Abstract
Flowing blood exerts a frictional force, fluid shear stress (FSS), on the endothelial cells that line the blood and lymphatic vessels. The magnitude, pulsatility, and directional characteristics of FSS are constantly sensed by the endothelium. Sustained increases or decreases in FSS induce vessel remodeling to maintain proper perfusion of tissue. In this review, we discuss these mechanisms and their relevance to physiology and disease, and propose a model for how information from different mechanosensors might be integrated to govern remodeling.
INTRODUCTION
The circulatory system consists of a fluid (blood), a pump (the heart), and the vessels through which the blood circulates. Vertebrates also have a parallel system of lymphatic vessels that drain excess fluid from the tissues and return it to the blood. These structures are lined by a specialized epithelium, the endothelium, which is supported by mural cells (pericytes in capillaries and smooth muscle cells in arteries and veins) and extracellular matrix. Flowing blood and lymph exert a frictional force parallel to the endothelial surface termed fluid shear stress (FSS). FSS magnitude is directly proportional to the fluid velocity and viscosity and inversely proportional to the vessel diameter. Typical magnitudes in human blood vessels are between 0.5 and 5 Pa (Lipowsky et al., 1978) and ∼10 times lower in lymphatic vessels (Dixon et al., 2006). For comparison, typical traction forces from endothelial cells (ECs) in vitro are ∼100 Pa (Krishnan et al., 2011), while circumferential stretch of the vessel wall during the cardiac cycle can be > 1000 Pa (Haga et al., 2007). Blood and lymph flow are also pulsatile, with distinct characteristics depending on location and conditions (Feaver et al., 2013). Flow can be laminar and unidirectional (i.e., flow smoothly); laminar with backflow (oscillatory) and multidirectional (different angles, including perpendicular); or turbulent (i.e., chaotic). Oscillatory flow is prominent in lymphatic vessels (Dixon et al., 2006); multidirectional flows occur at vessel bifurcations or other irregularities (Zhao et al., 2000), while true turbulence occurs just after the aortic valve or in more severe irregularities associated with disease (Gülan et al., 2012).
Despite its comparatively low magnitude, FSS is a major determinant of both developmental and postnatal remodeling of vasculature and lymphatics. In this review, we discuss recent views on the different mechanisms of flow sensing and their roles in physiology and disease.
MECHANOTRANSDUCTION THROUGH THE JUNCTIONAL COMPLEX
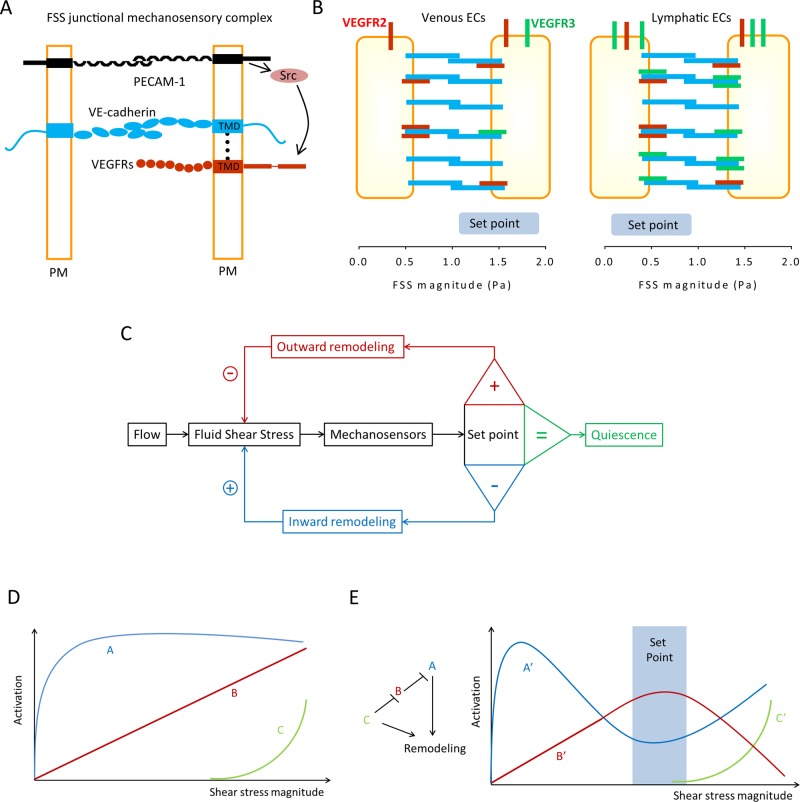
While flow sensing has been attributed to different mechanosensors, including G protein–coupled receptors, glycocalyx, and primary cilium and ion channels (Hahn and Schwartz, 2009), the best-studied FSS mechanoreceptor is the endothelial-specific junctional complex comprising PECAM1, VE-cadherin, VEGFR2 (Tzima et al., 2005), and more recently VEGFR3 (Coon et al., 2015) (Figure 1A). Pulling on these receptors with magnetic beads demonstrated direct mechanotransduction by PECAM1 (Tzima et al., 2005; Collins et al., 2012). Measurements using fluorescence-based tension sensors confirmed that flow induced an increase in force on PECAM1, unexpectedly mediated by de novo connection of PECAM1 to the vimentin cytoskeleton and transmission of force from myosin (Conway et al., 2013). VE-cadherin bears force constitutively but does not transduce forces from flow, instead functioning as an adaptor. This function is mediated at least in part by the binding to VEGF receptors 2 and 3 through their respective transmembrane domains (Coon et al., 2015). Our current model for mechanotransduction is that flow first acts on an as yet unidentified upstream sensor, which triggers cytoskeletal association of PECAM1 and transmission of myosin-derived force to this molecule (Conway et al., 2013); force on PECAM1 triggers activation of a Src family kinase, probably fyn (Chiu et al., 2008); in the presence of VE-cadherin, Src phosphorylates and transactivates VEGFRs, which mediate downstream signaling (Figure 1A).
FIGURE 1:
Control of vascular remodeling by fluid shear stress sensing. (A) Endothelial mechanosensitive junctional complex. Conceptual model for the assembly of the junctional mechanosensory complex. VEGF receptors (both VEGFR2 and VEGFR3) and VE-cadherin associate through their transmembrane domains (TMDs) within the plasma membrane (PM). FSS triggers force on PECAM-1, which leads to activation of a src family kinase (Src), which phosphorylates and activates VEGFRs. (B) Blood vs. lymphatic ECs. Higher VEGFR3 (green) expression in lymphatic ECs increases their sensitivity to FSS, resulting in a lower FSS set point in lymphatic compared with blood ECs. VEGFR2 is in red. (C) Classical control theory. In this model, ECs sense FSS magnitude and compare it with a pre-existing value, the FSS set point. Deviations from this value activate remodeling mechanisms to alter vessel diameter and return FSS to the steady-state level. This model is unlikely to describe a biological mechanism. (D) Biological pathways. We speculate that a more realistic mechanism for the FSS set point requires several mechanosensitive elements, denoted A, B, and C, that have different sensitivities. Pathway A is activated and reaches a maximum at low FSS, while the other(s) may require higher FSS. (E) Integration to determine the set point. The outputs from A, B, and C are denoted A′, B′, and C′. These pathways would be organized into a network such that B′ is maximal at an intermediate FSS level, the FSS set point (blue rectangle). B′ stabilizes the vessel and inhibits remodeling. The ratio of A′ and B′ determine the direction of remodeling, where high A′/low C′ gives inward remodeling and high A′/high C′ gives outward remodeling. This model is highly hypothetical and is meant only to illustrate the general class of theories that might explain the set point.
SENSING FLOW MAGNITUDE IN VESSEL REMODELING: THE SHEAR STRESS SET POINT THEORY
During adult life, blood vessels remodel to maintain optimal perfusion of tissues. Tissue growth or metabolic activity increases nutrient and oxygen demand, which triggers peripheral arteriole relaxation and decreased resistance, increasing blood flow and hence FSS in the upstream arteries (Michiels, 2004; Segal, 2005; Padilla et al., 2011). It has long been observed that changing flow magnitude and thus shear stress leads to proportional changes of vessel diameter to restore the initial shear stress level (Thoma, 1893; Kamiya and Togawa, 1980; Kamiya et al., 1984; Langille, 1996; Langille and O’Donnell, 1986; Langille et al., 1989; Tronc et al., 1996; Tuttle et al., 2001). These observations suggested that ECs induce remodeling to maintain FSS within a desired range, essentially a set point. Sustained deviation outside this range then triggers readjustment of vessel diameter (Rodbard, 1975). We recently obtained evidence that ECs have an in vitro FSS set point at which flow activates pathways that promote blood vessel stabilization, whereas higher or lower FSS triggers pathways characteristic of remodeling (Baeyens et al., 2015). We propose that FSS at the set point promotes EC quiescence and vessel maturation, whereas FSS outside that range triggers remodeling: inward for low and outward for high FSS (Figure 1C). The specific signaling or gene expression pathways that distinguish low flow/inward versus high flow/outward remodeling have not been determined, though production of nitric oxide by endothelial nitric oxide synthase (eNOS) has been implicated (Rudic et al., 2000; Dumont et al., 2007). PECAM1 is implicated in this process, since PECAM1−/− mice are defective in both inward and outward flow-dependent remodeling (Chen and Tzima, 2009; Chen et al., 2010). Interestingly, PECAM1−/− mice show constitutive activation of eNOS but loss of flow responses (Fleming et al., 2005; McCormick et al., 2011), consistent with the junctional complex regulating eNOS activity in this process.
Corresponding to the large differences in flow magnitudes and patterns for different types of vessels (Lipowsky et al., 1978; Dixon et al., 2006), vascular and lymphatic ECs showed distinct set points in vitro that matched their FSS levels in vivo (Baeyens et al., 2015). This difference was largely accounted for by levels of VEGFR3, which is highly expressed in lymphatic ECs compared with blood vascular cells (Kaipainen et al., 1995). It was shown that increasing VEGFR3 levels increased cells’ sensitivity to FSS, that is, the set point shifted to lower values. Identification of VEGFR3 as an element of the FSS junctional mechanosensor (Coon et al., 2015) thus appears to explain the distinct FSS levels in veins versus lymphatic vessels (Figure 1B).
How might flow sensors encode a set point? Classical control theory requires only a single measurement that is compared with a fixed value (Carpenter, 2004; Figure 1B). However, signal transduction pathways in cells seldom operate that way. In the absence of digital measurements, a more likely mechanism is the existence of several molecular sensors with different sensitivities whose outputs are integrated to determine the response. To illustrate the concept, one possible mechanisms of integration is proposed in Figure 1, D and E. Identification of these mechanosensors and characterization of their FSS dose–response characteristics and mechanisms of integration is a major area for future research.
SENSING FLOW FREQUENCY
Arterial and lymphatic flow patterns are highly pulsatile, whereas venous flow is nearly steady (Dixon et al., 2006; Huo and Kassab, 2006). These time-varying components can strongly influence endothelial responses. Compared with steady flow with similar time-averaged magnitudes, pulsatile flow stimulates Erk activity more strongly (Kadohama et al., 2007), while AMPK activity is sensitive to pulse frequency (Zhang et al., 2006). Comparison of proinflammatory oscillatory flow with anti-inflammatory pulsatile-flow patterns at atherosclerosis-prone versus atherosclerosis-resistant sites in the human carotid artery identified specific frequency components that exerted pro- or anti-inflammatory effects (Feaver et al., 2013). Of clinical interest, left ventricle assist devices for heart failure patients generate nonpulsatile flow that induces arterial-venous malformations (Islam et al., 2013), suggesting that pulsatility is important for maintaining arterial identify. Erk may mediate these effects, since it is strongly linked to arterial specification (Deng et al., 2013).
These results imply that endothelial mechanosensors must decode subsecond-frequency characteristics, which necessitates rapid rates of both activation and inactivation. These kinetics, particularly the required inactivation rates, exclude even G protein signaling; however, ion channels can have very rapid activation and inactivation kinetics. Indeed, stretch-activated channels (SACs) have been implicated in FSS-induced calcium entry (Mendoza et al., 2010; Bubolz et al., 2012; Li et al., 2014; Ranade et al., 2014). Mechanosensitive Piezo channels have very rapid activation and inactivation rates (Coste et al., 2010; Gottlieb et al., 2012) and are required for vascular development (Li et al., 2014; Ranade et al., 2014), and thus are prime candidates. However, the nonselective SACs blocker gadolinium did not prevent EC alignment in response to FSS (Malek and Izumo, 1996) or other flow responses (Malek et al., 1999; Traub et al., 1999), potentially separating FSS-induced polarity from mechanosensitive calcium influx.
SENSING FLOW DIRECTION
The correlation between atherosclerosis-prone regions of arteries and disturbed flow led to the idea that oscillatory shear, where flow reverses during part of the cardiac cycle, promotes plaque formation (Ku et al., 1985; Chiu and Chien, 2011). But how ECs sense flow direction is very poorly understood. Interestingly, ECs in athero-prone regions fail to elongate and align in the direction of flow, and cells therefore experience flow at many angles, including perpendicular, whereas in athero-resistant regions of blood vessels, flow is parallel to the cell axis. In fact, recent studies suggest that plaque localization correlates more strongly with the perpendicular or transverse wall shear stress component than with oscillations (Peiffer et al., 2013; Mohamied et al., 2015). Interestingly, for elongated ECs in vitro, flow perpendicular to the cells’ axis promotes inflammatory responses, while flow parallel to the axis stimulates primarily anti-inflammatory pathways (Wang et al., 2012, 2013). These effects correlate with the ability of steady laminar or unidirectional pulsatile FSS to induce cell elongation and alignment in the flow direction in conjunction with suppression of inflammation, whereas low or oscillatory flow fails to induce alignment and promotes inflammatory pathways (Mohan et al., 1997; Chiu and Chien, 2011; Wang et al., 2013). The fortuitous observation that the proteoglycan syndecan 4 (Sdc4) is required for EC alignment in flow provided a way to test this idea. When Sdc4−/− mice (in which ECs fail to align) were crossed into a high-cholesterol strain, atherosclerotic plaque not only increased but formed in normally resistant areas of laminar flow (Baeyens et al., 2014). Yet in vitro, Sdc4 was not required for other flow responses, and thus appears to be specifically required for sensing flow direction, which is separable from sensing flow magnitude. Together, these results demonstrate that loss of alignment is causal for athero-susceptibility and that Sdc4 is a component of the flow direction sensor.
CONCLUSION AND PERSPECTIVES
Hemodynamic forces control vessel specification and maturation during development and continue to regulate vessel remodeling during postnatal and adult life. The exquisite ability of ECs to sense multiple aspects of fluid shear stress patterns allows them to coordinate the complex ballet of events involving different cell types and signaling pathways, to constantly adjust vessel structure and function. Identifying molecular mechanosensors behind the distinct sensing of FSS magnitude, direction, and pulsatility are major directions for future research. Further, characterizing how downstream signaling pathways and gene regulation events are integrated in the context of growth and disease is required to better understand the amazing plasticity of our blood vessels. The finely tuned biomechanical responses discussed here govern health and disease in nearly every tissue from our first to our last heartbeat. Understanding these processes is a major, exciting challenge for biomedical research in the coming years.
Abbreviations used:
- ECs
endothelial cells
- eNOS
endothelial nitric oxide synthase
- FSS
fluid shear stress
- SAC
stretch-activated channel
- Sdc4
syndecan 4.
Footnotes
REFERENCES
- Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, Schwartz MA. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci USA. 2014;111:17308–17313. doi: 10.1073/pnas.1413725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL, et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife. 2015;4:04645. doi: 10.7554/eLife.04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol. 2012;302:H634–H642. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH. Homeostasis: a plea for a unified approach. Adv Physiol Educ. 2004;28:180–187. doi: 10.1152/advan.00012.2004. [DOI] [PubMed] [Google Scholar]
- Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circulation Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Larrivee B, Zhuang ZW, Atri D, Moraes F, Prahst C, Eichmann A, Simons M. Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood. 2013;121:3988–3996. doi: 10.1182/blood-2012-12-474601. S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27:317–324. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaver RE, Gelfand BD, Blackman BR. Human haemodynamic frequency harmonics regulate the inflammatory phenotype of vascular endothelial cells. Nat Commun. 2013;4:1525. doi: 10.1038/ncomms2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels. 2012;6:282–289. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülan U, Lüthi B, Holzner M, Liberzon A, Tsinober A, Kinzelbach W. Experimental study of aortic flow in the ascending aorta via particle tracking velocimetry. Exp Fluids. 2012;53:1469–1485. [Google Scholar]
- Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomechanics. 2007;40:947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Kassab GS. Pulsatile blood flow in the entire coronary arterial tree: theory and experiment. Am J Physiol Heart Circulatory Physiol. 2006;291:H1074–H1087. doi: 10.1152/ajpheart.00200.2006. [DOI] [PubMed] [Google Scholar]
- Islam S, Cevik C, Madonna R, Frandah W, Islam E, Islam S, Nugent K. Left ventricular assist devices and gastrointestinal bleeding: a narrative review of case reports and case series. Clinical Cardiol. 2013;36:190–200. doi: 10.1002/clc.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadohama T, Nishimura K, Hoshino Y, Sasajima T, Sumpio BE. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J Cell Physiol. 2007;212:244–251. doi: 10.1002/jcp.21024. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Bukhari R, Togawa T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46:127–137. doi: 10.1007/BF02463726. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol. 2011;300:C146–C154. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol. 1996;74:834–841. [PubMed] [Google Scholar]
- Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256:H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky HH, Kovalcheck S, Zweifach BW. The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circulation Res. 1978;43:738–749. doi: 10.1161/01.res.43.5.738. [DOI] [PubMed] [Google Scholar]
- Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- Malek AM, Zhang J, Jiang J, Alper SL, Izumo S. Endothelin-1 gene suppression by shear stress: pharmacological evaluation of the role of tyrosine kinase, intracellular calcium, cytoskeleton, and mechanosensitive channels. J Mol Cell Cardiol. 1999;31:387–399. doi: 10.1006/jmcc.1998.0873. [DOI] [PubMed] [Google Scholar]
- McCormick ME, Goel R, Fulton D, Oess S, Newman D, Tzima E. Platelet-endothelial cell adhesion molecule-1 regulates endothelial NO synthase activity and localization through signal transducers and activators of transcription 3-dependent NOSTRIN expression. Arterioscler Thromb Vasc Biol. 2011;31:643–649. doi: 10.1161/ATVBAHA.110.216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164:1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamied Y, Rowland EM, Bailey EL, Sherwin SJ, Schwartz MA, Weinberg PD. Change of direction in the biomechanics of atherosclerosis. Ann Biomed Eng. 2015;43:16–25. doi: 10.1007/s10439-014-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Mohan N, Sprague EA. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol. 1997;273:C572–C578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology. 2011;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer V, Sherwin SJ, Weinberg PD. Computation in the rabbit aorta of a new metric—the transverse wall shear stress—to quantify the multidirectional character of disturbed blood flow. J Biomech. 2013;46:2651–2658. doi: 10.1016/j.jbiomech.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard S. Vascular caliber. Cardiology. 1975;60:4–49. doi: 10.1159/000169701. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Bucci M, Fulton D, Segal SS, Sessa WC. Temporal events underlying arterial remodeling after chronic flow reduction in mice: correlation of structural changes with a deficit in basal nitric oxide synthesis. Circulation Res. 2000;86:1160–1166. doi: 10.1161/01.res.86.11.1160. [DOI] [PubMed] [Google Scholar]
- Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- Thoma R. Untersuchungen über die Histogenese und Histomechanik des Gefäß-systems. Stuttgart, Germany: Enke; 1893. [Google Scholar]
- Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J Biol Chem. 1999;274:20144–20150. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol. 2013;33:2130–2136. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lu H, Schwartz MA. A novel in vitro flow system for changing flow direction on endothelial cells. J Biomechanics. 2012;45:1212–1218. doi: 10.1016/j.jbiomech.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- Zhao SZ, Xu XY, Hughes AD, Thom SA, Stanton AV, Ariff B, Long Q. Blood flow and vessel mechanics in a physiologically realistic model of a human carotid arterial bifurcation. J Biomech. 2000;33:975–984. doi: 10.1016/s0021-9290(00)00043-9. [DOI] [PubMed] [Google Scholar]