Abstract
Predicting the outcome of future climate change requires an understanding of how alterations in multiple environmental factors manifest in natural communities and affect ecosystem functioning. We conducted an in situ, fully factorial field manipulation of CO2 and temperature on a rocky shoreline in southeastern Alaska, USA. Warming strongly impacted functioning of tide pool systems within one month, with the rate of net community production (NCP) more than doubling in warmed pools under ambient CO2 levels relative to initial NCP values. However, in pools with added CO2, NCP was unaffected by warming. Productivity responses paralleled changes in the carbon-to-nitrogen ratio of a red alga, the most abundant primary producer species in the system, highlighting the direct link between physiology and ecosystem functioning. These observed changes in algal physiology and community productivity in response to our manipulations indicate the potential for natural systems to shift rapidly in response to changing climatic conditions and for multiple environmental factors to act antagonistically.
Introduction
Recent climatic changes have been small relative to those expected in the future [1] yet have altered biological systems worldwide [2]. However, despite the increasing confidence with which climate modelers are making prognoses for future climate change [1], ecologists lack crucial biological data necessary to forecast future impacts on the earth’s species. In marine systems, few studies have explored the impacts of alterations in multiple environmental factors on natural communities, and even fewer have assessed the consequences of such changes for rates of ecosystem functioning; we address both of these issues in this study.
Species can be affected by climate change both directly (e.g., if mortality is elevated under increasingly stressful climatic conditions) and/or indirectly via changes in the abundance or per capita effects of interacting species. A recent meta-analysis showed that marine species’ responses to acidification differed when they were measured in studies with single versus multiple species [3]. Furthermore, interacting species often respond differently when subjected to changes in multiple climate factors at the same time [4]. Temperature and pH can affect species interactively, either amplifying or reducing both positive and negative responses. The few previous studies of these multiple stressors in marine systems have, more often than not, demonstrated an interaction between increased temperature and increased CO2 / decreased pH [4], and interactive effects have been highly variable in both magnitude and direction [3]. Thus, in order to predict future impacts of climate change, we need to consider responses to multiple, interacting environmental factors, and how these responses manifest in situ, in the context of a natural community.
Field manipulations of multiple environmental factors have the potential to provide some of the best predictions of how climate change will impact coastal systems. Empirical, community-level field data are notably lacking in marine systems as compared to the more numerous results from terrestrial warming and FACE (Free-Air CO2 Enrichment) experiments [5]. The majority of marine climate-change studies have (i) used observational (rather than experimental) methods, (ii) examined a single environmental factor, and (iii) focused on a single species [6]. A more recent review of marine climate-change studies conducted between 2000 and 2009 showed that these same gaps remain [7]. Specifically, only 35% of studies reviewed included multiple climate variables, 19% quantified impacts at the community level, and 11% included some type of field assessment (transplant or space-for-time techniques were most common; none were replicated manipulative experiments of environmental factors). The few temperature manipulations in coastal systems have used passive warmers [8] or heated tiles [9]. CO2 has been manipulated in a few subtidal systems (e.g., [10]) and, as far as we know, only two intertidal communities [11,12]. Thus, whereas climate change has been identified as one of the primary drivers of global biodiversity loss [13], impacts on marine ecosystems remain poorly understood.
Here, we describe a fully factorial field experiment in which we manipulated environmental conditions by increasing CO2 and temperature in natural tide pool habitats in southeastern Alaska (Fig 1). We measured biological metrics from the organismal (i.e., seaweed nutrient ratios) to the ecosystem (i.e., net community production) level both before and after warming and CO2 addition to test the hypothesis that these climate factors would have individual and combined effects on the composition, diversity, and productivity of a natural marine community. We predicted that adding CO2 would enhance net community production (due to potential carbon limitation in tide pools) and that warming effects would be either positive (due to increased metabolic rates) or negative (due to increased thermal stress).
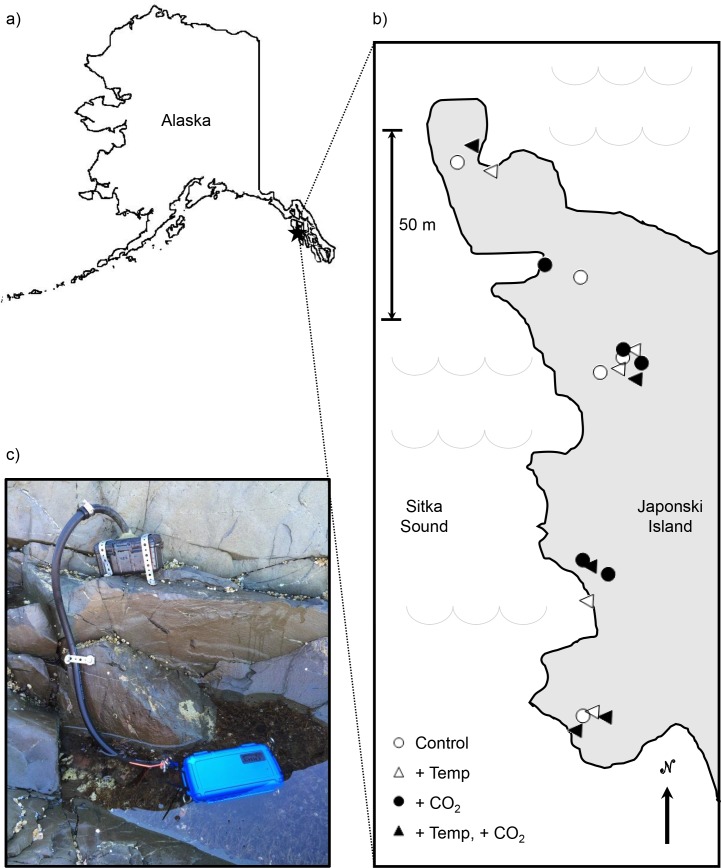
Fig 1. Field site and experimental design.
(a) Location of field site in Sitka, Alaska, USA (map redrawn from the USGS TNM 2.0 viewer [public domain]). (b) Tide pools (n = 20) were randomly assigned to treatment: control (open circle), +Temp (open triangle), +CO2 (closed circle), and both (closed triangle). (c) Representative tide pool. The submerged OtterBox® contained a warmer, and the OtterBox® outside the pool contained a yeast solution that generated CO2 which bubbled into the pool.
Materials and Methods
Our field site was John Brown’s Beach on Japonski Island, Sitka, Alaska, USA (57.06°N, 135.37°W; Fig 1A). Our research and collections at the site were conducted with the permission of the Alaska Department of Fish and Game (Permit CF-14-098). The site is characterized by a maximum tidal amplitude of 6.48 m. We identified 20 pools in the mid-to-high intertidal zone (Fig 1B). For each pool, we determined tide height (2.51 ± 0.05 m above mean lower low water; all values given as mean ± 1 SE), volume (11.31 ± 1.7 L), perimeter (2.43 ± 0.21 m), and maximum depth (12.3 ± 1.0 cm). Pools were randomly assigned to one of the four treatments (n = 5 per treatment): control (un-manipulated), +CO2 (CO2 added), +Temp (warmed), and both (CO2 added and warmed). Assignments were re-randomized until the following pool attributes did not differ significantly between treatments: volume, surface area, and initial values for sessile species cover, sessile species richness, and mobile species richness (for all, ANOVA p > 0.1).
Tide pool temperatures and CO2 levels were manipulated between 17 July and 01 August 2014. To simulate a climate-related temperature increase during the most stressful part of the tidal cycle–the midday low tide–when pools are naturally warmest, tide pool temperature was manipulated using rechargeable hand warmers (EnerHandz®) packaged in waterproof OtterBoxes® and attached to the bottom of the pools. Unwarmed pools contained empty OtterBoxes® to control for potential effects of shading or disturbance. Warmers were replaced daily, and pool temperatures were recorded every 10 min by TidbiT® data loggers (Onset®, Bourne, Massachusetts, USA). CO2 was delivered to each elevated CO2 pool via tubing from a yeast reactor [14]: a watertight plastic box (Drybox 2500, OtterBox, Fort Collins, Colorado, USA) containing 500 mL of water, 75 g of sugar, 2 g of yeast, and 2 g of NaHCO3 to buffer the internal pH of the reactor (Fig 1C). CO2 is the most abundant gas produced during fermentation of baker’s yeast and is produced by this mixture at a rate of approx. 140 ml CO2 per min [15]. The reactor solutions were replaced every 3–4 days, and tide pool pH was measured daily (while gently stirring the pool) with a handheld pH meter (pH10A, YSI, Yellow Springs, Ohio, USA). Measurements of pH are presented on the total hydrogen ion concentration scale after cross-calibration with buffers prepared according to Dickson [16].
Both before and after the experimental manipulations, we conducted community surveys, measured rates of productivity, and collected samples of Odonthalia floccosa (Esper) Falkenberg (hereafter, Odonthalia), the most common seaweed species (covering 48.27 ± 0.06% of available space, Fig 2) and the only seaweed species present in all pools. Algal samples (one thallus per pool) were washed in deionized water, blotted dry, and stored at -25°C. For analyses of internal C:N, samples were dried to constant mass (60°C for 72 h), ground to a fine powder (Retsch Mixer Mill MM 400, Verder Scientific, Newtown, Pennsylvania, USA) and analyzed on a Flash 2000 Elemental Analyzer (Thermo Fisher, Cambridge, UK).
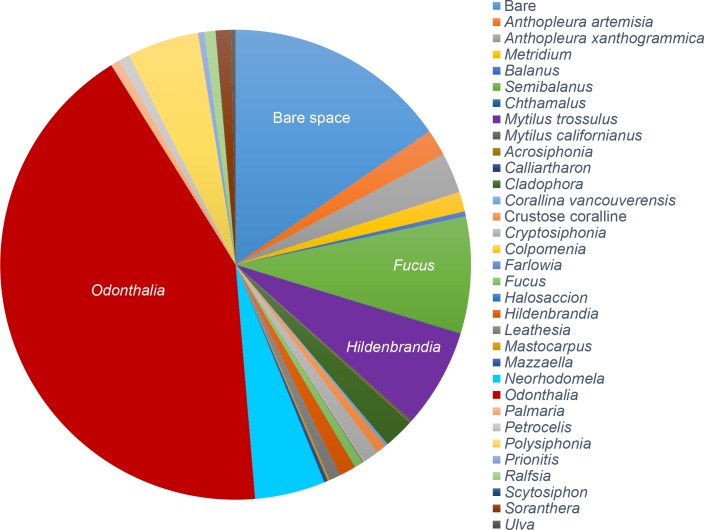
Fig 2. Relative cover of sessile species in experimental tide pools.
The red alga Odonthalia floccosa was the most abundant species, covering 48.27% (± 0.06 SE) of available space. 17.49% (± 0.04 SE) of space was bare. Values are mean percent cover across the n = 20 pools.
On 10–12 July 2014 and 02 August 2014, we quantified percent cover of sessile species (macroalgae and sessile invertebrates) and counted mobile invertebrates in emptied pools by laying a flexible mesh quadrat across the bottom surface area. Water was retained in an adjacent bucket and replaced within approx. 10 min to limit stress to tide pool organisms. We measured net community production on 15 and 31 July 2014 by quantifying the change in O2 concentrations (using a Professional Plus Multiparameter Meter, YSI, Yellow Springs, Ohio, USA) in the pools in the light according to published methods [17]. To ensure that initial oxygen concentrations were low, we took our first sample after pools were covered with dark plastic for ~30 min. We took a second sample after ~30 min of exposure to daylight at saturating irradiance levels (703 ± 115 [mean ± SE] μmol photons m-2 s-1). Pools were gently stirred with the multimeter probe prior to O2 measurements to prevent stratification. Net community production rates (NCP) were calculated as follows:
| (1) |
where Δ[O 2] is the change in the O2 concentration (mg O2 L-1) and Δt is the change in time.
To compare temperatures in experimentally warmed tide pools with those in un-warmed, ambient pools, we determined the 90th percentile of temperatures recorded by our TidbiT® data loggers each day in each tide pool. We calculated the difference between the average temperatures of ambient and warmed pools on each day of our experiment and used linear regression (PROC GLM in SAS v. 9.4, Cary, North Carolina, USA) to examine this difference as a function of the 90th percentile of air temperatures for each day (recorded by the weather station at the Sitka Airport, < 1 km from our study site). To compare pH in our ambient and CO2 addition pools, we used a two-sample t-test of the mean daily pH values recorded in every pool 1.4 ± 0.3 h before the daytime low tide. Note that because CO2 was bubbled continuously into pools, differences between ambient and CO2 addition pools were likely to increase over the course of a low tide, with maximum differences immediately prior to re-submersion by the incoming tide.
We used general linear models (ANOVA, repeated measures ANOVA) to evaluate effects of our experimental treatments on NCP, C:N, and Odonthalia cover. Other analyses included t-tests to evaluate temperature and pH treatments. Data were evaluated for normality (Shapiro-Wilk test) and homogeneity of variances (Levene’s test), and no transformations were necessary. We calculated the percentage change in NCP between the initial (pre-manipulation) and final (post-manipulation) values as a function of temperature (ambient vs. warmed), CO2 (ambient vs. added), and the interaction between temperature and CO2 (temperature × CO2). Similarly, we evaluated the change in the internal C:N ratio of the seaweed Odonthalia during our experiment as a function of temperature, CO2, and temperature × CO2. Effects of experimental treatments on community composition were evaluated using PERMANOVA (PRIMER v. 6.1.13 & PERMANOVA + v. 1.0.3, PRIMER-E, Ltd., Ivybridge, UK), and effects on diversity (species richness, Shannon diversity, and evenness as Pielou’s J) were examined using general linear models (ANOVA).
Results
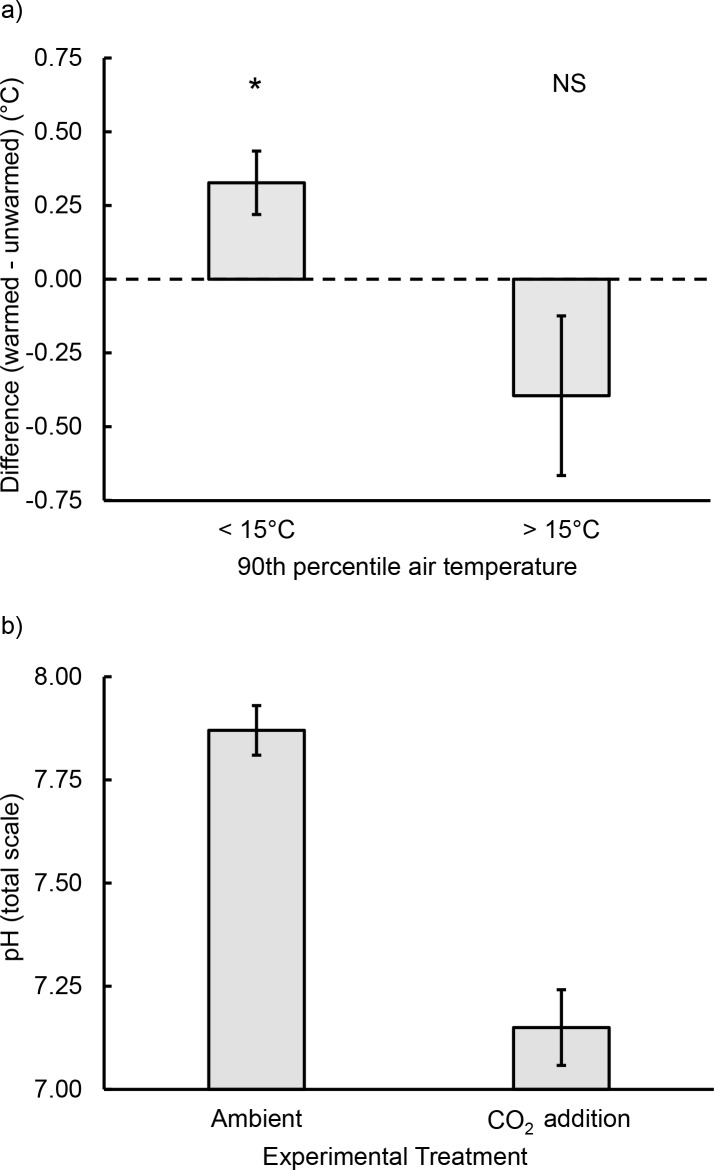
The effects of experimental warming depended on air temperature (F1,13 = 8.5, p = 0.012; Fig 3A). On cooler days, when the 90th percentile of air temperatures was ≤ 15°C (the median temperature during our experiment), the 90th percentile of temperatures in warmed pools averaged 0.33 (± 0.11°C higher than in ambient pools (t = 3.1, df = 6, p = 0.023). On warmer days, when temperatures were ≥ 15°C, there was no effect of warming on tide pool temperatures (t = 1.5, df = 7, p = 0.188). Effects of warming on the 90th percentile of water temperatures therefore differed with time (repeated measures ANOVA, within-subject effect: F14,224 = 2.6, p = 0.002). However, the effect of temperature did not differ between pools with and without added CO2, either overall (between-subjects effect: F1,16 = 0.8, p = 0.387) or over time (within-subjects effect: F14,224 = 0.1, p = 0.999). Furthermore, daily temperatures did not differ between warmed pools with and without CO2 added (p > 0.15 on all days after Tukey adjustment). CO2 addition caused pH levels to decrease by approx. 0.7 units in CO2 addition pools (7.19 ± 0.09) relative to ambient pools (7.88 ± 0.06) (t = 6.4, df = 18, p < 0.001; Fig 3B). This difference is similar to the change in pH observed in one un-manipulated tide pool during a single daytime low-tide, where pH increased from 7.51 to 8.15 due to passive warming and photosynthetic draw-down of CO2. pH levels varied by more than 3 units in un-manipulated pools based on daily measurements during our experiment.
Fig 3. Effects of experimental warming and CO2 addition on tide pools.
(a) Effects of warming on tide pool temperatures depended on air temperature. Experimental warming increased pool temperatures on cooler days (≤ 15°C) but not when air temperatures were ≥ 15°C. Values are the 90th percentiles of air temperatures (x-axis) and difference between 90th percentile temperatures in warmed vs. ambient pools (y-axis) on each day. Statistical significance is indicated: p < 0.05 (*), p > 0.05 (NS). (b) Compared to pools at ambient CO2 levels, experimental manipulations led to decreases in average pH (total hydrogen ion scale). Values are means ± SE.
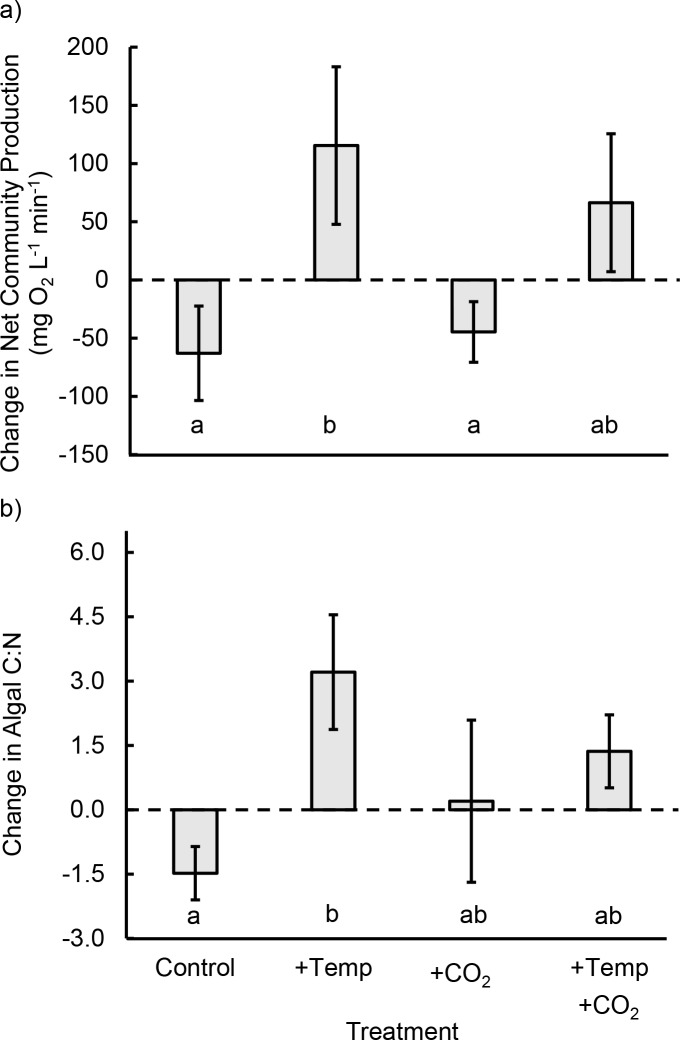
We found a strong effect of experimental warming on NCP (F1,16 = 8.0, p = 0.012), but no effect of CO2 addition (F1,16 = 0.1, p = 0.767) or temperature × CO2 interaction (F1,16 = 0.4, p = 0.518; Fig 4A). Initially, NCP did not differ significantly between assigned treatments. After the 16-day manipulation, the percentage change in NCP was higher in the warmed treatment than in the control under ambient CO2 conditions (p = 0.025 after Tukey adjustment for multiple comparisons), but warming did not affect NCP when CO2 was added (p = 0.144 after Tukey adjustment). At the end of our 16-d experiment, tide pools where CO2 was added had higher O2 concentrations than pools with no CO2 added (F1,16 = 4.73, p = 0.045); however, our NCP results did not change when we incorporated O2 concentrations as a covariate.
Fig 4. Effects of warming and CO2 addition on net community production (NCP) and algal C:N.
(a) At ambient CO2 levels, NCP increased in warmed pools. However, when CO2 was added, warming did not affect NCP. Values are the percentage change between initial (pre-manipulation) and final (post-manipulation) NCP measurements. (b) At ambient CO2 levels, C:N of the seaweed Odonthalia increased in warmed pools. However, when CO2 was added, warming did not affect C:N. Values are means ± SE. Letters indicate statistically significant differences after Tukey adjustment for multiple comparisons.
These productivity responses paralleled changes in the physiology of the seaweed Odonthalia (Fig 4B). Warming resulted in an increase in the carbon-to-nitrogen ratio (C:N) over the course of our experiment (F1,16 = 5.3, p = 0.035) due to a tendency toward both increases in %C and declines in %N in Odonthalia collected from warmed pools. There was no effect of CO2 on C:N (F1,16 < 0.1, p = 0.949), and the effect of temperature on C:N did not change when CO2 was added (‘temperature × CO2’ interaction; F1,16 = 1.9, p = 0.185). The main effect of temperature was driven entirely by a strong effect of warming on C:N under ambient CO2 conditions (p = 0.019 after Tukey adjustment) and not by an effect of warming on C:N when CO2 was added to pools (p = 0.527 after Tukey adjustment). Thus, as with the NCP results (Fig 3A), there was no effect of temperature on C:N when CO2 was added. On average, Odonthalia cover in tide pools did not change during our experiment, either overall (t = 1.3, df = 19, p = 0.218) or in response to our experimental manipulations (p > 0.30 for all factors [i.e., temperature, CO2, temperature × CO2, irradiance, and pool volume]). Furthermore, the strong effect of warming on C:N (F1,17 = 6.3, p = 0.023) remained after accounting for variation between pools in Odonthalia growth (i.e., change in percent cover; F1,17 = 4.2, p = 0.056).
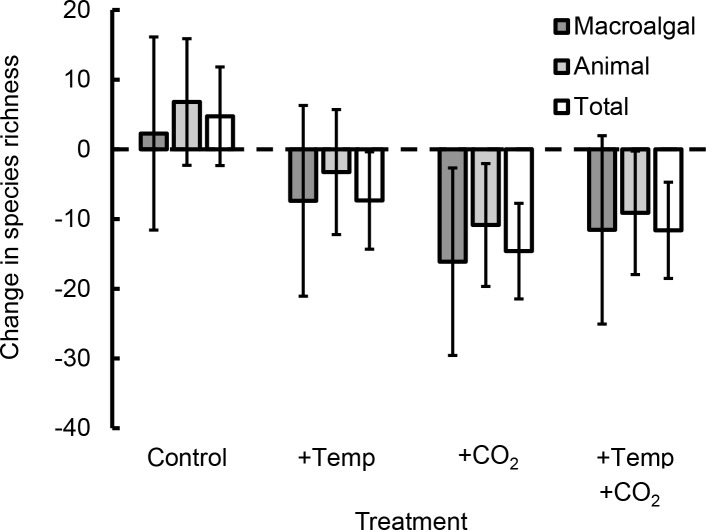
There was a tendency toward declines in species richness in +CO2 treatments (Fig 5). However, we did not detect any significant responses to our manipulations in any of the community-level metrics, including community composition (PERMANOVA, p > 0.1 for all factors [i.e., temperature, CO2, and temperature × CO2] and comparisons [i.e., sessile species, mobile species, and algal species]), or richness, evenness, or Shannon diversity (p > 0.1 for all factors and comparisons).
Fig 5. Percentage change in animal, macroalgal, and total species richness in response to experimental treatments.
Whereas there was a tendency toward declines in species richness in +CO2 treatments, species richness did not respond significantly to our manipulations (p > 0.10 in all cases). Values are least-squares means (± SE) after accounting for tide pool surface area to volume ratio.
Discussion
Based on our manipulation of a natural tide pool system, we found that effects of short-term warming and CO2 addition occurred in less than a month at the organismal (algal C:N) and ecosystem (NCP) levels. These responses were related, with both algal C:N and NCP affected by warming but not by addition of CO2. A strong response to warming but not to CO2 addition is somewhat counterintuitive given that our warming treatments were conservative relative to predicted temperature increases at high latitudes while our CO2 additions resulted in a decline in average pH substantially greater than that predicted by the year 2100 [2]. These results also differ from our initial prediction that adding CO2 would enhance NCP. The organismal and ecosystem-level impacts of warming and CO2 addition likely share a common origin in the physiological responses of individual species to the experimental treatments, which translated directly to effects on NCP. When there is a direct link between organismal physiology and ecosystem function [18], very rapid responses of the system to climatic changes can occur.
At the organismal level, we found that C:N of the most abundant and widespread seaweed species in our experimental tide pools, the red alga Odonthalia, responded to experimental warming. These physiological changes occurred during a low growth period for Odonthalia. Odonthalia growth typically peaks in the winter and spring, with reproduction occurring in late spring and early summer and individuals then senescing through the summer and fall [19]. Our results were consistent with this seasonality: Odonthalia cover did not change during our experiment, suggesting that it was transitioning toward senescence. Furthermore, we found a strong effect of warming on C:N after accounting for change in Odonthalia cover, suggesting that this effect was growth-independent.
We can, thus, envision two mechanisms to explain warming-driven increases in algal C:N: loss of proteins and/or increases in carbon accumulation. Warming may have accelerated senescence, which in red algae is associated with a loss of soluble proteins and a corresponding increase in C:N [20]. Higher C:N could also be due to higher rates of carbon accumulation associated with increased NCP in warmed pools. Moderate levels of warming often enhance macroalgal photosynthetic rates (e.g., [21,22]), and an increase in NCP combined with no growth would lead to accumulation of internal C relative to N. In addition to seasonal changes, Odonthalia growth also varies with nutrient availability. Algal C:N averaged 13.6 during our experiment, a value that exceeded the threshold of ~10 for nitrogen limitation in red algae [23] and was much higher than the ratio of ~7.5 measured when Odonthalia were actively growing in the spring [24].
The increase in NCP in warmed pools was not evident when CO2 was added, suggesting that CO2 addition depressed the warming-related increase in NCP. Potential negative effects of increased CO2 concentrations on seaweeds include reduced C affinity, lower HCO3 - utilization, reduced Rubisco content, and decreased pigment concentrations [25,26], all of which could contribute to decreased NCP. Furthermore, these negative effects of CO2 addition can interact with temperature. In an indoor mesocosm experiment, Olabarria et al. [27] found that whereas NCP was typically higher in warmed mesocosms, the warming effect was more pronounced under ambient CO2 conditions and was lower when CO2 was elevated. Similarly, C:N was not increased by warming when CO2 was added to pools, suggesting that CO2 addition limited the effect of warming on C:N. This may reflect a negative effect of CO2 addition on NCP [25,26], described above, which could reduce rates of carbon accumulation.
Although we detected responses only at the organismal and ecosystem levels, there are several reasons to expect that altering multiple environmental factors could impact community structure (e.g., diversity) and dynamics (e.g., consumption rates) over longer time scales than considered in this study. First, changes in C:N of basal species can affect community dynamics and ecosystem functioning [28]. This represents a mechanism by which organism-level responses to climate change could scale up to successively affect populations, communities, and ecosystems. Second, previous studies suggest that in a longer-term experiment we might expect to see effects of warming and/or CO2 addition on rates of herbivory [29,30], predation [31,32], or competition [33,34] or on biodiversity [12,35]. These community-level phenomena have the potential to influence ecosystem-level processes. For example, changes in the diversity of seaweeds on rocky shores can directly affect NCP [36]. The importance of these community-mediated effects, relative to the organism-mediated ones we report here, remains unknown, especially in the field.
Given anticipated future changes in both temperature and CO2 levels, it is essential to understand not only the independent effects of these factors, but also the potential interactions between them. Factorial manipulations of CO2 and temperature in field settings have become an important tool for understanding the effects of climate change on terrestrial communities and ecosystems (e.g., [5]). However, similar factorial manipulations in marine systems have been limited to laboratory and mesocosm studies [27,30,35]. Here, we demonstrate that it is possible to manipulate both temperature and CO2 under field conditions and reveal important, interactive effects on both organismal and ecosystem-level processes (e.g., [37]). Further fine-tuning of both CO2 delivery and warmers will allow us to match manipulations to regional predictions of future pH and warming. We show that such manipulations are possible, and biological responses are measurable, within the context of a highly dynamic natural system.
Supporting Information
(XLSX)
Acknowledgments
We thank L. Tang for capable and cheerful field assistance; G. Bernatchez for help with sample processing and data analyses; and family, friends, and members of the public who joined us in the field. We appreciate the Sitka Sound Science Center hosting us as part of the Scientist-in-Residency Fellowship Program (funded by NSF award PLR 1023704 to J. Straley) and the University of Alaska Southeast for providing office space. We thank the anonymous reviewers for helpful comments that improved the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by the National Science Foundation, Office of Polar Programs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. IPCC (2013) Climate change 2013: the physical science basis Cambridge University Press, Cambridge, UK. [Google Scholar]
- 2. IPCC (2014) Climate change 2013: impacts, adaptation, and vulnerability Cambridge University Press, Cambridge, UK. [Google Scholar]
- 3. Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, et al. (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315. 10.1111/j.1461-0248.2008.01253.x [DOI] [PubMed] [Google Scholar]
- 5. Dieleman WIJ, Vicca S, Dijkstra FA, Hagedorn F, Hovenden MJ, Larsen KS, et al. (2012) Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob Change Biol 18:2681–2693. [DOI] [PubMed] [Google Scholar]
- 6. Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, et al. (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241. [DOI] [PubMed] [Google Scholar]
- 7. Wernberg T, Smale DA, Thomsen MS (2012) A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob Change Biol 18:1491–1498. [Google Scholar]
- 8. Gedan KB, Bertness MD (2009) Experimental warming causes rapid loss of plant diversity in New England salt marshes. Ecol Lett 12:842–848. 10.1111/j.1461-0248.2009.01337.x [DOI] [PubMed] [Google Scholar]
- 9. Smale DA, Wernberg T, Peck LS, Barnes DKA (2011) Turning on the heat: ecological response to simulated warming in the sea. PLoS ONE 6:e16050 10.1371/journal.pone.0016050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kline DI, Teneva L, Schneider K, Miard T, Chai A, Marker M, et al. (2012) A short-term in situ CO2 enrichment experiment on Heron Island (GBR). Scientific Reports 2:413 10.1038/srep00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langley JA, Megonigal JP (2010) Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 466:96–99. 10.1038/nature09176 [DOI] [PubMed] [Google Scholar]
- 12.Gillis BC (2014) The effects of increased oceanic CO2 on tide pool communities. M.S. Thesis. Northeastern University, USA.
- 13. Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen O, Andersen T, Christensen C (2007) CO2 in planted aquaria. Aquat Gardener 20:24–33. [Google Scholar]
- 15. Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, et al. (2010) Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae . Malar J 9:10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickson AG (1993) pH buffers for sea water media based on the total hydrogen ion concentration scale. Deep Sea Res I 40:107–118. [Google Scholar]
- 17. Noël LM-L, Griffin JN, Thompson RC, Hawkins SJ, Burrows MT, Crowe TP, et al. (2010) Assessment of a field incubation method estimating primary productivity in rockpool communities. Estuar Coast Shelf Sci 88:153–159. [Google Scholar]
- 18. Shachak M, Jones CG (1995) Ecological flow chains and ecological systems: concepts for linking species and ecosystem perspectives In: Jones CG, Lawton JH (eds) Linking species and ecosystems. Chapman and Hall, New York, USA, pp. 280–294. [Google Scholar]
- 19. Ruesink JL (1998) Diatom epiphytes on Odonthalia floccosa: the importance of extent and timing. J Phycol 34:29–38. [Google Scholar]
- 20. Hernández I, Corzo A, Gordillo FJ, Robles MD, Saez E, Fernández JA, et al. (1993) Seasonal cycle of the gametophytic form of Porphyra umbilicalis: nitrogen and carbon. Mar Ecol Prog Ser 99:301–311. [Google Scholar]
- 21. Davison IR, Greene RM, Podolak EJ (1991) Temperature acclimation of respiration and photosynthesis in the brown alga Laminaria saccharina . Mar Biol 110:449–454. [Google Scholar]
- 22. Colvard NB, Carrington E, Helmuth B (2014) Temperature-dependent photosynthesis in the intertidal alga Fucus gardneri and sensitivity to ongoing climate change. J Exper Mar Biol Ecol 458:6–12. [Google Scholar]
- 23. D'Elia CF, DeBoer JA (1978) Nutritional studies of two red algae. II. Kinetics of ammonium and nitrate uptake. J Phycol 14:266–272. [Google Scholar]
- 24. Bracken MES (2004) Invertebrate-mediated nutrient loading increases growth of an intertidal macroalga. J Phycol 40:1032–1041. [Google Scholar]
- 25. Johnston AM, Raven JA (1990) Effects of culture in high CO2 on the photosynthetic physiology of Fucus serratus . Brit Phycol J 25:75–82. [Google Scholar]
- 26. Andría J, Vergara J, Perez-Llorens JL (1999) Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cádiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur J Phycol 34:497–504. [Google Scholar]
- 27. Olabarria C, Arenas F, Viejo RM, Gestoso I, Vaz-Pinto F, Incera M, et al. (2013) Response of macroalgal assemblages from rockpools to climate change: effects of persistent increase in temperature and CO2 . Oikos 122:1065–1079. [Google Scholar]
- 28. Bracken MES, Hillebrand H, Borer ET, Seabloom EW, Cebrian J, Cleland EE, et al. (2015) Signatures of nutrient limitation and co-limitation: responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos 124:113–121. [Google Scholar]
- 29. O'Connor MI (2009) Warming strengthens an herbivore-plant interaction. Ecology 90:388–398. [DOI] [PubMed] [Google Scholar]
- 30. Alsterberg C, Eklöf JS, Gamfeldt L, Havenhand JN, Sundbäck K (2013) Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc Natl Acad Sci USA 110:8603–8608. 10.1073/pnas.1303797110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrari MCO, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt Ö, et al. (2011) Putting prey and predator into the CO2 equation–qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol Lett 14:1143–1148. 10.1111/j.1461-0248.2011.01683.x [DOI] [PubMed] [Google Scholar]
- 32. Kordas RL, Harley CDG, O'Connor MI (2011) Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J Exper Mar Biol Ecol 400:218–226. [Google Scholar]
- 33. Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony KRN (2011) High CO2 enhances the competitive strength of seaweeds over corals. Ecol Lett 14:156–162. 10.1111/j.1461-0248.2010.01565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorte CJB, White JW (2013) Competitive and demographic leverage points of community shifts under climate warming. Proc Roy Soc B 280:20130572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hale R, Calosi P, McNeill L, Mieszkowska N, Widdicombe S (2011) Predicted levels of future ocean acidification and temperature rise could alter community structure and biodiversity in marine benthic communities. Oikos 120:661–674. [Google Scholar]
- 36. Bracken MES, Williams SL (2013) Realistic changes in seaweed biodiversity affect multiple ecosystem functions on a rocky shore. Ecology 94:1944–1954. [DOI] [PubMed] [Google Scholar]
- 37. Pfister CA, Esbaugh AJ, Frieder CA, Haumann H, Bockmon EE, White MM, et al. (2014) Detecting the unexpected: a research framework for ocean acidification. Environ Sci Technol 48:9982–9994. 10.1021/es501936p [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.