||
| G1–3 | 5 | 3,082 | 0.58 (0.48–0.70) | <0.001 | Fixed | 0.268 | 22.1 | 5 | 1,842 | 0.71 (0.62–0.81) | <0.001 | Fixed | 0.084 | 48.4 |
| G1–4 | 4 | 2,703 | 0.73 (0.58–0.92) | 0.008 | Random | 0.099 | 52.1 | 2 | 1,491 | 0.79 (0.69–0.90) | <0.001 | Fixed | 0.112 | 40.4 |
| Cut-off value | ||||||||||||||
| >3 | 9 | 4,964 | 0.63 (0.54–0.73) | <0.001 | Random | 0.023 | 55.0 | 5 | 4,211 | 0.76 (0.70–0.83) | <0.001 | Fixed | 0.283 | 20.7 |
| ≤3 | 7 | 2,592 | 0.53 (0.46–0.61) | <0.001 | Fixed | 0.431 | 0.0 | 3 | 687 | 0.51 (0.37–0.68) | <0.001 | Fixed | 0.417 | 0.0 |
| Cancer type | ||||||||||||||
| Digestive system cancer | 6 | 1,745 | 0.63 (0.49–0.81) | <0.001 | Random | 0.024 | 61.4 | 4 | 1,016 | 0.76 (0.58–0.98) | 0.037 | Random | 0.073 | 57.0 |
| Urinary tract tumor | 3 | 985 | 0.66 (0.52–0.84) | 0.001 | Fixed | 0.716 | 0.0 | 0 | – | – | – | – | – | – |
| NPC | 3 | 2,475 | 0.50 (0.43–0.57) | <0.001 | Fixed | 0.459 | 0.0 | 1 | 1,547 | 0.67 (0.53–0.84) | <0.001 | – | – | – |
| Lung cancer | 3 | 2,011 | 0.62 (0.54–0.72) | <0.001 | Fixed | 0.349 | 5.1 | 1 | 1,453 | 0.75 (0.65–0.87) | <0.001 | – | – | – |
| Others | 1 | 340 | 0.60 (0.30–0.85) | 0.01 | Fixed | 0.180 | 44.4 | 2 | 882 | 0.60 (0.46–0.78) | <0.001 | Fixed | 0.203 | 37.3 |
Note:
Represents a mixed group including both no-metastatic and metastatic diseases.
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NPC, nasopharyngeal carcinoma; OS, overall survival.
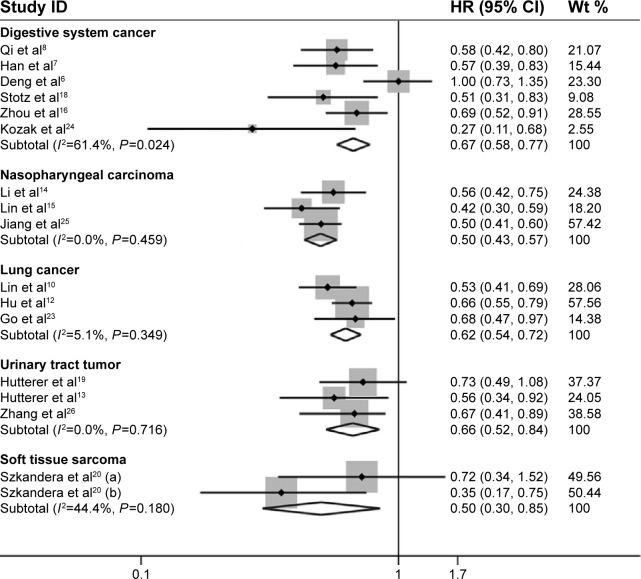
To minimize the influence of heterogeneity, we further conducted seven subgroup analyses based on ethnicity, LMR cut-off value, sample size, median age, type of cancers, and tumor stage/grade (Table 2). In the subgroup of ethnicity, a pooled analysis of nine studies in Asians demonstrated that increased LMR was significantly associated with enhanced OS (HR=0.61, 95% CI: 0.53–0.69, random-effects model, I2=55.5%). Enhanced OS in Caucasians with elevated pretreatment LMR was also found by merging five studies with pooled HR of 0.56 (95% CI: 0.45–0.71, fixed effect model; I2=19.4%). When stratified by cancer type, the result indicated that LMR was a favorable prognostic marker in patients with digestive system carcinomas (HR=0.63, 95% CI: 0.49–0.81, random-effects model; I2=61.4%), urinary tract tumors (HR=0.66, 95% CI: 0.52–0.84, fixed-effects model; I2=0.0%), lung cancer (HR=0.62, 95% CI: 0.54–0.72, fixed-effects model; I2=5.1%), and nasopharyngeal carcinoma (HR=0.50, 95% CI: 0.43–0.57, fixed-effects model; I2=0.0%) (Figure 3). In the subgroup of the LMR cut-off value, merging eight studies of cut-off value >3 indicated that increased LMR was significantly associated with enhanced OS (HR=0.63, 95% CI: 0.54–0.73, random-effects model; I2=55.0%). The same outcome was also shown in meta-analysis of studies of cut-off value ≤3 (HR=0.53, 95% CI: 0.46–0.61, fixed-effects model; I2=0.0%). In the subgroup of sample size, we found that no matter the sample size >300 or <300, high LMR level was still a favorable predictor for OS (HR=0.63, 95% CI: 0.54–0.73, HR=0.55, 95% CI: 0.48–0.64, respectively). When stratified by tumor stage, we found that high levels of LMR predict a better OS for non-metastatic diseases (HR=0.62, 95% CI: 0.55–0.69), metastatic diseases (HR=0.49, 95% CI: 0.43–0.57), and mixed group (HR=0.72, 95% CI: 0.56–0.93). Similarly, subgroup analyses stratified by median age and tumor grade did not alter the prognostic role of LMR in OS (Table 2).
Figure 3.
Forest plots for the subgroup analysis of OS in different malignant diseases.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; wt, weight.
Relationship between LMR and DFS
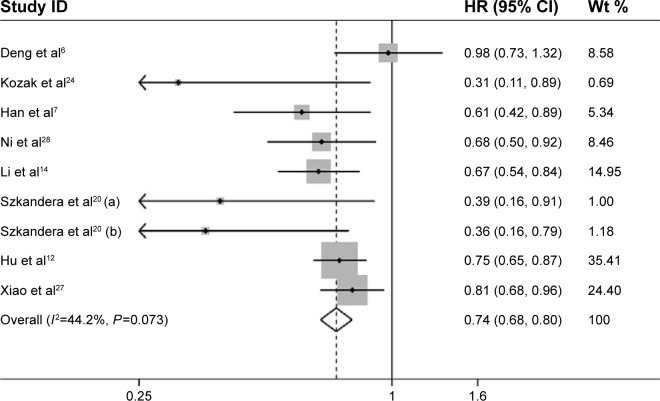
For eight studies evaluating DFS, a fixed-effects model was used in that there was no significant heterogeneity among them (I2=44.2, P=0.073). The pooled HR was 0.74 (95% CI: 0.68–0.80) (Figure 4), which indicated that high LMR level was significantly connected with favorable DFS. Similar to OS analyses, subgroup analyses stratified by ethnicity, LMR cutoff value, sample size, median age, type of cancers, and tumor stage/grade were also conducted. As shown in Table 2, the pooled outcomes indicated that high LMR was also significantly associated with favorable DFS in all subgroup analyses.
Figure 4.
Forest plots of studies evaluating the association between LMR and DFS in solid tumor patients.
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; wt, weight.
Heterogeneity analysis
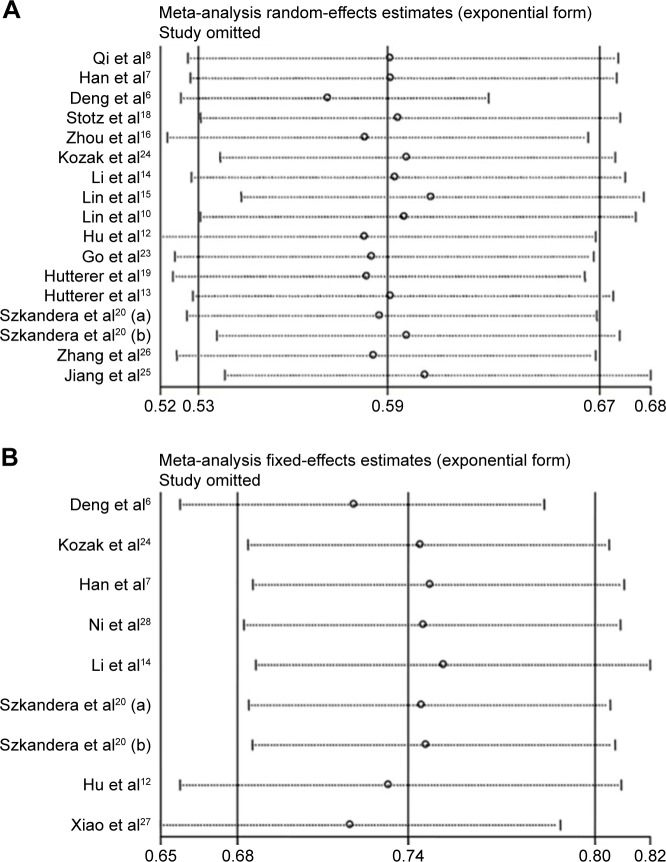
To explore the most possible sources of heterogeneity in the OS meta-analysis, a sensitivity analysis was performed by removing one study each time and evaluating the pooled HRs again. We found that Deng et al’s6 study distinctively influenced the overall results (Figure 5A). When we removed this study from the analysis, the heterogeneity became insignificant (P=0.298, I2=13.6%), whereas the pooled outcome still indicated that LMR was a favorable predictor for OS (HR=0.58, 95% CI: 0.53–0.62). Consequently, we considered that Deng et al’s study was responsible for the heterogeneity. We also performed a meta-regression to explore the potential factors that lead to heterogeneity in the OS meta-analysis. As a result, factors including published year (P=0.72), ethnicity (P=0.52), tumor type (P=0.92), median age (P=0.23), tumor stage (P=0.52), tumor grade (P=0.55), and sample size (P=0.28) did not significantly contribute to the heterogeneity, whereas LMR cutoff value (P=0.07) was perhaps the source of heterogeneity. In the DFS studies,6,7,12,14,20,24,27,28 we also conducted a sensitivity analysis. The result showed that no individual study significantly affected the pooled outcome (Figure 5B).
Figure 5.
Sensitivity analysis for meta-analysis of LMR.
Notes: (A) Effect of single study on the pooled HR for LMR and OS of patients. (B) Effect of single study on the pooled HR for LMR and DFS of patients.
Abbreviations: DFS, disease-free survival; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; OS, overall survival.
Publication bias
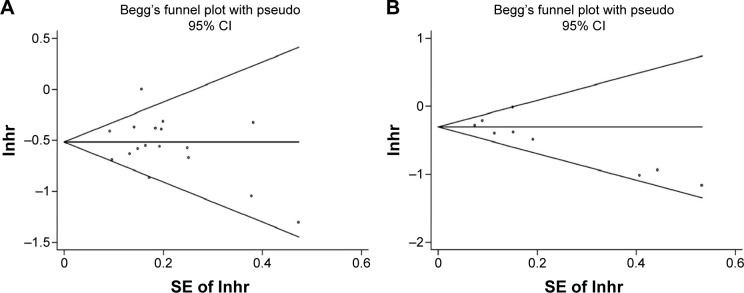
We assessed a publication bias of the enrolled studies by using the Begg’s funnel plot and Egger’s test. In the analyses of OS, the funnel plots seemed symmetrical (Figure 6A). The P-value of Begg’s and Egger’s tests was 0.34 and 0.54, respectively. Hence, no proof of significant publication bias was detected for OS. However, the asymmetric funnel plots (Figure 6B) and the result of Egger’s test (P=0.03) indicate a potential publication bias for DFS.
Figure 6.
Publication bias in this meta-analysis.
Notes: (A) Begg’s funnel plots of publication bias for merged analysis of OS. (B) Begg’s funnel plots of publication bias for merged analysis of DFS.
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival; SE, standard error.
Discussion
During the past few years, many kinds of predictors have been found and applied for predicting solid tumors outcomes, including inflammatory factors. Many tumors may rise from the sites of chronic irritation, infection, and inflammation.29 Inflammation is a key component of cancer progression. Cancer-related inflammation also aids malignant cells proliferation and promotes tumor-correlated angiogenesis and metastasis.4 As important inflammatory markers, lymphocytes and monocytes have been independently connected with the prognosis of various malignant neoplasms.30,31 The pre-treatment LMR potentially balances the effects of lymphocytes, and monocytes makes it a useful prognostic factor. Moreover, because pre-treatment hematological tests are routinely measured in the clinic, LMR seems to be an easily obtained, inexpensive, and a robust predictor.
To the best of our knowledge, our meta-analysis is the first systematic study to determine the prognostic value of LMR in patients with solid tumors. Our combined results showed that elevated LMR was associated with favorable OS and DFS. Furthermore, subgroup analyses were performed based on types of cancers, ethnicity, cut-off value, median age, sample size, and tumor stage/grade. We found that elevated LMR was a favorable predictor for both Asians and Caucasians, but the association in Caucasians was stronger than that in Asians. We also showed that high LMR was connected with favorable OS and DFS in patients diagnosed with digestive system cancers, as well as nasopharyngeal carcinoma and lung cancer. Similar results were also shown in other six subgroup analyses. Therefore, LMR is a promising prognostic biomarker that can be used to estimate survival outcomes of solid tumor patients. To find the potential sources of heterogeneity in studies for OS, sensitivity analysis and meta-regression were conducted. Result of the sensitivity analysis indicated that the study of Deng et al6 was responsible for the heterogeneity. Deng et al’s study assessed the prognostic value of NLR, derived (dNLR), PLR, and LMR in patients with gastric cancer. It found that elevated LMR was significantly associated with enhanced OS in univariate analysis but insignificantly in multivariate analysis. The insignificant prognostic effect of elevated LMR in this study perhaps induced a negative result in our meta-analysis. The result of meta-regression showed that LMR cut-off value may be the potential sources of heterogeneity. Studies included using different methods to explore the LMR cut-off value. However, subgroup analyses demonstrated that LMR was an effective prognostic factor, regardless of the cut-off value >3 or ≤3.
At present, the exact mechanism of the prognostic value of LMR in solid tumor patients is poorly understood. Several underlying mechanisms for this association are listed as follows. First, lymphocytes as crucial components of host immunity play important role in the antitumor immunological reaction by inducing cytotoxic cell death and inhibiting tumor cell proliferation and migration.4,29 Previous studies have reported that tumor-infiltrating lymphocytes are associated with favorable prognosis in patients with various carcinomas.31,32 Infiltrated CD4+ and CD8+ T cells are essential to the antitumor immunological reaction and can induce tumor cell apoptosis via interaction among each other.33,34 However, systemic inflammation response from malignant cells could cause immunosuppression, for which tumor cells can escape host immune surveillance.35 A low lymphocyte count has been found in many human neoplasms, and it is often associated with worse clinical outcomes, which may be due to the depressed lymphocyte-mediated immune response to the tumor.36 On the other hand, macrophages derived from circulating monocytes show specific phenotypic characteristics. Increasing evidence demonstrated the high density of tumor-associated macrophages in a wide spectrum of tumors is associated with increased tumor-correlated angiogenesis, invasiveness, and poor outcomes.37 Macrophages may enhance tumor development and angiogenesis by releasing TNF-α, vascular endothelial growth factor, and epidermal growth factor.38,39 As mentioned earlier, LMR combined with the effects of lymphocyte and monocyte may be a stronger predictor of prognosis for cancer patients.
Although the present meta-analysis showed that high LMR level was associated with favorable outcomes, the results should be interpreted cautiously for several limitations identified in our study. First, there was some heterogeneity among included studies. Heterogeneity might be caused by the differences in age, sex, ethnicity, sample sizes, types of the tumors, disease stage, treatment received, cut-off values of LMR, durations of follow-up, and other factors. Despite a random-effects model was applied, the factor and study found responsible for heterogeneity should be paid attention to while considering the related conclusions. Second, several HRs and 95% CI were calculated from available numerical data in the studies, which may bring minor deviations. Third, the cut-off value of LMR applied in the enrolled studies was not unified. Fourth, the eligible studies were all retrospective analysis. Finally, although no evidence of significant publication bias was noted in OS meta-analysis, there was significant publication bias in DFS meta-analysis. Several reasons may lead to the publication bias. On the one hand, our study included only published articles and meanwhile they were all written in English. On the other hand, studies with positive results were more easily to be published than those with negative results. Even though published, they were usually unassessable due to the concise reports of results.
Conclusion
In summary, our study demonstrated that increased pretreatment LMR is correlated with significantly favorable outcomes in patients with solid tumors. We consider that LMR, a widely available, inexpensive, and robust inflammatory biomarker, could be widely used to evaluate the prognosis of solid tumors. However, because all studies included in our meta-analysis were retrospective, larger prospective, and multicenter studies should be performed to confirm the predictive value of LMR. Future studies based on large sample to explore a definitive LMR cut-off value are also recommended. Additionally, specific therapies or interventions to modify a low pre-treatment LMR level may be proven to be beneficial in improving the prognosis of patients with solid tumors. Targeting systemic inflammation may be an effective treatment for tumor patients with low LMR. All of these possibilities are necessary to be addressed in the near-future studies.
Acknowledgments
The work was supported by the Foundation of Health and the Family Planning Commission of Shandong Province (Grant No. 2013WSC02041 and Grant No. YG201519).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 6.Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han LH, Jia YB, Song QX, Wang JB, Wang NN, Cheng YF. Prognostic significance of preoperative lymphocyte–monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2015;16(6):2245–2250. doi: 10.7314/apjcp.2015.16.6.2245. [DOI] [PubMed] [Google Scholar]
- 8.Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15(2):145–150. doi: 10.1016/j.pan.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Koh YW, Jung SJ, Yoon DH, et al. The absolute lymphocyte to monocyte ratio is associated with poor prognosis in classical Hodgkin lymphoma patients younger than 60 years of age. Hematol Oncol. 2014;33(3):133–140. doi: 10.1002/hon.2155. [DOI] [PubMed] [Google Scholar]
- 10.Lin GN, Peng JW, Xiao JJ, Liu DY, Xia ZJ. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doublet. Med Oncol. 2014;31(7):70. doi: 10.1007/s12032-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 11.Anand M, Chodda SK, Parikh PM, Nadkarni JS. Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis. Hematol Oncol. 1998;16(4):143–154. doi: 10.1002/(sici)1099-1069(199812)16:4<143::aid-hon628>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte–monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(9):e108062. doi: 10.1371/journal.pone.0108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutterer GC, Sobolev N, Ehrlich GC, et al. Pretreatment lymphocyte–monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol. 2015;68(5):351–355. doi: 10.1136/jclinpath-2014-202658. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Jiang R, Liu WS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One. 2013;8(12):e83069. doi: 10.1371/journal.pone.0083069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin GN, Peng JW, Liu DY, Xiao JJ, Chen YQ, Chen XQ. Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumour Biol. 2014;35(11):10849–10854. doi: 10.1007/s13277-014-2362-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Du Y, Xu J, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcomes in patients with stage II/III gastric cancer. Tumour Biol. 2014;35(11):11659–11666. doi: 10.1007/s13277-014-2504-x. [DOI] [PubMed] [Google Scholar]
- 17.Wei X, Huang F, Wei Y, et al. Low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in non-germinal center type diffuse large B-cell lymphoma. Leuk Res. 2014;38(6):694–698. doi: 10.1016/j.leukres.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte–monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol. 2014;32(7):1041–1048. doi: 10.1016/j.urolonc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Szkandera J, Gerger A, Liegl-Atzwanger B, et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer. 2014;135(2):362–370. doi: 10.1002/ijc.28677. [DOI] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go SI, Kim RB, Song HN, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014;31(12):323. doi: 10.1007/s12032-014-0323-y. [DOI] [PubMed] [Google Scholar]
- 24.Kozak MM, von Eyben R, Pai JS, et al. The prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancer. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000183. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Jiang R, Cai XY, Yang ZH, et al. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer. 2015;34(1):23. doi: 10.1186/s40880-015-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte–monocyte and platelet–lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol. 2015 Jun 2; doi: 10.1007/s13277-015-3613-x. Epub. [DOI] [PubMed] [Google Scholar]
- 27.Xiao WW, Zhang LN, You KY, et al. A low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in pathological T3N0 rectal cancer patients following total mesorectal excision. J Cancer. 2015;6(7):616–622. doi: 10.7150/jca.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni XJ, Zhang XL, Ou-Yang QW, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9(11):e111886. doi: 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SD, Kim SH, Kim YK, Lee SA, Park SJ. Prognostic significance of preoperative peripheral blood monocyte ratio in patients with hepatocellular carcinoma. World J Surg. 2014;38(9):2377–2385. doi: 10.1007/s00268-014-2545-8. [DOI] [PubMed] [Google Scholar]
- 31.Balermpas P, Rodel F, Weiss C, Rodel C, Fokas E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. Oncoimmunology. 2014;3(1):e27403. doi: 10.4161/onci.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171(1):1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 34.Zikos TA, Donnenberg AD, Landreneau RJ, Luketich JD, Donnenberg VS. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother. 2011;60(6):819–827. doi: 10.1007/s00262-011-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102(7):742–747. doi: 10.1002/jso.21725. [DOI] [PubMed] [Google Scholar]
- 36.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 38.Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153(2):587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(2):177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]