Abstract
Drug-induced liver injury (DILI) remains a significant clinical challenge and is the leading cause of acute liver failure in most countries. An aging population that uses more medications, a constant influx of newly developed drugs and a growing risk from unfamiliar herbal and dietary supplements will make DILI an increasing part of clinical practice. Currently, the most effective strategy for disease management is rapid identification, withholding the inciting agents, supportive care and having a firm understanding of the expected natural history. There are resources available to aid the clinician, including a new online “textbook” as well as causality assessment tools, but a heightened awareness of risk and the disease’s varying phenotypes and good history-taking remain cornerstones to diagnosis. Looking ahead, growing registries of cases, pharmacoepidemiology studies and translational research into the mechanisms of injury may produce better diagnostic tools, markers for risk and disease, and prevention and therapeutics.
Keywords: Drug induced liver injury, Hepatotoxicity, Diagnosis, Epidemiology, Herbal and dietary supplements
INTRODUCTION
Among Western countries, drug-induced liver injury (DILI) remains the leading cause of acute liver failure (ALF).1 Thus DILI has profound implications on healthcare, affecting patient morbidity and mortality, healthcare expenditures, drug development, and clinical practice. DILI remains one of the top reasons for drug withdrawal from the marketplace2 creating cost and medication availability ramifications that reach well beyond the individual having liver injury. The primary challenge in DILI care and research continues to be the rare, idiosyncratic and varied nature of disease presentation leading to difficult recognition and accrual of cases for study. This review will cover incidence, risk factors, clinical presentations and diagnostic strategies, and highlight some common culprit agents. Possible clinical advancements arising from current research will also be covered. Nonidiosyncratic liver injury such as that seen with acetaminophen will not be covered.
INCIDENCE
DILI incidence is difficult to determine but is commonly reported to be 1 in 10,000 to 1 in 100,000.3–5 Such estimates are undermined by under-reporting and varying diagnostic accuracy of cases. Additionally, clinical trials are typically underpowered to detect small incidences of toxicity.6 However, recent population based studies are shedding better light on the true incidence. A study from Iceland7 suggested an overall incidence of 19 per 100,000 and a much higher risk than expected for certain medications (e.g., 1 in 133 for azathioprine). This study was unique for its completeness of prescription data and vetting of DILI cases, and represented a rise in incidence compared to prior population based studies.4 Moreover incidence increased with age (Table 1), suggesting DILI incidence will rise in countries with aging populations.
Table 1.
Crude Annual Incidence of Drug Induced Liver Injury and Mean Prescription Rate in Iceland
| DILI per 100,000 | Mean prescription rate | |
|---|---|---|
| Overall | 19.1 | NA |
| Age, yr | ||
| 15–24 | 8.5 | 0.9 |
| 25–39 | 12.6 | 1.2 |
| 40–59 | 18.8 | 2.4 |
| 60–69 | 32.6 | 4.8 |
| 70–79 | 39.9 | 7.3 |
| 80–106 | 41.0 | 9.3 |
DILI, drug induced liver injury; NA, not available.
Adapted from Björnsson ES, et al. Gastroenterology 2013;144:1419–1425.e3, with permission from Elsevier.7
RISK FACTORS
At this time, there are only a few clinically useful risk factors. As mentioned, age increases the overall risk in part due to polypharmacy. A few individual medications, such as isoniazid (INH), flucloxacillin, halothane, amoxicillin-clavulanate, and nitrofurantoin carry increased risk with older age.8–12 There are some trends in the patterns of injury, with hepatocellular as well as autoimmune like injury being more common in females.3,13,14 With respect to patients with chronic liver disease (CLD), it is hypothesized that this population would be at higher risk, given the inherent altered pharmacokinetics of medications metabolized by the liver.15 However, the data regarding this population is mixed and hindered by the vagaries of diagnosing DILI in patients with inherent competing causes for elevated liver biochemistries (e.g., flares in autoimmune, viral, or alcoholic hepatitis).16–18
While not yet clinically useful, other recently identified risk factors are beginning to elucidate the pathophysiology of DILI. Several human leukocyte antigen (HLA) serotypes have been identified as risk factors for DILI from specific agents (e.g., amoxicillin/clavulanate and flucloxacillin), suggesting an important immunologic component to DILI. Interestingly, drugs whose daily dose is 50 mg or more account for over 70% to 80% of cases and there are several medications reported to cause DILI after a dosing increase. These data suggest idiosyncratic DILI may have a dose dependent component similar to acetaminophen thus blurring the lines between nonidiosyncratic and idiosyncratic.19,20 Also lipophilicity has been shown to be a strong predictor of hepatotoxicity. In fact, when drugs have both a dosage over 100 mg per day as well as lipophilicity, there is a marked increased risk of toxicity, as shown by Chen et al.21
PRESENTATION, DIAGNOSIS AND OUTCOME
Clinical presentation will usually consist of nonspecific (nausea, fatigue, and abdominal pain), and occasionally liver specific symptoms (jaundice, pruritus, encephalopathy, and ascites) in severe cases. Occasionally, rash, eosinophilia or a drug related eosinophilic systemic syndrome (DRESS) presentation will make drug hepatotoxicity obvious, but typically symptoms will not point specifically to DILI. Fever may be present with DILI as well as other competing diagnoses (gallstone disease and viral infection). Therefore, diagnosis is heavily reliant on obtaining a detailed history and careful selection of diagnostic tests.
In the history, it is crucial to obtain the timing of exposure as precisely as possible, the biochemical pattern of injury, and a complete medication history, including prescribed, over the counter, and herbal or dietary supplements (HDS). Latencies for certain medications can be long, so patients will often not remember taking an agent let alone when they took it. Calling the patient’s pharmacy, interviewing family members and using memory cues can be quite helpful. Many times cases can be diagnosed by simply obtaining or retaking a meticulous history.
The pattern of injury is often grouped by the R-ratio at presentation:22
R-ratios greater than 5 are considered hepatocellular injuries, 2 to 5 mixed, and less than 2 cholestatic. While these cutoffs were set arbitrarily, they have proven useful clinically and in drug development. Determining the R-ratio can help decide the likelihood of DILI because agents will often have a propensity toward certain patterns of injury (Table 2). However, it is important to note that the pattern of injury can change over time, particularly from hepatocellular to cholestatic.23 Therefore, patients presenting late, may have moved through an undetected hepatocellular injury. Also, the calculated pattern of injury does not always match the histologic picture.24 The pattern of injury will also help narrow the list of competing diagnoses that need to be considered (e.g., cholestatic injury and gallstone disease, hepatocellular injury and viral infection).
Table 2.
Common Medications Implicated in Drug Induced Liver Injury with Timing and Pattern of Injury
| Drug | Latency | Pattern of injury |
|---|---|---|
| Antimicrobials | ||
| Isoniazid | 1–6 mo | Hepatocellular, resembling viral hepatitis |
| Rifampin | 0–3 mo | Hepatocellular, sometimes mixed |
| Pyrazinamide | 1–3 mo | Hepatocellular, resembling viral hepatitis |
| Amoxicillin-clavulanate | 1–4 wk | Cholestatic, more hepatocellular in children |
| Sulfonamides | 1–6 wk | Cholestatic, sometimes mixed; immunoallergic |
| Nitrofurantoin, minocycline | 1 wk to months or years | Both an acute and chronic injury form of injury seen. Immunoallergic, autoimmune features |
| Ketoconazole | 0–3 mo | Hepatocellular, sometimes mixed |
| Antiretrovirals | ||
| NRTIs | 1–6 mo | Cholestatic or mixed with lactic acidosis (e.g., stavudine) |
| nNRTIs | 0–3 mo | Initial hepatocellular evolving to cholestatic. Immunoallergic (e.g., nevirapine) |
| Protease inhibitors | 1–3 mo | Hepatocellular, sometimes mixed (ritonavir) |
| Antiepileptics | ||
| Phenytoin | 0–3 mo | Hepatocellular, cholestatic or mixed; immunoallergic |
| Carbamezapine | 1–3 mo | Hepatocellular, cholestatic or mixed; immunoallergic |
| Valproic acid | 1–6 mo | Hepatocellular or mixed, rise in ammonia, sometimes acidosis |
| Analgesics | ||
| NSAIDs | 1–6 mo | Hepatocellular |
| Lipid lowering agents | ||
| Statins | 1–6 mo | Hepatocellular, cholestatic or mixed; autoimmune |
| Immunologics | ||
| TNF antagonists | 1–6 mo | Hepatocellular, autoimmune |
| Herbal and dietary supplements | ||
| Ephedra | 1–3 mo | Hepatocellular, resembling viral hepatitis |
| Green tea extract | 0–6 mo | Hepatocellular |
| Muscle enhancers | 1–6 mo | Cholestatic (anabolic steroids) |
NRTIs, nucleoside reverse transcriptase inhibitors; nNRTIs, non-NRTIs; NSAIDs, nonsteroidal anti-inflammatory drugs; TNF, tumor necrosis factor.
While not always necessary, a liver biopsy can be very helpful. As with the R-ratio there are certain histologic findings associated with particular agents (Table 3). The biopsy can be particularly helpful in ruling out other causes for liver injury13 or pointing toward other etiologies such as autoimmune hepatitis (AIH) that often competes diagnostically with DILI. AIH diagnostic criteria include histology, and unlike DILI, AIH typically requires immunosuppression.25–27 One should also consider a biopsy the longer the liver biochemistries stay elevated. DILI usually improves with time, so lack of resolution tends to erode the likelihood of DILI, and other etiologies need to be reconsidered. Generally, if the difference of the peak alanine aminotransferase (ALT) from the ULN in a hepatocellular injury does not reduce by greater than 50% by 60 days, a biopsy should be considered.28,29 For cholestatic injury, the cutoff is approximately 180 days for a greater than 50% drop in alkaline phosphatase or bilirubin levels.30
Table 3.
Biopsy Reported Phenotypes of Drug Induced Liver Injury and Agents
| Phenotype | Histologic feature | Example |
|---|---|---|
| Acute hepatic necrosis | Collapse and centrolobular necrosis (Fig. 3) | Acetaminophen, isoniazid |
| Acute fatty liver with lactic acidosis | Microvesicular hepatic steatosis; later with macrovesicular steatosis | Didanosine, valproate |
| Acute viral hepatitis like | Inflammatory infiltrates | Isoniazid, flutamide |
| Autoimmune-like hepatitis | Plasma cell infiltration and interface hepatitis | Minocycline, nitrofurantoin |
| Bland cholestasis | Ballooned hepatocytes with minimal inflammation | Anabolic steroids |
| Cholestatic hepatitis | Ballooned hepatocytes with inflammation | Amoxicillin-clavulanate, phenytoin |
| Cirrhosis | Fibrosis without inflammation | Methotrexate, amiodarone |
| Immunoallergic hepatitis | Eosinophilic infiltration | Phenytoin, trimethoprim-sulfamethoxazole |
| Nodular regeneration | Microscopic or macroscopic liver nodules without fibrosis | Azathioprine, oxaliplatin |
| Nonalcoholic fatty liver | Micro/macrosteatosis, ballooned hepatocytes, periportal inflammation | Methotrexate, tamoxifen |
| Sinusoidal obstruction syndrome | Obliteration of central veins with inflammation | Busulfan |
| Vanishing bile duct syndrome | Loss of small inter- and intralobular bile ducts (Fig. 1) | Sulfonamides and β-lactams |
The Roussel Uclaf Causality Assessment Model (RUCAM) is a diagnostic scoring algorithm used to estimate the likelihood of DILI.23,28 It factors in variables such as the pattern of injury, timing, risk factors, competing causes, and rechallenge. Scores are grouped into five categories: “excluded” if score less than or equal to 0, “unlikely” if 1 to 2, “possible” if 3 to 5, “probable” if 6 to 8, and “highly probable” if greater than 8. The main limiting factor in its use is the ambiguity in defining some of the scoring variables. Alcohol use merits additional points, but the RUCAM does not clearly define “alcohol use.” It does not specify how much or how recent. Implicated agents that are “known hepatotoxins” are given points but the definition of what constitutes a known hepatotoxin is not completely clear either. For example, it is not clear whether mere mention of elevated liver enzymes in a package insert as a rare side effect makes the agent a known hepatotoxin. Given these imprecisions, the RUCAM has a fairly low interobserver reliability (reliability coefficient of 0.51, upper 95% confidence limit 0.76).31 On the other hand, it is a useful starting point for the clinician, providing a framework for a diagnostic work-up and reminding one to think of cholestatic and hepatocellular injuries differently. It is also fairly accurate at the extreme categories (e.g., “unlikely” and “highly probable”).31
Recently, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Library of Medicine (NLM) created LiverTox, an online, updated textbook for DILI (http://www.livertox.nih.gov/). It contains over 750 agents with plans to expand to over 1,000. It has quickly become an invaluable resource with over 115,000 unique visitors per month.32,33 Information is presented concisely, emphasizing incidence, clinical presentation and outcome. Direct links to a robust set of references and illustrative cases are provided. Very commonly used medications are also included even if the risk of liver injury is quite low (e.g., omeprazole, ibuprofen, and amlodipine).
Clinical course and prognosis of DILI can be quite variable. Based on clinical experience, the late Hy Zimmerman, suggested a 10% mortality risk in DILI cases with the following: (1) aspartate aminotransferase (AST) or ALT >3 times the ULN; (2) serum total bilirubin >2 times the ULN without elevation of alkaline phosphatase; and (3) no other reason for the rise in transaminases or bilirubin (absence of acute or CLD).34,35 Hy’s Law has proven remarkably robust, based on registries from the United States, Sweden, and Spain totaling about 1,500 patients.13,35,36 Therefore, patients meeting these criteria need careful follow-up and consideration for early referral to a transplant center if any signs of hepatic decompensation arise (e.g., elevated international normalized ratio (INR) and mental status changes).
For the vast majority of cases (80%–90%), the prognosis for DILI is favorable with full recovery. In fact, DILI outcome was often viewed primarily as either fatal or having full recovery. However, recent data suggest that chronic DILI can occur in up to 10%–15% of cases. At the moment, there is no set diagnostic criteria, but it is broadly interpreted as persistent (>6–12 months) elevations in liver enzymes despite cessation of the offending agent. Whether this is simply very slow to resolve injury, a true chronic immune mediated entity or superimposed background liver disease (e.g., nonalcoholic fatty liver disease) remains unknown. However, clear cases of severe chronicity such as vanishing bile duct syndrome (VBDS) are well described (Fig. 1).
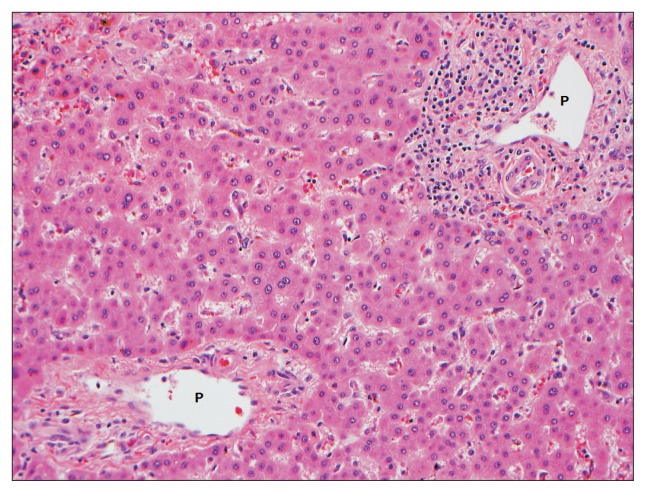
Fig. 1.
A native hepatectomy specimen from a 27-year-old male with vanishing bile duct syndrome due to allopurinol. This patient required liver transplantation 10 months after clinical presentation. A histologic section of the liver demonstrates a paucity of interlobular bile ducts. Two portal tracts shown here are devoid of interlobular bile ducts. Overall, approximately two-thirds of the small portal tracts showed bile duct loss (H&E stain, ×200).
P, portal tract.
SPECIFIC MEDICATIONS
We highlight the following classes of agents because they are commonly implicated in DILI and/or contain commonly prescribed agents. For a more extensive list and coverage of agents, the reader is directed to other reviews, textbooks or LiverTox.37
1. Antimicrobials
Antimicrobials are by far the most commonly implicated drugs in DILI (45.5% of the cases in the U.S. DILIN registry, and 32% in the Spanish registry).13,36 β-Lactams make up the largest class of antibiotics prescribed in the United States.38 and amoxicillin-clavulanate consistently tops the list of causal agents in DILI registries.13,36 The injury is felt to be from the clavulanate.39,40 The latency is usually between 2 and 6 weeks. The injury pattern is variable, with hepatocellular patterns more commonly seen in those under 55 years of age, and cholestatic/mixed patterns in those over 55.11 There are also reports of immunoallergic reactions as well as VBDS.41,42 Other β-lactams implicated include flucloxacillin and oxacillin (cholestasis) as well as cephalosporins.9,43,44
Antituberculosis drugs, including INH, rifampin, and pyrazinamide probably carry some of the highest DILI risk of any medication, but are less commonly prescribed compared to amoxicillin-clavulanate. Of the tuberculosis medications, INH has been the most studied. The mechanism has classically been thought to result from the toxic metabolite hydrazine, but more recent data suggest the injury is more complex including some immunoallergic component.45 INH causes asymptomatic transaminase elevation in 20% of patients, symptomatic hepatitis in 1% and severe injury in 0.01%.46 Injury resolves rapidly if the drug is held before onset of liver failure. For cases of mild injury, reintroduction of therapy has been done with success.47 Rifampin typically will cause a mild hepatocellular injury pattern. Pyrazinamide has a dose dependent hepatotoxic effect, and it has been shown to actually potentiate hepatotoxicity in INH-rifampin combinations.48,49
Sulfonamides are another common antimicrobial implicated in DILI, with an incidence of hepatotoxicity approximated as high as 1 per 1,000 users.5 The pattern of injury is usually a cholestatic or mixed picture, with hypersensitivity symptoms (e.g., rash) being common. Indeed, DRESS is well described with this agent.24,50 VBDS has also been reported.51 Histology often confirms cholestasis with centrolobular hepatocyte swelling and bile accumulation (Fig. 2).
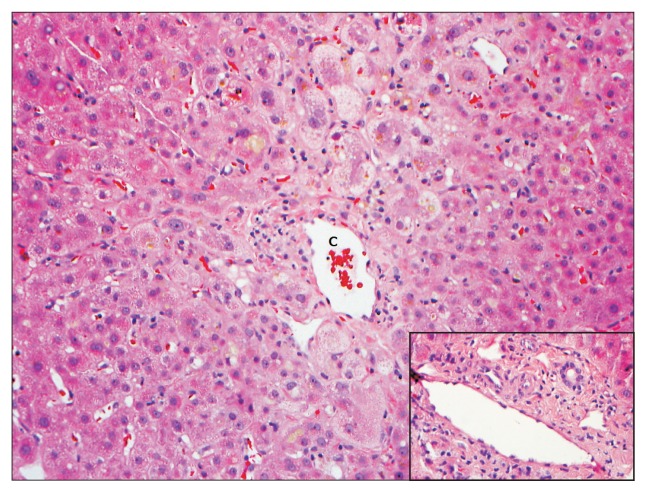
Fig. 2.
A liver biopsy from a 69-year-old male patient with a cholestatic pattern of liver injury following administration of trimethoprim-sulfamethoxazole. The hepatic lobules showed predominantly centrizonal hepatocanalicular cholestasis and marked swelling of the perivenular hepatocytes. In this case, there was no evidence of ongoing significant biliary epithelial injury in the portal tracts (inset). The antibiotic had been held for several weeks. A biopsy was conducted for persistent jaundice and pruritus. Eventually, the patient had a full recovery (H&E stain, ×200).
C, central vein.
Hepatotoxicity from antifungals is well-described. The azoles (ketoconazole, fluconazole, itraconazole, and voriconazole) are particularly common culprits. Ketoconazole is perhaps the most notorious, with risk approaching 134 per 100,000 patient-months.52 The pattern of injury is usually hepatocellular, with a latency of around a month.53 Fluconazole will usually cause a transient, asymptomatic rise of transaminases, with higher risk among bone marrow transplant patients.54,55 Cross reactivity between the azoles is unclear with some successful switches being reported. If such a switch is necessary careful monitoring of liver biochemistries is warranted.
DILI from antiretrovirals (ARVs) for treatment of human immunodeficiency virus infection has been well reviewed in the literature.56,57 Generally, ARVs are of four classes: nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (nNRTIs), protease inhibitors (PIs), and integrase inhibitors. NRTIs tend to cause mitochondrial injury, leading to microvascular steatosis with lactic acidosis. Despite severe injury (e.g., jaundice and coagulopathy), elevation in transaminases can be relatively modest. nNRTIs can induce a hypersensitivity reaction, with fever, eosinophilia and rash. PI hepatotoxicity is uncommon, but acute hepatitis, rarely leading to ALF has been reported with ritonavir.58 Risk of DILI from ritonavir is higher at full dosage compared to lower dosing. Otherwise, PIs are known to induce asymptomatic hyperbilirubinemia secondary to inhibition of uridine diphosphate glucuronosyl transferase, inducing a clinical Gilbert’s syndrome.59 Integrase inhibitors are newer to ARV therapy, and relatively little hepatotoxicity has been reported.
2. Antiepileptics
Phenytoin can induce an asymptomatic elevation of γ-glutamyl transferase and alkaline phosphatase. However, more severe hepatocellular or cholestatic hepatitis and hypersensitivity reactions including DRESS are well described.60,61 Phenytoin’s aromatic ring structure is similar to that found in carbamezapine which can cause similar hepatotoxicity presentations.60 Thus it recommended that both agents be avoided when such DILI occurs. Indeed, phenobarbital, lamotrigine and ethosuximide also carry similar aromatic ring structures and probably carry some cross-reactivity risk as well. Switching to levetiracetam, or gabapentin may be safer.
Valproic acid, a nonaromatic antiepileptic, is the most common antiepileptic causing DILI in the United States.13 It is unique in that it can lead to three different hepatotoxicity presentations: (1) hyperammonemic encephalopathy or coma with relatively modest liver enzyme abnormalities; (2) acute hepatocellular injury; and (3) Reye’s like picture, reported usually in children.33 Histology typically suggests mitochondrial injury with microvesicular steatosis and variable inflammation or necrosis.24,62
3. Nonsteroidal anti-inflammatory drugs
Although much less common than acetaminophen liver injury, nonsteroidal anti-inflammatory drugs (NSAIDs) do pose a hepatotoxicity risk that is frequently overlooked, particularly in women and the elderly.63,64 Given the diverse chemical structures of NSAIDs, the pattern of injury and risk are variable. Ibuprofen rarely causes significant hepatotoxicity, but because of its widespread use, it was a common agent reported in the Spanish registry.36 However, population incidence is probably quite low (1.6 per 100,000).65 Clinical presentations vary, including asymptomatic elevation in ALT and AST, acute hepatitis, ALF, and VBDS.66,67 Diclofenac, the most commonly prescribed NSAID, carries a higher risk of DILI, with an estimated incidence as high as 1 per 9,148 users.7 It typically induces an acute hepatocellular or mixed injury pattern, with some cases of an AIH-like presentation.68 The mechanism of injury is thought to be from metabolites (diclofenac acyl glucuronide and diclofenac-2,5-quinone imine) causing direct cellular damage or autoimmunity.69 Cyclooxygenase 2 inhibitors (i.e., celecoxib) did not show significantly more liver enzyme elevation than placebo in clinical trials. Nevertheless, rare cases of acute cholestatic hepatitis and need of liver transplant are reported.70,71
4. Statins
Much has been written about statin hepatotoxicity and in many ways, this class is one of the best examples of the difficulties in balancing benefit and risk when it comes to DILI. The statin benefit in such an important health threat as cardiovascular disease makes their long-term use widespread and highly recommended. Yet rare instances of hepatotoxicity raise concerns for patients, clinicians and the pharmaceutical industry, and this benefit versus risk tension is unlikely to go away. Monitoring liver enzymes is often done, but largely unsupported by data. In fact, the frequency of liver injury in statin exposure is estimated at 1 in 100,000 patient-years.72 About 3% of patients taking a statin will develop a rise in transaminases, but these will typically normalize with continued use.72,73 Multiple studies have demonstrated statin safety in patients with chronic liver disease and they should not be withheld from these patients, particularly in those where the cardiovascular benefit heavily outweighs the risk of DILI (e.g., NAFLD patients).74,75 Nevertheless statins can cause a spectrum of liver injury (cholestatic and hepatocellular) which can rarely be severe or fatal (Fig. 3).76–79 A better means of identifying those rare injuries early on and those at risk for DILI from these commonly used and needed agents is clearly needed.
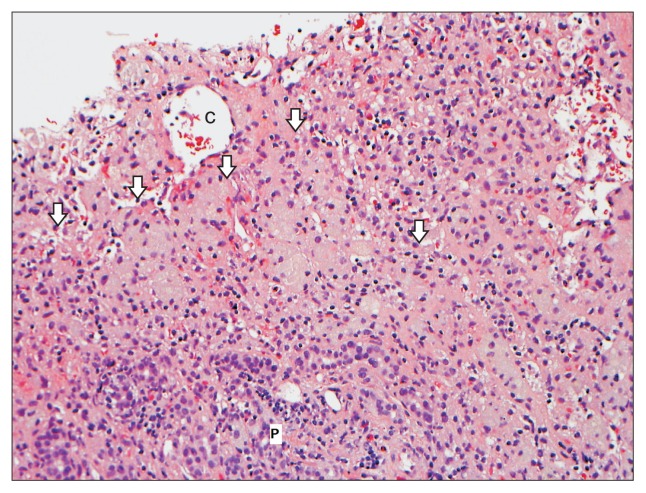
Fig. 3.
A liver biopsy from a 55-year-old female who developed a submassive hepatocyte necrosis and dropout 2 to 3 months after starting rosuvastatin. This photomicrograph shows large areas of hepatocyte dropout with multiple aggregates of reactive ceroid-laden macrophages (arrows). No viable hepatocytes are present in this picture (H&E stain, ×200).
C, central vein; P, portal tract.
5. Antagonists of tumor necrosis factor
Antagonists of tumor necrosis factor (aTNFs; infliximab, adalimumab, and certoluzimab) have become a mainstay in the treatment of inflammatory bowel disease (IBD) and rheumatoid arthritis. There are two liver injury issues with aTNFs: reactivation of underlying chronic hepatitis B and direct hepatotoxicity. Screening for hepatitis B virus (HBV) infection is recommended. If hepatitis B surface antigen (HBsAg) is positive then prophylactic therapy with oral HBV medications should be given.80,81 If HBsAg is negative but antihepatitis B core antibody is positive (i.e., past exposure), then monitoring is recommended, with prompt application of HBV therapy if reactivation occurs.
While all the aTNFs can cause hepatotoxicity, infliximab was most frequently cited in the U.S. DILIN case series.82 The presentation varies including acute hepatitis, bland cholestasis, autoimmune-like injury and ALF.24,82 Interestingly, there are reports of safely transitioning from infliximab to etanercept in rheumatoid arthritis and infliximab to adalimumab in IBD.83–85 This may be due to fundamental differences in protein structure, as etanercept is made of soluble TNF-α receptors linked to the Fc portion of human IgG1. Infliximab is a humanized mouse monoclonal IgG1 antibody, and adalimumab is a fully human monoclonal IgG1 antibody.82
6. Herbal and dietary supplements
HDS use has grown steadily with 54% of U.S. adults using them by 2006 versus 40% between 1988 and 1994.86 The true incidence of hepatotoxicity from any particular HDS is impossible to estimate, as the total population of patients using the agent is unknown and there are no prescription numbers to monitor. However, the U.S. DILIN recently reported an increase in the percentage of HDS cases from 7% to 20% over 9 years.87 We will mention just a few remarkable HDS hepatotoxicities to highlight some of the clinical features as well as challenges. More comprehensive reviews are published.88,89
With the rise in obesity, there is a growing market for weight loss supplements. Ma-Huang (Ephedra sinica), a Chinese herbal, is commonly taken for weight loss. Such products can cause a hepatitis with autoimmune features, sometimes with massive necrosis leading to ALF.88,90,91 Ephedra was felt to be the causal component, and in 2004, the Food and Drug Administration prohibited the sale of ephedra containing products. However, this ban has occasionally been circumvented.92
Green tea or Camellia sinensis extracts has been marketed as “ephedra free” alternative in weight loss supplements. However, the catechins and their gallic acid esters in such extracts can cause oxidative stress in the liver.88,89,93 The pattern of injury is typically hepatocellular, however, there are reports of mixed injury and AIH.94–96 Camellia Sinensis continues to be a major component of many weight loss supplements sold in the United States today.97
Muscle enhancers are frequently implicated in liver injury particularly those containing anabolic steroids.87,98 By the time most patients present, they typically have a bland cholestatic pattern of injury (high bilirubin with relatively low liver enzymes) occurring within 6 months of starting therapy.24 Deep jaundice (e.g., bilirubin over 20 mg/dL) can occur with weight loss, nausea, and pruritus that can last for months. The vast majority of cases recover, but cases of chronic ductopenia have been reported.99,100 Additionally, anabolic steroids are linked to tumors of the liver, particularly hepatic adenomas.101
FUTURE DIRECTIONS
DILI research is poised to make significant discoveries that will translate to clinical practice over the next decade. Several DILI registries are now growing and maturing worldwide. They will provide rich repositories for translational and clinical research. Based on the clinical data alone in these registries, newer diagnostic algorithms to improve upon the RUCAM will be forthcoming. Consolidation of large medical groups and systems in the United States along with the use of large electronic medical records (EMR) will provide a rich data source for pharmacoepidemiologic studies that will help define incidence and risk factors. Such “big data” EMRs may also identify cases for enrollment in studies. With increasing availability of tissue and blood from well-defined DILI cases, the chance of identifying biomarkers for DILI diagnosis and risk will increase. Already, genome-wide association studies (GWAS) are providing insight into DILI pathophysiology. Several HLA associations with DILI from a variety of agents strongly suggests an immune component to the injury.102–105 Such immune components may lend themselves to targeted therapies which may truncate DILI and prevent ALF. Other genetic and drug metabolism markers also show promise. Right now, none of the GWAS associations are common or specific enough for clinical use, but next generation sequencing technology and increasing sample sizes will bring some markers to diagnostic testing and risk assessment in the years to come.106,107
CONCLUSIONS
DILI remains a clinical challenge. Its iatrogenic nature and potential for severe or fatal outcome can be unnerving for clinician and patient alike. While relatively uncommon to rare for any specific agent, the overall incidence may be higher than previously thought and will probably rise with the aging of the general population and increasing polypharmacy. Useful diagnostic biomarkers will be forthcoming, but for now, diagnosis hinges on good old-fashioned history taking and efficient exclusion of competing diagnoses. Being aware of commonly implicated agents, their patterns of injury, and diagnostic resources (e.g., LiverTox and RUCAM) are also essential. The risks of ALF and chronicity require vigilant follow-up once the diagnosis has been made.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Wilke RA, Lin DW, Roden DM, et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115–122. doi: 10.1055/s-0034-1375953. [DOI] [PubMed] [Google Scholar]
- 4.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 5.de Abajo FJ, Montero D, Madurga M, Garcia Rodriguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58:71–80. doi: 10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33:155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- 7.Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425.e3. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–123. doi: 10.1378/chest.128.1.116. [DOI] [PubMed] [Google Scholar]
- 9.Olsson R, Wiholm BE, Sand C, Zettergren L, Hultcrantz R, Myrhed M. Liver damage from flucloxacillin, cloxacillin and dicloxacillin. J Hepatol. 1992;15:154–161. doi: 10.1016/0168-8278(92)90029-O. [DOI] [PubMed] [Google Scholar]
- 10.Stock JG, Strunin L. Unexplained hepatitis following halothane. Anesthesiology. 1985;63:424–439. doi: 10.1097/00000542-198510000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Lucena MI, Andrade RJ, Fernandez MC, et al. Determinants of the clinical expression of amoxicillin-clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–856. doi: 10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- 12.Stricker BH, Blok AP, Claas FH, Van Parys GE, Desmet VJ. Hepatic injury associated with the use of nitrofurans: a clinicopathological study of 52 reported cases. Hepatology. 1988;8:599–606. doi: 10.1002/hep.1840080327. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934.e4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–966. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 15.Morgan DJ, McLean AJ. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease: an update. Clin Pharmacokinet. 1995;29:370–391. doi: 10.2165/00003088-199529050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis. A practical guide. Aliment Pharmacol Ther. 2013;37:1132–1156. doi: 10.1111/apt.12324. [DOI] [PubMed] [Google Scholar]
- 17.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 18.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182–189. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 19.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 20.Lucena MI, Andrade RJ, Kaplowitz N, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001–2009. doi: 10.1002/hep.22895. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58:388–396. doi: 10.1002/hep.26208. [DOI] [PubMed] [Google Scholar]
- 22.Bénichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-A. [DOI] [PubMed] [Google Scholar]
- 23.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs: II. an original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 24.Chitturi S, Farrell GC. Drug-induced liver disease. In: Schiff ER, Maddrey WC, Sorrell MF, editors. Schiff’s diseases of the liver. 11th ed. Chichester: Wiley-Blackwell; 2012. pp. 703–783. [Google Scholar]
- 25.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 26.Czaja AJ. Corticosteroids or not in severe acute or fulminant autoimmune hepatitis: therapeutic brinksmanship and the point beyond salvation. Liver Transpl. 2007;13:953–955. doi: 10.1002/lt.21088. [DOI] [PubMed] [Google Scholar]
- 27.Ichai P, Duclos-Vallée JC, Guettier C, et al. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl. 2007;13:996–1003. doi: 10.1002/lt.21036. [DOI] [PubMed] [Google Scholar]
- 28.Danan G, Benichou C. Causality assessment of adverse reactions to drugs: I. a novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 29.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134–144. doi: 10.1055/s-0034-1375955. [DOI] [PubMed] [Google Scholar]
- 31.Rochon J, Protiva P, Seeff LB, et al. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoofnagle JH, Serrano J, Knoben JE, Navarro VJ. LiverTox: a website on drug-induced liver injury. Hepatology. 2013;57:873–874. doi: 10.1002/hep.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Library of Medicine. LiverTox [Internet] Bethesda: National Library of Medicine; c2015. [cited 2015 Jan 14]. Available from: http://www.LiverTox.nih.gov. [Google Scholar]
- 34.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 35.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 36.Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Bahirwani R, Reddy KR. Drug-induced liver injury due to cancer chemotherapeutic agents. Semin Liver Dis. 2014;34:162–171. doi: 10.1055/s-0034-1375957. [DOI] [PubMed] [Google Scholar]
- 38.Hicks LA, Taylor TH, Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 39.Larrey D, Vial T, Micaleff A, et al. Hepatitis associated with amoxycillin-clavulanic acid combination report of 15 cases. Gut. 1992;33:368–371. doi: 10.1136/gut.33.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García Rodríguez LA, Stricker BH, Zimmerman HJ. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med. 1996;156:1327–1332. doi: 10.1001/archinte.156.12.1327. [DOI] [PubMed] [Google Scholar]
- 41.Reddy KR, Brillant P, Schiff ER. Amoxicillin-clavulanate potassium-associated cholestasis. Gastroenterology. 1989;96:1135–1141. doi: 10.1016/0016-5085(89)91633-8. [DOI] [PubMed] [Google Scholar]
- 42.Richardet JP, Mallat A, Zafrani ES, Blazquez M, Bognel JC, Campillo B. Prolonged cholestasis with ductopenia after administration of amoxicillin/clavulanic acid. Dig Dis Sci. 1999;44:1997–2000. doi: 10.1023/A:1026610015899. [DOI] [PubMed] [Google Scholar]
- 43.Eckstein RP, Dowsett JF, Lunzer MR. Flucloxacillin induced liver disease: histopathological findings at biopsy and autopsy. Pathology. 1993;25:223–228. doi: 10.3109/00313029309066576. [DOI] [PubMed] [Google Scholar]
- 44.Peker E, Cagan E, Dogan M. Ceftriaxone-induced toxic hepatitis. World J Gastroenterol. 2009;15:2669–2671. doi: 10.3748/wjg.15.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther. 2011;89:911–914. doi: 10.1038/clpt.2010.355. [DOI] [PubMed] [Google Scholar]
- 46.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 47.Sharma SK, Singla R, Sarda P, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010;50:833–839. doi: 10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 48.Tahaoğlu K, Atac G, Sevim T, et al. The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2001;5:65–69. [PubMed] [Google Scholar]
- 49.Durand F, Bernuau J, Pessayre D, et al. Deleterious influence of pyrazinamide on the outcome of patients with fulminant or subfulminant liver failure during antituberculous treatment including isoniazid. Hepatology. 1995;21:929–932. doi: 10.1002/hep.1840210407. [DOI] [PubMed] [Google Scholar]
- 50.Mainra RR, Card SE. Trimethoprim-sulfamethoxazole-associated hepatotoxicity: part of a hypersensitivity syndrome. Can J Clin Pharmacol. 2003;10:175–178. [PubMed] [Google Scholar]
- 51.Yao F, Behling CA, Saab S, Li S, Hart M, Lyche KD. Trimethoprim-sulfamethoxazole-induced vanishing bile duct syndrome. Am J Gastroenterol. 1997;92:167–169. [PubMed] [Google Scholar]
- 52.García Rodríguez LA, Duque A, Castellsague J, Pérez-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48:847–852. doi: 10.1046/j.1365-2125.1999.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stricker BH, Blok AP, Bronkhorst FB, Van Parys GE, Desmet VJ. Ketoconazole-associated hepatic injury: a clinicopathological study of 55 cases. J Hepatol. 1986;3:399–406. doi: 10.1016/S0168-8278(86)80495-0. [DOI] [PubMed] [Google Scholar]
- 54.Ikemoto H. A clinical study of fluconazole for the treatment of deep mycoses. Diagn Microbiol Infect Dis. 1989;12(4 Suppl):239S–247S. doi: 10.1016/0732-8893(89)90143-0. [DOI] [PubMed] [Google Scholar]
- 55.Fischer MA, Winkelmayer WC, Rubin RH, Avorn J. The hepatotoxicity of antifungal medications in bone marrow transplant recipients. Clin Infect Dis. 2005;41:301–307. doi: 10.1086/431586. [DOI] [PubMed] [Google Scholar]
- 56.Núñez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology. 2010;52:1143–1155. doi: 10.1002/hep.23716. [DOI] [PubMed] [Google Scholar]
- 57.Surgers L, Lacombe K. Hepatoxicity of new antiretrovirals: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37:126–133. doi: 10.1016/j.clinre.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18:2277–2284. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 59.Sulkowski MS. Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S90–S97. doi: 10.1086/381444. [DOI] [PubMed] [Google Scholar]
- 60.Eshki M, Allanore L, Musette P, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67–72. doi: 10.1001/archderm.145.1.67. [DOI] [PubMed] [Google Scholar]
- 61.Mullick FG, Ishak KG. Hepatic injury associated with diphenylhydantoin therapy: a clinicopathologic study of 20 cases. Am J Clin Pathol. 1980;74:442–452. doi: 10.1093/ajcp/74.4.442. [DOI] [PubMed] [Google Scholar]
- 62.Zimmerman HJ, Ishak KG. Valproate-induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2:591–597. doi: 10.1002/hep.1840020513. [DOI] [PubMed] [Google Scholar]
- 63.Lacroix I, Lapeyre-Mestre M, Bagheri H, et al. Nonsteroidal anti-inflammatory drug-induced liver injury: a case-control study in primary care. Fundam Clin Pharmacol. 2004;18:201–206. doi: 10.1111/j.1472-8206.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 64.Johnson AG, Day RO. The problems and pitfalls of NSAID therapy in the elderly (Part I) Drugs Aging. 1991;1:130–143. doi: 10.2165/00002512-199101020-00005. [DOI] [PubMed] [Google Scholar]
- 65.Manoukian AV, Carson JL. Nonsteroidal anti-inflammatory drug-induced hepatic disorders: incidence and prevention. Drug Saf. 1996;15:64–71. doi: 10.2165/00002018-199615010-00005. [DOI] [PubMed] [Google Scholar]
- 66.Riley TR, 3rd, Smith JP. Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol. 1998;93:1563–1565. doi: 10.1111/j.1572-0241.1998.00484.x. [DOI] [PubMed] [Google Scholar]
- 67.Andrade RJ, Lucena MI, García-Cortés M, García-Ruiz E, Fernández-Bonilla E, Vázquez L. Chronic hepatitis C, ibuprofen, and liver damage. Am J Gastroenterol. 2002;97:1854–1855. doi: 10.1111/j.1572-0241.2002.05874.x. [DOI] [PubMed] [Google Scholar]
- 68.Scully LJ, Clarke D, Barr RJ. Diclofenac induced hepatitis: 3 cases with features of autoimmune chronic active hepatitis. Dig Dis Sci. 1993;38:744–751. doi: 10.1007/BF01316809. [DOI] [PubMed] [Google Scholar]
- 69.Daly AK, Aithal GP, Leathart JB, Swainsbury RA, Dang TS, Day CP. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology. 2007;132:272–281. doi: 10.1053/j.gastro.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 70.Maddrey WC, Maurath CJ, Verburg KM, Geis GS. The hepatic safety and tolerability of the novel cyclooxygenase-2 inhibitor celecoxib. Am J Ther. 2000;7:153–158. doi: 10.1097/00045391-200007030-00003. [DOI] [PubMed] [Google Scholar]
- 71.El Hajj I, Malik SM, Alwakeel HR, Shaikh OS, Sasatomi E, Kandil HM. Celecoxib-induced cholestatic liver failure requiring orthotopic liver transplantation. World J Gastroenterol. 2009;15:3937–3939. doi: 10.3748/wjg.15.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tolman KG. Defining patient risks from expanded preventive therapies. Am J Cardiol. 2000;85:15E–19E. doi: 10.1016/S0002-9149(00)00946-2. [DOI] [PubMed] [Google Scholar]
- 73.McKenney JM, Davidson MH, Jacobson TA, Guyton JR National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 74.Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 75.Avins AL, Manos MM, Ackerson L, et al. Hepatic effects of lovastatin exposure in patients with liver disease: a retrospective cohort study. Drug Saf. 2008;31:325–334. doi: 10.2165/00002018-200831040-00006. [DOI] [PubMed] [Google Scholar]
- 76.Grimbert S, Pessayre D, Degott C, Benhamou JP. Acute hepatitis induced by HMG-CoA reductase inhibitor, lovastatin. Dig Dis Sci. 1994;39:2032–2033. doi: 10.1007/BF02088142. [DOI] [PubMed] [Google Scholar]
- 77.Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. Am J Gastroenterol. 1999;94:1388–1390. doi: 10.1111/j.1572-0241.1999.01091.x. [DOI] [PubMed] [Google Scholar]
- 78.Pelli N, Setti M, Ceppa P, Toncini C, Indiveri F. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol. 2003;15:921–924. doi: 10.1097/00042737-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 79.Tolman KG. The liver and lovastatin. Am J Cardiol. 2002;89:1374–1380. doi: 10.1016/S0002-9149(02)02355-X. [DOI] [PubMed] [Google Scholar]
- 80.Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703–711. doi: 10.1002/hep.27609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 82.Ghabril M, Bonkovsky HL, Kum C, et al. Liver injury from tumor necrosis factor-alpha antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11:558–564.e3. doi: 10.1016/j.cgh.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becker H, Willeke P, Domschke W, Gaubitz M. Etanercept tolerance in a patient with previous infliximab-induced hepatitis. Clin Rheumatol. 2008;27:1597–1598. doi: 10.1007/s10067-008-1000-3. [DOI] [PubMed] [Google Scholar]
- 84.Thiéfin G, Morelet A, Heurgué A, Diebold MD, Eschard JP. Infliximab-induced hepatitis: absence of cross-toxicity with etanercept. Joint Bone Spine. 2008;75:737–739. doi: 10.1016/j.jbspin.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 85.Parekh R, Kaur N. Liver injury secondary to anti-TNF-alpha therapy in inflammatory bowel disease: a case series and review of the literature. Case Rep Gastrointest Med. 2014;2014:956463. doi: 10.1155/2014/956463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Navarro VJ, Lucena MI. Hepatotoxicity induced by herbal and dietary supplements. Semin Liver Dis. 2014;34:172–193. doi: 10.1055/s-0034-1375958. [DOI] [PubMed] [Google Scholar]
- 89.Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17. doi: 10.1111/apt.12109. [DOI] [PubMed] [Google Scholar]
- 90.Skoulidis F, Alexander GJ, Davies SE. Ma huang associated acute liver failure requiring liver transplantation. Eur J Gastroenterol Hepatol. 2005;17:581–584. doi: 10.1097/00042737-200505000-00017. [DOI] [PubMed] [Google Scholar]
- 91.Neff GW, Reddy KR, Durazo FA, Meyer D, Marrero R, Kaplowitz N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J Hepatol. 2004;41:1062–1064. doi: 10.1016/j.jhep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 92.Food and Drug Administration (FDA) FDA issues regulation prohibiting sale of dietary supplements containing ephedrine alkaloids and reiterates its advice that consumers stop using these products [Internet] Silver Spring: FDA; 2004. [cited 2015 Nov 9]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108242.htm. [Google Scholar]
- 93.Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58:2682–2690. doi: 10.1007/s10620-013-2687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Ann Intern Med. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 95.Javaid A, Bonkovsky HL. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): a growing concern. J Hepatol. 2006;45:334–335. doi: 10.1016/j.jhep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Vanstraelen S, Rahier J, Geubel AP. Jaundice as a misadventure of a green tea (camellia sinensis) lover: a case report. Acta Gastroenterol Belg. 2008;71:409–412. [PubMed] [Google Scholar]
- 97.Dara L, Hewett J, Lim JK. Hydroxycut hepatotoxicity: a case series and review of liver toxicity from herbal weight loss supplements. World J Gastroenterol. 2008;14:6999–7004. doi: 10.3748/wjg.14.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh V, Rudraraju M, Carey EJ, et al. Severe hepatotoxicity caused by a methasteron-containing performance-enhancing supplement. J Clin Gastroenterol. 2009;43:287. doi: 10.1097/MCG.0b013e31815a5796. [DOI] [PubMed] [Google Scholar]
- 99.Martin DJ, Partridge BJ, Shields W. Hepatotoxicity associated with the dietary supplement N.O.-XPLODE. Ann Intern Med. 2013;159:503–504. doi: 10.7326/0003-4819-159-7-201310010-00019. [DOI] [PubMed] [Google Scholar]
- 100.Glober GA, Wilkerson JA. Biliary cirrhosis following the administration of methyltestosterone. JAMA. 1968;204:170–173. doi: 10.1001/jama.1968.03140150074026. [DOI] [PubMed] [Google Scholar]
- 101.Westaby D, Portmann B, Williams R. Androgen related primary hepatic tumors in non-Fanconi patients. Cancer. 1983;51:1947–1952. doi: 10.1002/1097-0142(19830515)51:10<1947::AID-CNCR2820511034>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 102.Monshi MM, Faulkner L, Gibson A, et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727–739. doi: 10.1002/hep.26077. [DOI] [PubMed] [Google Scholar]
- 103.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 104.Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am J Respir Crit Care Med. 2002;166:916–919. doi: 10.1164/rccm.2108091. [DOI] [PubMed] [Google Scholar]
- 105.Martin AM, Nolan D, James I, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–99. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 106.Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis. 2014;34:123–133. doi: 10.1055/s-0034-1375954. [DOI] [PubMed] [Google Scholar]
- 107.Bamshad MJ, Ng SB, Bigham AW, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]