Abstract
In addition to its classical role in mediating responses to pain, the opioid system is strongly implicated in the regulation of social behavior. In young laboratory animals, low doses of opioid analgesic drugs reduce responses to isolation distress and increase play behavior. However, little is known about how opioid drugs affect responses to social stimuli in humans. Here we examined the effects of buprenorphine, a mu-opioid partial agonist and kappa-antagonist, on three dimensions of social processing; i) responses to simulated social rejection, ii) attention to emotional facial expressions, and iii) emotional responses to images with and without social content. Healthy adults (N = 36) attended two sessions during which they received either placebo or 0.2mg sublingual buprenorphine in randomized order, under double-blind conditions. Ninety minutes after drug administration, they completed three behavioral tasks: i) a virtual ball-toss game in which they were first included and then excluded by the other players; ii) an attention task in which they were shown pairs of faces (one emotional and one neutral), while the direction of their gazes was recorded using electrooculography, and iii) a picture-viewing task, in which they rated standardized images with and without social content. During the ball-toss game, buprenorphine decreased perceived social rejection. During the attention task, the drug reduced initial attention to fearful facial expressions, without influencing attention to angry, happy, and sad faces. Finally, during the picture-viewing task, buprenorphine increased ratings of positivity of images with social content, without affecting ratings of nonsocial images. These results suggest that even at low doses, opioid analgesic drugs reduce responses to some types of negative social stimuli, while enhancing positive responses to social stimuli. This provides further support for the role of the opioid system in mediating responses to social rejection and social reward.
Keywords: Opioid, social, psychophysiology, attention, buprenorphine
1. Introduction
The opioid system has been shown to play an important role in mediating socio-emotional responses in humans and other species (Herman and Panksepp, 1981; Kalin et al., 1988; Keverne et al., 1989; Panksepp et al., 1980; Panksepp and DeEskinazi, 1980; Panksepp et al., 1981). Positron emission tomography (PET) studies have shown that the opioid system mediates responses to acute social rejection and social loss in humans (Hsu et al., 2013; Zubieta et al., 2003), and low doses of exogenously administered mu-opioid agonists dampen responses to social isolation distress in a variety of species, including young guinea pigs, chicks, and dogs (Herman and Panksepp, 1978; Panksepp et al., 1978; Stein et al., 2007). Conversely, blocking opioid signaling with an opioid antagonist enhances responses to isolation distress and increases motivation for social contact (Fabre-Nys et al., 1982; Kalin et al., 1988; Martel et al., 1993; Martel et al., 1995; Panksepp et al., 1978). However, the effects of exogenously administered opioid drugs on responses to social rejection and responses to other negative social stimuli in humans have not been fully explored.
One opioid drug that has received some interest in clinical studies is the mu-opioid partial agonist and kappa antagonist buprenorphine (BP). There is some evidence that BP reduces symptoms of depression (Bodkin et al., 1995; Emrich et al., 1982; Nyhuis et al., 2008), and recent laboratory studies indicate that low doses of BP reduce responses to negative affective stimuli: BP reduces the ability to recognize fearful faces in an emotion recognition task (Ipser et al., 2013) and decreases physiological and subjective responses to acute social stress (Bershad et al., 2015). BP has also been shown to affect responses to positive social stimuli, bolstering short-term memory for social reward cues (Syal et al., 2015). Interestingly, these findings in humans are consistent with its antidepressant and anxiolytic-like effects in animal models (Falcon et al., 2014). However, many questions remain about the pharmacological basis of these effects, and about the behavioral processes that underlie the effects of BP on positive or negative emotional responses.
In this study, we investigated the effects of BP on several forms of responses to negative social stimuli in healthy young adults. We studied its effects on responses to simulated social rejection, attention to visual stimuli with emotional content, and psychophysiological responses to standardized affective images. We evaluated the effects of a low, but clinically relevant dose of BP on i) perceived rejection in a social rejection task, ii) attention to facial expressions of positive and negative emotions using electrooculography (EOG) and iii) emotional responses to positive and negative, as well as social and nonsocial images using facial electromyography (EMG) and subjective ratings. The three measures provide qualitatively distinctive indices of subjective and physiological reactivity to negative affective stimuli. We selected a very low dose of BP that was expected to produce subtle changes in emotional reactivity without producing significant subjective effects (e.g., “euphoria” or nausea) that might complicate the interpretation. We hypothesized that BP would dampen responses to simulated social rejection, reduce initial attention to negative emotional expressions and decrease affective responses to negative social stimuli.
2. Materials and Methods
2.1 Study Design
The within-subject, double-blind design consisted of two sessions wherein healthy young adults received, in counterbalanced order, 0.2 mg sublingual BP or placebo (sucralose tablets) before completing tasks assessing emotional reactivity. Subjective mood states and physiological measures were recorded before and 30, 90, 180, and 210 minutes after drug administration. At each session, subjects completed the behavioral tasks in counterbalanced order 90 minutes after drug administration.
2.2 Participants
Healthy subjects (N=36, 12 women) ages 18–40 were recruited through flyers and online advertisements. Screening consisted of a physical examination, electrocardiogram, modified Structural Clinical Interview for DSM-IV (SCID; First et al., 2012) and self-reported health and drug-use history. Inclusion criteria were: English fluency; high school education; BMI of 19–26; no current or past year DSM-IV Axis 1 disorders; no past year drug or alcohol dependence; and no history of opioid abuse, or regular use of opioid pain killers. To minimize variability related to the menstrual cycle, women were included only if they were taking oral contraceptives. Subjects were primarily Caucasian (N=23, 66%), in their 20s (mean± SD=21.9 ± 3.3), with some college education (mean± SD =14.9 ± 1.5), and light to moderate drug use (see Table 1).
Table 1.
Demographic and Substance Abuse Characteristics
| N (%) or M (SD) | ||
|---|---|---|
| Demographic variables | ||
| Sex (M/F) | 24/12 (66% / 33%) | |
| Race | 23 (66%) | Caucasian |
| 7 (19%) | African American | |
| 3 (8%) | Asian | |
| 3 (8%) | Other | |
| Age | 21.9 (3.3) | |
| Education in years | 14.9 (1.5) | |
| Current substance use | ||
| Alcoholic drinks/week | 3.4 (1.2) | |
| Cigarettes in past month | 0.9 (2.4) | |
| Lifetime recreational use | ||
| Cannabis | 3 (8%) | Never |
| 7 (19%) | 1–10x | |
| 12 (33%) | 11–50x | |
| 3 (8%) | 51–100x | |
| 11 (30%) | >100x | |
| Tranquilizers | 29 (80%) | Never |
| 6 (16%) | 1–10x | |
| 1 (2%) | 11–50x | |
| Stimulants | 22 (61%) | Never |
| 9 (25%) | 1–10x | |
| 5 (13%) | 11–50x | |
| Opiates | 30 (83%) | Never |
| 5 (13%) | 1–10x | |
| 1 (2%) | 51–100x | |
| Hallucinogens | 21 (58%) | Never |
| 14 (38%) | 1–10x | |
| 1 (2%) | 11–50x | |
| MDMA | 27 (75%) | Never |
| 8 (22%) | 1–10x | |
| 1 (2%) | 11–50x | |
| Other drugs | 33 (91%) | Never |
| 2 (5%) | 1–10x | |
| 1 (2%) | 11–50x | |
Subjects were required to abstain from recreational drugs for 48 hours before each session. They were instructed to avoid alcohol, prescription, and over-the-counter medications for 24 hours before each session, and to consume their normal amounts of caffeine the morning of the session. Compliance was verified by urinalysis (CLIAwaived Instant Drug Test Cup (IDTC), San Diego, CA) and breath alcohol testing (Alcosensor III, Intoximeters, St. Louis, MO). Female subjects provided urine samples for pregnancy tests. Subjects were told they might receive a placebo, stimulant, sedative or opioid drug. All participants provided informed consent prior to beginning the study procedures, which were approved by the University of Chicago Institutional Review Board.
2.3 Drug
Participants received 0.2mg sublingual BP (Temgesic®, Reckitt Benckiser Pharmaceuticals) with a sucralose tablet to disguise the taste of the drug or placebo (two Splenda® sucralose tablets) in counterbalanced order at two sessions. BP is a mu-opioid partial agonist and kappa-opioid antagonist that is used to treat moderate to severe pain and opioid dependence. The dose we administered in this study was less than one twentieth that used in opioid replacement therapy. This dose has been shown to affect memory for social reward (Syal et al., 2015) and emotion recognition (Ipser et al., 2013) without producing appreciable subjective effects or nausea. In our previous study (Bershad et al., 2015) a higher dose of BP (0.4 mg) produced appreciable nausea in the majority of participants. Peak plasma concentrations of the drug occur 90–360 minutes after ingestion (Mendelson et al., 1997).
2.4 Procedure
At a 1-hour orientation session, a research assistant described study tasks and psychophysiology procedures and subjects provided informed consent. Subjects then attended two 4.5 hour experimental sessions beginning at 9:00 AM and separated by at least 48 hours. After compliance was confirmed, baseline measures of subjective state and cardiovascular function were obtained. Sublingual tablets containing 0.2 mg BP or placebo were administered at 9:30 AM. Subjects then relaxed for 1 hour, reading or watching a movie. Subjective and cardiovascular measures were taken at 10:00 AM and 10:45 AM the psychophysiology sensors were applied. At 11 AM, participants completed subjective ratings, followed by the social rejection, attention bias task, and emotional responsiveness task in randomized, counterbalanced order. At 12:30 PM psychophysiology sensors were removed and subjective and cardiovascular measures were reassessed. At 1:00 PM, final measures were taken and subjects were discharged.
2.5 Subjective and Cardiovascular Drug Effects
Subjective and cardiovascular measures were obtained to monitor the effects of the drug. The primary measure of subjective drug effect was the Drug Effects Questionnaire DEQ; (Fischman and Foltin, 1991). The DEQ consists of five visual analog scales (VAS; 0–100) on which subjects rate how much they like, dislike and feel the effects of a drug, how “high” they feel, and how much they want more of the drug. Subjects also completed single-item VAS scales to indicate how nauseous they felt. Heart rate and blood pressure were monitored with portable monitors (Omron 10 Plus, Omron Healthcare, Kyoto, Japan). Questionnaires and cardiovascular measures were obtained at baseline (−15), 30, 90, 180 and 210 minutes after tablet administration. At the end of each session, participants were asked to guess which class of drug they had received.
2.6 Behavioral Tasks and Psychophysiological Measures
Simulated Social Rejection Task
The “Cyberball” task is widely used in simulating social acceptance and exclusion (Williams and Jarvis, 2006). In two 4-minute games, participants played “catch” with two differentially labeled, if otherwise identical, computer avatars. The behavior of these avatars was predetermined such that in each game subjects were either included (60 ± 3% of the tosses were to him/her) or rejected (15 ± 3% of the tosses were to him/her). At each session, subjects participated in one inclusion and one exclusion version of the game. Immediately after each, they completed questionnaires assessing their mood. Additionally, subjects were asked to estimate the percentage of tosses (0–100%) they received.
Attention Bias Task
In the Attention Bias Task, subjects viewed images of actors (8 male, 8 female) expressing anger, fear, happiness, sadness and emotional neutrality. Images were taken from the Karolinska Directed Emotional Faces Set (Goeleven et al., 2008). During each trial subjects viewed a 1,000 ms pre-image fixation mark, followed by a 2,000 ms pairs of images of single actor expressing neutrality and an emotion, placed adjacently on the screen. Immediately after this presentation, a visual probe icon (circle or square) was presented in place of one of the two images. Subjects were instructed to identify the probe as a circle or square as quickly as possible by responding on the keyboard. The probe identification part of the task was included to distract the participants from the purpose of the task. Trials were separated by 750–1,250 ms intervals. The 64-trial presentations were counterbalanced for icon shape, icon location, and emotional face location. The primary measure of attentional bias to emotional faces was the number of times participants looked toward each emotional face, as compared to the neutral one. These measures were recorded using electrooculography (EOG), achieved through the application of 4-mm Ag/AgCl sensors just outside the outer canthus of each eye. Trials were excluded if baseline gaze direction was not centrally fixed, or signal-noise obscured eye movements.
Emotional Images Task
Positive, negative, and neutral emotional images with and without social content were taken from the International Affective Picture System (Lang et al., 1999), as described in Wardle et al., 2012. Two different sets of these images matched for valence and arousal were prepared, one for each session, to minimize habituation across sessions. Social stimuli depicted two people or parts of people interacting, whereas nonsocial stimuli contained no people. Thus each set consisted of six subsets: social/negative, social/neutral, social/positive, nonsocial/negative, nonsocial/neutral, nonsocial/positive. Further, each subset contained nine images, three each of mild, moderate and extreme intensity. Pictures were presented in randomized order with no more than two pictures of consistent valence appearing consecutively. Images were presented for 6 seconds, preceded and followed by 3 seconds of a fixation mark. Subjects rated each image immediately after its presentation using an evaluative space grid (Larsen et al., 2009), which allowed for independent evaluations of emotional valence in both positive and negative dimensions (“0”: not at all; “4” extreme). Emotional reactivity to images was determined through electromyography (EMG) of corrugator and zygomatic musculature. EMG measures of facial muscle activity (corrugator and zygomatic muscles) are indicative of affective responses to emotional stimuli, and can provide a more objective measure of emotional reactivity than subjective reports alone (Dimberg et al., 2000). Highly positive stimuli increase activity in the zygomatic, or “smile” muscle and relax the corrugator (“frown”) muscle. Negative stimuli activate the corrugator muscle. EMG was measured with 4mm Ag/AgCl electrodes precisely placed on the cheek and left brow. An 8mm Ag/AgCl ground sensor was placed upon the subject’s forehead. Signals were amplified, filtered through a 10–500 Hz band pass, digitized, re-filtered, rectified and then integrated over 20 ms using EMG100C amplifiers, an MP150 Data Acquisition System and Acqknowledge software from Biopac Systems (Goleta, CA, USA).
2.7 Statistical Analyses
To analyze the data, we used paired t-tests or within-subjects repeated measures analysis of variance to compare responses during the drug session to the placebo session (RMANOVA). Any significant main effects and interactions were followed with paired t-tests, corrected for multiple comparisons. For all analyses, p values were considered statistically significant at less than 0.05.
3. Results
3.1 Subjective Effects of Buprenorphine
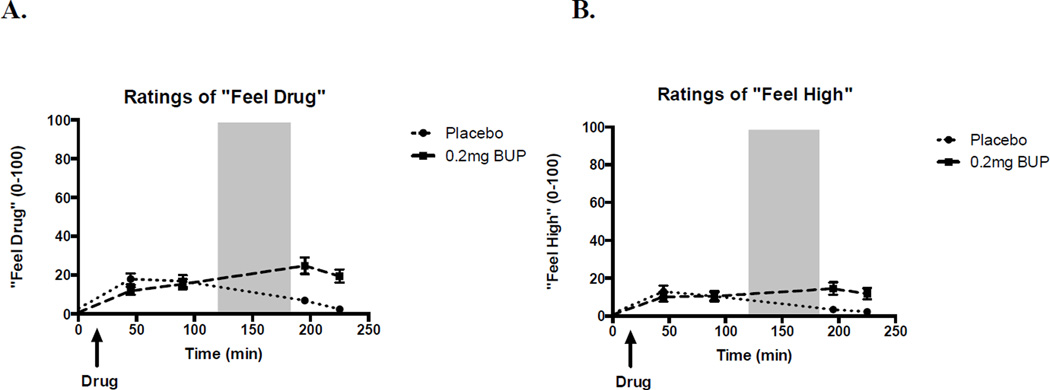
Five subjects reported nausea after BP, which interrupted their performance of the behavioral tasks. These subjects were excluded from behavioral analyses, even though responses on other subjective measures of drug effect did not significantly differ when these subjects were excluded or included in the analyses. BP tended to increase ratings of “feel drug” (Figure 1; t[36] = −1.87, p = 0.07), but did not increase ratings of “like effects”, “dislike effects”, “feel high”, “want more” or any of the other adjectives selected to detect subjective effects of the drug. Thus, the behavioral effects of BP could not be attributed to feelings of drug high or “euphoria”.
Figure 1. Effects of buprenorphine and placebo on subjective reports of a) “feel drug” and b) “feel high”.
Effects measured with visual analogue ratings. Shaded area indicates the time during which the tasks took place. Bars depict mean ± SEM.
3.3 Simulated Social Rejection
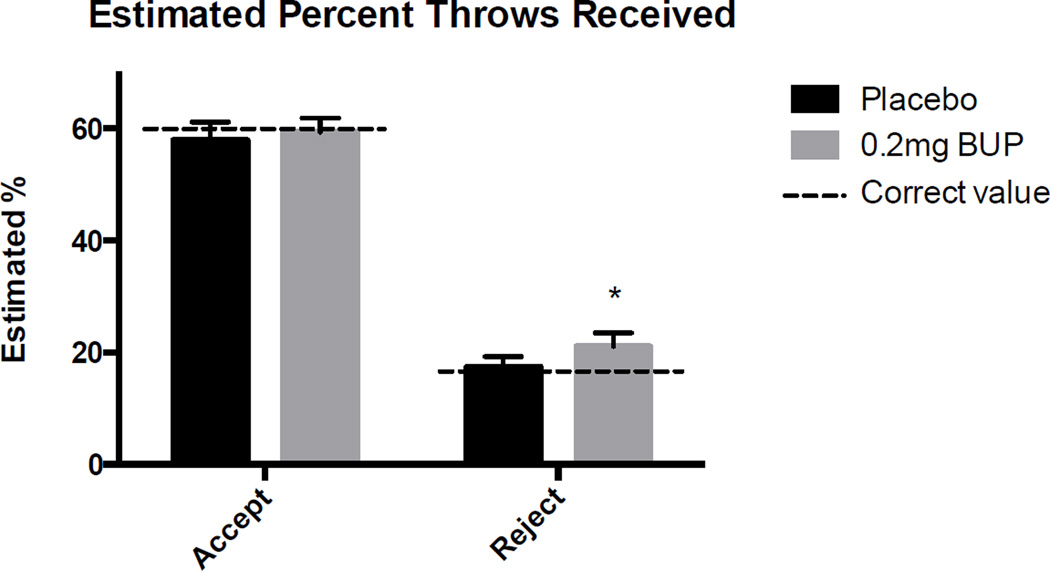
Rejection significantly increased self-reported negative mood and decreased positive mood during the placebo session (t[30] = −6.6, p <0.001, t[30] = 6.4, p <0.001), and decreased estimated percent throws received (t[30] = 13.3, p <0.001). BP significantly increased participants’ estimation of the degree to which they were included (Figure 4A; t[30] = −2.2, p <0.05), and tended to reduce negative mood in response to rejection (Figure 4B; t[30] = 1.7, p = 0.1). The drug did not affect ratings of positive mood in response to rejection, nor did it affect any measures of responses during the inclusion portion of the task.
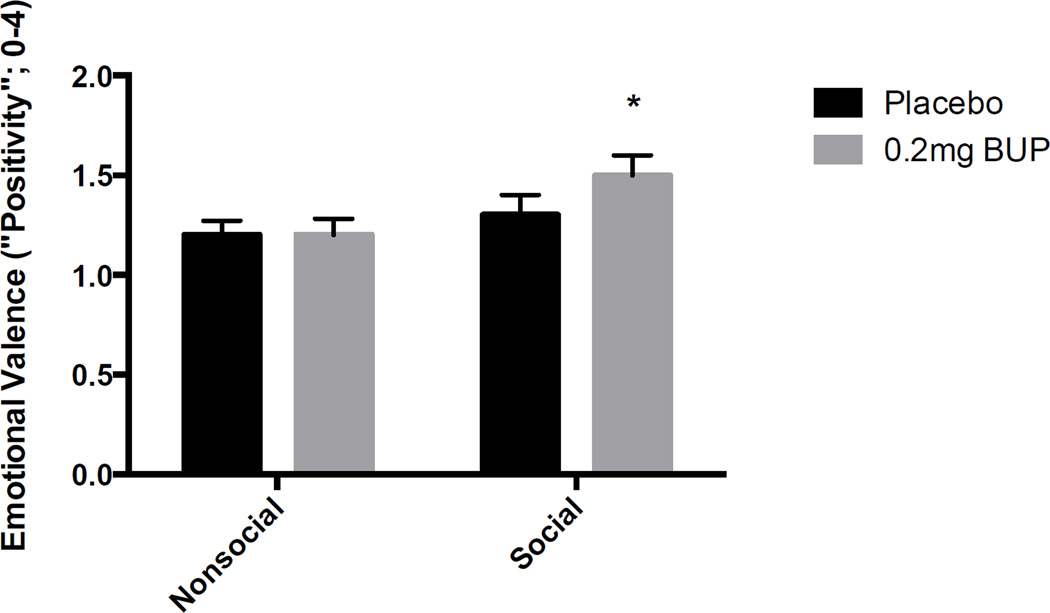
Figure 4. Mean (±SEM) ratings of ‘positivity’ of images with social and nonsocial content after buprenorphine or placebo.
Asterisk indicates a significant difference between drug and placebo, p<0.05.
3.4 Attention Bias Task
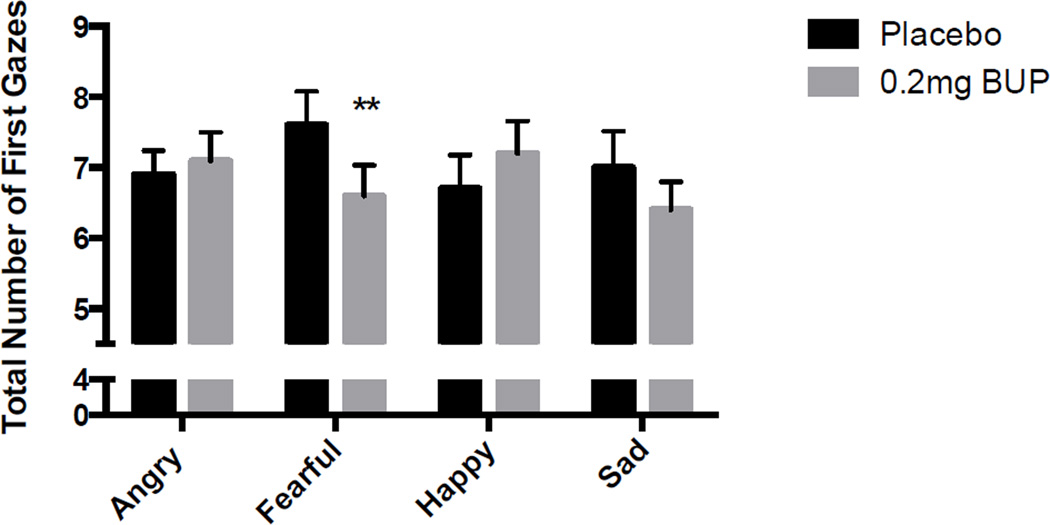
BP reduced the number of first gazes toward fearful facial expressions as compared to other emotions (Dose × Emotion Type F[2,28] = 3.61, p<0.05; Fear 0mg vs. 0.2 mg p<0.05). Overall, neither the drug nor the type of emotion affected the direction of first gaze (Figure 2; Dose F[2,27]=0.02, p = 0.9, Emotion Type F[2,27] = 0.41, p = 0.8).
Figure 2. Effects of buprenorphine on perceived social rejection during the accept and reject conditions.
Values shown are mean (± SEM) estimated percent throws received after acceptance and rejection. Dashed line indicates the actual percentage of throws received. Asterisks indicate significant difference between drug and placebo, p<0.05.
3.5 Emotional images task
As expected, subjects rated the positive images with the highest valence ratings and negative images the least (F[2, 29] = 280.3, p<0.001; positive vs. neutral p<0.05, negative vs. neutral p<0.05, positive vs. negative p<0.001). BP increased valence ratings only for images with social content (Figure 3; Dose × Social Type F[2, 29] = 4.5, p<0.05). BP did not significantly affect ratings of arousal for any type of image.
Figure 3. Mean (± SEM) number of initial gazes toward emotional facial expressions after buprenorphine and placebo, shown for each emotion depicted.
Asterisks indicate a significant difference between drug and placebo, p<0.01.
Measures of zygomatic reactivity were not normally distributed, so the square roots of the data were used in analyses. Facial EMG of the corrugator and zygomatic muscles showed expected main effects of picture type (Corrugator; Type F[2, 29] = 7.6, p<0.001, negative vs. neutral p=0.08, negative vs. positive p<0.01, Zygomatic; Type F[2, 29] = 4.0, p<0.05, negative vs. positive p<0.05); corrugator activity increased in response to negative images and zygomatic activity increased in response to positive images. No drug effects on EMG measures were observed.
3.6 Drug Identifications
Only 17% of participants correctly identified BP as an opioid following the session. The rest guessed they had taken a stimulant (11%), sedative (44%) or placebo (28%). After receiving the placebo dose, 36% percent of subjects correctly identified it as such. The remaining individuals guessed that they had received a stimulant (8%), sedative (36%) or opioid (19%) drug.
4. Discussion
In this study, we aimed to determine the effects of the mu-opioid partial agonist and kappa antagonist BP on responses to three different types of negative social stimuli, including simulated social rejection, emotional facial expressions, and images with social and nonsocial content. In accordance with our hypothesis, we found that BP decreased perceived social rejection and attention to fearful facial expressions, and increased ratings of positivity for images with social content. These effects occurred at a low dose of the drug, and appeared to be independent of any subjective experience of euphoria.
One of the results of this study was that BP reduced perceived social rejection in a simulated social rejection task. This result aligns with studies in young rodents and other species showing that exogenously administered opioids reduce behavioral measures of distress in response to social isolation (Bos et al., 2012; Herman and Panksepp, 1978; Panksepp et al., 1978; Stein et al., 2007; Sufka et al., 1994), perhaps by reducing the perception of the social rejection itself. In chicks, this distress-reducing effect was blocked by the administration the opioid antagonist naloxone (Panksepp et al., 1980). Studies using PET imaging in humans have suggested a role for the opioid system in mediating responses to social rejection (Hsu et al., 2013), and indirect evidence implicating the opioid system in responses to perceived social isolation has been obtained by measuring pain thresholds. Individuals with strong social support systems report less cardiac (King et al., 1993), chronic (Phillips and Gatchel, 2000), and post-operative pain (Kulik and Mahler, 1989), and this phenomenon is thought to be mediated in part by increases in endogenous opioid tone as a result of social contact (Dunbar, 2010; Odendaal and Meintjes, 2003). One study saw an increase in pain sensitivity in socially isolated rats, providing further support for a relationship between pain thresholds and opioid tone (Panksepp, 1980). There is some evidence that opioid users may use the drugs in part to restore low endogenous opioid levels resulting from diminished social interactions. Rats given a choice between morphine and water solutions consumed significantly more morphine when they had been singly-housed, compared to their group-housed conspecifics (Alexander et al., 1978). In a study with nonhuman primates, the concentration of β-endorphin in cerebral spinal fluid increased significantly after grooming (Keverne et al., 1989). Thus in opioid-dependent populations, where social relationships are often disrupted (O'donnell et al., 1967), opioids may be used in a compensatory manner (Albertin and Íniguez, 2008). Here we report results consistent with this idea, showing that an exogenous mu-partial agonist dampens responses to simulated social rejection.
The observed effects of BP on attention to emotional expressions are in line with a body of evidence suggesting the drug attenuates responses to fearful stimuli. One recent study showed that BP selectively reduces the ability to recognize fearful facial expressions (Ipser et al., 2013), and our results may suggest a behavioral mechanism through which BP has this effect. BP may act to direct attention away from faces expressing fear, reducing the ability to detect the emotion. Furthermore, BP’s effects on emotion processing are consistent with preclinical and clinical evidence in support of the anti-depressant properties of the drug (Bodkin et al., 1995; Emrich et al., 1982; Falcon et al., 2014). A negative bias in emotion processing has been observed in patients with mood and anxiety disorders, and this bias is sensitive to standard anti-depressants (Harmer et al., 2009), which show similar effects as those observed in this study.
BP acts at both the mu opioid receptor (MOR) and kappa opioid receptor (KOR), and its actions at both these sites may contribute to the effects observed here. Both actions have been implicated in anxiolytic effects: mu-opioid agonists reduce responses to fearful stimuli in rodents and non-human primates (Davis, 1979; Morris and Gebhart, 1978; Shepherd et al., 1992; Vivian and Miczek, 1993; Winslow et al., 2007), whereas mice lacking the KOR and those pretreated with a KOR antagonist fail to develop conditioned place aversion associated with repeated physical stressors such as foot shocks (Land et al., 2008). Further, administration of a kappa-antagonist reduces responses to stress, including stress-induced immobility and analgesia, and stress-induced reinstatemnt of alcohol seeking (Funk et al., 2014; McLaughlin et al., 2006). The combined actions of the drug at both receptors may have contributed to our observed results with responses to negative social stimuli. Interestingly, these same actions and effects may also contribute to the efficacy of BP in treating opioid use disorders, by reducing responses to stressors that may lead to relapse. The future availability of a selective kappa-antagonist for use in humans will help to clarify the pharmacological mechanisms of these effects.
Finally, we observed that BP selectively increases ratings of positivity for images with social content. This suggests that, in addition to its ability to decrease reactions to negative social stimuli (rejection and perception of fear), BP may also enhance reactivity to positive social stimuli. This is consistent with studies in nonhuman animals that opioids are involved in socially rewarding activities such as play behavior (Trezza et al., 2010; Vanderschuren et al., 1995a; Vanderschuren et al., 1995b; Panksepp and Bishop, 1981; Panksepp et al., 1985) and suggests that in humans opioid agonists enhance perceptions of social reward, as well as decreasing perceived negative social stimuli (Syal et al., 2015). The so-called “empathogen”, 3,4-Methylenedioxymethamphetamine (MDMA), has also been shown to selectively enhance positivity ratings for images with social content (Wardle et al., 2014), which supports the idea that opioids may also act to enhance social reward. For reasons that are not yet clear, the effect of BP on social stimuli was limited to subjective ratings of positivity, and was not detected in the electropsychophysiological indices of positive emotional responses (i.e., zygomatic muscle activity). The degree of association between physiological and subjective ratings of emotional state is in an interesting direction for future research.
One concern about using opioid drugs to dampen responses to negative social stimuli is that they may do so only by producing the subjective experience of euphoria. In this study, however, we detected effects on emotional responses at a very low dose (twenty times lower than the dose used in opioid replacement therapy), which did not produce appreciable subjective effects of any kind, and no euphoria. Thus, in this study, we showed BP produced its effects on responses to social stimuli independently of any direct positive mood effects of the drug.
The study presented here has several limitations. First, the small sample size limited our power and made it difficult to detect subtle effects. The sample size also made it difficult to examine individual differences in responsiveness to the drug. Also, in this study, we only tested a single low dose of BP. This is the lowest clinically available dose, but it is possible that similar effects may be present at even lower doses. Finally, we studied the effects of the drug in healthy adult volunteers, leaving unanswered the question of whether BP would more strongly affect responses to social stimuli in symptomatic populations, including drug users or other psychiatric populations.
The findings we report here align with a body of preclinical evidence and a handful of studies in humans showing that opioids reduce responses to various types of negative social stimuli. Questions for future investigation include the pharmacological mechanisms through which BP exerts its effects on social processing (e.g., mu or kappa), and how these effects might be leveraged in a clinical setting (Panksepp and Yovell, 2014).
Highlights.
► We show that buprenorphine dampens responses to simulated social rejection ► We show that buprenorphine reduces attention to fearful facial expressions, but does not affect attention to happy, sad, or angry faces ► We show that buprenorphine selectively increases ratings of positivity for emotional images with social content ► We observed these stress-blunting effects at a relatively low dose of the drug that does not produce significant euphoria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertin P, Íniguez L. Using drugs: The meaning of opiate substances and their consumption from the consumer perspective. Addiction Research & Theory. 2008;16:434–452. [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Bershad AK, Jaffe JH, Childs E, de Wit H. Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology. 2015;52:281–288. doi: 10.1016/j.psyneuen.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. Journal of clinical psychopharmacology. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluth R-M, Honk Jv. Acute effects of steroid hormones and neuropeptides on human social, emotional behavior: A review of single administration studies. Frontiers in Neuroendocrinology. 2012;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Davis M. Morphine and naloxone: Effects on conditioned near as measured with the potentiated startle paradigm. European journal of pharmacology. 1979;54:341–347. doi: 10.1016/0014-2999(79)90063-3. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Dunbar RI. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neuroscience & Biobehavioral Reviews. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Emrich H, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Annals of the new York Academy of Sciences. 1982;398:108–112. doi: 10.1111/j.1749-6632.1982.tb39483.x. [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, Meller RE, Keverne E. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacology Biochemistry and Behavior. 1982;16:653–659. doi: 10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2014:1–9. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub. 2012 [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective effects measurements in assessing abuse liability of drugs in humans. British journal of addiction. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Lê A. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain and Behavior. 2014;4:356–367. doi: 10.1002/brb3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska directed emotional faces: a validation study. Cognition and Emotion. 2008;22:1094–1118. [Google Scholar]
- Harmer CO, Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin G, Cowen P. Effect of acute antidepressant administration on negative affective bias in depressed patients. American Journal of Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacology Biochemistry and Behavior. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Ascending endorphin inhibition of distress vocalization. Science. 1981;211:1060–1062. doi: 10.1126/science.7466377. [DOI] [PubMed] [Google Scholar]
- Hsu D, Sanford B, Meyers K, Love T, Hazlett K, Wang H, Ni L, Walker S, Mickey B, Korycinski S. Response of the mu-opioid system to social rejection and acceptance. Molecular psychiatry. 2013;18:1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Panksepp J, Malcolm-Smith S, Thomas K, Stein DJ, van Honk J. Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology. 2013;38:166–170. doi: 10.1016/j.psyneuen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain research. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- King KB, Reis HT, Porter LA, Norsen LH. Social support and long-term recovery from coronary artery surgery: Effects on patients and spouses. Health psychology. 1993;12:56. doi: 10.1037//0278-6133.12.1.56. [DOI] [PubMed] [Google Scholar]
- Kulik JA, Mahler HI. Social support and recovery from surgery. Health Psychology. 1989;8:221. doi: 10.1037//0278-6133.8.2.221. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. Anglais. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: A single-item measure of positivity and negativity. Cognition and Emotion. 2009;23:453–480. [Google Scholar]
- Martel FL, Nevison CM, Rayment FD, Simpson MJ, Keverne EB. Opioid receptor blockade reduces maternal affect and social grooming in rhesus monkeys. Psychoneuroendocrinology. 1993;18:307–321. doi: 10.1016/0306-4530(93)90027-i. [DOI] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Simpson MJ, Keverne EB. Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Developmental psychobiology. 1995;28:71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J, Upton RA, Everhart ET, Jones RT. Bioavailability of sublingual buprenorphine. The Journal of Clinical Pharmacology. 1997;37:31–37. doi: 10.1177/009127009703700106. [DOI] [PubMed] [Google Scholar]
- Morris MD, Gebhart G. The effect of morphine on fear extinction in rats. Psychopharmacology. 1978;57:267–271. doi: 10.1007/BF00426749. [DOI] [PubMed] [Google Scholar]
- Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. Journal of clinical psychopharmacology. 2008;28:593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- O'donnell JA, Besteman KJ, Jones JP. Marital history of narcotics addicts. Substance Use & Misuse. 1967;2:21–38. [Google Scholar]
- Odendaal J, Meintjes R. Neurophysiological correlates of affiliative behaviour between humans and dogs. The Veterinary Journal. 2003;165:296–301. doi: 10.1016/s1090-0233(02)00237-x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Bean NJ, Bishop P, Vilberg T, Sahley TL. Opioid blockade and social comfort in chicks. Pharmacology Biochemistry and Behavior. 1980;13:673–683. doi: 10.1016/0091-3057(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Bishop P. An autoradiographic map of (3 H) diprenorphine binding in rat brain: Effects of social interaction. Brain research bulletin. 1981;7:405–410. doi: 10.1016/0361-9230(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Panksepp J, DeEskinazi FG. Opiates and homing. Journal of comparative and physiological psychology. 1980;94:650. doi: 10.1037/h0077708. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott J. The biology of social attachments: opiates alleviate separation distress. Biological Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman B, Vilberg T, Bishop P, DeEskinazi F. Endogenous opioids and social behavior. Neuroscience & Biobehavioral Reviews. 1981;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec J, DeEskinazi F, Bishop P. Opiates and play dominance in juvenile rats. Behavioral Neuroscience. 1985;99:441. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Yovell Y. Preclinical Modeling of Primal Emotional Affects (SEEKING, PANIC and PLAY): gateways to the development of new treatments for depression. Psychopathology. 2014;47:383–393. doi: 10.1159/000366208. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Brief social isolation, pain responsivity, and morphine analgesia in young rats. Psychopharmacology. 1980;72:111–112. doi: 10.1007/BF00433816. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Gatchel RJ. Extraversion–introversion and chronic pain. In: Weisberg RJGJN, editor. Personality characteristics of patients with pain. Washington, DC, US: American Psychological Association; 2000. pp. 181–202. [Google Scholar]
- Shepherd JK, Blanchard DC, Weiss SM, Rodgers RJ, Blanchard RJ. Morphine attenuates antipredator ultrasonic vocalizations in mixed-sex rat colonies. Pharmacology Biochemistry and Behavior. 1992;41:551–558. doi: 10.1016/0091-3057(92)90372-m. [DOI] [PubMed] [Google Scholar]
- Stein DJ, van Honk J, Ipser J, Solms M, Panksepp J. Opioids: from physical pain to the pain of social isolation. CNS spectrums. 2007;12:669. doi: 10.1017/s1092852900021490. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Hughes RA, McCormick TM, Borland JL. Opiate effects of isolation stress in domestic fowl. Pharmacology Biochemistry and Behavior. 1994;49:1011–1015. doi: 10.1016/0091-3057(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Syal S, Ipser J, Terburg D, Solms M, Panksepp J, Malcolm-Smith S, Bos PA, Montoya ER, Stein DJ, van Honk J. Improved memory for reward cues following acute buprenorphine administration in humans. Psychoneuroendocrinology. 2015;53:10–15. doi: 10.1016/j.psyneuen.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends in pharmacological sciences. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. μ-and κ-opioid receptor-meiated opioid effects on social play in juvenile rats. European journal of pharmacology. 1995a;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Spruijt BM, Hol T, Niesink RJ, Van Ree JM. Sequential analysis of social play behavior in juvenile rats: effects of morphine. Behavioural brain research. 1995b;72:89–95. doi: 10.1016/0166-4328(96)00060-5. [DOI] [PubMed] [Google Scholar]
- Vivian J, Miczek K. Morphine attenuates ultrasonic vocalization during agonistic encounters in adult male rats. Psychopharmacology. 1993;111:367–375. doi: 10.1007/BF02244954. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Garner MJ, Munafò MR, de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology. 2012;223:199–210. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Kirkpatrick MG, de Wit H. ‘Ecstasy’as a social drug: MDMA preferentially affects responses to emotional stimuli with social content. Social cognitive and affective neuroscience, nsu035. 2014 doi: 10.1093/scan/nsu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior research methods. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biological psychiatry. 2007;61:389–395. doi: 10.1016/j.biopsych.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic μ-opioid neurotransmission. Archives of general psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]