Abstract
Objective
Most comprehensive treatments for PBC include UDCA, combination of methotrexate (MTX), corticosteroids (COT), colchicine (COC) or bezafibrate (BEF), cyclosporin A (CYP), D-penicillamine (DPM), methotrexate (MTX), or azathioprine (AZP). Since the optimum treatment regimen remains inconclusive, we aimed to compare these therapies in terms of patient mortality or liver transplantation (MOLT) and adverse event (AE).
Methods
We searched PubMed, Embase, Scopus and the Cochrane Library for randomized controlled trials until August 2014. We estimated HRs for MOLT and ORs for AE. The sensitivity analysis based on dose of UDCA was also performed.
Results
The search identified 49 studies involving 12 different treatment regimens and 4182 patients. Although no statistical significance can be found in MOLT, COT plus UDCA was ranked highest for efficacy outcome amongst all the treatment regimes. While for AEs, compared with OBS or UDCA, monotherapy with COC (OR 5.6, P < 0.001; OR 5.89, P < 0.001), CYP (OR 3.24, P < 0.001; OR 3.42, P < 0.001), DPM (OR 8.00, P < 0.001; OR 8.45, P < 0.001) and MTX (OR 5.31, P < 0.001; OR 5.61, P < 0.001) were associated with statistically significant increased risk of AEs. No significant differences were found for other combination regimes. Effect estimates from indirect comparisons matched closely to estimates derived from pairwise comparisons. Consistently, in the sensitivity analysis, results closely resembled our primary analysis.
Conclusions
COT plus UDCA was the most efficacious among treatment regimens both for MOLT and AEs.
Keywords: primary biliary cirrhosis, UDCA-based therapy, adverse events, network meta-analysis, indirect comparison
INTRODUCTION
Primary biliary cirrhosis (PBC) is a chronic progressive inflammatory autoimmune- mediated liver disease that primarily affects middle-aged women with a gender ratio of 1:10 [1]. The annual incidence of primary biliary cirrhosis ranges from 1 to 49 persons per million, and the prevalence was estimated between 7 to 402 persons per million [2].
So far, some single or combined therapies have been studied by randomized controlled trials (RCTs). However, which treatment regime is optimal for patients with PBC remains controversial. Although UDCA is the only medical treatment that has received U.S. Food and Drug Administration approval [3], the effects of UDCA evaluated by several clinical studies have yielded conflicting results. Some randomized clinical trials (RCT) have confirmed that UDCA is an effective medical treatment for the disease [4–7]. Also, UDCA has been shown to improve not only symptoms, liver enzymes, and liver histology, but also patient survival [8–9]. Conversely, two comprehensive traditional meta-analyses which include 16 recent RCTs concluded that the use of UDCA did not demonstrate any benefit on mortality and mortality or live transplantation (MOLT) [10–11]. Furthermore, PBC disease continued to progress in many patients who do not show complete response during UDCA therapy, hence, additional medical treatment or other drugs are urgently required.
Other drugs for the treatment of PBC have been studied for the past decade as single agents or adjuvant medications, which are mainly immunomodulatory and other agents, such as colchicine (COC), cyclosporin A (CYP), D-penicillamine (DPM), methotrexate (MTX), corticosteroids (COT), bezafibrate (BEF), or azathioprine (AZP). Although some of these drugs when administered as monotherapy showed little benefit for patients with PBC in clinical outcomes from several conventional meta-analyses, we are still unable to identify the clinical effects of drugs evaluated in traditional meta-analysis with a small number of included studies. Moreover, addition of these treatments to UDCA was evaluated by some studies. Combination therapy with UDCA and COC in patients with PBC has been evaluated in a small study and in a large double blind, placebo-controlled study [12–13]. Results from the Japanese study (12) indicate no benefits from the addition of COC to UDCA, whilst the French RCT (13) suggests that the addition of COC to UDCA provides only a marginal advantage over UDCA monotherapy. The role of combination therapy with MTX and UDCA were evaluated by Leung et al, which showed a clinical improvement compared with that predicted by the Mayo model [3, 14]. However, another RCT evaluating a combination of MTX plus UDCA demonstrated that there was no improvement in symptoms and the regime may have been associated with substantial toxicity. Consistently, the clinical outcomes of other additional therapies (COT or BEF) to UDCA still remain unclear or yield inconsistent results [2, 15].
Therefore, in order to attempt to resolve this issue, which theoretically may be answered by conducting a very large clinical trial with multiple comparator arms, of conflicting results in PBC treatments for patients, we performed the network meta-analysis, which allows us to integrate direct and indirect comparisons to simultaneously compare several treatments [16–18]. The objective of network meta-analysis was to apply the established methodology used in network meta-analysis to an area of clinical practice where no such previous studies have existed. In such cases, our aims were to summarize a much broader evidence base and to indirectly compare the relative efficacy and safety of these treatments with most comprehensive therapies (AZP, MTX, COT, COC, CYP, DPM, UDCA, MTX plus UDCA, COT plus UDCA, COC plus UDCA or BEF plus UDCA) for patients with PBC.
RESULTS
Study characteristics
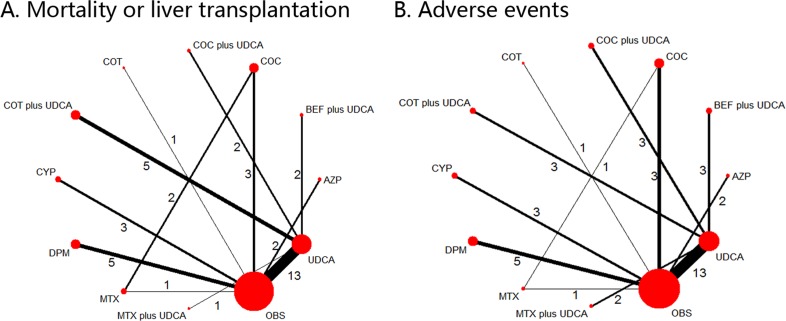
3133 studies were identified for review of title and abstract (Supplementary 1). After the initial screening, we retrieved the full text of potentially eligible articles for detailed assessment, 3084 articles were excluded. We included forty-nine eligible studies for meta-analysis, with a total of 4182 patients who received one of the eleven treatment strategies including monotherapy with UDCA, AZP, MTX, COT, COC, CYP, DPM, combination of MTX plus UDCA, COT plus UDCA, COC plus UDCA, BEF plus UDCA or observation (Figure 1). The duration of treatment ranged from three months to ten years, and the mean age of trial participants was 55.3 years and ranged from 24 to 79 years. Most trials (47 [96%] of 31) were two-grouped studies and only two [19–20] were three-grouped studies and mean study sample was 41.8 patients per group (minimum-maximum 4-176). For the primary outcome, twelve unique comparisons were available for 40 [2, 4–5, 7, 15, 20–37] different trials. In terms of outcome of safety, there were also 40 [2–5, 7, 12–13, 20–32, 34–35, 38–55] trials providing data for twelve unique comparisons. Table 1 summarizes the characteristics of the included forty-nine studies. Supplementary 2 shows the quality assessment parameter assessed by the Cochrane Risk of Bias tool, which was rated as good, even though some studies did not reported details regarding randomization and allocation concealment.
Figure 1. Network of the comparisons for the Bayesian network meta-analysis.
The numbers along the link lines indicate the number of trials or pairs of trial arms. Lines connect the interventions that have been studied in head-to-head (direct) comparisons in the eligible controlled trials. The width of the lines represents the cumulative number of trials for each comparison and the size of every node is proportional to the number of enrolled participants (sample size). COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; OBS: observation. A. Mortality or liver transplantation; B. Adverse events.
Table 1. Characteristics of included studies.
| Author (Year) | Country | Mean age (range) | Treatment/Control | Dose | Treatment duration | Study size | Mortality or liver transplantation (%) | Adverse events (%) |
|---|---|---|---|---|---|---|---|---|
| Treatment/Control | Treatment/Control | |||||||
| Itakura (2004) | Japan | 57 (47–67) | BEF plus UDCA/UDCA | BEF: 400 mg per day; UDCA: 600 mg per day | 6 months | 16 | 8/6 | 11/14 |
| Iwasaki (a) (2008) | Japan | 56 (45–67) | BEF plus UDCA/UDCA | BEF: 400 mg per day; UDCA: 600 mg per day | 52 weeks | 22 | 0/0 | 42/10 |
| Iwasaki (b) (2008) | Japan | 56(28–75) | BEF/UDCA | BEF :400 mg daily orallyUDCA;600 mg daily | 52 weeks | 45 | NR/NR | |
| Kanda (2003) | Japan | 57 (52–72) | BEF plus UDCA/UDCA | BEF: 400 mg per day; UDCA: 600 mg per day | 6 months | 22 | 11/11 | 18/9 |
| Leuschner (1999) | German | 50 (40–60) | COT plus UDCA/UDCA | COT: 3 mg three times daily; UDCA: 10–15 mg/kg/day in three divided doses | 2 years | 40 | 5/5 | 10/5 |
| Combes (2005) | United States | 53 (42–60) | MTX plus UDCA/UDCA | MTX: in four doses over 48 hours (total dose 10 mg/week); UDCA: 500 mg per day | 117 months | 265 | NR/NR | 10/9 |
| Gonzalez-Koch (1997) | Chile | 53 (45–75) | MTX plus UDCA/UDCA | UDCA: 13–15mg/kg/day MTX: 0.25mg/kg/week | 48 weeks | 25 | 12/10 | 25/8 |
| Lindor (1995) | United States | 59.5 (40–75) | MTX plus UDCA/UDCA/OBS | UDCA: 13–15mg/kg/day MTX: 0.25mg/kg/week | 2 years | 121 | NR/NR/NR | 22/NR/NR |
| Leung (2011) | United States | 54 (52–56) | MTX plus UDCA/COC plus UDCA | UDCA:13–15mg/kg/day; MTX:0.25mg/kg/week; COC: 1 mg/day | 10 years | 29 | NR/NR | NR/NR |
| Battezzati (1993) | Italy | 57 (42–60) | UDCA/OBS | 500 mg per day | 1 year | 88 | 43/43 | 9/2 |
| Combes (1995) | United States | 49 (52–56) | UDCA/OBS | 10 to 12 mg/kg/day | 2 years | 151 | 70/70 | 17/16 |
| Eriksson (1997) | Sweden | 57 (46–70) | UDCA/OBS | 500 mg per day | 2 years | 116 | 58/52 | 12/7 |
| Heathcote (1994) | Canada | 56 (50–62) | UDCA/OBS | 14mg/kg/day | 2 years | 222 | 91/94 | 16/24 |
| Hwang (1993) | China | 58 (50–66) | UDCA/OBS | 600 mg/day. | 3 months | 12 | 6/6 | 17/17 |
| Leuschner (1989) | German | 53 (53–73) | UDCA/OBS | 10 mg/kg/day | 9 months | 20 | 9/9 | 10/10 |
| Lindor (1994) | United States | 53 (51–55) | UDCA/OBS | 13 to 15mg/kg/day | 4 years | 180 | 75/68 | 8/13 |
| Oka (1990) | Japan | 59 (51–67) | UDCA/OBS | 600 mg/day | 24 weeks | 52 | 25/25 | 15/4 |
| Papatheodoridis (2002) | Greece | 54 (44–64) | UDCA/OBS | 12 to 15 mg/kg/day | 92 months | 86 | 26/29 | 53/40 |
| Pares (2000) | Spain | 54 (44–64) | UDCA/OBS | 14 to 16 mg/kg/day | 2 years | 192 | 89/89 | 26/18 |
| Poupon (1991) | France | 56 (47–65) | UDCA/OBS | 13 to 15 mg/kg/day | 2 years | 146 | 67/63 | 15/26 |
| Turner (1994) | England | 57 (54–60) | UDCA/OBS | 10mg/kg/day | 2 years | 46 | 21/21 | 27/29 |
| Vuoristo (1995) | Finland | 55 (50–60) | UDCA/OBS | 12 to 15 mg/kg/day | 2 years | 59 | 29/26 | 3/29 |
| Chazouilleres (1998) | France | 50 (47–53) | UDCA/COT plus UDCA | UDCA: 13 to 15 mg/kg/day; COT: 0.5 mg/kg/day | 23 months | 11 | 3/5 | 20/33 |
| Gunsar (2002) | Turkey | 44 (40–55) | UDCA/COT plus UDCA | UDCA: 13 mg/kg/day; COT: 0.5 mg/kg/day | 28 months | 20 | 12/5 | 15/14 |
| Chazouilleres (2006) | France | 41 (39–57) | UDCA/COT plus UDCA | UDCA: 13 to 15 mg/kg/day; COT: 0.5 mg/kg/day | 90 months | 17 | NR/NR | NR/NR |
| Heurgue (2007) | France | 44 (41–56) | UDCA/COT plus UDCA | UDCA: 13 to 15 mg/kg/day; COT: 0.5 mg/kg/day | 60 months | 13 | NR/NR | NR/NR |
| Ozaslan (2010) | Turkey | 44 (40–51) | UDCA/COT plus UDCA | UDCA: 13 to 15 mg/kg/day; COT: 0.5 mg/kg/day | 31 months | 12 | 2/6 | NR/NR |
| Tanaka (2011) | Japan | 54 (50–60) | UDCA/COT plus UDCA | UDCA: 10 mg/kg/day; COT: 0.5 mg/kg/day | 73 months | 25 | 14/8 | NR/NR |
| Ikeda (1996) | Japan | 57 (55–60) | COC plus UDCA/UDCA | COC: 1 mg/kg/day; UDCA: 600 mg/day | 30 months | 22 | NR/NR | 30/8 |
| Poupon (1996) | France | 53 (51–55) | COC plus UDCA/UDCA | COC: 1 mg/d, 5 days per week; UDCA: 13–15 mg/day | 2 years | 74 | NR/NR | 5/3 |
| Almasio (2000) | Italy | 55 (45–65) | COC plus UDCA/UDCA | COC: 1 mg/daily; UDCA: 500 mg/daily | 3 years | 90 | 35/41 | 4/2 |
| Battezzati (2001) | Italy | 58 (48–68) | COC plus UDCA/UDCA | COC: 1 mg/daily; UDCA: 500 mg/daily | 10 years | 44 | 14/8 | NR/NR |
| Christensen(1985) | Denmark | 55 (25–78) | AZP/OBS | 300 to 700 mg/week | 11 years | 248 | 58/72 | 6/7 |
| Heathcote(1976) | UK | 51 (24–79) | AZP/OBS | 2 mg/kg/day | 5 years | 45 | 36/52 | 16/0 |
| Lombard(1993) | UK | 54 (44–64) | CYP/OBS | 3 mg/kg/day | 928 days | 349 | 17/18 | 56/42 |
| Minuk(1988) | Canada | 51 (41–61) | CYP/OBS | 2.5 mg/kg/day | 1year | 12 | 0/17 | 100/17 |
| Wiesner(1990) | USA | 46 (37–57) | CYP/OBS | 4 mg/kg/day | 2.7 years | 29 | 5/30 | 79/50 |
| Dickson(1985) | USA | 46 (36–56) | DPM/OBS | 250 mg/day | 10 years | 227 | 40/40 | 53/22 |
| Epstein(1981) | UK | 53 (43–63) | DPM/OBS | over 8 to 10 weeks from 150 mg/day to 600 mg/day | 6 years | 98 | 30/43 | 31/3 |
| Matloff(1982) | USA | 52 (42–62) | DPM/OBS | 1g/day | 28 months | 52 | 62/12 | 35/4 |
| Neuberger(1985) | UK | 53 (41–62) | DPM/OBS | 1.2 g/day, increased from 300 mg by 300 mg each fortnight until 1.2 g | 4 years | 189 | 54/32 | 36/8 |
| Taal(1983) | Netherlands | 51 (43–62) | DPM/OBS | 1 g/day (increased from 250 mg every month until 1 g for the first 6 months. After that, decreased to 500 mg/day for the remaining 6 months | 1 years | 24 | 18/31 | 100/85 |
| Mitchison (1992) | England | 52 (44–60) | COT/OBS | initially 30 mg/day then reduced by 5 mg/day every two weeks until a maintenance dose of 10 mg/day was reached. | 3 years | 36 | 16/29 | 79/24 |
| Hendrickse(1999) | England | 57 (55–59) | MTX/OBS | 7.5 mg/week. | 6 years | 60 | 40/37 | 83/73 |
| Kaplan(2003) | USA | 51 (50–53) | MTX plus COC | MTX: 15 mg/week, 5 mg every 12 hours 3 times.COC: 0.6 mg twice daily | 2 years | 85 | 57/44 | 17/7 |
| Kaplan(1999) | USA | 51 (50–53) | COC plus MTX | COC: 0.6 mg twice dailyMTX: 15 mg/wk, taken as 5 mg every 12 hours 3 times | 24 months | 85 | 23/29 | NR/NR |
| Kaplan(1986) | USA | 48 (40–68) | COC/OBS | 0.6 mg twice daily. | 2 years | 60 | 20/47 | 13/3 |
| Warnes(1987) | UK | 53 (47–64) | COC/OBS | 1 mg/day | 18 months | 64 | 15/30 | 56/7 |
| Bodenheimer(1988) | USA | 52 (34–59) | COC/OBS | 0.6 mg twice daily. | 5 years | 57 | NR/NR | 14/3 |
| Zifroni (1991) | USA | 57 (50–65) | COC/OBS | 0.6 mg twice daily. | 4 years | 57 | 46/48 | 11/0 |
Notes: COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; OBS: observation; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; NR: not reported.
Results from pair-wise comparisons
Seven different comparisons were accomplished in pairwise meta-analysis. The weighted HRs for MOLT and ORs for AEs, respectively, were calculated for each comparison. The geometric distribution of controlled trials on MOLT (Figure 1A) and AEs (Figure 1B) were displayed. The weighted hazard ratios and odds ratios for the two outcomes, MOLT and AEs, were calculated for each comparison. Statistical heterogeneity was assessed using the I2 statistic, which was assessable in two of the comparisons. Overall, statistical heterogeneity was moderate, although for some comparisons 95% CIs were wide and included values indicating very high or no heterogeneity (Table 2). In the meta-analyses of direct comparisons for efficacy, I² values higher than 75% were recorded for only two comparisons: COC plus UDCA versus UDCA (86.9%), with two studies in the meta-analysis, and DPM versus OBS, with five studies included. While in terms of AEs, I² values higher than 50% were recorded for only one comparison: CYP versus OBS (52.6%), with three studies in the meta-analysis, and the remaining comparisons were all lower than 40%. In addition, all P values for Begg's rank correlation test and Egger's test were greater than 0.05, indicating that no publication bias was found amongst those pairwise comparisons of different treatment regimens. (Table 3).
Table 2. Comparison of outcomes between pair-wise meta-analysis and network meta-analysis.
| Treatment comparisons | Results of pair-wise meta-analysis | Results of network meta-analysis |
|---|---|---|
| Clinical improvement | ||
| MTX vs OBS | 1.15 (0.41, 3.26) | 0.95 (0.32, 2.78) |
| DPM vs OBS | 1.51 (0.63, 3.61) | 1.54 (0.76, 3.17) |
| CYP vs OBS | 0.57 (0.17, 1.88) | 0.53 (0.16, 1.46) |
| COT vs OBS | 0.45 (0.09, 2.26) | 0.43 (0.05, 3.34) |
| COC vs OBS | 0.50 (0.24, 1.01) | 0.57 (0.25, 1.26) |
| MTX vs COC | 1.51 (0.80, 2.88) | 1.67 (0.64, 4.29) |
| AZP vs OBS | 0.54 (0.33, 0.88) | 0.53 (0.18, 1.56) |
| UDCA vs OBS | 0.93 (0.65, 1.31) | 0.78 (0.45, 1.27) |
| UDCA vs UDCA plus COT | 0.68 (0.20, 2.27) | 0.38 (0.09, 1.39) |
| UDCA vs UDCA plus BEF | 0.75 (0.04, 14.58) | 0.77 (0.06, 9.89) |
| MTX plus UDCA vs UDCA | 0.42 (0.03, 5.30) | 0.74 (0.24, 2.44) |
| COC plus UDCA vs UDCA | 1.17 (0.09, 14.55) | 1.05 (0.33, 3.26) |
| Adverse events | ||
| MTX VS OBS | 1.82 (0.52, 6.38) | 5.31 (1.21, 24.83) |
| COC vs MTX | 2.67 (0.64, 11.11) | 1.07 (0.23, 4.65) |
| DPM VS OBS | 5.27 (3.34, 8.30) | 8.00 (3.50, 22.46) |
| CYP VS OBS | 3.24 (0.90, 11.64) | 3.24 (1.21, 13.42) |
| COT VS OBS | 12.19 (2.53, 58.72) | 6.08 (0.80, 47.58) |
| COC VS OBS | 8.91 (3.11, 25.56) | 5.60 (1.95, 18.04) |
| AZP VS OBS | 1.78 (0.25, 12.56) | 1.60 (0.42, 7.30) |
| UDCA vs OBS | 0.96 (0.64, 1.44) | 0.95 (0.59, 1.56) |
| UDCA vs COT plus UDCA | 1.08 (0.24, 4.89) | 1.42 (0.17, 11.86) |
| UDCA vs UDCA plus BEF | 2.58 (0.58, 11.57) | 3.16 (0.59, 20.67) |
| UDCA vs UDCA plus MTX | 1.30 (0.58, 2.94) | 1.54 (0.50, 5.96) |
| UDCA vs UDCA plus COC | 2.66 (0.65, 10.92) | 3.12 (0.63, 18.75) |
Notes: COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; OBS: observation; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; NA: not available;
Table 3. Assessment of heterogeneity and publication bias for trials included in the traditional meta-analysis.
| Treatment comparisons | I2 (%) | P values of Begg's test | P values of Egger's test |
|---|---|---|---|
| Mortality or liver transplantation | |||
| MTX vs OBS | NR | NR | NR |
| DPM vs OBS | 79.8 | 0.62 | 0.84 |
| CYP vs OBS | 29.5 | 0.60 | 0.28 |
| COT vs OBS | NR | NR | NR |
| COC vs OBS | 16.5 | 0.60 | 0.44 |
| MTX vs COC | 0.0 | 0.32 | NR |
| AZP vs OBS | 0.0 | 0.32 | NR |
| UDCA vs OBS | 1.0 | 0.89 | 0.53 |
| UDCA vs UDCA plus COT | 0.0 | 0.33 | 0.08 |
| UDCA vs UDCA plus BEF | 0.0 | NA | NA |
| MTX plus UDCA vs UDCA | 0.0 | NA | NA |
| COC plus UDCA vs UDCA | 86.9 | 0.32 | NA |
| Adverse events | |||
| UDCA vs OBS | 35.8 | 0.90 | 0.77 |
| MTX vs OBS | NR | NR | NR |
| DPM vs OBS | 0.0 | 0.62 | 0.13 |
| CYP vs OBS | 52.6 | 0.12 | 0.39 |
| COT vs OBS | NR | NR | NR |
| COC vs OBS | 0.0 | 1.00 | 0.23 |
| MTX vs COC | NR | NR | NR |
| AZP vs OBS | 45.7 | 0.32 | NR |
| UDCA vs COT plus UDCA | 0.0 | 0.12 | 0.06 |
| UDCA vs UDCA plus BEF | 0.0 | 0.12 | 0.12 |
| UDCA vs UDCA plus MTX | 3.3 | 0.32 | NA |
| UDCA vs UDCA plus COC | 0.0 | 0.12 | 0.30 |
Notes: COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; OBS: observation; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; NA: not available.
Results from the network meta-analysis of primary and secondary outcomes
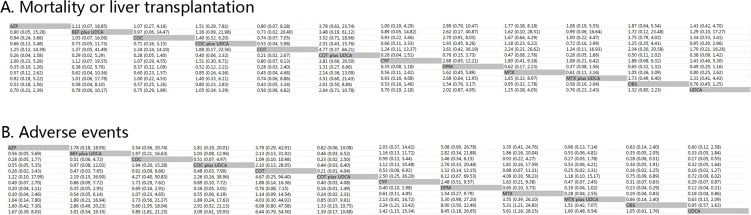
Figure 2A and Figure 2B illustrates the HRs and ORs for MOLT and AEs respectively and 95% confidence intervals obtained from the indirect comparisons of the included regimens. Following Figure 2A from left to right, although differing not significantly, there was a trend that combination of COT plus UDCA was most effective in reducing the risk of MOLT than AZP (HR 3.78, 95%CI 0.63 to 23.74), COC (HR 3.52, 95%CI 0.71 to 18.96), COT (HR 4.77, 95%CI 0.37 to 66.21), CYP (HR 3.81, 95%CI 0.66 to 26.50), DPM (HR 1.31, 95%CI 0.27 to 6.66), MTX (HR 2.14, 95%CI 0.36 to 13.09), OBS (HR 2.01, 95%CI 0.50 to 8.84), monotherapy with UDCA (HR 2.63, 95%CI 0.72 to 10.75), COC plus UDCA (HR 2.55, 95%CI 0.44 to 15.03), MTX plus UDCA (HR 3.58, 95%CI 0.61 to 21.96) or BEF plus UDCA (HR 3.48, 95%CI 0.21 to 56.93). Consistently, Figure 2B shows that for AEs, when compared with OBS, COC (HR 0.18, 95%CI 0.06 to 0.51), CYP (HR 0.31, 95%CI 0.07 to 0.83), DPM (HR 0.13, 95%CI 0.04 to 0.29) and MTX (HR 0.19, 95%CI 0.04 to 0.83) yielded a significant difference in causing AEs. In addition, compared with UDCA, a statistical significance was also achieved in COC (HR 0.17, 95%CI 0.05 to 0.55), CYP (HR 0.29, 95%CI 0.07 to 0.87), DPM (HR 0.12, 95%CI 0.04 to 0.31) and MTX (HR 0.18, 95%CI 0.04 to 0.86).
Figure 2. Clinical efficacy and safety of all treatments according to network meta-analysis.
Treatments are reported in alphabetical order. The ORs were estimated in upper and lower triangle comparing column-defining with row-defining treatment. A. Mortality or liver transplantation; B. Adverse events. For mortality or liver transplantation, HRs higher than 1 favor the column-defining treatment, while for adverse effects, ORs lower than 1 favor the row-defining treatment. COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; OBS: observation. A. Mortality or liver transplantation; B. Adverse events.
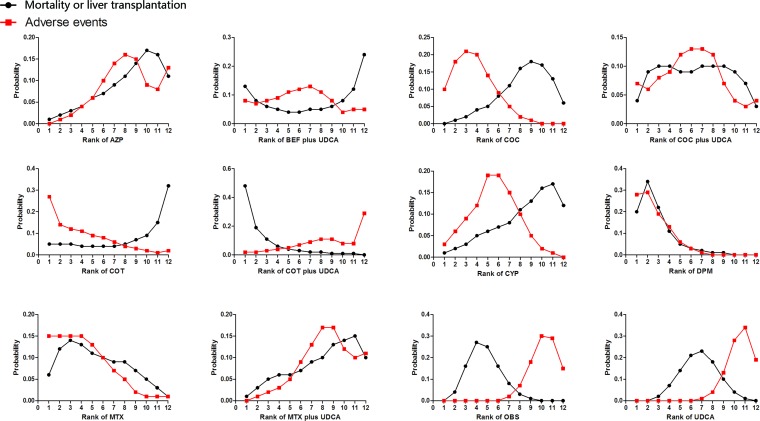
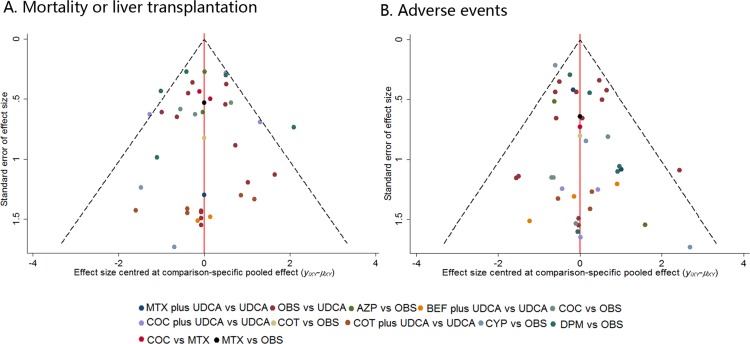
Figure 3 shows the distribution of probabilities for each treatment being ranked at different positions for the outcome of MOLT or AEs. Although the efficacy effects of combined therapy of COT plus UDCA compared with OBS compared with placebo was not statistically significant, COT plus UDCA had the greatest probability (48%) for being the best treatment option on reducing risk of MOLT. While in terms of AEs, DPM had the highest probabilities (28%) of reduction in AEs (Figure 3), suggesting DPM was more likely to cause AEs than remaining treatments. On the other hand, COC or COT plus UDCA demonstrated the least adverse events, the cumulative probabilities of being ranked first and second in with respect to the safety profile was combination of COC or COT and UDCA. Figure 4 presents comparison-adjusted funnel plot for UDCA-based therapies network, without the evidence of asymmetry.
Figure 3. Rankograms showing probability of each strategy having each specific rank (1-6) for mortality or liver transplantation and adverse events.
Ranking indicates the probability to be the best treatment, the second best, the third best and so on. Rank 1 is worst and rank N is best. COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; OBS: observation.
Figure 4. Comparison-adjusted funnel plot for the treatment network in terms of mortality or liver transplantation and adverse events.
The red line represents the null hypothesis that the study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates. Different colors correspond to different comparisons. Estimates below one indicate that the benefit of the experimental intervention is more pronounced in the trial than the pooled estimate. Observations from small studies missing on the right side of the line of null effect (ratio of rate ratios > 1) indicate that small studies tend to exaggerate the effectiveness of experimental treatments. COC: colchicine; BEF: bezafibrate; COT: corticosteroids; MTX: methotrexate; UDCA: ursodeoxycholic acid; CYP: cyclosporin A; DPM: D-penicillamine; AZP: azathioprine; OBS: observation. A. Mortality or liver transplantation; B. Adverse events.
Comparisons between traditional pairwise and network meta-analyses
Table 2 shows the results of traditional pairwise and network meta-analyses. Although the pooled estimates showed small differences, the confidence intervals from traditional pairwise meta-analyses and the credible intervals from Bayesian network meta-analyses in general overlapped. The P-values show no significant difference between the direct and indirect effects (Table 4). In general, the node splitting method showed no significant inconsistency within the networks for any of the two outcomes.
Table 4. Assessment of inconsistency between direct and indirect evidence.
| Treatment comparisons | P value of node-splitting method |
|---|---|
| Mortality or liver transplantation | |
| COC vs MTX | 0.76 |
| COC vs OBS | 0.21 |
| COC vs UDCA | 0.10 |
| MTX vs OBS | 0.73 |
| COC plus UDCA vs MTX plus UDCA | 0.69 |
| COC plus UDCA vs UDCA | 0.67 |
| MTX plus UDCA vs UDCA | 0.69 |
| Adverse events | |
| COC vs MTX | 0.07 |
| COC vs OBS | 0.07 |
| MTX vs OBS | 0.07 |
Notes: COC: colchicine; MTX: methotrexate; UDCA: ursodeoxycholic acid; OBS: observation
Sensitivity analysis
In the sensitivity analysis, there were eleven trials [2, 12, 22, 24–25, 27–28, 31, 33, 35, 39] excluded which reported patients administrated by high (more than 500 mg/kg/day) dose of UDCA (BEF plus UDCA excluded). Thirty-eight independent studies were performed for the primary outcome, MOLT. Overall, the sensitivity analysis showed that omitting those trials with high dose UDCA did not impact on the ranking order and statistical significance of the remaining treatments. Supplementary 3A indicates that combination of COT and UDCA was the top-ranked treatments, although differ not significantly. Similar findings were also observed for the outcome of AEs. DPM, COC or CYP were associated with significant effects in causing AEs compared with OBS. Besides, COT or COC plus UDCA were ranked the least possibility to cause AEs (Supplementary 3B).
DISCUSSION
In this network meta-analysis reviewing the efficacy and safety of most comprehensive interventions in patients with PBC to date, we found that combined therapy with COT and UDCA was the most effective in reducing the risk of MOLT with a weighted benefit-risk ratio for patients with PBC. Monotherapy with DPM, CYP, AZP or MTX received statistical significance in causing AEs where treatment with DPM showed higher probabilities of being at the superior ranking positions in AEs. These estimates are fairly robust and changed little in sensitivity analyses.
Our study has several strengths. Firstly, our network meta-analysis, until more evidences of direct active comparisons are reported, provides a useful and complete picture for propensity of most comprehensive treatments associated with efficacy and safety outcomes among patients with PBC. Network meta-analysis allowed comparison of all available strategies in a single analysis to give a combined total 4182 patients of treatments, rather than separate and disconnected meta-analyses for individual pairs of treatments. Secondly, we were able to provide a formal rank order for all treatment strategies by their capacity to reduce the risk of MOLT or AEs by conducting a network meta-analysis, and this is only attainable by using a Bayesian approach. Finally, in order to reduce concerns on potential inconsistency, we performed inconsistency diagnostic for all triangular and quadrilateral loops. In addition, statistical heterogeneity was moderate, although for some I² values of comparisons, heterogeneity was high (Table 2). In order to test the robustness of our results due to high heterogeneity values in some comparisons, we performed sensitivity analysis regarding to patients administrated by high (more than 500 mg/kg/day) dose of UDCA (BEF plus UDCA excluded), which showed that the results was similar to our main analysis. Furthermore, we analyzed the effects of AEs to obtain a favorable benefit-risk ratio for patients with PBC based on all treatments.
We do also have to acknowledge several limitations in the analysis. Firstly, it may be argued that the inclusion of trial patients with optimum dose of UDCA and trials with high dose may have biased results. However, a sensitivity analysis excluding trials in trials where patients were administrated by high dose of UDCA yielded much the same results as the primary analysis. Secondly, the study size assigned to different comparisons was overall small in most included studies in this analysis. However, our study had established the largest PBC treatment sample size for trials performed to date in the world. Thirdly, Factors such as trial heterogeneity, bias and inconsistency can affect the estimates reported in the study [56]. For instance, this analysis was performed on the assumption of consistency where the validity of indirect comparisons was determined by the extent of clinical and methodological trial similarity. However, inconsistency remains a methodological issue of multiple treatment comparisons, as it arises from pooling the data and small number of trials available for the different comparisons resulting in discrepancies between the direct and indirect comparisons, and consequently threatening the validity of the results [56–58]. Furthermore, we are unable to provide comparisons of drug efficacy based on disease duration, MELD, and the presence of cirrhosis due to lack of the above information reported from included trials. Hence, further studies need to be conducted on disease duration, MELD, and the presence of cirrhosis. In the case of our network meta-analysis, the indirect estimates were often very similar to those obtained in the direct comparisons because only single comparisons were available for the majority of the cases. This resulted in a less conventional geometry where our network of trials did not have any closed loops. Finally, our analysis was that not all AE outcomes of interest were reported consistently across trials. Furthermore, there were cases where no events had occurred for the outcome of interest resulting in the requirement to add a continuity correction to the results [58]. In general, missing data resulted in wider credible intervals due to greater uncertainty around the estimates. However, despite these limitations, this network meta-analysis provides the largest scale comparative information on the major clinical outcome profiles of different interventions in current use.
Our study results are consistent with some of previous pairwise meta-analyses, but the network meta-analysis incorporates both direct and indirect comparisons of treatment strategies, including those that have never been compared directly. UDCA appears a safe regimen and may be useful for preventing the progression of PBC, which is the only therapy approved by the U.S. Food and Drug Administration [59]. However, one traditional meta-analysis in 2008 [10] and another published in 2012 [11] of 16 RCTs both reported that, although differing not significantly, they did not demonstrate any benefit of UDCA on mortality and MOLT of patients with PBC. These findings are consistent with our results. In addition, some trials have also concluded that UDCA has no beneficial effect on patient survival, but may be a safe option for patients with PBC. As previously reported in a Canadian Multicenter RCT (reference here), UDCA, when administered to patients with PBC, led to an improvement in clinical outcomes. However, a larger data sample is needed to determine whether UDCA therapy has a beneficial effect on PBC patient survival. Hence, one possible explanation for seemingly inconsistent results, was that the small patient population in those trials which supported UDCA was beneficial to patients.
Although our analysis did not demonstrate that combination therapy of COT with UDCA had a significant risk reduction in MOLT, we found the evidence favoring COT plus UDCA compared with OBS (HR 2.01, 95%CI 0.5 to 8.84), and further network meta-analysis results also revealed similar non-significant relationship. The results of our analysis on efficacy are remarkably similar with several studies. A previous meta-analysis in 2013 [60] was performed of RCTs concluded that the combination therapy of UDCA and COT was more effective in comparison to monotherapy with UDCA for patients with PBC, which we also found in both our direct and indirect comparisons. Similarly, two RCTs [61–62] of the analysis showed that COT plus UDCA appeared to be the best therapeutic option for PBC patients. In addition, COT plus UDCA had the probability of being ranked second with respect to safety outcome. Few AEs were reported, which included osteoporosis, bleeding, aggravated itching, and diarrhea in only two included RCTs [26, 29] associated with COT plus UDCA.
Besides, other single therapies, such as DPM, CYP, AZP or MTX, were also evaluated by several studies. The results of our analysis showed compared with OBS, CYP, DPM or MTX were significantly more likely to cause AEs. Further, no survival benefits can also be found in treatments with CYP, AZP, MTX or MTX. As reported by AASLD in 2009 [59], the clinical guidelines do not recommend any of above treatments. Some head-to-head meta-analyses [63–65] all found that treatments with AZP, CYP or MTX did not appear to reduce the risk of MOLT, and led to more AEs for patients with PBC, which are consistent with our results. As is showed in our analysis, treatment with DPM had the highest probabilities of being ranked top in adverse-effects, which included rash, thrombocytopenia, myasthenia, arthralgia, etc [42–43, 48, 52]. In addition, two following traditional meta-analyses [66–67] showed that treatment with DPM was associated with little clinical benefits over OBS, and increased the risk of causing the AEs. Therefore, based on our analysis incorporating direct and indirect evidences, there was little evidence favoring the use of these single drugs in clinical efficacy or safety profile.
In conclusion, the study suggests that the superiority of using COT plus UDCA in the treatment of PBC with weighted benefit-risk ratio in MOLT and AEs. For outcome of AEs, UDCA-based therapies, such as UDCA, COT plus UDCA or COC plus UDCA, all showed well-tolerated in adverse effects. While single treatments with CYP, DPM or MTX all have achieved statistically significant as compared with OBS, increased risk of AEs where DPM was most likely to cause AEs.
MATERIALS AND METHODS
Search strategy
We adhered to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement for reporting systematic reviews and meta-analyses in healthcare interventions (Supplementary 4) [68]. Four electronic databases (PubMed, Scopus, Embase, and the Cochrane Library) were searched until the end of August 2014 for randomized controlled trials investigating any treatments for patients with PBC, with the key terms “treatments/therapies, primary biliary cirrhosis, randomized clinical trial” without any language or date restrictions. We also searched the additional studies in the reference lists of all identified publications, including relevant meta-analyses and systematic reviews. Two reviewers (Zhu GQ, Huang S) independently assessed the eligibility of all potential abstracts and titles. Any disagreements were resolved through discussion and repeat extraction by a third reviewer (Zheng MH).
Selection criteria
We included randomized, placebo, or untreated controlled clinical trials comparing the effects of any single or combination of treatments with observation or other classes of active treatments in patients with PBC older than 18 years old. Included studies had to report at least one of two outcomes: MOLT and adverse events (AEs). PBC was diagnosed according to established diagnostic criteria (at least three of the following criteria: alkaline phosphatase and (or gamma glutamyl transpeptidase above the upper limit of normal; antimitochondrial antibodies positive at a titer of 1:40; absence of biliary obstruction by ultrasonography or other radiological tests; or compatible liver biopsy). Other exclusions were trials that comprised a non-randomized design, reviews or pooled-analyses and assessments of other therapies and studies with no usable outcomes data. Duplications were eliminated for the same title, author list or publication date. Eligible studies had to be published as full length articles.
Data extraction
Two investigators (Zhu GQ, Shi KQ) independently extracted data from each study and enter it into a database. Any discrepancies regarding the extraction of data were resolved by additional investigator (Zheng MH). Data were extracted from each study with a predesigned electronic database, including publication data (the first author's name, year of publication, and country of population studied), treatment protocols that were compared and number of patients assigned to each group, the number of events of interest in each group. When relevant information on design or outcomes was unclear, or when doubt existed about duplicate publications, we contacted the original authors for clarification. We choose two clinically meaningful events to estimate efficacy and safety separately of treatments for network meta-analysis: MOLT, which was commonly considered as the most important evaluation, and AEs, including serious events (defined as any untoward medical occurrence that was life threatening, resulted in death, or was persistent or led to significant disability) or non-serious events (that is, any medical occurrence not necessarily having a causal relationship with the treatment). When relevant information on design or outcomes was unclear, or when some needed data was unavailable directly from the study, the original authors were contacted for clarifications and assistance by email.
Study quality
Two independent reviewers assessed the quality of the methodology by using the Cochrane Risk of Bias Tool, an established tool based on assessing sequence generation for the randomization of subjects, allocation concealment of treatment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias [69]. Trials with high or unclear risk for bias for any one of the first three components were regarded as trials with high risk of bias. Otherwise, they were considered as trials with low risk of bias.
Data analysis
All data from each eligible study were extracted and entered into a standardized spreadsheet (Microsoft Excel 2007; Microsoft, Redmond, WA). We analyzed two treatment outcomes separately (MOLT, AE). Firstly, traditional pairwise meta-analyses were conducted for studies that directly compared different treatment arms. Then we performed Bayesian network meta-analyses to compare different PBC therapies.
Firstly, we performed traditional pairwise meta-analyses for studies that directly compared different treatment arms with STATA 12.0 (Stata Corporation, College Station, Texas, USA). We calculated, by using the method of DerSimonian and Laird random effects model, of hazards ratios, the pooled estimates of odds ratios and 95% confidence intervals of direct comparisons between two strategies according to Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Clinical heterogeneity was first assessed through clinical judgment with input from experts in the field. Statistical heterogeneity was then assessed by the P values of Egger's and Begg's test from pair-wise meta-analysis, which was higher than 0.05 suggesting heterogeneity. A formal assessment of heterogeneity was then accomplished by referring to the I2 statistic. According to the standard guidelines, I2 values greater than 50% are considered high heterogeneity levels, between 25-50% moderate and less than 25% considered low heterogeneity levels. Once the heterogeneity was suspected, sensitivity analysis was employed [70]. It was conducted based on the important covariate, the dose of UDCA, to investigate the robustness of our main analyses. The sensitivity analysis for dose of UDCA included trials that patients administrated by a dose of 13-15 mg/kg/day, according to AASLD practice guidelines in 2009 [59].
In addition to the direct comparison meta-analyses, we performed multiple-treatment meta-analysis with a random-effects model within a Bayesian framework using Markov chain Monte Carlo methods in WinBUGS (MRC Bio- statistics Unit, Cambridge, UK). The advantages of using a bayesian analytical approach are that direct probability statements on treatment comparisons can be made and that all evidence for a specific problem can be taken into account as it includes evidence on both indirect and direct comparisons and as such allows estimation of the comparisons between interventions that have not been examined directly in previous trials [16, 18]. The pooled estimates were obtained using the Markov chains Monte Carlo method. As we have described in our previous published network meta-analysis [2, 26, 30, 39], analyses were based on non-informative priors for relative-effect parameters (flat normal with mean of 0 and precision of 0.001) and between-study SD (a flat uniform distribution between 0 and 2). Convergence and lack of autocorrelation were checked and confirmed after a 5000-simulation burn-in phase without any thinning and using 4 chains with different initial values. Then, a burn-in phase of 20 000 iterations was used, followed by 50 000 iterations to estimate parameters. This method combined direct and indirect evidence for any given pair of treatments in one joint analysis [71–72].
We compared the pooled HRs from the network meta-analysis with corresponding HRs from pair-wise random-effects meta-analysis of direct comparisons to assess whether there was inconsistency between direct and indirect comparisons. Odds ratios (ORs) were calculated from the number of total patients and the number of patients in each trial for network meta-analysis for AEs. In addition, to estimate inconsistency, we used the node splitting method to calculate the inconsistency of the model, which separated evidence on a particular comparison into direct and indirect evidence [72]. Bayesian P value was also reported and evaluated between the direct and indirect evidence [72].
The treatments were ranked for each outcome in each simulation on the basis of their posterior probabilities. We assessed the probability that each treatment was the most efficacious regimen, the second best, the third best and so on, by calculating the HR for each treatment compared with an arbitrary common group and counting the proportion of iterations of the Markov chain in which each treatment had the highest HR, the second highest, and so on. Even though the differences in effect size among treatments may be small, clinical decisions about the choice of treatments can still be suggested based on the probabilities of treatment ranking [26, 73]. The outcome of AEs was also reported as odds ratios with corresponding 95% credible intervals, as well as the probabilities of ranking by treatment. Therefore, the network meta-analysis increased statistical power by incorporating evidence from both direct and indirect comparisons across all treatments.
SUPPLEMENTARY MATERIAL
Acknowledgments
Author contributions: Zhu GQ, Huang S, Shi KQ, Chen YP and Zheng MH designed the study. Zhu GQ, Huang S, Huang GQ and Wang JT screened studies and extracted data. Zhu GQ, Huang GQ, Zhou ZR did the statistical analyses. Lin YQ, Wang LR, Wu YM prepared figures. Zhu GQ, Shi KQ, Huang S, Braddock M, Zhou MT and Zheng MH reviewed the results, interpreted data, and wrote the manuscript. All authors have made an intellectual contribution to the manuscript and approved the submission.
Footnotes
CONFLICTS OF INTEREST
The authors report no declarations of interest.
FUNDING INFORMATION
This work was supported by grants from the Scientific Research Foundation of Wenzhou, Zhejiang Province, China (H20090014, Y20090269, Y20140696), Health Bureau of Zhejiang Province (2010KYB070), Research Foundation of Education Bureau of Zhejiang Province (Y201009942), Fresh Talent Program for Science and Technology Department of Zhejiang Province (2013R413018, 2013R413035 and 2013R413015), Research Funds for Tian Qing Liver Diseases (TQGB20120057) and Project of New Century 551 Talent Nurturing in Wenzhou, Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents, Ministry of health of China-the major medicine science and technology project in Zhejiang province (No. WKJ2012-2-033), National Natural Science Foundation of China (81370563), Zhejiang Provincial Natural Science Foundation of China (LR14H030001).
REFERENCES
- 1.Hohenester S, Oude-Elferink RP, Beuers U. Primary biliary cirrhosis. Seminars in immunopathology. 2009;31:283–307. doi: 10.1007/s00281-009-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu GQ, Shi KQ, Huang S, Huang GQ, Lin YQ, Zhou ZR, Braddock M, Chen YP, Zheng MH. Network meta-analysis of randomized controlled trials: efficacy and safety of UDCA-based therapies in primary biliary cirrhosis. Medicine. 2015;94:e609. doi: 10.1097/MD.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan MM, Cheng S, Price LL, Bonis PA. A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology (Baltimore, Md.) 2004;39:915–923. doi: 10.1002/hep.20103. [DOI] [PubMed] [Google Scholar]
- 4.Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, Michieletti P, Minuk GY, Pappas SC, Scully LJ, et al. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology (Baltimore, Md.) 1994;19:1149–1156. [PubMed] [Google Scholar]
- 5.Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 6.Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. The New England journal of medicine. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 7.Combes B, Carithers RL, Jr., Maddrey WC, Lin D, McDonald MF, Wheeler DE, Eigenbrodt EH, Munoz SJ, Rubin R, Garcia-Tsao G, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology (Baltimore, Md.) 1995;22:759–766. [PubMed] [Google Scholar]
- 8.Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110:1515–1518. doi: 10.1053/gast.1996.v110.pm8613058. [DOI] [PubMed] [Google Scholar]
- 9.Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 10.Gong Y, Huang ZB, Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. The Cochrane database of systematic reviews. 2008:CD000551. doi: 10.1002/14651858.CD000551.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. The Cochrane database of systematic reviews. 2012;12:CD000551. doi: 10.1002/14651858.CD000551.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda T, Tozuka S, Noguchi O, Kobayashi F, Sakamoto S, Marumo F, Sato C. Effects of additional administration of colchicine in ursodeoxycholic acid-treated patients with primary biliary cirrhosis: a prospective randomized study. Journal of hepatology. 1996;24:88–94. doi: 10.1016/s0168-8278(96)80191-7. [DOI] [PubMed] [Google Scholar]
- 13.Poupon RE, Huet PM, Poupon R, Bonnand AM, Nhieu JT, Zafrani ES. A randomized trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. UDCA-PBC Study Group. Hepatology (Baltimore, Md.) 1996;24:1098–1103. doi: 10.1002/hep.510240520. [DOI] [PubMed] [Google Scholar]
- 14.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology (Baltimore, Md.) 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Harada K, Ebinuma H, Komori A, Yokokawa J, Yoshizawa K, Abe M, Miyake Y, Kikuchi K, Ohira H, Zeniya M, Yamamoto K, Ishibashi H, et al. Primary biliary cirrhosis - Autoimmune hepatitis overlap syndrome: A rationale for corticosteroids use based on a nation-wide retrospective study in Japan. Hepatology research : the official journal of the Japan Society of Hepatology. 2011;41:877–886. doi: 10.1111/j.1872-034X.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 16.Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, Lu G. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24:1–19. doi: 10.2165/00019053-200624010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26:753–767. doi: 10.2165/00019053-200826090-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lindor KD, Dickson ER, Jorgensen RA, Anderson ML, Wiesner RH, Gores GJ, Lange SM, Rossi SS, Hofmann AF, Baldus WP. The combination of ursodeoxycholic acid and methotrexate for patients with primary biliary cirrhosis: the results of a pilot study. Hepatology (Baltimore, Md.) 1995;22:1158–1162. doi: 10.1016/0270-9139(95)90624-x. [DOI] [PubMed] [Google Scholar]
- 20.Vuoristo M, Farkkila M, Karvonen AL, Leino R, Lehtola J, Makinen J, Mattila J, Friman C, Seppala K, Tuominen J, et al. A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology. 1995;108:1470–1478. doi: 10.1016/0016-5085(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 21.Leuschner U, Fischer H, Hubner K. [Ursodeoxycholic acid in the treatment of primary biliary cirrhosis: results of a controlled study] Zeitschrift fur Gastroenterologie. Verhandlungsband. 1989;24:133. [PubMed] [Google Scholar]
- 22.Oka H, Toda G, Ikeda Y, Hashimoto N, Hasumura Y, Kamimura T, Ohta Y, Tsuji T, Hattori N, Namihisa T, et al. A multi-center double-blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis. Gastroenterologia Japonica. 1990;25:774–780. doi: 10.1007/BF02779195. [DOI] [PubMed] [Google Scholar]
- 23.Poupon RE, Balkau B, Eschwege E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. The New England journal of medicine. 1991;324:1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 24.Battezzati PM, Podda M, Bianchi FB, Naccarato R, Orlandi F, Surrenti C, Pagliaro L, Manenti F. Ursodeoxycholic acid for symptomatic primary biliary cirrhosis. Preliminary analysis of a double-blind multicenter trial. Italian Multicenter Group for the Study of UDCA in PBC. Journal of hepatology. 1993;17:332–338. doi: 10.1016/s0168-8278(05)80214-4. [DOI] [PubMed] [Google Scholar]
- 25.Hwang SJ, Chan CY, Lee SD, Wu JC, Tsay SH, Lo KJ. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a short-term, randomized, double-blind controlled, cross-over study with long-term follow up. Journal of gastroenterology and hepatology. 1993;8:217–223. doi: 10.1111/j.1440-1746.1993.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu GQ, Shi KQ, You J, Zou H, Lin YQ, Wang LR, Braddock M, Chen YP, Zheng MH. Systematic review with network meta-analysis: adjuvant therapy for resected biliary tract cancer. Alimentary pharmacology & therapeutics. 2014;40:759–770. doi: 10.1111/apt.12900. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson LS, Olsson R, Glauman H, Prytz H, Befrits R, Ryden BO, Einarsson K, Lindgren S, Wallerstedt S, Weden M. Ursodeoxycholic acid treatment in patients with primary biliary cirrhosis. A Swedish multicentre, double-blind, randomized controlled study. Scandinavian journal of gastroenterology. 1997;32:179–186. doi: 10.3109/00365529709000190. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Koch A, Brahm J, Antezana C, Smok G, Cumsille MA. The combination of ursodeoxycholic acid and methotrexate for primary biliary cirrhosis is not better than ursodeoxycholic acid alone. Journal of hepatology. 1997;27:143–149. doi: 10.1016/s0168-8278(97)80294-2. [DOI] [PubMed] [Google Scholar]
- 29.Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology (Baltimore, Md.) 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 30.Zhu GQ, You J, Shi KQ, He SY, Wang LR, Chen YP, Braddock M, Zheng MH. Systematic review with network meta-analysis: adjuvant chemotherapy for resected colorectal liver metastases. Medicine. 2015;94:e379. doi: 10.1097/MD.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almasio PL, Floreani A, Chiaramonte M, Provenzano G, Battezzati P, Crosignani A, Podda M, Todros L, Rosina F, Saccoccio G, Manenti F, Ballardini G, Bianchi FP, et al. Multicentre randomized placebo-controlled trial of ursodeoxycholic acid with or without colchicine in symptomatic primary biliary cirrhosis. Alimentary pharmacology & therapeutics. 2000;14:1645–1652. doi: 10.1046/j.1365-2036.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 32.Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza A, Berenguer J, Rodriguez-Martinez D, Mercader J, Velicia R. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. Journal of hepatology. 2000;32:561–566. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 33.Battezzati PM, Zuin M, Crosignani A, Allocca M, Invernizzi P, Selmi C, Villa E, Podda M. Ten-year combination treatment with colchicine and ursodeoxycholic acid for primary biliary cirrhosis: a double-blind, placebo-controlled trial on symptomatic patients. Alimentary pharmacology & therapeutics. 2001;15:1427–1434. doi: 10.1046/j.1365-2036.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- 34.Papatheodoridis GV, Hadziyannis ES, Deutsch M, Hadziyannis SJ. Ursodeoxycholic acid for primary biliary cirrhosis: final results of a 12-year, prospective, randomized, controlled trial. The American journal of gastroenterology. 2002;97:2063–2070. doi: 10.1111/j.1572-0241.2002.05923.x. [DOI] [PubMed] [Google Scholar]
- 35.Kanda T, Yokosuka O, Imazeki F, Saisho H. Bezafibrate treatment: a new medical approach for PBC patients? Journal of gastroenterology. 2003;38:573–578. doi: 10.1007/s00535-002-1102-7. [DOI] [PubMed] [Google Scholar]
- 36.Ozaslan E, Efe C, Akbulut S, Purnak T, Savas B, Erden E, Altiparmak E. Therapy response and outcome of overlap syndromes: autoimmune hepatitis and primary biliary cirrhosis compared to autoimmune hepatitis and autoimmune cholangitis. Hepato-gastroenterology. 2010;57:441–446. [PubMed] [Google Scholar]
- 37.Leung J, Bonis PA, Kaplan MM. Colchicine or methotrexate, with ursodiol, are effective after 20 years in a subset of patients with primary biliary cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:776–780. doi: 10.1016/j.cgh.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Combes B, Emerson SS, Flye NL, Munoz SJ, Luketic VA, Mayo MJ, McCashland TM, Zetterman RK, Peters MG, Di Bisceglie AM, Benner KG, Kowdley KV, Carithers RL, Jr., et al. Methotrexate (MTX) plus ursodeoxycholic acid (UDCA) in the treatment of primary biliary cirrhosis. Hepatology (Baltimore, Md.) 2005;42:1184–1193. doi: 10.1002/hep.20897. [DOI] [PubMed] [Google Scholar]
- 39.Zhu GQ, Shi KQ, Huang S, Wang LR, Lin YQ, Huang GQ, Chen YP, Braddock M, Zheng MH. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Alimentary pharmacology & therapeutics. 2015;41:624–635. doi: 10.1111/apt.13122. [DOI] [PubMed] [Google Scholar]
- 40.Bodenheimer H, Jr., Schaffner F, Pezzullo J. Evaluation of colchicine therapy in primary biliary cirrhosis. Gastroenterology. 1988;95:124–129. doi: 10.1016/0016-5085(88)90300-9. [DOI] [PubMed] [Google Scholar]
- 41.Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, Doniach D, Ranek L, Tygstrup N, Williams R. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology. 1985;89:1084–1091. doi: 10.1016/0016-5085(85)90213-6. [DOI] [PubMed] [Google Scholar]
- 42.Dickson ER, Fleming TR, Wiesner RH, Baldus WP, Fleming CR, Ludwig J, McCall JT. Trial of penicillamine in advanced primary biliary cirrhosis. The New England journal of medicine. 1985;312:1011–1015. doi: 10.1056/NEJM198504183121602. [DOI] [PubMed] [Google Scholar]
- 43.Epstein O, Jain S, Lee RG, Cook DG, Boss AM, Scheuer PJ, Sherlock S. D-penicillamine treatment improves survival in primary biliary cirrhosis. Lancet. 1981;1:1275–1277. doi: 10.1016/s0140-6736(81)92456-9. [DOI] [PubMed] [Google Scholar]
- 44.Heathcote J, Ross A, Sherlock S. A prospective controlled trial of azathioprine in primary biliary cirrhosis. Gastroenterology. 1976;70:656–660. [PubMed] [Google Scholar]
- 45.Hendrickse MT, Rigney E, Giaffer MH, Soomro I, Triger DR, Underwood JC, Gleeson D. Low-dose methotrexate is ineffective in primary biliary cirrhosis: long-term results of a placebo-controlled trial. Gastroenterology. 1999;117:400–407. doi: 10.1053/gast.1999.0029900400. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan MM, Alling DW, Zimmerman HJ, Wolfe HJ, Sepersky RA, Hirsch GS, Elta GH, Glick KA, Eagen KA. A prospective trial of colchicine for primary biliary cirrhosis. The New England journal of medicine. 1986;315:1448–1454. doi: 10.1056/NEJM198612043152304. [DOI] [PubMed] [Google Scholar]
- 47.Lombard M, Portmann B, Neuberger J, Williams R, Tygstrup N, Ranek L, Ring-Larsen H, Rodes J, Navasa M, Trepo C, et al. Cyclosporin A treatment in primary biliary cirrhosis: results of a long-term placebo controlled trial. Gastroenterology. 1993;104:519–526. doi: 10.1016/0016-5085(93)90422-9. [DOI] [PubMed] [Google Scholar]
- 48.Matloff DS, Alpert E, Resnick RH, Kaplan MM. A prospective trial of D-penicillamine in primary biliary cirrhosis. The New England journal of medicine. 1982;306:319–326. doi: 10.1056/NEJM198202113060602. [DOI] [PubMed] [Google Scholar]
- 49.Minuk GY, Bohme CE, Burgess E, Hershfield NB, Kelly JK, Shaffer EA, Sutherland LR, Van Rosendaal G. Pilot study of cyclosporin A in patients with symptomatic primary biliary cirrhosis. Gastroenterology. 1988;95:1356–1363. doi: 10.1016/0016-5085(88)90373-3. [DOI] [PubMed] [Google Scholar]
- 50.Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three-year results. Journal of hepatology. 1992;15:336–344. doi: 10.1016/0168-8278(92)90065-w. [DOI] [PubMed] [Google Scholar]
- 51.Neuberger J, Christensen E, Portmann B, Caballeria J, Rodes J, Ranek L, Tygstrup N, Williams R. Double blind controlled trial of d-penicillamine in patients with primary biliary cirrhosis. Gut. 1985;26:114–119. doi: 10.1136/gut.26.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taal BG, Schalm SW, Ten Kate FW, Van Berge Henegouwen GP, Brandt KH. Low therapeutic value of D-penicillamine in a short-term prospective trial in primary biliary cirrhosis. Liver. 1983;3:345–352. doi: 10.1111/j.1600-0676.1983.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 53.Warnes TW, Smith A, Lee FI, Haboubi NY, Johnson PJ, Hunt L. A controlled trial of colchicine in primary biliary cirrhosis. Trial design and preliminary report. Journal of hepatology. 1987;5:1–7. doi: 10.1016/s0168-8278(87)80053-3. [DOI] [PubMed] [Google Scholar]
- 54.Wiesner RH, Ludwig J, Lindor KD, Jorgensen RA, Baldus WP, Homburger HA, Dickson ER. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. The New England journal of medicine. 1990;322:1419–1424. doi: 10.1056/NEJM199005173222003. [DOI] [PubMed] [Google Scholar]
- 55.Zifroni A, Schaffner F. Long-term follow-up of patients with primary biliary cirrhosis on colchicine therapy. Hepatology (Baltimore, Md.) 1991;14:990–993. [PubMed] [Google Scholar]
- 56.Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: Application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Statistics in medicine. 2009;28:1861–1881. doi: 10.1002/sim.3594. [DOI] [PubMed] [Google Scholar]
- 57.Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. Journal of clinical epidemiology. 2010;63:875–882. doi: 10.1016/j.jclinepi.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC medical research methodology. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. American Association for Study of Liver D. Primary biliary cirrhosis. Hepatology (Baltimore, Md.) 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Lu J, Dai W, Wang F, Shen M, Yang J, Zhu R, Zhang H, Chen K, Cheng P, He L, Wang C, Xu L, et al. Combination therapy of ursodeoxycholic Acid and corticosteroids for primary biliary cirrhosis with features of autoimmune hepatitis: a meta-analysis. Gastroenterology research and practice. 2013;2013:490731. doi: 10.1155/2013/490731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chazouilleres O, Wendum D, Serfaty L, Rosmorduc O, Poupon R. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. Journal of hepatology. 2006;44:400–406. doi: 10.1016/j.jhep.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 62.Heurgue A, Vitry F, Diebold MD, Yaziji N, Bernard- Chabert B, Pennaforte JL, Picot R, Louvet H, Fremond L, Geoffroy P, Schmit JL, Cadiot G, Thiefin G. Overlap syndrome of primary biliary cirrhosis and autoimmune hepatitis: a retrospective study of 115 cases of autoimmune liver disease. Gastroenterologie clinique et biologique. 2007;31:17–25. doi: 10.1016/s0399-8320(07)89323-7. [DOI] [PubMed] [Google Scholar]
- 63.Giljaca V, Poropat G, Stimac D, Gluud C. Methotrexate for primary biliary cirrhosis. The Cochrane database of systematic reviews. 2010:CD004385. doi: 10.1002/14651858.CD004385.pub3. [DOI] [PubMed] [Google Scholar]
- 64.Gong Y, Christensen E, Gluud C. Azathioprine for primary biliary cirrhosis. The Cochrane database of systematic reviews. 2007:CD006000. doi: 10.1002/14651858.CD006000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gong Y, Christensen E, Gluud C. Cyclosporin A for primary biliary cirrhosis. The Cochrane database of systematic reviews. 2007:CD005526. doi: 10.1002/14651858.CD005526.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gong Y, Frederiksen SL, Gluud C. D-penicillamine for primary biliary cirrhosis. The Cochrane database of systematic reviews. 2004:CD004789. doi: 10.1002/14651858.CD004789.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gong Y, Klingenberg SL, Gluud C. Systematic review and meta-analysis: D-Penicillamine vs placebo/no intervention in patients with primary biliary cirrhosis—Cochrane Hepato-Biliary Group. Alimentary pharmacology & therapeutics. 2006;24:1535–1544. doi: 10.1111/j.1365-2036.2006.03164.x. [DOI] [PubMed] [Google Scholar]
- 68.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England) 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Lumley T. Network meta-analysis for indirect treatment comparisons. Statistics in medicine. 2002;21:2313–2324. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- 73.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.