Abstract
Background
Small GTPase Rap1 has been implicated in a number of basic cellular functions, including cell-cell and cell-matrix adhesion, proliferation and regulation of polarity. Evolutionarily conserved, Rap1 has been studied in model organisms: yeast, Drosophila and mice. Mouse in vivo studies implicate Rap1 in the control of multiple stem cell, leukocyte and vascular cell functions. In vitro, several Rap1 effectors and regulatory mechanisms have been proposed. In particular, Rap1 has been implicated in maintaining epithelial and endothelial cell junction integrity and linked with cerebral cavernous malformations.
Rationale
How Rap1 signaling network controls mammalian development is not clear. As a first step in addressing this question, we present phenotypes of murine total and vascular-specific Rap1a, Rap1b and double Rap1a and Rap1b (Rap1) knockout (KO) mice.
Results and Conclusions
The majority of total Rap1 KO mice die before E10.5, consistent with the critical role of Rap1 in epithelial morphogenesis. At that time point, about 50% of Tie2-double Rap1 KOs appear grossly normal and develop normal vasculature, while the remaining 50% suffer tissue degeneration and show vascular abnormalities, including hemorrhages and engorgement of perineural vessels, albeit with normal branchial arches. However, no Tie2-double Rap1 KO embryos are present at E15.5, with hemorrhages a likely cause of death. Therefore, at least one Rap1 allele is required for development prior to the formation of the vascular system; and in endothelium–for the life-supporting function of the vasculature.
Introduction
The evolutionarily conserved and ubiquitously expressed small GTPase Rap1 is at the crossroads of signaling pathways that govern key cellular processes. Downstream from multiple receptors, Rap1 activity is spatiotemporally regulated by a network of guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) acting in a tissue-specific manner [1]. In a GTP-bound form, activated Rap1 interacts with a number of effectors to control cell-substrate adhesion, cell-cell adhesion and junction formation [2], and cell polarity [3]. In vitro studies have implicated KRIT1/CCM1, a protein mutated in cerebral cavernous malformations (CCM), RASIP1 and an actin-cytoskeleton linker Canoe/Afadin as Rap1 effectors controlling cell-cell junction formation and maintenance.
In particular, Rap1 interaction with KRIT1 [4–7] has raised interest due to its potential significance for human disease [8]. KRIT1, one of three proteins whose autosomal mutations have been linked with CCM; a neurovascular malformation syndrome that leads to seizures and lethal stroke [9–12], is a multi-domain protein that links cortical actin cytoskeleton with integral membrane proteins, and interacts with CCM2 [13]. In vitro, in endothelial cells (ECs), Rap1 facilitates localization of KRIT1 to cell-cell junctions and interaction with junctional proteins [5, 14, 15]. However, whether Rap1-KRIT1 interaction plays a physiological role in the development of CCM is unknown.
Rap1 functions in vivo have been studied in several model organisms. In lower organisms, a single Rap1 ortholog plays a central role in the development of cell polarity: in budding yeast S. cerevisiae, Rap1 ortholog Bud1/Rsr controls positioning of the bud [16] and in Drosophila, Rap1 controls apico-basal polarity during mesoderm formation and dorsal closure via its interactions with a protein network involving atypical PKC (aPKC) and Canoe/Afadin [17–19]. In higher organisms, two highly homologous isoforms of Rap1 exist: Rap1a and Rap1b. The two Rap1 isoforms are encoded by separate genes [20, 21] and murine genetic deletion models of both have been described [8]. Deletion of either isoform leads to partial embryonic lethality and bleeding [22, 23]. While deletion of either Rap1 isoform does not limit the lifespan of surviving adult mice, several defects in neurological [24] and immune responses [23, 25–28] and hematopoiesis [29] have been described. Some of the most significant defects observed in Rap1 knockout (KO) mice involve their cardiovascular functions: platelet function [22], angiogenesis [30, 31], smooth muscle contractility and vessel tone [32]. The similarity of some of the phenotypes of the two Rap1 isoforms KOs suggested functional redundancy. To determine if the two isoforms have similar functions, we attempted to generate double Rap1a, Rap1b KO mice. In this paper, for the first time to our knowledge, we report on the phenotype of these mice.
Because of the bleeding phenotype in total Rap1-deficient embryos we have been particularly interested in the role of Rap1 in the vasculature. We have made endothelial lineage restricted Rap1 KO mice and demonstrated a critical role of Rap1 in endothelial cells in angiogenesis [32, 33] and, more recently, regulation of endothelial function and blood pressure [34]. Interestingly, molecular mechanisms underlying these defects in adult mice implicate Rap1 in regulation of the signaling aspect of adhesion receptors [32, 33], rather than in their role in promoting cell adhesion. However, the role of Rap1 in vasculature during morphogenesis and development is not known. To address this knowledge gap, here we describe the phenotype of Rap1 endothelial-specific KO mice and analyze it in the context of Rap1 effectors, KRIT1 and Afadin.
Methods
Animal generation and husbandry
All mouse procedures were performed according to approved Medical College of Wisconsin or Indiana University School of Medicine Institutional Animal Use and Care Committee protocols. Generation of Rap1b -/- mice and endothelial-specific Rap1 KO mice (EC-Rap1 KO; Tie2-Cre+/0 Rap1af/f Rap1bf/f) (with a mixed 129SvEv/C57BL6, 50%/50% average, background) has been previously described [22, 33]. Rap1b +/- mutant mice were back-crossed with C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) for at least 8 successive generations. Double KO Rap1a -/- Rap1b -/- mice were generated by intercrossing Rap1b -/- with Rap1a -/- mice [23]. Mouse genotypes were determined by PCR on tail DNA as described previously [22, 23, 33].
Determination of embryonic lethality
Timed pregnancies were set up with mid-day of vaginal plug defined as E0.5. Embryos were collected at specified times and genotypes were determined by PCR of tissue samples. Expected embryo numbers (NE) were determined using Mendelian ratios, based on 100% survival of WT embryos. Embryo survival was defined as observed (NO) vs. expected (NO/NE*100%); embryo lethality was defined as (1-NO/NE)*100%.
Histology
Embryos were collected and bright-field images were obtained using Zeiss stereoscope (SteREO Lumar.V12, Carl Zeiss MicroImaging GmbH, Germany) at 6.4x magnification. Histological staining was performed as previously described [35, 36]. Briefly, paraffin sections were stained with hematoxilin and eosin (H&E) or with antibodies to CD31 (PECAM-1, at 1:250 dilution, clone MEC13.3, BD Biosciences). Improved visualization on paraffin sections was obtained using a biotinylated tyramide signal amplification (TSA) kit (PerkinElmer) according to the manufacturer’s instructions.
Results
Partial embryonic lethality of total Rap1b KO mice
Two highly homologous Rap1 isoforms exist encoded by separate Rap1a and Rap1b genes [20, 21]. We reported partial embryonic and perinatal lethality of Rap1b -/- mice on mixed background [22]. While Rap1b -/- females (on mixed genetic background) were fertile, litter size was reduced (4 ± 0.28, s.e.m., surviving pups/litter; n = 40 litters from Rap1b -/- intercrosses) compared to WT litter size (7.2 ± 0.37, s.e.m., surviving pups/litter; n = 40 litters from Rap1b +/+ intercrosses). In about 20% of embryos, starting at E13.5 we observed dispersed subcutaneous bleeding, and hemorrhages on the side of the head and in liver, accompanied by edema [22]. To better determine the time and cause of embryo lethality, we performed systematic analysis of Rap1b -/- embryos from staged pregnancies. We found that at E13.5 and E15.5, Rap1b -/- embryo number (on mixed genetic background) was not reduced compared to WT embryos (80–90 embryos analyzed), however about 30–50% Rap1b -/- embryos appeared abnormal, containing hemorrhages or clots. At E18.5 about 20% of Rap1b -/- embryos were found resorbing. Furthermore, we found Rap1b -/- embryo body weight significantly decreased at E15.5 (0.233 ± 0.013 vs. 0.273 ± 0.010 g, WT; p<0.05) and at E18.5, (1.119 ± 0.036 g vs. 1.242 ± 0.030 g, WT; p<0.05). The difference in body weight persisted after birth [32]. Observed growth restriction of embryos is consistent with a systemic defect, such as in vascular development, and may contribute to decreased viability at weaning to 28% of expected numbers [22], (Table 1).
Table 1. Survival of Rap1b -/- and Rap1a -/- mice at weaning.
| Mixed C57Bl6/129SvEv background | C57Bl6 background | C57Bl6 background | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | WT | Rap1b+/- | Rap1b-/- | Total | WT | Rap1b+/- | Rap1b-/- | Total | WT | Rap1a+/- | Rap1a-/- | Total |
| Mice at weaning | 357 | 667 | 100 | 1124 | 392 | 522 | 12 | 926 | 92 | 188 | 59 | 339 |
| Expected */ | 357 | 714 | 357 | 392 | 784 | 392 | 92 | 184 | 92 | |||
| Viable/Expected | 93% | 28% | 67% | 3% | ≥100% | 64% | ||||||
Number of adult mice from Rap1b +/- or Rap1a +/- intercrosses on mixed and C57Bl6 backgrounds.
*/Expected number = Total number * Mendelian frequency (1:2:1), based on 100% survival of WT mice.
Initial observations of Rap1a -/- mice on mixed genetic background did not suggest lethality, however upon backcross onto C57Bl6 background (F7-11), partial embryonic lethality occurred [23], see Table 1. We hypothesized that Rap1b -/- mice on pure C57Bl6 background may reveal additional phenotype that may not be apparent in the mixed background due to modifier genes. Upon back-crossing Rap1b -/- mice to F8 onto C57Bl6 background we examined survival of Rap1b -/- mice. We found further decreased survival of mice at weaning (Table 1). To determine the time of embryonic death, we examined homozygous KO embryos from staged pregnancies (Fig 1). No overt morphological defects were seen before E10.5 Rap1b -/-; however, at that time, Rap1b -/- embryos were smaller in size (Fig 1A).
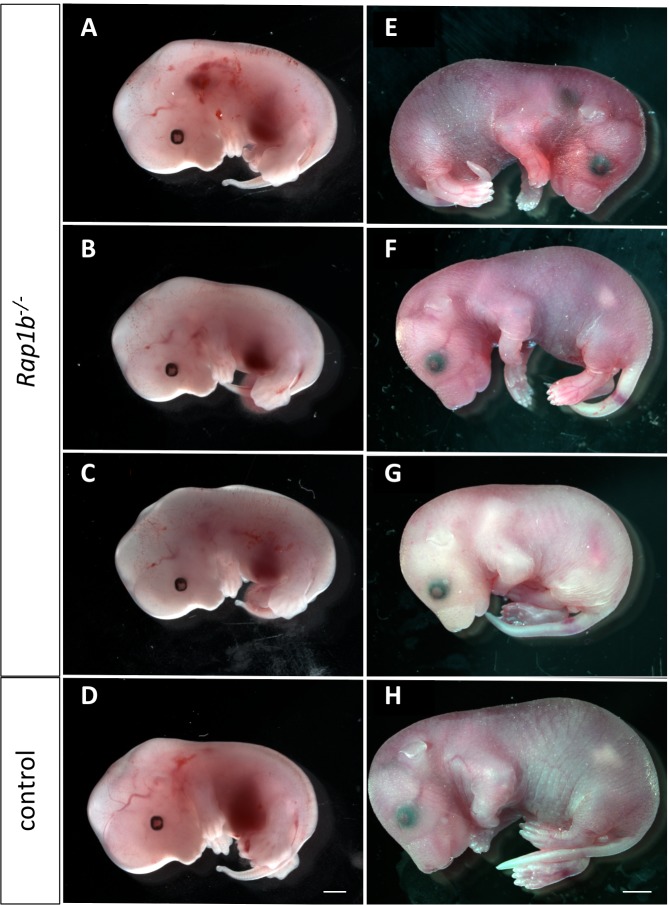
Fig 1. Vascular abnormalities in Rap1b -/- embryos on C57Bl6 genetic background.
Embryos from pregnancies resulting from Rap1b +/- intercrosses at E13.5 (A-D, left panel) and E18.5 (E-H, right panel) on C57/BL6 genetic background. Rap1b-deficiency leads to cranial hemorrhage in about 20–50% of embryos (A, E), edema (C), pale pallor (G), and a smaller body size compared to WT littermate controls (D, H). Stereoscopic images are representative of 4–6 analyzed pregnancies. Scale bars: 1 mm (A-D) and 2 mm (E-H).
At E13.5 and E15.5, about 20–50% of Rap1b -/- embryos on C57Bl6 background displayed prominent hemorrhage on the side of the head, indicative of cardiovascular defects, while other Rap1b -/- had no overt defects (Fig 1B). Similarly, at E18.5 (Fig 1C) this bleeding phenotype was only displayed in about 25% of Rap1b -/- embryos. With 97% lethality at weaning (Table 1), we conclude that most Rap1b -/- mice die perinatally and Rap1b is not absolutely required for embryonic development.
Early embryonic lethality of Rap1a, Rap1b KO mice
In lower organisms Rap1 is critical for morphogenesis [18] and deletion of the only isoform of Rap1 present in these organisms leads to lethality [37]. Since deletion of either Rap1a or Rap1b genes is not essential for development, as some KOs survive to adulthood, we hypothesized that there is a redundancy in Rap1a and Rap1b function in vertebrates. To test if either Rap1 gene is essential for development, we attempted to generate a double Rap1a, Rap1b KO (“Rap1 KO”) by intercrossing Rap1a +/- and Rap1b +/- mice, but no viable Rap1 KO mice were obtained (Table 2). We therefore examined the effect of Rap1 deletion on embryonic development. We found that at E8.5 all expected embryos were recovered and appeared grossly normal, which indicates that Rap1 is not absolutely required for early development. However at E10.5, a reduced number of Rap1 KO embryos was recovered and only about 20% Rap1 KO embryos appeared viable (Table 3), while the remaining 80% of Rap1 KO embryos appear grossly underdeveloped and abnormal (Fig 2). Thus, we conclude that, unlike in lower organisms [38], Rap1 in mice is not critical for early embryonic events, such as gastrulation; but it is required for further development.
Table 2. Survival of offspring from Rap1a +/-,Rap1b +/- intercrosses,(on mixed genetic background).
| Genotype | Total | Expected */ | Viable/Expected |
|---|---|---|---|
| Rap1a+/+Rap1b+/+ | 13 | 13 | 100% |
| Rap1a+/+ Rap1b+/- | 32 | 26 | ≥100% |
| Rap1a+/+ Rap1b-/- | 6 | 13 | 46% |
| Rap1a+/- Rap1b+/+ | 47 | 26 | ≥100% |
| Rap1a+/- Rap1b+/- | 76 | 52 | ≥100% |
| Rap1a+/- Rap1b-/- | 0 | 26 | 0% |
| Rap1a-/- Rap1b+/+ | 22 | 13 | ≥100% |
| Rap1a-/- Rap1b+/- | 3 | 26 | 12% |
| Rap1a-/- Rap1b-/- | 0 | 13 | 0% |
Number of adult mice from Rap1a+/-, Rap1b+/- intercrosses, at weaning.
Total animal number = 199
*/ Expected number = Total number * Mendelian frequency (1:2:1;2:4:2;1:2:1), based on 100% survival of WT mice.
Table 3. Embryonic lethality of double Rap1 KO mice.
| Genotype | Total embryo number | Expected */ embryo number | Abnormal (resorbing) | Viable embryos | Viable/Expected |
|---|---|---|---|---|---|
| Rap1a+/+Rap1b+/+ | 8 | 8 | 1 | 7 | 92% |
| Rap1a+/+ Rap1b+/- | 16 | 15 | 4 | 12 | 79% |
| Rap1a+/+ Rap1b-/- | 7 | 8 | 2 | 5 | 66% |
| Rap1a+/- Rap1b+/+ | 16 | 15 | 3 | 13 | 85% |
| Rap1a+/- Rap1b+/- | 36 | 31 | 7 | 29 | 95% |
| Rap1a+/- Rap1b-/- | 15 | 15 | 7 | 8 | 52% |
| Rap1a-/- Rap1b+/+ | 10 | 8 | 3 | 7 | 92% |
| Rap1a-/- Rap1b+/- | 9 | 15 | 4 | 5 | 33% |
| Rap1a-/- Rap1b-/- | 5 | 8 | 4 | 1 | 13% |
Number of E10.5 embryos obtained from Rap1a+/-Rap1b+/- intercrosses per genotype.
Total embryo number = 122.
*/Expected number = Total number * Mendelian frequency (1:2:1; 2:4:2; 1:2:1)
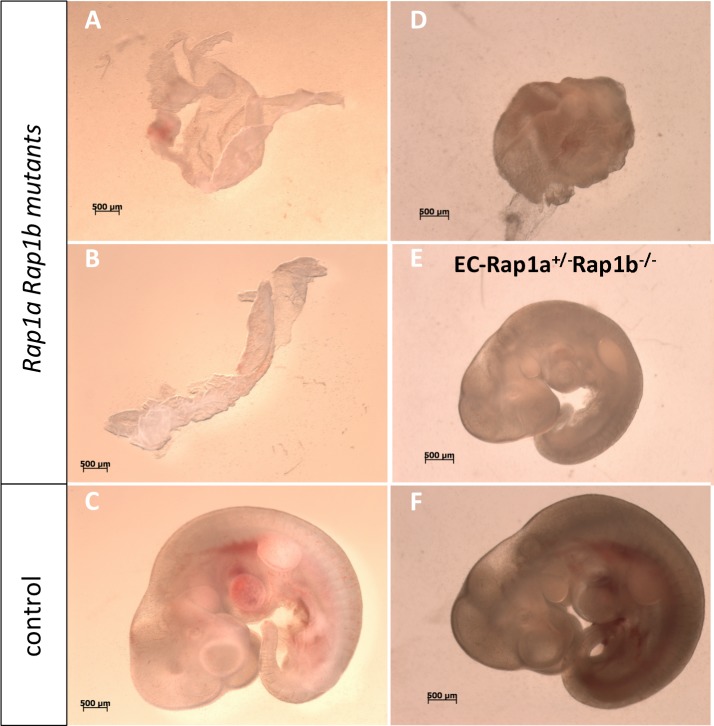
Fig 2. Double KO of Rap1a and Rap1b leads to lethality of majority of embryos before E10.5.
Stereoscopic images from two (A-C; D-F) of eight analyzed pregnancies resulting from Rap1a +/-, Rap1b +/- intercrosses. About 80% of Rap1a -/- ; Rap1b -/- embryos (A, B, D) fail to develop and pregnancy products are grossly malformed and non-viable. (E) Deletion of both Rap1b and one Rap1a allele leads to severe growth restriction; (C, F) littermate Rap1a +/-, Rap1b +/+ controls. Scale bar: 0.5 mm.
Endothelial-specific double KOs are lethal; either Rap1 isoform is redundant, partial Rap1 deletion leads to partial lethality
Because of the hemorrhaging found in total Rap1-KO embryos (Fig 1) and reduced survival of Rap1-KO mice (Table 2), we hypothesized that vascular defects contribute to lethality in Rap1-KO embryos. To address the role of Rap1 in endothelium during development, we intercrossed Tie2-Cre+/0; Rap1af/+, Rap1bf/+ mice [33] to generate endothelial-specific Rap1KO (Tie2-Cre+/0; Rap1af/f, Rap1bf/f, EC-Rap1 KO) mice. Tie2-driven Cre recombination leads to efficient Rap1 protein loss in endothelium and hematopoietic cells [33]. We found that deletion of either Rap1b alone (EC-Rap1b KO) or Rap1a alone (EC-Rap1a KO) did not lead to lethality. However, we did not obtain any double Rap1a, Rap1b KO (EC-Rap1 KO) mice (Table 4), similarly to total Rap1-KO mice (Table 2). Interestingly, mice with only one Rap1a allele (EC-Rap1a+/-, Rap1b-/-) exhibited significantly reduced viability (to 55%) at weaning. In contrast, deletion of all but one Rap1b allele (EC-Rap1a-/-, Rap1b+/-) minimally impacted mouse survival (Table 4).
Table 4. Embryonic lethality of endothelial-specific (EC)-Rap1 KO mice.
| Genotype | Total embryo number | Expected */ | Abnormal | Viable | Viable/Expected | Comments |
|---|---|---|---|---|---|---|
| Tie2-Cre+/0 Rap1af/f Rap1bf/f | 2 | 4.75 | 2 | 0 | 0% | Smaller size, hemorrhage at base of skull |
| Tie2-Cre0/0 Rap1af/f Rap1bf/f | 7 | 4.75 | 1 | 6 | ≥100% | |
| Tie2-Cre+/0 Rap1af/f Rap1bf/+ | 3 | 4.75 | 1 | 2 | 42% | Hemorrhage, blood clots, edema |
| Tie2-Cre0/0 Rap1af/f Rap1bf/+ | 5 | 4.75 | 0 | 5 | ≥100% | |
| Tie2-Cre+/0 Rap1af/+ Rap1bf/f | 7 | 4.75 | 2 | 5 | ≥100% | |
| Tie2-Cre0/0 Rap1af/+ Rap1bf/f | 7 | 4.75 | 1 | 6 | ≥100% | |
| Tie2-Cre+/0 Rap1af/+ Rap1bf/+ | 5 | 4.75 | 0 | 5 | ≥100% | |
| Tie2-Cre0/0 Rap1af/+ Rap1bf/+ | 4 | 4.75 | 0 | 4 | 84% |
Number of E15.5 embryos obtained from Rap1af/f Rap1bf/f x Tie2 Cre+/0; Rap1af/+ Rap1b f/+ crosses, per genotype.
Total number of embryos = 38.
*/ Expected number = Total number * 1/8 frequency
Endothelial-specific double KOs suffer from hemorrhage but branchial arch formation appears normal
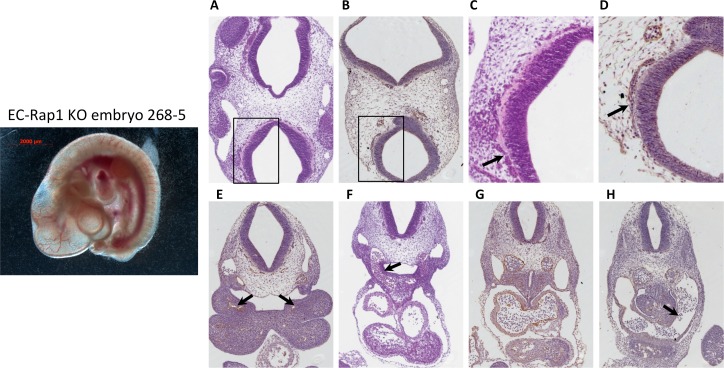
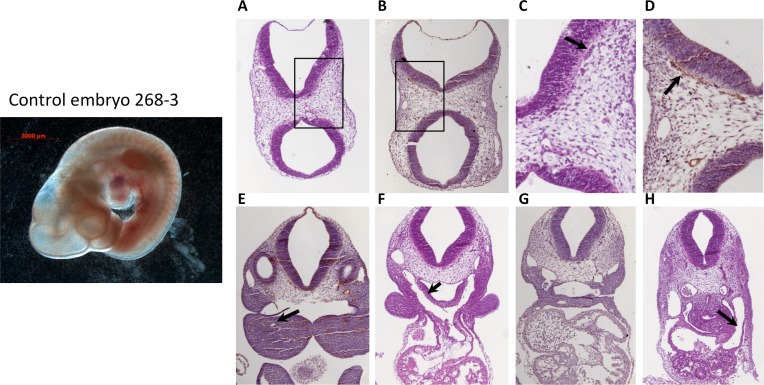
To determine the cause of death of EC-Rap1 KO mice, we examined embryos from staged Tie2-Cre+/0: Rap1af/+, Rap1bf/+ intercrosses. At E10.5 we found that a significant fraction of EC-Rap1 KO embryos were defective compared to WT E10.5 embryos (Figs 3 and 4) with a different embryo size suggestive of different times of death. The defective embryos displayed tissue degeneration (Fig 3A–3F) and cranial hemorrhage on their right side (Fig 3D–3F), with vascular rapture in or around branchial arches as likely cause of death. Furthermore, some EC-Rap1 KO embryos displayed dilated microvessels in the vicinity of the neural tube and vascular engorgement of perineural vessels (Fig 4A and 4B). However, the remaining 50% of EC-Rap1 KO embryos appeared viable without obvious vascular or cardiac lesions (Fig 5; normal EC-Rap1 KO). Interestingly, branchial arch formation appeared normal (Fig 5E and 5F) and similar to WT E10.5 mice (Fig 6E and 6F). This is significantly different from KRIT1 or CCM2 KO mice [35, 36].
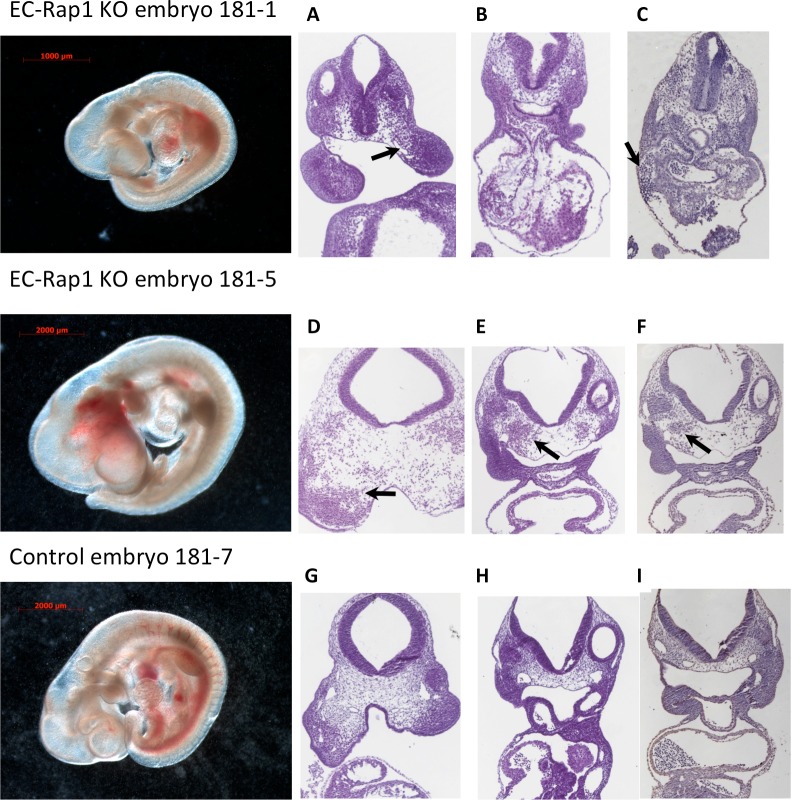
Fig 3. Tissue degeneration and hemorrhages in E10.5 EC-Rap1 KO embryos.
Embryos were generated by crossing Tie2-Cre+/0 Rap1a f/+ Rap1bf/+♂ and Rap1af/f Rap1bf/f♀. Left: whole mount images, left. Right, (A-F): Tissue degeneration (top row) and hemorrhages on the right side of EC-Rap1 KO embryo (middle row), with vascular rapture in or around branchial arches (D-F, arrows) are likely cause of death. Comparable images of control (Tie2-Cre0; Rap1af/f, Rap1bf/f) embryo (G-I, bottom row).
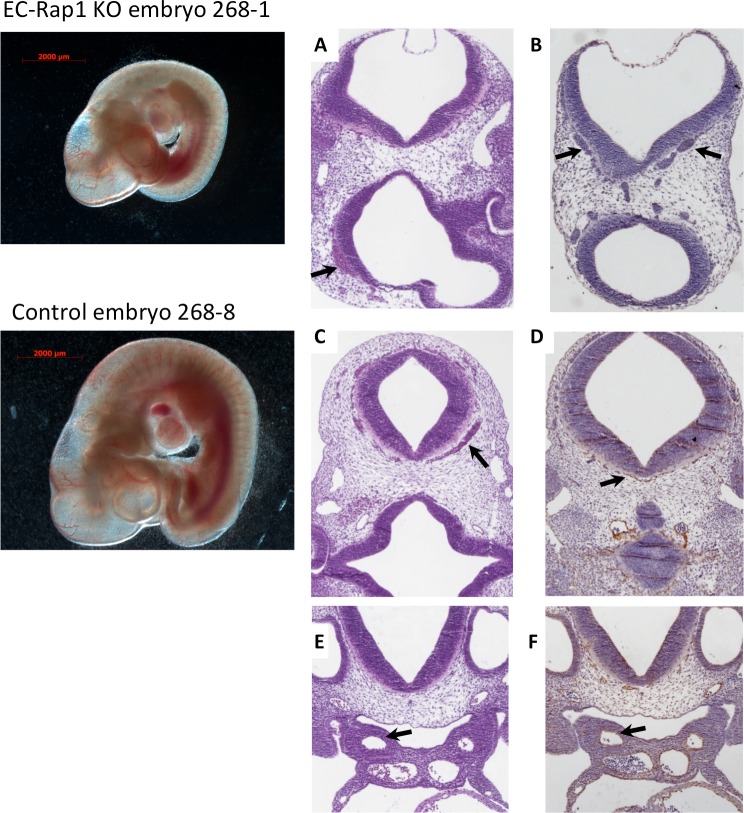
Fig 4. Normal branchial arches in EC-Rap1 KO embryos.
Left: whole mount images. Right: (A, B, top row): Dilated microvessels in E10.5 EC-Rap1 KO embryo heads near neural tube (arrows). C-F: Control (Tie2-Cre0; Rap1af/f, Rap1bf/f, E10.5) embryo sections. Blood-packed perineural vessels (C, D, arrows) are seen in fewer sections than in EC-Rap1KO embryos (B). (E, F, arrows) normal branchial arch arteries. Staining shown is H&E (A, C, E) and CD31 (B, D, F).
Fig 5. 50% of E10.5 EC-Rap1 KO embryos appear grossly normal.
Left: whole mount image. Right, A-D: normal perineural vasculature; C and D: enlargement of boxed areas in A and B; (C, arrow) blood-filled vessels; (D, arrow) ECs. E: 1st branchial arch artery, normal (arrow); F: larger 3rd branchial arch artery (arrow); G: aortae and cardinal veins with normal pericardial space at the level of atrium; (H, arrow) sinus venosus. Staining shown is H&E (A, C, F) and CD31 (B, D, E, G and H).
Fig 6. Normal vasculature in control, Tie2-Cre0/0; Rap1f/f E10.5 embryos.
Left: whole mount image. Right: A-D: Normal perineural vasculature; C and D: enlargement of boxed areas in A and B; (C, arrow) blood-filled vessels; (D, arrow) ECs. (E, arrow) 1st branchial arch artery, normal (arrow); (F, arrow): larger 3rd branchial arch artery; (G): aortae and cardinal veins at the level of arterio-venous canal of the heart, with normal pericardial space; H: venous entrance to the heart (arrow: sinus venosus). Staining shown is H&E (A, C, F and H) and CD31 (B, D, G and H).
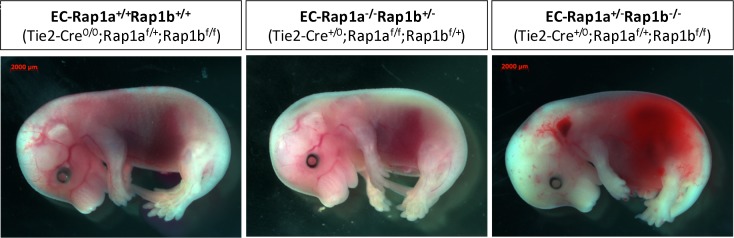
The analysis at E15.5 did not reveal any viable EC-Rap1 KO embryos (Table 4). Considering the vascular phenotype at E10.5 (Fig 3), death likely resulted from vascular defects. Furthermore, viability of Rap1 mutants containing only one of four Rap1 alleles was reduced (Table 4). We observed that about 30% of E15.5 EC-Rap1a +/- Rap1b -/- embryos displayed cranial hemorrhage, which contributed to increased lethality (45%) at weaning (Fig 7). No such hemorrhage was observed in EC-Rap1a -/- Rap1b +/- embryos. Considering that Rap1b is the major Rap1 isoform in ECs in mice (EC-Rap1a+/-Rap1b-/- contains less Rap1 protein than EC-Rap1a-/-Rap1b+/- mutant) [33], and that neither Rap1 isoform is absolutely required in endothelium for survival (Table 4), this suggests that it may be a quantitative effect and that a certain minimal level of Rap1 is critical for vessel development.
Fig 7. Vascular abnormalities in partial EC-Rap1 KO embryos.
Cranial hemorrhage is present in approximately 30% of EC-Rap1a+/-Rap1b-/- embryos (right panel) at E15.5 and contributes to increased lethality (44.7%) at weaning. No bleeding was observed in EC-Rap1a-/-Rap1b+/- embryos at E15.5 (center). Stereoscopic images are representative of n = 6 analyzed pregnancies resulting from Tie2-Cre+/0; Rap1af/+, Rap1bf/+ x Tie2-Cre0/0; Rap1af/f, Rap1bf/f crosses. Number of animals obtained (NO) and expected (NE), based on Mendelian distribution, as determined at weaning. Lethality was calculated using the following formula: (1-NO/NE)*100%.
Discussion
Rap1 is a positive regulator of adhesion and a critical regulator of polarity-dependent morphogenesis in lower organisms [16, 17, 19]. In this paper we demonstrate that Rap1 is essential for early morphogenesis in mice. Deletion of Rap1a and Rap1b separately has only a small effect on initial development and is not required for embryonic morphogenesis (Table 3). Global deletion of both Rap1 genes leads to major malformation and death before mid-gestation (E10.5). This phenotype is consistent with Rap1 playing a key role in adhesion and polarity in vivo also in higher organisms. Furthermore, we demonstrate that Rap1 in endothelium is critically required for vessel formation, as endothelial-specific deletion of both Rap1 isoforms leads to engorgement of perineural vessels, hemorrhage and embryonic lethality between E10.5 and E13.5. Therefore Rap1 is critical for both: tissue and vessel morphogenesis.
Earlier studies in lower organisms demonstrated the importance of the single Rap1 ortholog in tissue morphogenesis through its critical role in the regulation of polarity. Epithelial polarity, required for collective cell migration and tissue morphogenesis, is maintained by cell-cell junctions: tight junctions and cadherin-based adherens junctions, and, physically connected to them, polarized cytoskeleton networks [39]. Rap1 functional interaction with actin cytoskeleton linker Canoe/Afadin is essential to Drosophila morphogenesis [40]. Mechanistically, Rap1 localizes Afadin to cadherin in adherens junctions [19, 41]. Global deletion of Afadin leads to developmental defects during gastrulation and embryonic lethality after E9.5, with loss of structures derived from ectoderm and mesoderm and improper adherens junctions’ organization [42]. The severity of Afadin-/- phenotype exceeds that of Rap1 KO, suggesting the existence of other regulatory mechanisms in the absence of Rap1.
Vessel stability is critical for embryo growth and development. Stabilization of vessels to allow circulation and withstand shear stress forces coincides with dynamic regulation of growth and remodeling. Thus, vascular permeability is tightly regulated and changes in permeability lead to pathologies, including hemorrhage and edema [43]. Our studies show that Rap1 is a critical regulator of vascular stability. Endothelial KO of both Rap1 isoforms leads to localized hemorrhage and tissue degeneration in 50% of double EC-Rap1 KO at mid-gestation. Several Rap1 effectors including KRIT1, RASIP1 and Afadin have been implicated in regulation of vascular permeability by controlling cell-cell junctions. Endothelial deletion of Afadin leads to a defect in postnatal angiogenesis in vivo and reduced VEGFR2 signaling [44], similarly to what we reported in EC-Rap1 KO mice [33]. Reduced viability of EC-Afadin KO mice at weaning has been attributed to angiogenesis defect during development [44], however no specific developmental vascular phenotypes have been described. RASIP1, another proposed junctional effector of the EPAC-Rap1 signaling axis, has been implicated in vascular lumen formation [45–47] and more recently, in stabilization of cell-cell junctions required for formation of normal vasculature [48]. RASIP1-KO vessels initially form but are unable to sustain circulation beyond E10.5 in mice, leading to focal hemorrhage and lethality [48]. Similarity of RASIP1 and Afadin KO phenotypes to that of EC-Rap1 KO mice suggests that functional interaction of these molecules is important for murine vascular development. However, approximately half of EC-Rap1 KO embryos are able to form functional vessels and appear grossly normal at mid-gestation (Fig 5). This, again, suggests a regulatory rather than critical role of endothelial Rap1 in early vasculogenesis.
KRIT1 has been implicated as a cell-cell junction target of Rap1 required for vascular stability [6, 49]. Global deletion of KRIT1and CCM2 leads to early and severe vascular pathologies that result in embryo lethality mid-gestation. Vascular defects in KRIT1-null embryos include dilatation of brain vessels and a branchial arch formation defect [35, 50] and endothelial-specific deletion of KRIT1 leads to lethality before E12.5 due to failed vascular development [51]. Global or endothelial-specific CCM2 KO mice die of cardiovascular defects, such as: insufficient vascular lumen formation, defective arteriogenesis and heart malformation [36, 52]. All three CCM genes are essential for embryonic angiogenesis [14, 53, 54]. Interestingly, here we find that EC-Rap1 KO phenotype is distinct from that of KRIT1, as branchial arches form normally in these embryos (Fig 4). Therefore, Rap1 and CCM proteins may not act in the same signaling pathways during development. However, these interactions may still be pertinent in the regulation of cerebral vascular integrity; consequently, their disruption might result in postnatal development of brain hemorrhagic lesions [8, 55].
In addition to directly controlling the function of the above putative effectors, Rap1 may regulate vascular stability via additional mechanisms. We have recently shown that Rap1 is a critical regulator of mechanosensing complexes and shear stress-regulated vessel homeostasis in adult mice [34]. Interestingly, vascular defects observed in endothelial KRIT1-deficiency have also been associated with a defect in endothelial flow response [51]. It is possible that observed EC-Rap1 KO bleeding and embryo lethality might arise from physiologic/hemodynamic abnormality and defective mechanosensing functions, a hypothesis to address in future studies. In addition to the mechanosensing function in endothelium, Rap1b controls vascular tone and blood pressure by limiting smooth muscle contractility [32]. Interestingly, the phenotype of total Rap1b -/- mice: hypertension and cardiac hypertrophy, phenocopies that of EC-Rap1a+/-Rap1b-/- mice. This suggests that similar hemodynamic defects may contribute to the observed perinatal lethality of pups of the two genotypes.
This study has provided an insight into discrete and redundant functions of two Rap1 isoforms. During initial development, the functions of Rap1a and Rap1b are at least somewhat redundant, as development of the majority of embryos is supported upon single isoform deletion (Table 3) [22, 23]. However, later in development Rap1b -/-, but not Rap1a -/-, embryos develop hemorrhages (Fig 1) and Rap1a -/- embryos develop edema [23](and data not shown). This suggests that the Rap1b isoform may be a more critical regulator of vascular stability during development. While this bleeding phenotype is not observed in EC-Rap1b KO, additional deletion of one Rap1a allele results in a bleeding phenotype similar to that of global Rap1b KO (Fig 7). This suggests that Rap1 in other, non-endothelial cells is required for vessel homeostasis. Such a conclusion is supported by our findings of the importance of Rap1b in smooth muscle cells in maintenance of vascular tone [32]. Thus, additional studies of Rap1 in other vascular cells are required for full understanding of the role of this important molecule in vessel homeostasis.
Acknowledgments
We thank Ms. Anna Kraus, Ms. Jillian Dargatz and Dr. Sribalaji Lakshmikanthan for excellent technical assistance and Ms. Shana Maker for proof-reading the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by American Heart Association grant 0950118G (to M.C.-W.), the National Institutes of Health grants (HL111583 to M.C.-W. and NS075168 to K.J.W.) and a Biomedical Research Grant from Indiana University School of Medicine (to L.A.Q.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends Cell Biol. 2011;21(10):615–23. Epub 2011/08/09. 10.1016/j.tcb.2011.07.001 . [DOI] [PubMed] [Google Scholar]
- 2. Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120(Pt 1):17–22. Epub 2006/12/22. 10.1242/jcs.03306 . [DOI] [PubMed] [Google Scholar]
- 3. Carmena A. A big new job for small GTPases. Small GTPases. 2012;3(3):159–62. Epub 2012/06/01. 10.4161/sgtp.19631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA. Association of Krev-1/rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21-22. Oncogene. 1997;15(9):1043–9. Epub 1997/08/28. 10.1038/sj.onc.1201268 . [DOI] [PubMed] [Google Scholar]
- 5. Béraud-Dufour S, Gautier R, Albiges-Rizo C, Chardin P, Faurobert E. Krit 1 interactions with microtubules and membranes are regulated by Rap1 and integrin cytoplasmic domain associated protein-1. Febs J. 2007;274(21):5518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179(2):247–54. Epub 2007/10/24. 10.1083/jcb.200705175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X, Zhang R, Draheim KM, Liu W, Calderwood DA, Boggon TJ. Structural basis for small G protein effector interaction of ras-related protein 1 (Rap1) and adaptor protein krev interaction trapped 1 (KRIT1). Journal of Biological Chemistry. 2012;287(26):22317–27. 10.1074/jbc.M112.361295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chrzanowska-Wodnicka M. Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction. Exp Cell Res. 2013;319(15):2350–9. Epub 2013/08/06. 10.1016/j.yexcr.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laberge-le Couteulx S, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M, et al. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nature Genetics. 1999;23(2):189–93. . [DOI] [PubMed] [Google Scholar]
- 10. Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG, et al. Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1). Hum Mol Genet. 1999;8(12):2325–33. Epub 1999/11/05. . [DOI] [PubMed] [Google Scholar]
- 11. Liquori CL, Berg MJ, Siegel AM, Huang E, Zawistowski JS, Stoffer T, et al. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am J Hum Genet. 2003;73(6):1459–64. Epub 2003/11/19. 10.1086/380314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergametti F. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005;76:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zawistowski JS. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet. 2005;14:2521–31. [DOI] [PubMed] [Google Scholar]
- 14. Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15(2):169–76. Epub 2009/01/20. 10.1038/nm.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu JJ, Stockton RA, Gingras AR, Ablooglu AJ, Han J, Bobkov AA, et al. A mechanism of Rap1-induced stabilization of endothelial cell-cell junctions. Molecular Biology of the Cell. 2011;22(14):2509–19. 10.1091/mbc.E11-02-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71(1):48–96. Epub 2007/03/10. 10.1128/MMBR.00028-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asha H, De Ruiter ND, Wang MG, Hariharan IK. The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO Journal. 1999;18(3):605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295(5558):1285–8. Epub 2002/02/16. 10.1126/science.1067549 . [DOI] [PubMed] [Google Scholar]
- 19. Boettner B, Harjes P, Ishimaru S, Heke M, Fan HQ, Qin Y, et al. The AF-6 Homolog Canoe Acts as a Rap1 Effector During Dorsal Closure of the Drosophila Embryo. Genetics. 2003;165(1):159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56(1):77–84. Epub 1989/01/13. . [DOI] [PubMed] [Google Scholar]
- 21. Pizon V, Lerosey I., Chardin P., Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B). Nucleic Acids Res. 1988;16(15):7719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White Ii GC. Rap1b is required for normal platelet function and hemostasis in mice. Journal of Clinical Investigation. 2005;115(3):680–7. 10.1172/jci200522973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Yan JL, De P, Chang HC, Yamauchi A, Christopherson KW, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. Journal of Immunology. 2007;179(12):8322–31. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan BX, Vautier F, Ito W, Bolshakov VY, Morozov A. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci. 2008;28(9):2089–98. Epub 2008/02/29. 10.1523/JNEUROSCI.5156-07.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJT. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Molecular and Cellular Biology. 2006;26(2):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu H, Awasthi A, White GC 2nd, Chrzanowska-Wodnicka M, Malarkannan S. Rap1b regulates B cell development, homing, and T cell-dependent humoral immunity. J Immunol. 2008;181(5):3373–83. Epub 2008/08/21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Awasthi A, Samarakoon A, Chu H, Kamalakannan R, Quilliam LA, Chrzanowska-Wodnicka M, et al. Rap1b facilitates NK cell functions via IQGAP1-mediated signalosomes. The Journal of Experimental Medicine. 2010;207(9):1923–38. 10.1084/jem.20100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar S, Xu J, Kumar RS, Lakshmikanthan S, Kapur R, Kofron M, et al. The small GTPase Rap1b negatively regulates neutrophil chemotaxis and transcellular diapedesis by inhibiting Akt activation. Journal of Experimental Medicine. 2014;211(9):1741–58. 10.1084/jem.20131706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minato N, Kometani K, Hattori M. Regulation of immune responses and hematopoiesis by the Rap1 signal Advances in Immunology, Vol 93 Advances in Immunology 93. San Diego: Elsevier Academic Press Inc; 2007. p. 229–64. [DOI] [PubMed] [Google Scholar]
- 30. Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111(5):2647–56. Epub 2007/11/13. 10.1182/blood-2007-08-109710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol. 2008;28(18):5803–10. Epub 2008/07/16. 10.1128/MCB.00393-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, et al. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(7):1486–94. 10.1161/ATVBAHA.114.303678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, et al. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3). Blood. 2011;118(7):2015–26. Epub 2011/06/04. 10.1182/blood-2011-04-349282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lakshmikanthan S, Zheng X, Nishijima Y, Sobczak M, Szabo A, Vasquez-Vivar J, et al. Rap1 promotes endothelial mechanosensing complex formation, NO release and normal endothelial function. EMBO Rep. 2015;16(5):628–37. Epub 2015/03/27. 10.15252/embr.201439846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131(6):1437–48. [DOI] [PubMed] [Google Scholar]
- 36. Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nature Medicine. 2009;15(2):177–84. 10.1038/nm.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi W, Harris NJ, Sumigray KD, Peifer M. Rap1 and Canoe/afadin are essential for establishment of apical–basal polarity in the Drosophila embryo. Molecular Biology of the Cell. 2013;24(7):945–63. 10.1091/mbc.E12-10-0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai IC, Amack JD, Gao ZH, Band V, Yost HJ, Virshup DM. A Wnt-CKIÎμ-Rap1 Pathway Regulates Gastrulation by Modulating SIPA1L1, a Rap GTPase Activating Protein. Developmental Cell. 2007;12(3):335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9(9):1016–24. Epub 2007/09/01. 10.1038/ncb433 . [DOI] [PubMed] [Google Scholar]
- 40. Boettner B, Govek E-E, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. PNAS. 2000;97(16):9064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. Journal of Cell Biology. 2009;186(1):57–73. 10.1083/jcb.200904001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, et al. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146(5):1117–32. Epub 1999/09/09. 10.1083/jcb.146.5.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dejana E, Tournier-Lasserve E, Weinstein BM. The Control of Vascular Integrity by Endothelial Cell Junctions: Molecular Basis and Pathological Implications. Developmental Cell. 2009;16(2):209–21. 10.1016/j.devcel.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 44. Tawa H, Rikitake Y, Takahashi M, Amano H, Miyata M, Satomi-Kobayashi S, et al. Role of afadin in vascular endothelial growth factor- and sphingosine 1-phosphate-induced angiogenesis. Circ Res. 2010;106(11):1731–42. Epub 2010/04/24. 10.1161/CIRCRESAHA.110.216747 . [DOI] [PubMed] [Google Scholar]
- 45. Xu K, Chong DC, Rankin SA, Zorn AM, Cleaver O. Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Developmental Biology. 2009;329(2):269–79. 10.1016/j.ydbio.2009.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu K, Sacharidou A, Fu S, Chong D, Skaug B, Chen Z, et al. Blood Vessel Tubulogenesis Requires Rasip1 Regulation of GTPase Signaling. Developmental Cell. 2011;20(4):526–39. 10.1016/j.devcel.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitin NY, Ramocki MB, Zullo AJ, Der CJ, Konieczny SF, Taparowsky EJ. Identification and characterization of rain, a novel Ras-interacting protein with a unique subcellular localization. Journal of Biological Chemistry. 2004;279(21):22353–61. 10.1074/jbc.M312867200 [DOI] [PubMed] [Google Scholar]
- 48. Wilson CW, Parker LH, Hall CJ, Smyczek T, Mak J, Crow A, et al. RASIP1 regulates vertebrate vascular endothelial junction stability through EPAC1-RAP1 signaling. Blood. 2013;122(22):3678–90. 10.1182/blood-2013-02-483156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gore AV, Lampugnani MG, Dye L, Dejana E, Weinstein BM. Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. Dis Model Mech. 2008;1(4–5):275–81. 10.1242/dmm.000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA. Loss of p53 Sensitizes Mice with a Mutation in Ccm1 (KRIT1) to Development of Cerebral Vascular Malformations. Am J Pathol. 2004;165(5):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mleynek TM, Chan AC, Redd M, Gibson CC, Davis CT, Shi DS, et al. Lack of CCM1 induces hypersprouting and impairs response to flow. Human Molecular Genetics. 2014;23(23):6223–34. 10.1093/hmg/ddu342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cunningham K, Uchida Y, O'Donnell E, Claudio E, Li WL, Soneji K, et al. Conditional deletion of Ccm2 causes hemorrhage in the adult brain: a mouse model of human cerebral cavernous malformations. Human Molecular Genetics. 2011;20(16):3198–206. 10.1093/hmg/ddr225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boulday G, Blécon A, Petit N, Chareyre F, Garcia LA, Niwa-Kawakita M, et al. Tissue-specific conditional CCM2 knockout mice establish the essential role of endothelial CCM2 in angiogenesis: Implications for human cerebral cavernous malformations. DMM Disease Models and Mechanisms. 2009;2(3–4):168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. He Y, Zhang HF, Yu LY, Gunel M, Boggon TJ, Chen H, et al. Stabilization of VEGFR2 Signaling by Cerebral Cavernous Malformation 3 Is Critical for Vascular Development. Sci Signal. 3(116). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boulday G, Rudini N, Maddaluno L, Blécon A, Arnould M, Gaudric A, et al. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. Journal of Experimental Medicine. 2011;208(9):1835–47. 10.1084/jem.20110571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.