Abstract
Thymic maturation of T cells depends on the intracellular interpretation of αβTCR signals by processes that are poorly understood. In this study, we report that α-catenin/Tcf signaling was activated in double-positive thymocytes in response to TCR engagement and impacted thymocyte selection. TCR engagement combined with activation of β-catenin signaled thymocyte deletion, whereas Tcf-1 deficiency rescued from negative selection. Survival/apoptotis mediators including Bim, Bcl-2, and Bcl-xL were alternatively influenced by stabilization of β-catenin or ablation of Tcf-1, and Bim-mediated β-catenin induced thymocyte deletion. TCR activation in double-positive cells with stabilized β-catenin triggered signaling associated with negative selection, including sustained overactivation of Lat and Jnk and a transient activation of Erk. These observations are consistent with β-catenin/Tcf signaling acting as a switch that determines the outcome of thymic selection downstream the αβTCR cascade.
Productive rearrangement of the TCRα gene at the CD4+CD8+ double-positive (DP)6 stage of thymocyte development leads to the surface assembly of a mature αβTCR. This initiates a cascade of processes, ultimately leading to development of the various T cell lineages and shaping of the T cell repertoire. The outcome of thymocyte selection depends on the quality of αβTCR interactions with self-peptide-MHC complexes. Most DP thymocytes fail to engage in self-peptide-MHC interactions, and in the absence of TCR signals, they die by neglect. Interactions generating strong TCR signals delete autoreactive thymocytes, leading to negative selection, whereas interactions generating moderate TCR signals lead to positive selection and differentiation mainly toward CD4 or CD8 single-positive (SP) lymphocytes (1–10). The molecular mechanisms translating differences in TCR signaling into qualitatively different responses are still poorly understood.
Positive and negative selection have been correlated with a differential activation of the kinases Erk and Jnk, respectively, upon TCR stimulation (11, 12). Stronger TCR signals in DP thymocytes are needed for Jnk than for Erk activation (5). TCR-dependent signals shape the apoptotic response during negative selection. The BH3-only proapoptotic Bcl-2 family member Bim was shown to be essential for clonal deletion (13), and its up-regulation by the NF-κB-inducing kinase-related kinase (Mink) was proposed to be responsible for coupling TCR signals to Jnk activation and negative selection (14). The inability of Bcl-2 to fully reconstitute the Bim deficiency led to proposals that apoptotic responses in thymocyte selection are controlled by an intricate balance between positive and negative forces (15).
β-Catenin is the effector protein of the canonical Wnt pathway. In the absence of Wnt signals, β-catenin is constitutively phosphorylated in the cytoplasm by glycogen synthase kinase-3β (GSK-3β) and casein kinase-I (16, 17). This leads to its ubiquitination and degradation (18). Wnt signals inhibit GSK-3β-dependent phosphorylation of β-catenin, resulting in stabilization and increased levels of the protein in cytoplasm and nucleus (19). Consequently, nuclear β-catenin activates transcription by interaction with Tcf/Lef transcription factors and recruitment of chromatin-remodeling complexes (20).
Although Wnt signals have been identified in multiple stages of thymic development (21), the role of the Wnt signaling cascade has been mostly characterized in double-negative (DN) thymocytes. Reporter expression studies indicated that Tcf/Lef-mediated transcription is increased during the transition from the DN3 to DN4 stage (22), and mice deficient for the downstream effector of Wnt, Tcf-1, exhibit multiple defects in thymocyte development, including a partial block at the CD8+TCRβ− immature SP stage (23, 24). Superimposing the Lef-1 deficiency onto a Tcf-1 hypomorph leads to a complete block of embryonal thymocytes at the immature SP stage (25). This block can only be revoked by the transgenic expression of Tcf-1 mutants with an intact β-catenin binding domain, suggesting that the canonical Wnt/β-catenin pathway is required in early thymocyte development (26). By contrast, activation of β-catenin is sufficient to promote development of DN thymocytes to the DP stage in the absence of pre-TCR (27). A role for β-catenin signaling in the DP to SP transition is beginning to emerge as it was shown that β-catenin activity enhances the survival of DP cells (26) and predisposes them to transformation (28). Moreover, although conditional stabilization of β-catenin by excision of exon 3 impacts the DP to SP transition (28), transgenic expression of a stabilized β-catenin increases the generation of CD8+ SP thymocytes (29).
In this study, we show that β-catenin/Tcf is activated by αβTCR signaling and influences the developmental fate of DP thymocytes. Conditional stabilization of β-catenin leads to sensitization of TCR signaling and biases the cellular response to TCR signals toward negative selection in a selective MHC background. In contrast, Tcf-1 deficiency inhibits negative selection. These different outcomes upon activation of β-catenin vs ablation of Tcf-1 correlate with the expression levels of the proapoptotic mediator of negative selection, Bim, and the antiapoptotic Bcl-2 and Bcl-xL. We thus propose that the outcome of thymic selection is determined by the action of β-catenin/Tcf on TCR signaling.
Materials and Methods
Mice and cell lines
Conditional β-catenin stabilization Ctnnbex3 mice have been described previously (30). These mice were crossed onto the LckCre or CD4Cre transgenic strains to obtain LckCre-Ctnnbex3 and CD4Cre-Ctnnbex3 mice, respectively. The LckCre-Ctnnbex3-Rag2−/− mice have been described previously (27, 28). β-Catenin stabilization in TCR transgenic mice was achieved by crossing LckCre-Ctnnbex3-Rag2−/− mice onto the 6.5 TCR background (expressing the selective MHC class II (MHCII) (IEd)), or with CL4 mice that express the selective MHCI (H-2kd). TCR transgenic mice on the nonselective background were generated by crossing for seven generations LckCre-Ctnnbex3-Rag2−/−CL4 and LckCre-Ctnnbex3-Rag2−/−6.5 mice to C57BL/6 Rag2−/− mice. Expression of H-2kb and H-2kd haplotypes was confirmed by FACS analysis of blood lymphocytes. Tcf-1−/− mice were described previously (23). Top-Gal mice (31) were purchased from The Jackson Laboratory. MHCI/II double-deficient (4080 MM) mice were purchased from Taconic Farms. Signal-deficient Jurkat cell lines were described previously: lck−/− (32), Slp76−/− (33), and ZAP70−/− (34). All animal procedures were done in compliance with institutional guidelines.
Flow cytometry and Abs
FITC-, PE-, CyChrome-, or APC-conjugated Abs were purchased from BD Biosciences. Abs used included anti-CD4 (RM4-5), anti-CD8 (53.6.7), anti-TCRβ (H57), anti-CD5 (53-7.3), and anti-CD69 (H1.2F3). Thymocyte suspensions were stained in FACS buffer (PBS with 2% heat-inactivated FBS) for 30 min on ice with Abs as indicated. Samples were washed with FACS buffer and subjected to FACS analysis using a Cyan flow cytometer (DakoCytomation). Data were analyzed using the FlowJo software (TreeStar).
Unconjugated anti-CD3ε (145-2C11) and anti-CD28 (37.51) Abs for TCR stimulation were purchased from eBioscience. Intracellular staining for Bcl-2 levels was performed using a kit from BD Biosciences according to the manufacturer’s recommendations.
Intracellular β-galactosidase staining
Staining of thymocytes for intracellular β-galactosidase levels was performed according to the manufacturer’s instructions with variations. Briefly, 106 thymocytes suspended in FACS buffer were subjected to a hypotonic shock for 40 s at 37°C by adding an equal volume of a solution containing the fluorescent substrate fluorescein-di-β-D-galactopyranoside (FDG) (Invitrogen) in distilled water. Immediately after, cells were diluted 30-fold in ice-cold IMDM (10% FBS) and incubated for 12 h at 4°C before surface staining and FACS analysis.
Western blot analysis
Thymocytes were lysed in radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitor mixtures (Roche Applied Sciences and Santa Cruz Biotechnology), and 80 μg of protein was separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes (GE Healthcare Bio-Sciences). After blocking 2 h in PBST, 5% milk membranes were incubated overnight at 4°C with the first Abs in blocking buffer. Primary Abs used were anti-β-catenin (1/2000; BD Transduction Laboratories, BD Biosciences) and anti-GAPDH (1/3000; Abcam). Detection of phosphorylated proteins was performed using phospho-specific Abs in a 1/1000 dilution, anti-phospho-JNK (catalog no. 9251), anti-JNK (catalog no. 9252), anti-phospho-ERK (catalog no. 9101), anti-ERK (catalog no. 9107), anti-phospho-p38 (catalog no. 9211), and anti-p38 (catalog no. 9212) (all from Cell Signaling Technology). After washing, the membrane was incubated for 40 min at room temperature with the appropriate secondary Abs coupled to HRP in blocking buffer. The signal was detected using the ECL Plus kit (GE Healthcare Bio-Sciences).
Semiquantitative RT-PCR and real-time PCR
cDNA was prepared by reverse transcription using the Superscript RT kit (Invitrogen). Equilibration of cDNA levels between different samples was performed by real-time PCR for β-actin using the SYBR-green PCR master mix (Applied Biosystems) and an Opticon (Bio-Rad) PCR machine. Serial 1/5 dilutions were prepared, and PCR products were analyzed on 1.2% agarose gels. Quantitative PCR for Itk, Ctla-4, and Gadd45γ was performed using TaqMan assays (Applied Biosystems). For the Bim quantitative PCR, we used the 5′-cggagatacggattgcacagg-3′ as forward primer and 5′-tggtcttcagcctcgcggtaa-3′ as reverse primer. Quantitative analysis of cDNA amplification was assessed by incorporation of SYBR Green into dsDNA. PCR containing 1000 ng of cDNA template, 0.5 μM each of forward and reverse primers, and SYBR Green PCR Master Mix was performed in a total volume of 25 μl. Serial dilutions of a single standard cDNA were amplified to create a standard curve for genes of interest and the normalizer, and values of unknown samples were estimated relative to this standard curve. Normalization of samples was performed by dividing the value of the gene of interest by the value of the endogenous reference gene actin. All cDNA samples were tested in quadruplicate. Actin primers were 5′-tggaatcctgtggcatccatgaaac-3′ as forward and 5′-taaaacgcagctcag taacagtccg-3′ as reverse primer.
TUNEL assay and immunofluorescence
The TUNEL reaction was performed as indicated in the manufacturer’s protocol (Millipore). Briefly, unstained paraffin thymic sections were deparaffinized and rehydrated by sequential washings with xylene, ethanol, and PBS. Ag retrieval was performed by incubation with proteinase K (10 mg/ml in PBS) for 15 min at room temperature, followed by washing with PBS. Slides were incubated with TdT for 1 h at 37°C, and reaction was stopped with stop/wash buffer. At this time, slides were washed in PBS, blocked with PBS 3% normal goat serum for 1 h at room temperature, and incubated for 18 h at 4°C with rabbit polyclonal anti-keratin 5 Ab 1/1000 in PBS/1% normal goat serum (Covance). Keratin-5 staining was developed by 30 min of incubation at room temperature with anti-rabbit-FITC Ab, followed by development of TUNEL with the anti-dioxin-rhodamine Ab provided in the TUNEL detection kit (Covance). Slides were mounted and analyzed in a Nikon TE300 microscope (Nikon Americas).
Annexin V staining
Detection of apoptotic cells was done using the annexin V staining protocol (BD Biosciences) according to the manufacturer’s instructions. Fresh thymocyte suspensions were stained with surface Abs in FACS buffer as detailed above, washed, and resuspended in 100 μl of annexin V binding buffer containing 2 μl of Annexin VFITC. After 15 min of incubation at room temperature, samples were diluted by adding 400 μl of binding buffer and immediately analyzed by FACS.
In vitro stimulation of thymocytes
To elicit a strong stimulation, 24-well plates were coated with anti-CD3 Abs (1 μg/ml) in PBS and incubated at 4°C for 18 h. Sorted DP thymocytes were added to the anti-CD3-coated plates and incubated in IMDM supplemented with 10% FBS, glutamine, and antibiotics in the presence of 5 μg/ml purified anti-CD28 Ab. A weaker TCR stimulation was elicited by adding directly 1 μg/ml anti-CD3 and 5 μg/ml anti-CD28 Abs to the culture.
Results
Induction of β-catenin/Tcf activity upon TCR stimulation of DP thymocytes
Recent evidence involving two independent animal models has implicated β-catenin signaling in the DP to SP thymocyte transition (28, 29). We therefore measured Tcf/Lef activity in thymocytes undergoing selection, using a transgenic mouse strain in which a Tcf/Lef-responsive promoter drives the expression of bacterial β-galactosidase (Top-Gal) (31). Reporter activity was quantified by FACS using the substrate FDG.
Preselected TCRβ-low (TCRβlow) CD4+CD8+ DP thymocytes showed low FDG mean fluorescence intensity (MFI), indicating reduced Tcf/Lef activity. Among TCRβ-high (TCRβhigh) posts-elected DP thymocytes, a fraction (~16%) showed increased FDG MFI, indicating enhanced Tcf/Lef activity. Approximately 20% of CD4 and CD8 SP thymocytes showed elevated Tcf/Lef signaling (Fig. 1A). In agreement with this, mRNA levels of Tcf-1, which is a Tcf/Lef target, were elevated (5- to 10-fold) in postselected compared with preselected DP thymocytes (Fig. 1B). These observations suggested that Tcf/Lef signaling is activated concurrently with thymic selection.
FIGURE 1.
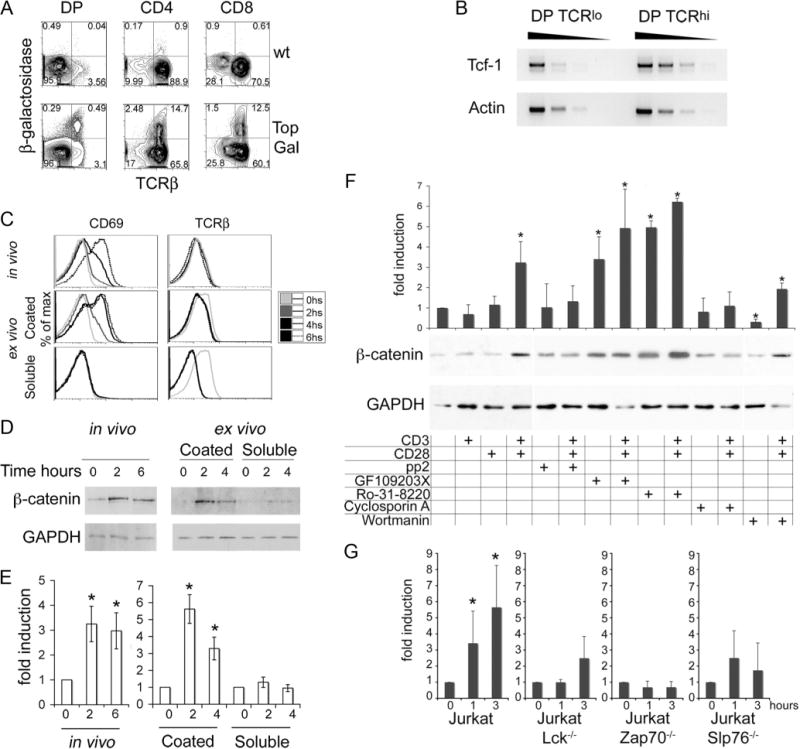
TCR signals activate the β-catenin/Tcf pathway in DP cells. A, TopGal and wild-type thymocytes were stained with a fluorescent β-galactosidase substrate (FDG) followed by surface staining for CD4, CD8, and TCRβ. Gated DP, CD4, and CD8 subpopulations were analyzed for β-galactosidase activity vs TCR surface expression by FACS. x- and y-axis represent log10 fluorescence. B, Semiquantitative RT-PCR for Tcf-1 mRNA on 1/5 serial dilutions of target cDNA extracted from sorted DP (TCRlow) and (TCRhigh) cells. MHCI-II double-deficient mice were stimulated with anti-CD3 Abs in vivo by a single i.p. injection (50 μg/mouse) or DP thymocytes from MHCI-II double-deficient mice were sorted for ex vivo stimulation either with plate-bound (coated) or soluble anti-CD3 (1 g/ml) plus anti-CD28 Abs (5 μg/ml). C, The kinetics of activation were followed by surface expression of CD69 and TCR at the indicated times and treatment by FACS (log10 fluorescence). D, Representative immunoblot for β-catenin and GADPH protein levels after in vivo or ex vivo stimulation of DP cells as for the indicated times. E, Densitometric quantification of β-catenin vs GADPH levels from three to five immunoblots as in D using the ImageJ software (average ± SE). t test in vivo 0 vs 2 or 6 h, p = 0.003 and p = 0.007, respectively; ex vivo (coated) 0 vs 2 or 4 h, p = 0.009 and p = 0.04, respectively.*, Statistically significant induction (p<0.05). F, Sorted DP thymocytes were stimulated as indicated for 2 h before processing for Western blot analysis. Histograms represent densitometric quantification of β-catenin levels relative to GAPDH from three independent immunoblots using the ImageJ software (average ± SD).*, A statistically significant induction (t test) compared with nonstimulated cells; p<0.05. The gel below the histograms shows a representative experiment. G, Jurkat cells and derivative cell lines were induced by plate-bound anti-CD3 plus anti-CD28 Abs as indicated. Whole-cell lysates were prepared at the indicated time points after TCR stimulation and used in Western blot analyses to detect β-catenin or GAPDH. Densitometric quantification of β-catenin levels relative to GAPDH of three to five independent blots (average ± SD).*, A statistically significant induction (t test) compared with nonstimulated cells at 1 h, p = 0.05, and at 3 h, p = 0.01.
To examine whether Tcf/Lef activity increased following TCR engagement and was mediated by β-catenin, we measured β-catenin protein levels in DP thymocytes after in vivo or ex vivo TCR stimulation. TCR signaling was induced in vivo in MHCI/II double-deficient mice by a single injection of anti-CD3 (50 μg/mouse). DP thymocytes were sorted from the treated mice at 2 or 6 h after injection, and β-catenin levels were monitored by Western blots. For ex vivo TCR stimulation, sorted DP thymocytes were incubated either with plate-bound anti-CD3 plus anti-CD28 to elicit strong TCR signals or with soluble anti-CD3 plus anti-CD28 to elicit weaker activation. As expected, strong ex vivo stimulation led to up-regulation of CD69 and a modest down-regulation of surface TCR expression (Fig. 1C). The weaker ex vivo stimulation failed to up-regulate CD69. Only the in vivo and strong but not the weaker ex vivo treatment increased significantly (~5 fold, p < 0.04) the β-catenin protein levels (Fig. 1, C–E), indicating that activation of β-catenin requires strong TCR signals.
To molecularly dissect the cross-talk between αβTCR and β-catenin, we measured TCR-dependent β-catenin levels in the presence of specific inhibitors of TCR signaling. Sorted DP thymocytes were left untreated or were treated ex vivo either with anti-CD3 (1 μg/ml) or anti-CD28 (5 μg/ml) Abs or both. Whole-cell lysates were prepared 2 h after TCR stimulation and analyzed by Western blot. β-Catenin was only stabilized by the combination of anti-CD3 plus anti-CD28 (p = 0.02) (Fig. 1F) but not by anti-CD3 or anti-CD28 alone. Two hours of treatment with anti-CD3 plus anti-CD28 was also performed in the presence of the Lck inhibitor PP2 (20 μM), the protein kinase C (PKC) inhibitors GF109203X (2.5 μM) and Ro-31–8220 (5 μM), the calcineurin inhibitor cyclosporine A (25 ng/ml), and the PI3K/Akt inhibitor wortmannin (30 nM). Analysis of cell lysates showed that inhibition of Lck and calcineurin abrogated the ability of TCR signaling to stabilize β-catenin. Interestingly, inhibition of PKC resulted in significant stabilization of β-catenin in untreated cells (p<0.03). Further TCR stimulation had no additional effect. This finding is in line with recent reports that PKC-α directly phosphorylates the N terminus of β-catenin, compromising its stability (35). Inhibition of PI3K/Akt by wortmanin significantly reduced β-catenin protein levels in unstimulated cells (p = 0.0008), whereas TCR stimulation of wortmanin-treated cells resulted in β-catenin stabilization (p = 0.004) (Fig. 1E). Thus, PI3K/Akt activity may be controlling the steady-state levels but does not influence the TCR-dependent accumulation of β-catenin. We then confirmed and expanded our observations using Jurkat-derived cell lines deficient for components of the TCR signaling cascade. Treatment of Jurkat cells with anti-CD3 plus anti-CD28 increased β-catenin levels (p = 0.05 after 1 h and p = 0.01 after 3 h). However, similar treatment of Jurkat-derived cell lines lacking ZAP70 (p = 0.1 after 1 h and p = 0.09 after 3 h), Slp76 (p = 0.2 after 1 h and p = 0.5 after 3 h), or Lck (p = 0.96 after 1 h and p = 0.13 after 3 h) failed to induce significant changes in the levels of β-catenin (Fig. 1G). Two recent reports (36, 37) indicated that β-catenin stability could be regulated by direct Jnk2-mediated phosphorylation or by p38-dependent phosphorylation and inactivation of GSK-3β. Both Jnk and p38 are activated upon TCR stimulation. To address the possibility that Jnk or p38 may be involved in TCR-mediated β-catenin, stabilization-sorted DP thymocytes were independently stimulated in the presence of the Jnk inhibitor IX (15 μM) or the P38 inhibitor SB202190 (20 μM). Despite effective inhibition of these kinases (data not shown), TCR-induced stabilization of β-catenin was not abrogated, indicating that this process is independent of Jnk and p38 (supplemental Fig. 1).7 These findings show that β-catenin/Tcf signaling is activated in DP thymocytes in response to TCR stimulation through a mechanism that requires Lck, ZAP70, PKC, calcineurin, and Slp76 but is independent of PI3K/Akt, Jnk, and p38 signaling.
β-Catenin/Tcf signaling impacts the generation of postselected DP thymocytes
To assess the impact of β-catenin/Tcf activity on thymocyte selection, we used two gain- and one loss-of-function approaches. In the gain-of-function models, conditional stabilization of β-catenin was achieved by independently breeding Ctnnb1ex3 (30) and APClox468 (38) mice, with mice expressing Cre recombinase under the control of the CD4 gene promoter. Deletion of exon 3 of β-catenin in Ctnnb1ex3 mice removes sites of phosphorylation by GSK-3 and produces a stable protein that escapes degradation (27, 30, 38). Conditional truncation of APC at codon 468 in APClox468 mice generates an APC protein that is unable to assemble in the β-catenin-APC-Axin-Gsk-3 complex leading to stabilization of β-catenin. Tcf-1−/− mice were used in the loss-of-function studies despite the availability of conditional β-catenin “ablation” alleles, because both available strains (39, 40) generate an N-terminally deleted mutant protein of ~50 kDa. The truncated protein is expected to retain over half of the Tcf interaction domain, as well as binding to ICAT, Brg1, and CBP/p300 while lacking α-catenin binding (41, 42). Tcf-1 ablation has been our choice, because it led to elimination Tcf-reporter activity in DP thymo-cytes whereas the double deletion of β- and γ-catenin did not.
Activation of β-catenin by CD4-Cre led to a partial developmental block in the DP to SP transition characterized by a reduction in the number and fraction of CD4 and CD8 SP thymocytes in both CD4-Cre-Ctnnbex3 and CD4-Cre-APClox468 mice (Ref. 28 and Fig. 2A). The developmental block coincided with the selection processes and resulted in a significantly reduced fraction and number of TCRβhigh postselected DP thymocytes compared with CD4-Cre controls (Fig. 2, B and C). In contrast, Tcf-1−/− mice had a reduced DP fraction and increased CD4 and CD8 SP fractions (Fig. 2, A–C). These mice, which have a significantly reduced thymic cellularity (23), had ~18-fold fewer TCRβlow DP thymocytes but a comparable number of TCRβhigh DP thymocytes to CD4-Cre-Ctnnbex3 and only three times fewer than CD4-Cre mice (Fig. 2C). Taken together, these observations suggest that β-catenin/Tcf signaling controls the generation of postselected DP thymocytes, potentially by being involved in the TCR-induced processes of thymocyte selection.
FIGURE 2.
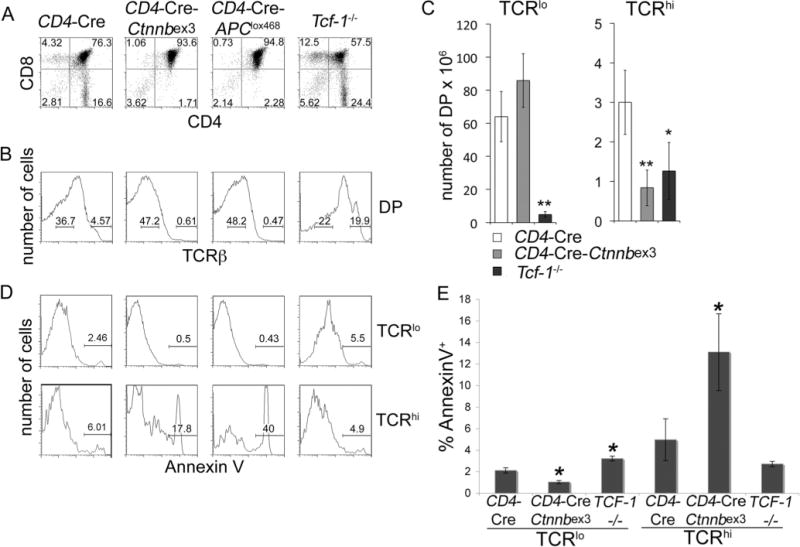
β-Catenin/Tcf signaling controls the generation and survival of postselected DP thynocytes. A, CD4/CD8 thymocyte profile of the indicated mice. B, FACS analysis of surface TCRβ expression in DP thymocytes from mice in A. Levels of surface TCR expression were used to distinguish preselected (TCRlow) from postselected (TCRhigh) DP subpopulations. C, Number of thymocytes per mouse for the indicated subset and mouse strain. Numbers are the average of five CD4-Cre, five CD4-Cre-Ctnnbex3, and three Tcf-1−/− mice error bars represent SD.*, p<0.05;**, p=0.01. D, Representative FACS analysis of Annexin V cells in preselected (TCRlow) and postselected (TCRhigh) DP subsets as indicated. Log10 fluorescence is represented in A (x- and y-axis), B, and C (x-axis). E, Quantification of the fraction of Annexin V cells in TCRlow or TCRhigh DP thymocytes from the indicated mice (average SE). Results are the pool of four independent experiments (n=4). Apoptosis of preselected CD4-Cre-Ctnnbex3 DP thymocytes was significantly reduced (p = 0.003) and of preselected Tcf-1−/− DP thymocytes was significantly increased (p=0.004) compared with CD4-Cre. Apoptosis of postselected CD4-Cre-Ctnnbex3 DP was significantly increased (p = 0.02) compared with CD4-Cre.*, p<0.05.
To examine the survival properties of preselected and posts-elected CD4-Cre-Ctnnbex3, CD4-Cre-APClox468, and Tcf-1−/− DP thymocytes, we measured their rate of apoptosis by annexin V staining (Fig. 2D). Preselected DP thymocytes with conditional β-catenin stabilization had a significantly reduced fraction of apoptotic cells compared with controls (two-tailed t test, p = 0.002) (Fig. 2E). The same subset from Tcf-1−/− mice showed increased apoptosis as previously reported for Tcf-1−/− DP thymocytes (p = 0.004) (43). By contrast, postselected CD4-Cre-Ctnnbex3 and CD4-Cre-APClox468 DP thymocytes were dramatically more apoptotic (p = 0.02 for CD4-Cre-Ctnnbex3), whereas apoptosis levels of Tcf-1−/− postselected DP thymocytes did not differ statistically (p = 0.12) from controls (Fig. 2, D and E). Thus, β-catenin/Tcf signals appear to regulate survival of both pre- and postselected DP thymocytes. These findings indicate that β-catenin signaling is induced in response to TCR signals, and its activity impacts thymocyte selection.
TCR-dependent deletion of DP thymocytes with stabilized β-catenin
The requirement for TCR signaling in β-catenin-mediated elimination of postselected DP thymocytes was examined in CD4 and CD8 TCR-transgenic mice that recognize epitopes of the influenza hemagglutinin (HA). Two TCR-transgenic strains were independently used, the Kd:HA512–520-specific Cl4-TCR (44) and the IEd: HA107–119-specific 6.5-TCR (45). These mice were independently backcrossed to Rag2-deficient (Rag−/−) BALB/c mice expressing the selective H-2kd MHC as well as to Rag2−/− C57BL/6 mice expressing the nonselective H-2kb MHC. The purpose was to differentiate the outcome of β-catenin signaling (nonselective MHC) from the combined action of β-catenin and TCR (selective MHC).
In H-2kd mice, Cl4-TCR and 6.5-TCR thymocytes were positively selected and developed to the CD8 and CD4 SP lineages, respectively (Fig. 3A). By contrast, Cl4- and 6.5-TCR-transgenic thymocytes in H-2kb mice showed an accumulation of DP cells and absence of SP cells, confirming the lack of selection (Fig. 3A). These mice were also deficient for CD4 and CD8 SP lymphocytes in peripheral lymphoid organs (data not shown).
FIGURE 3.
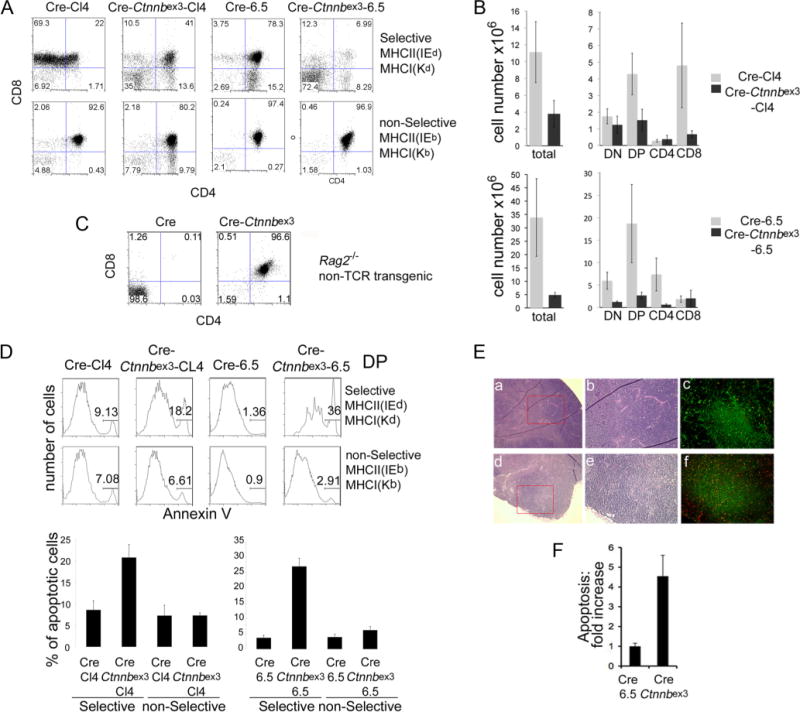
TCR-dependent deletion of TCR transgenic DP thymocytes with stabilized β-catenin. A, Dot plots of CD4 vs CD8 surface expression of thymocytes from the indicated mice. Top row, Mice in the selective MHC (H-2Kd/IEd); bottom row, mice in the nonselective (H-2Kb/IEb) MHC. B, Cell numbers of total thymocytes and each represented subset from the indicated mice. C, Dot plots of CD4 vs CD8 surface expression of non-TCR-transgenic Rag2-deficient thymocytes from Lck-Cre and Lck-Cre-Ctnnbex3 mice. Log10 fluorescence is represented in A and C (x- and y-axis). D, Representative histograms of annexin V staining in electronically gated DP thymocytes from the indicated mice and MHC backgrounds. Histograms are quantification of the percentage of Annexin V+ cells in electronically gated DP thymocytes (average±SE). Values are from six to eight independent experiments. Cre-Ctnnbex3-Cl4-TCR vs Cre-Cl4-TCR and Cre-Ctnnbex3-6.5-TCR vs Cre-6.5-TCR in the selective MHC t test; p=0.014 and p<0.001, respectively. E, H&E staining of Cre-6.5-TCR (a and b) and Cre-Ctnnbex3-6.5-TCR thymus (d and e) in ×4 (a and d) or ×10 (b and e) magnification. c and f are immunofluorescence of keratin-5 (green) and TUNEL (red) staining of Cre-6.5-TCR (c) and Cre-Ctnnbex3-6.5 (f) thymi in ×10 magnification. F, Quantitative analysis of TUNEL-positive cells in nine independent panels (n=9) as in C and F (average±SE), t test, p=0.002.
Stabilization of β-catenin in H-2kd mice led to thymic profiles that were reminiscent of clonal deletion in male HY-TCR-transgenic mice. Both Cre-Ctnnbex3-Cl4 TCR and Cre-Ctnnbex3-6.5 TCR-transgenic mice had an increased fraction of DN thymocytes and a decreased fraction of DP and SP thymocytes (Fig. 3, A and C). Stabilization of β-catenin in these mice led to a significantly reduced thymic cellularity. Postselection subsets including CD8 SP in Cre-Ctnnbex3-Cl4 TCR and the CD4 SP in Cre-Ctnnbex3-6.5 TCR were particularly affected, supporting the notion that they have undergone clonal deletion (Fig. 3B). The reduction of DP cells in Cre-Ctnnbex3-Cl4-TCR and Cre-Ctnnbex3-6.5-TCR-transgenic mice is unlikely the consequence of β-catenin stabilization, because we have shown that stabilization of β-catenin allows progression of Rag2−/− thymocytes to the DP stage in the absence of TCR signaling (Fig. 3C and Ref. 27). DP thymocytes were not deleted in Cre-Ctnnbex3-Cl4-TCR and Cre-Ctnnbex3-6.5-TCR H-2kb mice where most cells reached the DP stage (Fig. 3A). Thy-mic profiles in Cre-Ctnnbex3-Cl4-TCR and Cre-Ctnnbex3-6.5-TCR H-2kb mice were comparable to those of Cl4-TCR and 6.5-TCR mice. The cardinal difference between H-2kd (selective) and H-2kb (nonselective) Cl4-TCR or 6.5-TCR mice is that H-2b mice failed to activate TCR signaling in the absence of peptide-MHC engagement. Thus, the altered subset distribution in H-2kd (selective) mice can only be attributed to the combined action of TCR and β-catenin activities.
To determine whether reduction of Cre-Ctnnbex3-Cl4 TCR and Cre-Ctnnbex3-6.5 TCR DP thymocytes with stabilized β-catenin was the result of increased cell death, we compared their rate of apoptosis to that of Cl4-TCR and 6.5-TCR DP thymocytes in mice with selective or nonselective MHC. Annexin V staining showed that β-catenin stabilization increased apoptosis of Cre-Ctnnbex3-Cl4-TCR and Cre-Ctnnbex3-6.5-TCR DP thymocytes, only in the selective MHC (from an average of 8–20% and from an average of 3–26%, respectively). DP thymocytes from these same strains in the nonselective MHC had comparable apoptosis rates ranging on the average between 2.5 and 7%. This indicates that the increased cell death seen by stabilization of β-catenin at this stage is dependent on endogenous TCR signals (Fig. 3D). TUNEL assays confirmed that stabilization of β-catenin increased apoptosis in 6.5 TCR thymi. Apoptotic cells were evenly spread throughout the thymic lobe, in agreement with increased apoptosis of both DN and DP populations (Fig. 3, E and F).
HY-TCR+ thymocytes are positively selected in Tcf-1-deficient male mice
Because conditional activation of β-catenin synergizes with TCR signals in the clonal deletion of thymocytes, we examined the effect of ablating β-catenin/Tcf signaling on thymocyte selection. To this aim, we crossed the Tcf-1−/− allele onto the HY-TCR-transgenic mice. These mice express a TCR specific for a male Ag (46). In male HY mice, thymocytes are eliminated by negative selection before the DP stage, whereas in female mice, they are positively selected by MHC I. Tcf-1−/−-HY-TCR mice had comparable thymic cellularity with Tcf-1−/− littermate controls (~6×106 at 6 wk of age), reflecting already reported defects of early thymic development in Tcf-1−/− mice (23, 24). Tcf-1−/+-HY-TCR mice had comparable thymic cellularity to HY-TCR mice (~8×106 for the males and ~10×107 for the females). Earlier Tcf-1-dependent defects also accounted for an increased fraction of TCR− DN thymocytes in Tcf-1−/−-HY-TCR male and female mice (Fig. 4). To avoid including a disproportionate fraction of these immature cells, we focused our analyses on cells that have already expressed the mature TCR.
FIGURE 4.
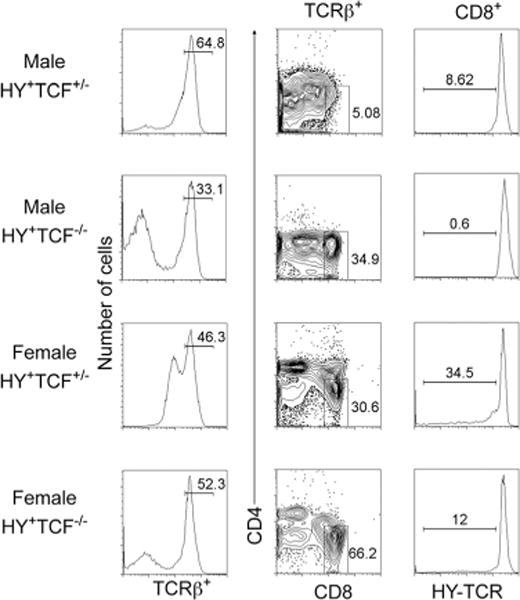
Tcf-1 deficiency enhances positive selection of HY-expressing thymocytes. Male and female Tcf-1+/− or Tcf-1−/− HY TCR transgenic mice as indicated were stained for surface expression of TCRβ, HY-TCR, CD4, and CD8 and analyzed by FACS. First column, Histograms of surface expression of TCRβ. Second column, Two-parameter dot plots of CD4 vs CD8 surface expression gated on TCRβ+ cells. Third column, Histograms of surface expression of HY-TCR electronically gated on TCRβ+CD8+ cells from the indicated mice. Numbers over the bars in histograms indicate the percentage of cells defined by the bar. Numbers in dot plots indicate the percentage of cells inside the gate.
Both male and female Tcf-1−/− HY-TCR mice contained an increased fraction of TCRβ+CD8+ SP cells compared with Tcf-1−/+-HY-TCR littermate controls (34.9% compared with 5% in males and 66.2% compared with 30% in females) (Fig. 4). Importantly, almost all of these TCRβ+CD8+ SP cells showed high levels of surface expression of the HY-TCR. The fraction of positively selected TCRβ+CD8+HY-TCR+ SP thymocytes in female Tcf-1−/−-HY-TCR compound mutant mice was also increased compared with heterozygote littermates (Fig. 4). Despite the presence of mature CD8+ SP cells, male Tcf-1−/−-HY-TCR mice did not have a distinct DP subset. This may be due to reduced levels of CD4 expression, a target of Tcf-1 (47), and/or increased DP stage apoptosis (Fig. 2). In support of this possibility, female HY-Tcf1−/− mice also had a reduced but detectable DP subset with low CD4 surface expression. Although the accumulation of the HY-TCR and Tcf1−/− thymic defects is challenging in the interpretation of analyses, the significantly increased generation of TCRβ+CD8+HY-TCR+ cells in male Tcf-1−/−-HY-TCR mice supports the notion that the Tcf-1 deficiency may weaken TCR signals, leading to rescue from clonal deletion.
Altogether, both gain- and loss-of-function analyses indicate that β-catenin/Tcf signaling downstream the TCR impacts thymocyte selection.
β-Catenin/Tcf signaling controls the levels of pro- and antiapoptotic genes involved in thymocyte selection
Survival of DP thymocytes before and after TCR stimulation is controlled by the interplay of pro- and antiapoptotic molecules, including Bim, Bcl-xL, and Bcl-2. In particular, Bim has been functionally linked to negative selection. To determine whether the observed changes in survival of DP thymocytes either by ablating Tcf-1 or activating β-catenin (Fig. 2, D and E) relate to mechanisms of thymocyte selection, we measured the expression levels of Bim, Bcl-xL, and Bcl-2.
Quantitative RT-PCR analyses of sorted DP thymocytes indicated that activation of β-catenin elevated expression of Bim, and ablation of Tcf-1−/− reduced levels of Bim mRNA compared with CD4-Cre and Tcf-1−/+ controls (Fig. 5A). Similar results were obtained by Western blot analyses of lysates from sorted preselected DP thymocytes (data not shown). To examine whether the increased expression of Bim in CD4-Cre-Ctnnbex3 mice impacted thymocyte selection, we ablated one Bim allele by crossing to Bim−/− mice. This led to a partial rescue of the CD4-Cre-Ctnnbex3 thymic profile generating an increased fraction of SP thymocytes (Fig. 5B) and a significantly increased fraction of postselected DP thymocytes (Fig. 5C). These findings indicated that apoptotic death of postselected CD4-Cre-Ctnnbex3 DP thymocytes was controlled by Bim.
FIGURE 5.
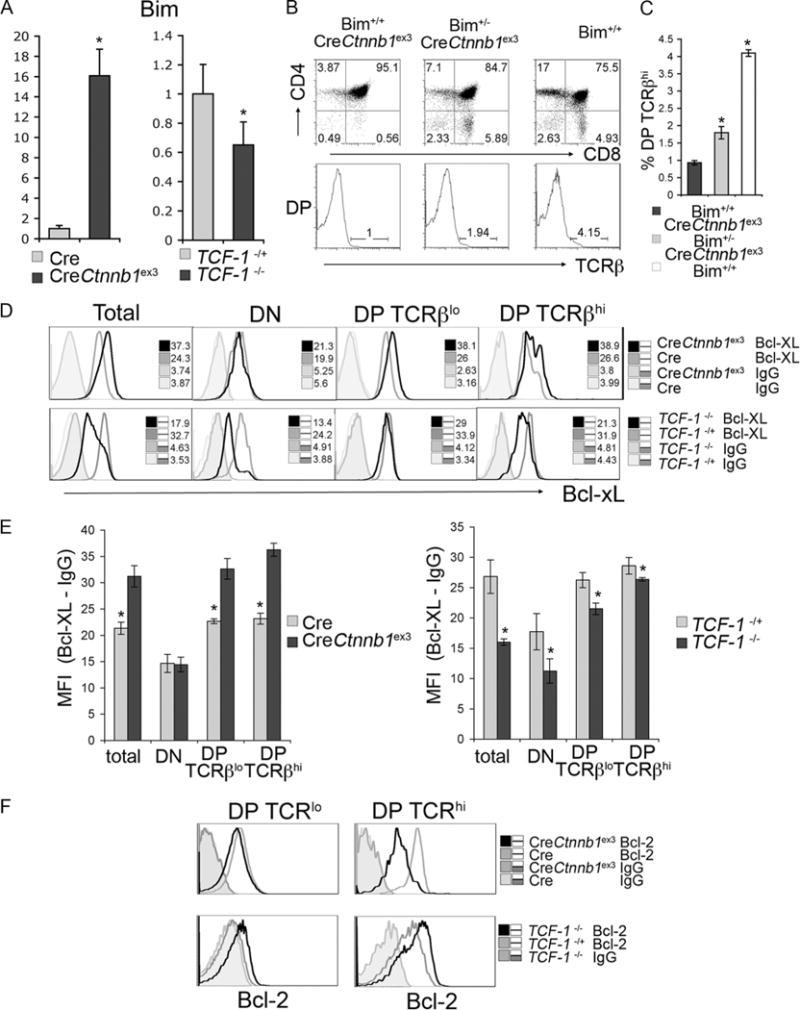
β-Catenin/Tcf signaling controls expression of mediators of negative selection. A, Quantitative RT-PCR of Bim in mRNA extracted from sorted DP thymocytes from CD4-Cre and CD4-Cre-Ctnnbex3 as well as Tcf-1+/− and Tcf-1−/− mice as indicated. B, Dot plots of CD4/CD8 surface expression in Bim+/+CreCtnnb1ex3, Bim−/+CreCtnnb1ex3, and Bim+/+ mice as indicated. Histograms below the dot plots show the levels of surface TCR expression in gated CD4+CD8+ DP thymocytes. Numbers over the bars in histograms indicate the percentage of TCR high cells. C, Quantification of the fraction of TCRβhigh (TCRβhigh) cells in Bim+/+CreCtnnb1ex3, Bim−/+CreCtnnb1ex3, and Bim+/+ mice (average ± SD).*, A statistically significant event (p<0.05). D, Flow cytometric analysis of intracellular Bcl-xL levels from CD4-Cre and CD4-Cre-Ctnnbex3 as well as Tcf-1+/− and Tcf-1−/− mice as indicated. x-axis represents log10 fluorescence. Histograms represent intracellular levels of Bcl-xL and isotype (IgG) controls as indicated. Numbers in the plots show the MFI. E, Quantification was by subtraction of the Bcl-xL from the IgG MFI in the indicated mouse strains and gated populations. Values from four independent mice were used. F, Flow cytometric analysis of intracellular Bcl-2 levels subsets from CD4-Cre and CD4-Cre-Ctnnbex3 as well as Tcf-1+/− and Tcf-1−/− mice as indicated.
Reciprocal changes in the expression of antiapoptotic Bcl-xL and Bcl-2 were also detected in pre- and postselected DP thymo-cytes with activated β-catenin or ablated Tcf-1 (Fig. 5, D and F). Intracellular staining for these molecules showed that β-catenin activation led to up-regulation, whereas Tcf-1 deficiency led to down-regulation of Bcl-xL in both pre- and postselected DP thymocytes (Fig. 5D). Bcl-2 expression was low in Cre-Ctnnbex3 and modestly elevated in Tcf-1−/− preselected DP thymocytes compared with Cre and Tcf-1+/− littermate controls, respectively (Fig. 5F). Although Bcl-2 was up-regulated in postselected control thymocytes, it remained low in the equivalent Cre-Ctnnbex3 cells and was consistently more elevated in Tcf-1−/− postselected thymocytes (Fig. 5F).
Expression changes of Bim, Bcl-xL, and Bcl-2 upon activation of β-catenin or ablation of Tcf-1 indicate that β-catenin/Tcf signals control the survival of thymocytes under selection.
Thymocytes with stabilized β-catenin are partially activated
We found that β-catenin/Tcf can modulate the outcome of thymocyte selection by impacting αβTCR signaling. If β-catenin acts downstream of the TCR, it would be expected that thymocytes with stabilized β-catenin have an activated phenotype. To examine this possibility, we determined the surface expression of activation markers CD69 and CD5, the phosphorylation status of p38 kinase, and the expression of Gadd45γ, Ctla4, and Itk, which are linked to TCR signaling.
As expected, CD69 and CD5 surface expression was elevated in Cl4 and 6.5 TCR transgenes in the selective MHC, as compared with the nonselective MHC. Stabilization of β-catenin caused an additional increase in the levels of CD5 and CD69 surface expression in both TCR-transgenic strains (Fig. 6A) as well as in DP thymocytes from CD4-Cre-Ctnnbex3 and CD4-Cre-APClox468 mice (data not shown). Western blots of DP thymocytes from CD4-Cre-Ctnnbex3 mice also showed significantly elevated levels of phos-phorylated p38 (Fig. 7A). Moreover, quantitative RT-PCR of mRNA from sorted preselected TCRβlow DP thymocytes from CD4-Cre or CD4-Cre-Ctnnbex3 mice showed significantly higher mRNA levels of Ctla4, Gadd45γ, and Itk (Fig. 6B). Likewise, postselected TCRβhigh DP CD4-Cre-Ctnnbex3 thymocytes expressed higher levels of these molecules, although in these cells, the difference from the equivalent wt population was reduced, and in the case of Itk, it was no more statistically significant (p<0.05). These results show that activation of β-catenin leads to partial activation of thymocytes before TCR stimulation, i.e., in preselected (TCRβlow) DP thymocytes (Fig. 6B).
FIGURE 6.
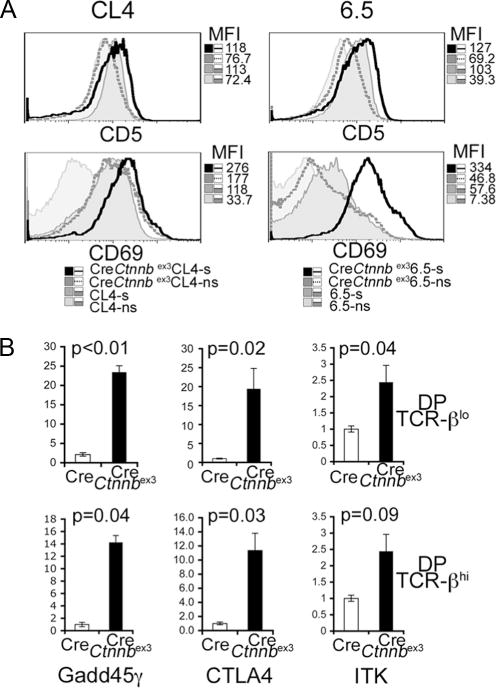
Thymocytes with stabilized β-catenin are activated. A, Histogram overlays of CD5 and CD69 surface-stained thymocytes from the indicated mice in selective (s) (H-2Kd/IEd) and nonselective (ns) (H-2Kb/IEb) MHC. The x-axis represents log10 fluorescence. The MFI of each plot is indicated. B, Q-PCR of cDNA prepared from preselected (TCRlow) and postselected (TCRhigh) DP thymocytes isolated from CD4-Cre and CD4-Cre-Ctnnbex3 mice. Values of p are indicated. Bars represent the average ± SD.
FIGURE 7.
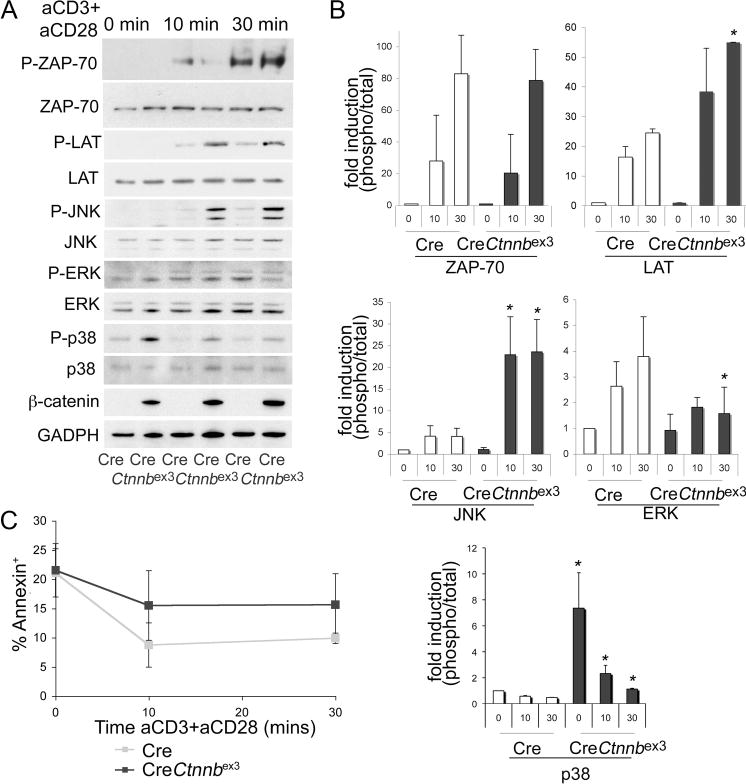
β-Catenin activation diverts TCR signals. DP thymocytes from CD4-Cre and CD4-Cre-Ctnnbex3 mice were stimulated with soluble anti-CD3 plus anti-CD28 Abs, and whole-cell lysates were prepared. A, Representative Western blots detecting phosphorylated and total ZAP70, Lat, Jnk, Erk, and p38 kinases, as well as β-catenin and GAPDH. B, Quantification of phosphorylated vs total ZAP70, Lat, Jnk, Erk, and p38 from three independent Western blots as in A (average ± SD). t test analysis indicated that p38 phosphorylation was significantly higher in CD4-Cre-Ctnnbex3 vs CD4-Cre DP thymocytes both before (p=0.01) and after TCR stimulation (at 10 min, p=0.04, and at 30 min, p=0.003). Jnk phosphorylation increased significantly in CD4-Cre-Ctnnbex3 vs CD4-Cre DP thymocytes at 10 min (p=0.019) after TCR stimulation. Both Lat (p=0.002) and Jnk (p=0.024) showed significantly increased phosphorylation in CD4-Cre-Ctnnbex3 vs CD4-Cre DP at 30 min after TCR stimulation. By contrast, Erk phosphorylation is significantly reduced (p=0.018) in CD4-Cre-Ctnnbex3 vs CD4-Cre DP thymocytes at 30 min after TCR stimulation. C, Plots of Annexin V+ DP thymocytes (including dead and apoptotic) treated as in A. Gray and black squares indicate Cre and CD4-Cre-Ctnnbex3 DP thymocytes, respectively. Each time point shows the average of three independent values ± SD. Differences between Cre and CD4-Cre-Ctnnbex3 were not statistically significant.
Collectively, our findings are consistent with β-catenin acting downstream and mediating at least part of the signaling associated with TCR activation at the DP stage. However, this is not sufficient to confer clonal deletion in the absence of additional TCR signals as indicated by the phenotype of the TCR transgenes in the non-selective MHC background.
β-Catenin activation diverts TCR signaling toward negative selection
We hypothesized that β-catenin alters TCR signaling. To test this hypothesis, we monitored TCR-dependent activation of ZAP70, Lat, Jnk, p38, and Erk in ex vivo-stimulated, preselected DP thymocytes of mice with stabilized β-catenin.
Thymocytes were sorted and stimulated ex vivo with anti-CD3 plus anti-CD28. TCR stimulation induced similar levels of ZAP70 phosphorylation in DP thymocytes with activated β-catenin compared with controls. By contrast, it induced significantly higher Lat, Jnk, and p38 phosphorylation in thymocytes with activated β-catenin. Surprisingly, the same treatment induced only a transient induction of Erk phosphorylation (Fig. 7, A and B). Interestingly, phosphorylation of p38 was also elevated in preselected DP thymocytes with activated β-catenin before ex vivo TCR stimulation. To ensure that the observed signaling differences were not related to overt death of cells with activated β-catenin, we measured the fraction of dying and dead cells in the course of TCR stimulation. Thus, CD4Cre-Ctnnbex3 and CD4Cre DP thymocytes were treated with anti-CD3 and anti-CD28 for 0–30 min before staining with annexin V+ to label dead and dying cells. CD4Cre-Ctnnbex3 DP thymocytes showed a modest increase in annexin V cells, which was, however, not statistically significant, indicating that signaling differences did not result from reduced survival of cells with activated β-catenin (Fig. 7C). These findings indicate that β-catenin activation sensitizes TCR signaling toward the activation of Jnk, a kinase implicated in the deletion of autoreactive thymocytes and negative selection (48, 49). The transient Erk activation and p38 activation before ex vivo TCR stimulation are also consistent with the notion that β-catenin/Tcf signaling is an inherent component of TCR that diverts TCR responses leading to negative selection.
Discussion
The molecular basis of thymocyte selection is still poorly understood. In this study, we provide evidence that β-catenin/Tcf signaling is activated downstream of the TCR and determines the outcome of thymocyte selection. We derived this conclusion by analyzing a transgenic reporter as well as gain and loss of β-catenin/Tcf function mice independently or after crossing to TCR transgenes and to the Bim-knockout allele. Our studies showed that conditional stabilization of β-catenin induced and ablation of Tcf-1 rescued from clonal deletion. Thymocytes with stabilized β-catenin were partially activated and had stronger response to ex vivo TCR stimulation. Bim was functionally responsible for β-catenin/Tcf-dependent deletion of DP thymocytes. Thus, β-catenin/Tcf signaling is a component of the TCR cascade that defines the outcome of thymic selection by modulating survival of cells undergoing selection.
β-Catenin/Tcf signaling is activated as a result of TCR signals during thymocyte selection. Increased levels of nuclear β-catenin upon anti-CD3 and anti-CD28 treatment of murine CD4 lymphocytes was reported previously (50). Our findings show that this process is cell intrinsic (stroma independent), because it is observed both by in vivo as well as ex vivo TCR stimulation. The rapid stabilization of β-catenin within 1 h of TCR stimulation suggests a direct and probably Wnt-independent response to TCR signals. Both proximal (Lck, ZAP70, and Slp76) and intermediate (PKC and calcineurin) TCR signaling is required, indicating that these signals act upstream of β-catenin. The dependence of TCR-induced stabilization of β-catenin on calcineurin is surprising, because this protein has only been implicated in positive selection (51, 52) and may suggest a more complex involvement of calcineurin in the selection processes. The independence of TCR-mediated stabilization of β-catenin from PI3K/Akt signaling was also surprising, because PI3K/Akt mediates phosphorylation and inactivation of GSK-3β (53), a kinase responsible for proteolytic targeting of β-catenin. By contrast, Akt, which has been recently reported to directly phosphorylate and activate β-catenin (54), may control the steady-state levels of β-catenin independently of the TCR. Similarly, the accumulation of β-catenin by inhibition of PKC indicates that PKC may have a role in steady-state removal of β-catenin. This is in line with a recent report (35) showing N-terminal phosphorylation of β-catenin and targeting for degradation by PKC-α. To our surprise, pharmacological inhibition of Jnk and p38 activities did not influence the TCR-dependent stabilization of β-catenin. This was in contrast to recent reports indicating the stability of β-catenin may be regulated by direct Jnk2-mediated phosphorylation (37) or by p38-mediated phosphorylation and inactivation Gsk3β (36).
In agreement with a role in TCR signaling, conditional activation of β-catenin led to partial activation of preselected DP thymocytes. This included increased phosphorylation of p38, up-regulation of activation markers such as CD69 and CD5, and elevated expression of Gadd45γ, a factor required for Jnk and p38 activation (55). Cells with activated β-catenin were sensitized to ex vivo TCR stimulation leading to enhanced phosphorylation of Lat, Jnk, and p38 as well as transient activation of Erk. These signaling responses have been previously linked to negative selection (5, 11, 56–58). Future studies will delineate the molecular mechanism by which β-catenin/Tcf elicit their effect on TCR signaling.
We propose that the physiological significance of TCR-induced β-catenin/Tcf signaling at the DP stage is to regulate the outcome of thymocyte selection. In support of this suggestion, genetic modulation of β-catenin/Tcf signaling by conditional stabilization of β-catenin or ablation of Tcf-1 influenced the generation of post-selected DP thymocytes and led to the selection of T cells with an altered TCR repertoire. The thymic profiles of TCR-transgenic mice with activated β-catenin or ablated Tcf-1 provide additional evidence. In particular, the increased apoptosis and clonal elimination of T cells only in the selective MHC in TCR transgenes with activated β-catenin show that β-catenin and TCR signals cooperate in this event. This clonal elimination is not likely to result from β-catenin “toxicity” at this stage, because stabilization of β-catenin in Rag−/− DN thymocytes promotes their transition to the DP stage (Ref. 27 and data herein). Similarly, rescue of a fraction of HY-TCR-expressing CD8 SP thymocytes in male HY Tcf-1−/− mice also points to a role for this pathway in thymocyte selection. The rescue of Tcf-1−/−-HY-TCR thymocytes is incomplete probably due to Tcf-1-dependent defects at earlier stages and/or the expression and redundant action of Lef-1 (25). These factors prevent more detailed conclusions from the loss-of-function analyses, allowing only the conclusion that ablation of Tcf-1 leads to partial rescue from clonal deletion.
Members of the Bcl-2 family of proteins are the likely downstream mediators of β-catenin/Tcf signaling in thymocyte selection. The reciprocal changes of Bim, Bcl-xL, and Bcl-2 expression in thymocytes with activated β-catenin vs ablated Tcf-1 indicate that the balance between these regulators may control the survival of thymocytes during selection in these mice. Earlier analyses suggested that reduced expression to Bcl-xL in DP thymocytes with down-regulated β-catenin/Tcf signaling results in increased apoptosis (26, 59). Our studies permitted a direct comparison of loss and gain of function models as well as pre- and postselected DP thymocytes and led to the conclusion that Bcl-xL is alternatively regulated by activation of β-catenin vs ablation of Tcf-1 in both DP subsets. The levels of apoptosis of preselected DP thymocytes with activated β-catenin or ablated Tcf-1 followed the pattern of Bcl-xL expression and indicated that this antiapoptotic molecule specifically controls the survival of these cells. This conclusion is also in agreement with earlier suggestions that survival of DP thy-mocytes before selection (60) is mediated by Bcl-xL.
Survival of postselected DP thymocytes with activated β-catenin or ablated Tcf-1 is likely to be influenced by the balance between Bim and Bcl-2. The proapoptotic Bim, which binds to and regulates Bcl-2 function, has been tightly linked to the regulation of negative selection (61). The levels Bcl-2 and Bim expression in thymocytes are alternatively regulated by activation of β-catenin or ablation of Tcf-1. Several observations indicate that β-catenin/Tcf signaling fine-tunes the selection processes and controls the survival of postselected DP thymocytes by modulating the levels of Bcl-2 and Bim expression. First, ablation of one Bim allele selectively rescued and increased the fraction of posts-elected DP and SP thymocytes with activated β-catenin from apoptotic death. Second, overexpression of Bcl-2 in Tcf-1−/− thymocytes has been reported to rescue thymic cellularity and developmental progression to the SP stage (43). Further studies will address whether Bim and/or Bcl-2 are direct targets of the β-catenin/Tcf signaling pathway.
In conclusion, our study indicates that strong activation of β-catenin directs signaling toward negative selection, whereas Tcf-1 ablation prevents this process. Our observations place β-catenin/Tcf signaling in DP thymocytes downstream of the TCR and propose the functional integration of this evolutionary conserved pathway with the more recent cell-specific TCR. A similar integration of the TCR with the Notch cascade has been proposed recently (62). Our findings indicate that the β-catenin/Tcf cascade determines the choice between positive and negative selection by modulating the intracellular strength of TCR signaling leading to altered expression of mediators of thymocyte survival.
Supplementary Material
Acknowledgments
We thank Matthew Morrin for excellent technical support. We thank A. Parmelee and S. Kwok at the Tufts Laser Cytometry facility for providing invaluable theoretical and practical help with cell sorting. We also thank Dr Stephen Bunnell for providing us with the LCK-, SLP76-, and ZAP70-deficient cell lines.
Footnotes
This work was supported by National Institute of Health Grants R01 AI059676-01 and R21 AI076720, American Cancer Society Grant LIB-113428, the Smith Family New Investigator Award from the Medical Foundation, and the GRASP Center P30 DK-34928 Award to F.G. Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant T32 was awarded to D.K.
Abbreviations used in this paper: DP, double positive; DN, double negative; FDG, fluorescein-di-β-D-galactopyranoside; GSK-3β, glycogen synthase kinase-3β; HA, hemagglutinin; MFI, mean fluorescence intensity; MHCII, MHC class II; PKC, protein kinase C; SP, single positive
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 3.Hayes SM, Love PE. Strength of signal: a fundamental mechanism for cell fate specification. Immunol Rev. 2006;209:170–175. doi: 10.1111/j.0105-2896.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 4.Singer A. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr Opin Immunol. 2002;14:207–215. doi: 10.1016/s0952-7915(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 5.Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 6.Love PE, Lee J, Shores EW. Critical relationship between TCR signaling potential and TCR affinity during thymocyte selection. J Immunol. 2000;165:3080–3087. doi: 10.4049/jimmunol.165.6.3080. [DOI] [PubMed] [Google Scholar]
- 7.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Rincon M, Whitmarsh A, Yang DD, Weiss L, Derijard B, Jayaraj P, Davis RJ, Flavell RA. The JNK pathway regulates the in vivo deletion of immature CD4+CD8+ thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Villunger A, V, Marsden S, Zhan Y, Erlacher M, Lew AM, Bouillet P, Berzins S, Godfrey DI, Heath WR, Strasser A. Negative selection of semimature CD4 8 HSA thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc Natl Acad Sci USA. 2004;101:7052–7057. doi: 10.1073/pnas.0305757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarty N, Paust S, Ikizawa K, Dan I, Li X, Cantor H. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymo-cytes. Nat Immunol. 2005;6:65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- 15.Sohn SJ, Thompson J, Winoto A. Apoptosis during negative selection of autoreactive thymocytes. Curr Opin Immunol. 2007;19:510–515. doi: 10.1016/j.coi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein diversin recruits casein kinase I to the β-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional coactivator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 20.Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR, van Dongen JJ, Herzenberg LA, Staal FJ. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci USA. 2006;103:3322–3326. doi: 10.1073/pnas.0511299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goux D, Coudert JD, Maurice D, Scarpellino L, Jeannet G, Piccolo S, Weston K, Huelsken J, Held W. Cooperating pre-T cell receptor and TCF-1-dependent signals ensure thymocyte survival. Blood. 2005;106:1726–1733. doi: 10.1182/blood-2005-01-0337. [DOI] [PubMed] [Google Scholar]
- 23.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 24.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 25.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCR gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 26.Ioannidis V, Beermann F, Clevers H, Held W. The β-catenin–TCF-1 pathway ensures CD4+CD8+ thymocyte survival. Nat Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 27.Gounari F, Aifantis I, Khazaie K, Hoeflinger S, Harada N, Taketo MM, von Boehmer H. Somatic activation of β-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat Immunol. 2001;2:863–869. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- 28.Guo Z, Dose M, Kovalovsky D, Chang R, O’Neil J, Look AT, von Boehmer H, Khazaie K, Gounari F. β-Catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109:5463–5472. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Q, Sen JM. β-Catenin regulates positive selection of thymocytes but not lineage commitment. J Immunol. 2007;178:5028–5034. doi: 10.4049/jimmunol.178.8.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 32.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 33.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 34.Williams BL, Schreiber KL, Zhang W, Wange RL, Samelson LE, Leibson PJ, Abraham RT. Genetic evidence for differential coupling of Syk family kinases to the T cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwak J, Cho M, Gong SJ, Won J, Kim DE, Kim EY, Lee SS, Kim M, Kim TK, Shin JG, Oh S. Protein kinase C-mediated β-catenin phosphorylation negatively regulates the Wnt/β-catenin pathway. J Cell Sci. 2006;119:4702–4709. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- 36.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3 inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E, Khazaie K. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol. 2005;6:800–809. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 40.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 41.De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB. Maternal β-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 42.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and β-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 43.Wolfer A, Bakker T, Wilson A, Nicolas M, Ioannidis V, Littman DR, Lee PP, Wilson CB, Held W, MacDonald HR, Radtke F. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 44.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8+ T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 45.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z, Xie H, Ioannidis V, Held W, Clevers H, Sadim MS, Sun Z. Transcriptional regulation of CD4 gene expression by T cell factor-1/β-catenin pathway. J Immunol. 2006;176:4880–4887. doi: 10.4049/jimmunol.176.8.4880. [DOI] [PubMed] [Google Scholar]
- 48.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. JNK2 is required for efficient T cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 49.Sabapathy K, Kallunki T, David JP, Graef I, Karin M, Wagner EF. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J Exp Med. 2001;193:317–328. doi: 10.1084/jem.193.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of β-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 51.Gallo EM, Winslow MM, Cante-Barrett K, Radermacher AN, Ho L, McGinnis L, Iritani B, Neilson JR, Crabtree GR. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–735. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 53.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 54.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45γ mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 56.Werlen G, Hausmann B, Palmer E. A motif in the αβ T cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 57.Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC, Ohashi PS. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 58.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 59.Hossain MZ, Yu Q, Xu M, Sen JM. ICAT expression disrupts β-catenin-TCF interactions and impairs survival of thymocytes and activated mature T cells. Int Immunol. 2008;20:925–935. doi: 10.1093/intimm/dxn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chao DT, Korsmeyer SJ. BCL-xL-regulated apoptosis in T cell development. Int Immunol. 1997;9:1375–1384. doi: 10.1093/intimm/9.9.1375. [DOI] [PubMed] [Google Scholar]
- 61.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 62.Laky K, Fowlkes BJ. Presenilins regulate αβT cell development by modulating TCR signaling. J Exp Med. 2007;204:2115–2129. doi: 10.1084/jem.20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


