Abstract
Background
The presence of nucleated red blood cells (NRBCs) in the peripheral blood of critically ill patients is associated with a poorer prognosis, though data on cardiovascular critical care patients is lacking. The aim of the present study was to assess the role of NRBCs as a predictor of intensive care unit (ICU) and in hospital all-cause mortality among cardiologic patients.
Methods
NRBCs were measured daily in consecutive cardiac ICU patients, including individuals with both coronary and non-coronary acute cardiac care. We excluded patients younger than 18 years, with cancer or hematological disease, on glucocorticoid therapy, those that were readmitted after hospital discharge and patients who died in the first 24 hours after admission. We performed a multiple logistic analysis to identify independent predictors of mortality.
Results
We included 152 patients (60.6 ± 16.8 years, 51.8% female, median ICU stay of 7 [4–11] days). The prevalence of NRBCs was 54.6% (83/152). The presence of NRBC was associated with a higher ICU mortality (49.4% vs 21.7%, P<0.001) as well as in-hospital mortality (61.4% vs 33.3%, p = 0.001). NRBC were equally associated with mortality among coronary disease (64.71% vs 32.5% [OR 3.80; 95%CI: 1.45–10.0; p = 0.007]) and non-coronary disease patients (61.45% vs 33.3% [OR 3.19; 95%CI: 1.63–6.21; p<0.001]). In a multivariable model, the inclusion of NRBC to the APACHE II score resulted in a significant improvement in the discrimination (p = 0.01).
Conclusions
NRBC are predictors of all-cause in-hospital mortality in patients admitted to a cardiac ICU. This predictive value is independent and complementary to the well validated APACHE II score.
Introduction
In healthy adults, peripheral blood is usually free of nucleated red blood cells (NRBCs) [1,2]. However, those cells may occur in some diseases, such as cancer, congestive heart failure, acute and chronic anemia and other hematological disorders [3,4]. Their presence in the peripheral blood has been associated with hypoxemia or infection in critical patients, owing to the high concentrations of erythropoietin, interleukin-3 and interleukin-6 [1,3,5–8] caused by local or systemic disorders, suggesting a reduction in oxidation of the tissue and/or inflammation.
Prior studies have also demonstrated that those cells may have significant prognostic implications, as their presence may occur in the three weeks prior to death [1–3]. In particular, Stachon et al. have demonstrated that NRBCs are a prognostic indicator in the Intensive Care Unit (ICU) environment, as its presence is associated with a higher in hospital mortality and higher ICU readmission rates, particularly when NRBC persist in the peripheral blood even after patients are clinically stable [1]. Although this has been demonstrated form general ICU patients, no data on patients admitted in the ICU for acute cardiovascular diseases exist. In the present study, we tested the hypothesis that the presence of NRBC may predict ICU (primary end-point) and in hospital (secondary end-point) all-cause mortality among patients admitted to a cardiac ICU.
Materials and Methods
Subjects and Protocol
All consecutive patients admitted in the cardiovascular ICU of the Pernambuco Cardiac Emergency Unit (PROCAPE), a specialized tertiary care cardiovascular teaching hospital with 250 beds, between May 2013 and January 2014 were included in the present study. This ICU is devoted to treat clinical patients with cardiovascular diseases. The study was approved by the Research Ethics Committee in the HOSPITAL COMPLEX HUOC/PROCAPE under number CAAE: 08412412.20000.5192 (Brazil Plataform). We excluded patients younger than 18 years, with cancer or hematological diseases, on glucocorticoid therapy, those that were readmitted after hospital discharge and patients who died in the first 24 hours after ICU admission. All patients included in the study signed a free and informed consent form.
The Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA) scores were calculated from all patients twenty-four hours after admission to ICU, as previously described [9,10].
In the first twenty-four hours of admission, the patients were also classified as septic or not, according to previous criteria [11]. At the same time, the patients were also classified according to the cardiovascular disease etiology as coronary (acute or chronic) [12,13] or non-coronary (valvulopathies, perimyocardiopathies, cardiac arrhythmias), according to clinical and laboratorial and echocardiographic parameters.
Laboratory tests
Blood samples were obtained daily in the morning until discharge from ICU. Blood parameters (NRBCs, leukocytes, neutrophils, hemoglobin and platelets) were measured using a Sysmex XE-2100 blood analyzer [14,15]. C-reactive protein was measured using a Roche Cobas Integra 400 analyzer.
For the NRBC measurement, we used the highest value during ICU admission for each individual. For the binary analysis, a positive NRBC was defined as any value above zero at any time during admission.
Statistical analysis
All continuous variables are expressed as means ± standard deviation, or median and quartiles, as appropriate. Categorical variables are presented as absolute values and percents. Categorical variables were compared using two-tailed Pearson’s chi-squared (X2) test with the Yates correlation or Fisher’s exact test. The comparison of means, to establish the normality of the distribution, was carried out using the Kolmogov-Smirnov test, followed by Student’s t test for normal distribution variables or Mann-Whitney’s non-parametric test form non-normal distribution variables. The relative mortality risk was calculated for clinical and laboratory variables, with confidence intervals of 95%. Logistic univariate regressions were performed to evaluate predictors of mortality analyzing gender, age, APACHE score, SOFA score, sepsis diagnosis, presence of coronary artery disease and laboratorial data (Table 1). A multivariate logistic regression model (forward) was carried out to identify independent predictors of mortality. Variables with P<0.1 on univariate analysis were entered into a multivariate analysis. Due to the highly skewed distribution of the NRBC, we chose to perform its analysis as a binary variable based on the presence or absence of NRBC in the peripheral blood. In order to evaluate the incremental value of NRBC beyond clinical predictor of mortality in the ICU, we constructed ROC curves and calculated the area under the curve for each model, and compared a model including only the APACHE II score with a model including the APACHE II score and the NRBCs. The level of statistical significance adopted was p < 0.05. Sample size was calculated to assess a mortality odds ratio between patients with and without NRBC of 5.2 according to previous study by Stachon et al [1], assuming an α-error of 5% and a statistical power of 95%. The minimum sample size was 60 patients. Because many patients from our sample did not present sepsis criteria and NRBCs in peripheral blood, we decided to recruit more patients than previously calculated. Statistical analyses were conducted using the Statistical Program for Social Sciences (SPSS), version 10.0 for Windows.
Table 1. Biological and clinical characteristics of patients in intensive care according to NRBC status.
| Characteristics | All patients | NRBC-positive (n = 83) | NRBC-negative (n = 69) | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 67 (44.1%) | 40 (48.2%) | 27 (22.0%) | 0.263 |
| Female | 85 (51.8%) | 43 (51.8%) | 42 (78.0%) | |
| Age (in years) | 60.6 ± 16.8 | 63.0 ± 16.1 | 57.6 ± 17.4 | 0.049 † |
| Skin color | ||||
| White | 59 (38.8%) | 30 (36.1%) | 29 (42.0%) | 0.707 |
| Mixed | 67 (44.1%) | 39 (47.0%) | 28 (40.6%) | |
| Black | 26 (17.1%) | 14 (16.9%) | 12 (17.4%) | |
| Origin | ||||
| Emergency | 123 (80.9%) | 65 (78.3%) | 58 (84.1%) | 0.568 |
| Wards | 22 (14.5%) | 13 (15.7%) | 9 (13.0%) | |
| CTRU | 7 (4.6%) | 5 (6.0%) | 2 (2.9%) | |
| Median duration of stay in ICU (in days)* | 7 (4; 11) | 10 (5; 13) | 4 (3; 7) | < 0.001 † |
| Mortality | ||||
| ICU | 56 (36.8%) | 41 (49.4%) | 15 (21.7%) | < 0.001 † |
| Hospital | 74 (48.7%) | 51 (61.4%) | 23 (33.3%) | 0,001 † |
| Median APACHE II* | 21 (14; 27.5) | 25 (19; 33) | 15 (11; 22) | < 0.001 † |
| APACHE II | ||||
| < 25 points | 56 (36.8%) | 39 (47.0%) | 57 (82.6%) | < 0.001 † |
| ≥ 25 points | 96 (63.2%) | 44 (53.0%) | 12 (17.4%) | |
| Median SOFA* | 5 (2; 9) | 8 (4; 11) | 3 (1; 7) | < 0.001 † |
| SOFA score | ||||
| < 7 points | 86 (56.6%) | 35 (42.2%) | 51 (73.9%) | < 0.001 † |
| ≥ 7 points | 66 (43.4%) | 48 (57.8%) | 18 (26.1%) | |
| SEPSIS | ||||
| Yes | 84 (55.3%) | 59 (71.1%) | 25 (36.2%) | < 0.001 † |
| No | 68 (44.7%) | 24 (28.9%) | 44 (63.8%) | |
| Coronary patient | ||||
| Yes | 74 (48.7%) | 34 (41.0%) | 40 (58.0%) | 0.037 † |
| No | 78 (51.3%) | 49 (59.0%) | 29 (42.0%) | |
| Coronary/Sepsis | ||||
| Sepsis and coronary | 30 (19.7%) | 19 (22.9%) | 11 (15.9%) | < 0.001 † |
| Sepsis and non-coronary | 54 (35.5%) | 40 (48.2%) | 14 (20.3%) | |
| No sepsis and coronary | 44 (29.0%) | 15 (18.1%) | 29 (42.0%) | |
| No sepsis and non-coronary | 24 (15.8%) | 9 (10.8%) | 15 (21.7%) |
NRBCs: nucleated red blood cells APACHE II: Acute Physiology and Chronic Health Evaluation II. SOFA: Sequential Organ Failure Assessment CTRU: Cardio-Thoracic Recovery Unit.
† Statistically significant association.
*Median (P25; P75).
Results
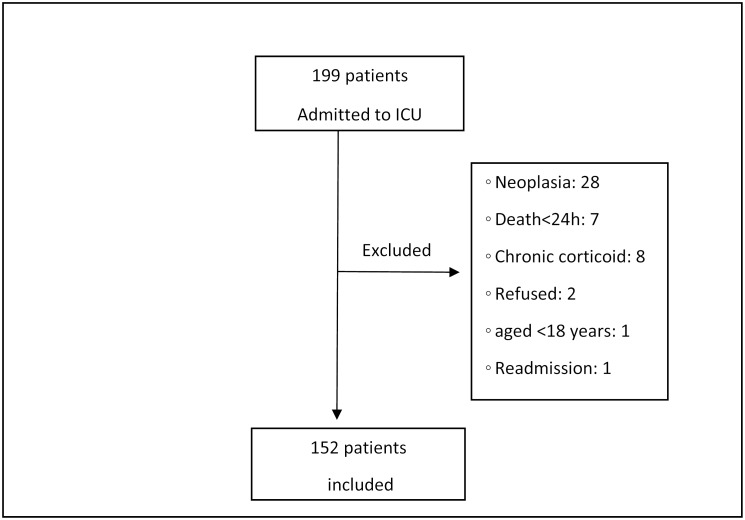
The study initially screened 199 patients, of whom 47 were excluded as shown in Fig 1. There were no follow-up losses and the final sample comprised 152 patients (60.6 ± 16.8 years, 51.8% female), with a median stay in ICU of 7 days (P25: 4; P75: 11) (Table 1). Approximately half of the sample presented NRBCs in the blood (83 patients; 54.6%) and the higher incidence of NRBC was noted after the ninth ICU day (Table 2). The presence of NRBCs was associated with older age, longer ICU stay, higher severity (SOFA and APACHE II) scores, sepsis and non-coronary cardiac etiology (Table 1). There was a negative correlation between levels of platelets and those of NRBCs (R = -0.25; p = 0.03). However, levels of C-reactive protein (R = 0.18; p = 0.14), leukocytes (R = 0.22; p = 0.06), neutrophils (R = 0.20; p = 0.09), and hemoglobin (R = -0.02; p = 0.87) were not associated with levels of NRBCs.
Fig 1. Flowchart of patients.
Table 2. Distribution of the measure of the maximum NRBC during the hospital stay in the ICU.
| NRBC | Statistics |
|---|---|
| Sample | 152 patients |
| NRBC maximum rating | |
| Zero | 69 (45.4%) |
| From 1 a 100 | 50 (32.9%) |
| From 101 a 200 | 18 (11.8%) |
| > 200 | 15 (9.9%) |
| Presence of NRBC per day of hospitalization | |
| 1° day | 34/152* (22.4%) |
| 2° day | 36/124 (29.0%) |
| 3° day | 30/110 (27.3%) |
| 4° day | 20/77 (25.9%) |
| 5° day | 24/84 (28.6%) |
| 6° day | 16/57 (28.1%) |
| 7° day | 15/67 (22.4%) |
| 8° day | 10/46 (21.7%) |
| 9° day | 17/44 (38.6%) |
| After 9° day | 11/35 (31.4%) |
* Number of patients with positive NRBC / Number of patients with measures NRBC.
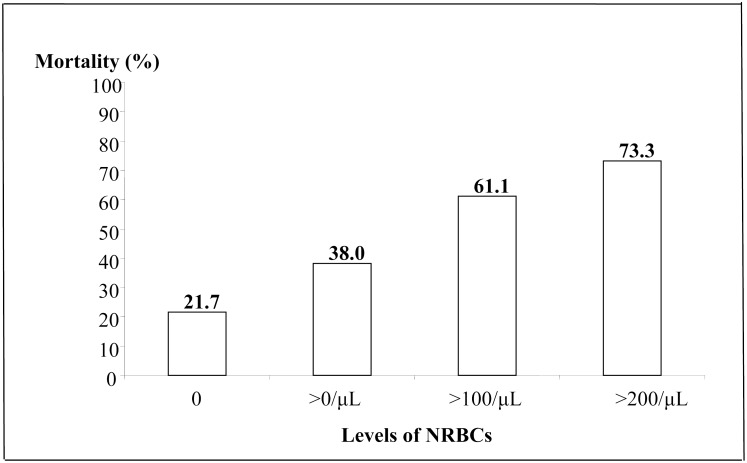
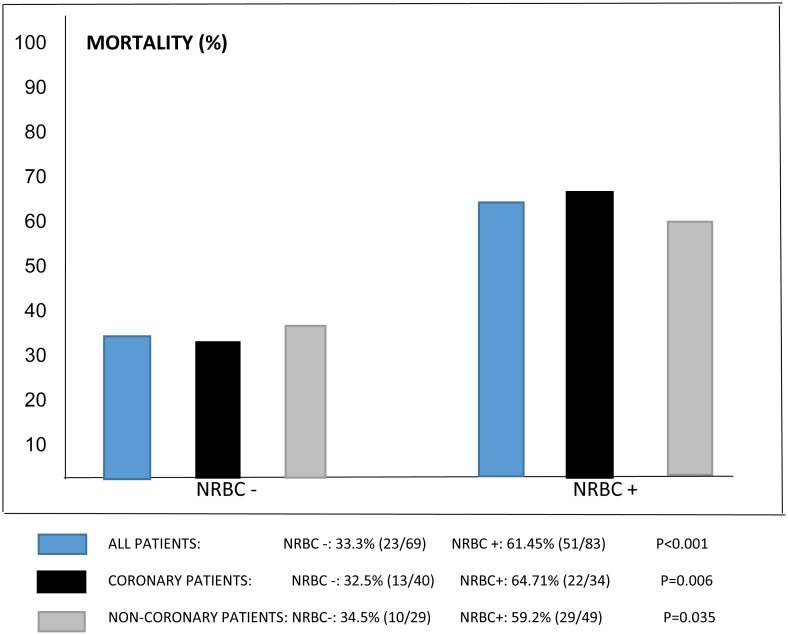
While the overall cardiovascular ICU mortality was 36.8% (56/152), increasing levels of NRBC were associated with a higher ICU mortality (Fig 2). Hospital mortality following discharge from ICU was 18.8% (18/96), with an in-hospital all-cause mortality of 48.7% (74/152). The presence of NRBC in the peripheral blood was associated with both ICU (49.4% vs. 21.7%, p<0.001) and in-hospital (61.4% vs. 33.3%, p = 0.001) all-cause mortality rates, respectively (Table 1) and was also associated with increased mortality among patients with coronary disease with 64.7% vs. 32.5% (OR 3.80; 95%CI: 1.45–10.0; p = 0.006), as well as among non-coronary disease patients with 59.2% vs 34.5% (OR 2.75; 95% CI: 1.06–7.15; p = 0.035) (Fig 3).
Fig 2. Mortality of ICU patients in relation to concentrations of nucleated red blood cells (NRBCs) P<0.001.
Fig 3. Mortality among coronary and non-coronary patients.
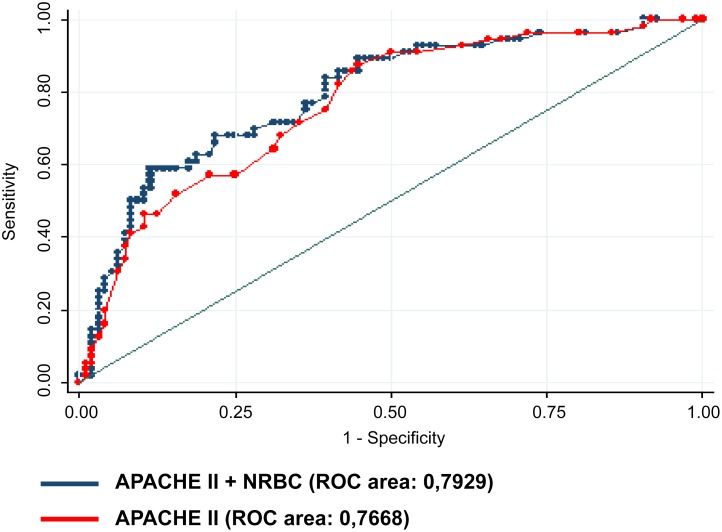
After adjustment, for the clinical mortality risk using the APACHE II score, sex and sepsis, NRBC remained associated with all-cause in hospital mortality (OR 3.96; 95% CI: 1.45–10.8; p = 0.007) (Table 3). The inclusion of NRBC in a model already including the APACHE II score resulted in a significant increase in the area under the ROC curve from 0.7668 to 0.7929, p = 0.011 (Fig 4).
Table 3. Univariable and multivariable predictors of ICU mortality.
| Variables | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| OR | CI (95%) | P | OR | CI (95%) | P | |
| Biological | ||||||
| Sex | ||||||
| Male | 1.0 | - | - | 1.0 | - | - |
| Female | 2.07 | 1.06–4.03 | 0.034 † | 1.69 | 0.81–3.55 | 0.162 |
| Clinical | ||||||
| APACHE II Classification | ||||||
| < 25 points | 1.0 | - | - | 1.0 | - | - |
| ≥ 25 points | 4.0 | 1.98–8.07 | 0.000 † | 2.41 | 1.08–5.37 | 0.031 |
| SOFA Classification | ||||||
| < 7 points | 1.0 | - | - | - | - | - |
| ≥ 7 points | 7.76 | 3.67–16.4 | 0.000 † | - | - | - |
| Sepsis | ||||||
| No | 1.0 | - | - | |||
| Yes | 3.37 | 1.64–6.89 | 0.001 † | 1.81 | 0.79–4.12 | 0.159 |
| Coronary patient | ||||||
| No | 1.0 | - | - | - | - | - |
| Yes | 0.69 | 0.36–1.34 | 0.270 | - | - | - |
| Laboratory | ||||||
| NRBC a | ||||||
| Zero | 1.0 | - | - | 1.0 | - | - |
| From 1 a 100 | 2.21 | 0.98–4.95 | 0.055 | 1.43 | 0.59–3.47 | 0.428 |
| > 100 | 7,20 | 2.86–18.1 | 0.000 | 3.96 | 1.45–10.8 | 0.007 |
| CRP b | 1.015 | 0.997–1.034 | 0.100 | - | - | - |
OR: Odds Ratio. NRBC: nucleated red blood cells. APACHE II: Acute Physiology and Chronic Health Evaluation II. SOFA: Sequential Organ Failure assessment.
a Divided into (0. 1–100. > 100).
b Calculated risk of 5 unit increase.
†Statistical significance.
Fig 4. Comparison of ROC curves, p = 0.01.
Discussion
In the present study, we have demonstrated that the presence and levels of NRBC in the peripheral blood are independent predictors of all-cause in-hospital mortality for patients admitted to a cardiac ICU. This association also resulted in an important improvement in the discrimination beyond the well-validated APACHE II score.
Our findings corroborate previous results in patients admitted to general ICUs, suggesting that NRBCs are equally predictive of events in both the cardiac and non cardiac population. [1–7]. In this population, coronary pathologies were not found to be associated with mortality (OR 0.90; p = 0.739) and the presence NRBCs, as in general ICUs, was the best indicator of higher mortality (adjusted OR 3.24; CI (95%) 1.64–6.38; p = 0.001). Possible mechanisms involved in higher mortality among patients with elevated NRBC are hypoxemia and systemic inflammation [1,3,5–7]. Interestingly, our results have a much higher prevalence of NRBC (54.6%) then previous studies such as Stachon et al. [1] (17.5%), Desai et al. [3] (17.5%), Kuert et al. [5] (28.6%), and Shah et al. [7] (24.8%). The higher frequency of the presence of NRBCs in the present study may be explained by the overall higher severity of disease in our population, as expressed by the fact that 63.2% of the patients had an APACHE II score ≥25.
When the severity of disease, represented by the APACHE II score, is taken into account, the prevalence of our study closely replicate those previous studies, as in patients with APACHE II score ≥25, in whom the majority were NRBC-positive (53%). This concurs with the findings of Stachon et al. [1] and Desai et al. [3]. Moreover, the median SOFA score in this study was 8 in NRBC-positive patients and 3 in those who were NRBC-negative (Table 1). Desai et al. [3] found a median SOFA score of 10 among NRBC-positive and 8 among the NRBC-negative. There would therefore appear to be an association between the presence of NRBCs in the bloodstream and the seriousness of the condition of patients in ICU.
Patients who are candidates for discharge from a general ICU who present with NRBCs should be carefully evaluated, since they have a higher mortality rate [1]. The present study corroborates these findings and suggests a possible new use for measurement of NRBC levels in ICU, since the mortality of NRBC-positive patients discharged from ICU was 61.4%.
Our study must, however, be read within the context of its design. First, we have not split the coronary group into acute and chronic cases, which may have led to bias in analysis of this sub-group and influenced other laboratory variables, such as platelets [16–18]. Second, the small number of patients in extreme NRBC levels may limit our analysis. However, despite the relatively small sample size, the power for detecting differences in mortality among patients with and without NRBC was 92%. The estimates presented in the study are influenced by random error and from that standpoint may not be dependable.
In conclusion, NRBC predict all-cause mortality in patients admitted to a cardiac ICU. The predictive value of NRBC is independent and complementary to the prediction provided by the well validated APACHE II score.
Acknowledgments
We would like to thank Paulo Fernando Leite Filho for the computer system, Ulisses Ramos Montarroyos for the statistical analysis and the whole team of the Coronary Unit and Laboratory of PROCAPE, Pernambuco, Brazil.
Data Availability
All relevant data are within the paper.
Funding Statement
These authors have no support or funding to report.
References
- 1. Stachon A, Segbers E, Holland-Letz T, Kempf R, Hering S, Krieg M. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Critical Care 2007, 11(3): R62 10.1186/cc5932 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stachon A, Holland-Letz T, Krieg M. High in-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin Chem Lab Med 2004, 42(8):933–38. . [DOI] [PubMed] [Google Scholar]
- 3. Desai S, Jones SL, Turner KL, Hall J, Moore LJ. Nucleated red blood cells are associated with a higher mortality rate in patients with surgical sepsis. Surgical Infections 2012, 13(6):360–5. 10.1089/sur.2011.089 . [DOI] [PubMed] [Google Scholar]
- 4. Danise P, Maconi M, Barella F, Palma AD, Avino D, Rovetti A, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med 2012, 50(2):357–60. 10.1515/CCLM.2011.766 . [DOI] [PubMed] [Google Scholar]
- 5. Kuert S, Holland-Letz T, Friese J, Stachon A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med 2011, 49(2):257–63. 10.1515/CCLM.2011.041 . [DOI] [PubMed] [Google Scholar]
- 6. Stachon A, Bolulu O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and levels of erythropoietin, interleukin-3, interleukin-6, and interleukin-12p70. Shock 2005, 24(1):34–9. . [DOI] [PubMed] [Google Scholar]
- 7. Shah R, Reddy S, Horst M, Stassnopoulos J, Lordan J, Rubinfeld I. Getting back to zero with nucleated red blood cells: following trends is not necessarily a bad thing. The American Journal of Surgery 2012. March; 203(3): 343–5. 10.1016/j.amjsurg.2011.10.002 . [DOI] [PubMed] [Google Scholar]
- 8. Roger HM, Xiaobing Y, Wen J, Smith R, Fibach E, Noguchi CT. Hypoxia Alters Progression of the Erythroid Program. Experimental Hematology 2008. January; 36(1):17–27. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985. January; 13(10):818–29. . [PubMed] [Google Scholar]
- 10. Vicent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis—Related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996. July; 22(7):707–10. . [DOI] [PubMed] [Google Scholar]
- 11. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: Internacional Guidelines for Management of Severe Sepsis and Septic Shock 2012. Intensive Care Med 2013. February; 39(2):165–228. 10.1007/s00134-012-2769-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistente ST-segment elevation of the European Society of Cardiology (ESC). European Heart jornal 2011. December; 32(23):2999–3054. 10.1093/eurheartj/ehr236 . [DOI] [PubMed] [Google Scholar]
- 13. Fraker TD, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, et al. 2007 Chronic angina focused update of the ACC/AHA 2002 Guidelines for the Management of patients with Chronic Stable Angina: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to Develop the Focused Update of the 2002 Guidelines for the Management of Patients with Chronic Stable Angina. Circulation 2007. December 4; 116(23): 2762–72. . [DOI] [PubMed] [Google Scholar]
- 14. Nakul-Aquaronne D, Sudaka-Sammarcelli I, Ferrero-Vacher C, Starck B, Bayle J. Evaluation of the Sysmex Xe-2100 hematology analyzer in hospital use. Journal of Clinical Laboratory Analysis 2003; 17(4): 113–23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pipitone S, Pavesi F, Testa B, Bardi M, Peri GB, Gennari D, et al. Evaluation of automated nucleated red blood cells counting on Sysmex XE5000 and Siemens ADVIA 2120. Clin Chem Lab Med 2012. October 1; 50(10):1857–9. 10.1515/cclm-2012-0148 . [DOI] [PubMed] [Google Scholar]
- 16. Núñez J, Núñez E, Bodí V, Sanchis J, Mainar L, Miñana G, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coronary Artery Disease 2010. January; 21(1):1–7. . [DOI] [PubMed] [Google Scholar]
- 17. Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST elevation myocardial infarction. The American Journal of Cardiology 2010. August 15; 106(4): 470–6. 10.1016/j.amjcard.2010.03.062 . [DOI] [PubMed] [Google Scholar]
- 18. Labriolle AD, Bonello L, Lemesle G, Roy P, Steinberg DH, Xue Z, et al. Decline in platelet count in patients treated by percutaneous coronary intervention: definition, incidence, prognostic importance and predictive factors. European Heart Journal 2010. May; 31(9): 1079–87. 10.1093/eurheartj/ehp594 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.