Abstract
Pharmacogenetic testing is increasingly available from clinical laboratories. However, only a limited number of quality control and other reference materials are currently available to support clinical testing. To address this need, the Centers for Disease Control and Prevention–based Genetic Testing Reference Material Coordination Program, in collaboration with members of the pharmacogenetic testing community and the Coriell Cell Repositories, has characterized 137 genomic DNA samples for 28 genes commonly genotyped by pharmacogenetic testing assays (CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, CYP4F2, DPYD, GSTM1, GSTP1, GSTT1, NAT1, NAT2, SLC15A2, SLC22A2, SLCO1B1, SLCO2B1, TPMT, UGT1A1, UGT2B7, UGT2B15, UGT2B17, and VKORC1). One hundred thirty-seven Coriell cell lines were selected based on ethnic diversity and partial genotype characterization from earlier testing. DNA samples were coded and distributed to volunteer testing laboratories for targeted genotyping using a number of commercially available and laboratory developed tests. Through consensus verification, we confirmed the presence of at least 108 variant pharmacogenetic alleles. These samples are also being characterized by other pharmacogenetic assays, including next-generation sequencing, which will be reported separately. Genotyping results were consistent among laboratories, with most differences in allele assignments attributed to assay design and variability in reported allele nomenclature, particularly for CYP2D6, UGT1A1, and VKORC1. These publicly available samples will help ensure the accuracy of pharmacogenetic testing.
Pharmacogenetic tests are used to predict or explain an individual's reaction to drugs by assaying for the presence or absence of known genetic polymorphisms in genes encoding drug metabolizing enzymes, drug transporters, drug receptors, or targets of drug action. These tests are used clinically to assist development of therapeutic strategies. For example, clopidogrel (Plavix) is a platelet inhibitor that is prescribed to patients with acute coronary syndromes to prevent blood clots. This drug is metabolized to its active form [2-{1-[1-(2-chlorophenyl)-2-methoxy-2-oxoethyl]-4-sulfanyl-3-piperidinylidene}acetic acid] by several cytochrome P (CYP) 450 enzymes, most notably CYP2C19. Individuals with loss-of-function CYP2C19 variants are not able to effectively metabolize clopidogrel and are at increased risk for adverse cardiovascular events, especially after receiving coronary artery stents. The boxed warning on clopidogrel (http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm203888.htm, last accessed September 12, 2014) notes that testing is available to determine the CYP2C19 genotype. The US Food and Drug Administration (FDA) requires that information about applicable pharmacogenetic tests be included in the labeling of certain FDA-approved drugs, including clopidogrel, warfarin, and abacavir.1 Pharmacogenetic tests are also used by researchers and pharmaceutical companies for discovery, drug development, and clinical trials.2
Clinical genetic testing laboratories are required by regulations and guided by professional or best practice standards to use reference materials (RMs) for assay development and validation, quality control, and proficiency testing [http://www.acmg.net/Pages/ACMG_Activities/stds-2002/g.htm; Washington State Legislature, http://app.leg.wa.gov/WAC/default.aspx?cite=246-338-090; College of American Pathologists, http://www.cap.org/apps/cap.portal (registration required); New York State Clinical Laboratory Evaluation Program, http://www.wadsworth.org/clep; all URLs last accessed April 17, 2015].3, 4, 5, 6, 7, 8, 9 Genetic testing laboratories often use genomic DNA samples, from cell lines or residual deidentified patient material, as RMs. Although clinical laboratories commonly offer pharmacogenetic tests, and many pharmacogenetic assays are being used for drug development and clinical trials, there are a limited number of quality control and other RMs that have been characterized using multiple methods. These materials cover some of the more commonly tested genes and alleles included in commercially available reagents and platforms, but no characterized RMs are currently available for most genes and alleles included in the more comprehensive assays, particularly for low-frequency variants or variants more commonly found in non-European populations. This lack of RMs hinders the ability of laboratories to develop and validate assays and perform necessary quality control. It also makes comparison of assays and standardization among laboratories difficult.
In 2010, the Centers for Disease Control and Prevention (CDC)–based Genetic Testing Reference Material Coordination (GeT-RM) Program, in collaboration with members of the pharmacogenetic testing community and Coriell Cell Repositories, characterized 107 publicly available genomic DNA samples for five commonly tested PGx genes: CYP2D6, CYP2C19, CYP2C9, VKORC1, and UGT1A1.10 This study confirmed the presence of a variety of the commonly tested polymorphisms in the five genes; however, many important variants were assayed but not identified among the samples tested. An additional study was published that characterized 48 samples for CYP2D6 only using multiple methods, including phenotyping. Two additional CYP2D6 alleles, *43 and *45, which were not tested in the GeT-RM study, were identified.11
During our initial study, clinical laboratories tested only a few pharmacogenetic genes for a limited number of variants. Today, a much larger number of pharmacogenetic genes and alleles are tested in clinical and research settings. Some commercially available platforms, such as the Affymetrix DMET Plus Array (Affymetrix, Santa Clara, CA) and the Agena Bioscience iPLEX ADME PGx Pro Panel (Agena Bioscience, San Diego, CA), and laboratory-developed assays that use massively parallel sequencing, commonly referred to as next-generation sequencing technology, can examine dozens to hundreds of pharmacogenetic genes. In addition, the number of variants that are associated with pharmacogenetic phenotypes has increased, and the knowledge about the effects of these variants on drug metabolism is actively being curated, leading to the development of practice guidelines for several gene-drug pairs (pharmGKB, Dosing Guidelines, Clinical Pharmacogenetics Implementation Consortium, http://www.pharmgkb.org/view/dosing-guidelines.do?source=CPIC#, last accessed February 12, 2015).12
To address the increasing need for characterized genomic DNA RMs for pharmacogenetic testing of additional PGx genes and alleles, the GeT-RM Program and the genetic testing community collaborated to characterize an additional 137 publicly available genomic DNA samples for 230 pharmacogenetic genes. The nine participating pharmacogenetic testing laboratories used a variety of commercially available platforms and laboratory-developed tests, including DNA sequencing assays, to genotype the samples. The findings from this study will be reported in two parts. The haplotype analysis of 28 pharmacogenetic genes (CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, CYP4F2, DPYD, GSTM1, GSTP1, GSTT1, NAT1, NAT2, SLC15A2, SLC22A2, SLCO1B1, SLCO2B1, TPMT, UGT1A1, UGT2B7, UGT2B15, UGT2B17, and VKORC1) are reported here; however, the results of the DNA sequence analyses and genotyping studies will be reported separately.
Materials and Methods
Cell Line and Laboratory Selection
One hundred thirty-seven cell lines were selected from the National Institute of General Medical Sciences and the National Human Genome Research Institute Repositories at the Coriell Cell Repositories for this study based on data from earlier partial genotype analysis and the varied ethnicities of the donors. For logistical reasons (related to cost of reagents, staffing, and batched run size), the 137 DNA samples were divided into two nonoverlapping sets: tier 1 (96 DNA samples) and tier 2 (41 DNA samples). Volunteer laboratories were selected based on assay, platform, and willingness to participate. Clinical and research laboratories as well as commercial assay manufacturing laboratories participated in the study. DNA samples were prepared by Coriell, and aliquots were sent to the volunteer laboratories for genotyping. Participants used a variety of commercially available tests, both FDA cleared and non–FDA cleared, as well as laboratory-developed tests. The alleles detected by each assay and the sample sets (tier 1 and 2) tested by each assay are given in Table 1. Reagents for the GenMark, Luminex, and Affymetrix assays were donated to the testing laboratories by the manufacturers.
Table 1.
Loci and Alleles Detected by Each Assay
| Assay (sample sets tested) | Affymetrix DMET (tier 1) | GenMark eSensor† (tier 1) | Luminex xTAG (tier 1) | LifeTech Taqman laboratory-developed tests (tiers 1 and 2) | Agena Bioscience iPLEX ADME PGx Pro (tiers 1 and 2) | Agena Bioscience CYP2D6, CYP2C9/VKORC1, CYP2C19, UGT1A1 (tiers 1 and 2) | Autogenomics CYP2D6, CYP3A4, CYP3A5, NAT2 (tier 1) |
|---|---|---|---|---|---|---|---|
| CYP1A1 | *2C, *3, *4, *5, *6, *7, *8, *9, *10, G45D, R279W, I286T, F381L, A463G | *2, *3, *4, *5, *6, *7, *8, *9 | |||||
| CYP1A2 | *1A, *1C, *1D, *1F, *1K, *1L, *2, *3, *4, *5, *6, *7, *8, *11, *15, *16 | *1A, *1C, *1F, *1K, *1L, *7 | |||||
| CYP2A6 | *2, *4, *6, *7, *8, *9, *11, *13, *17, *20, *28, 387FS | *2, *5, *6, *7, *8, *9, *11, *12, *17, *20, *26, *1X2b, CNV(*4) | |||||
| CYP2B6 | *2, *3, *4, *5, *6, *7, *8, *11, *12, *13, *14, *15, *16, *18, *19, *20, *21, *22, *26, *27, *28 | *6, *18 | *2, *6, *8, *13, *16, *28, CNV | ||||
| CYP2C8 | *1A, *2, *3, *4, *5, *7, *8, *12, L390S, P404A | *2, *3, *4 | *2, *3, *4, *5, *7, *8 | ||||
| CYP2C9 | *2, *3, *4, *5, *6, *9, *10, *11, *12, *13, *14, *15, *16, *25, Y358C | *2, *3 | *2, *3, *4, *5, *6 | *2, *3, *5, *6, *8, *11 | *2, *3, *4, *5, *6, *8, *9, *10, *11, *12, *13, *15, *25, *27 | *1A, *1B, *1C, *1D, *2A, *2B, *2C, *3A, *3B, *4, *5, *6, *7, *8, *9, *10, *11A, *11B, *12, *13, *14, *15, *16, *17, *18, *19, *20, *21, *22, *23, *24, *25, *26, *27, *28, *29, *30, *31, *32, *33, *34, *35 | |
| CYP2C19 | *2A, *2B, *3, *4, *5, *6, *7, *8, *9, *10, *12, *13, *14, *15, *17, 439FS, 241FS, V331I | *2, *3, *4, *5, *6, *7, *8, *9, *10, *13, *17 | *2, *3, *4, *5, *6, *7, *8, *9, *10, *17 | *2, *3, *4, *4B, *6, *8, *17 | *1B, *2, *3, *4, *5A, *5B, *6, *7, *8, *12, *17 | *1A, *1B, *1C, *2, *2B, *3A, *3B (*20), *4A, *4B, *5A, *5B, *6, *7, *8, *9, *10, *11, *12, *13, *14, *15, *16, *17, *18, *19, *20, *21, *22, *23, *24, *25, *26, *27, *28 | |
| CYP2D6 | *2, *3, *4, *5, *6, *7, *8, *9, *10, *11, *12, *14A, *14B, *15, *17, *18, *19, *20, *21, *29, *38, *40, *41, *42, *44, *56A, *56B, *64, S486T | *2, *3, *4, *5, *6, *7, *8, *9, *10, *11, *15, *17, *29, *35, *41, DUP | *2, *3, *4, *5, *6, *7, *9, *10, *17, *29, *41, XN, 1XN, 2XN, 4XN | *2A, *2L, *3, *4, *4M, *5, *6, *7, *8, *9, *10, *11, *12, *14A, *14B, *15, *17, *18, *19, *20, *21A, *21B, *30, *35?, *38, *40, *41, *42, *44, *56A, *56B, *58, *64, *69, CNV | *2, *2A, *2D, *2L, *2M, *3, *4, *4B, *4J, *4K, *4M, *4N;P, *5, *6, *6C, *7, *8, *9, *10A, *10B, *11, *12, *14A, *14B, *15, *17, *18, *19, *20, *21A, *21B, *27, *29, *30, *34, *35, *36, *38, *39, *40, *41, *42, *44, *45A, *56A, *56B, *57, *58, *63, *64, *65, *68, *69, *70, *71, *82, *83, *84 | *2, *3, *4, *5, *6, *7, *8, *9, *10, *12, *14A, *14B, *17, *29, *41, *XN | |
| CYP2E1 | *2, *3, *4, *5, *7A, *7B, *7C | *2, *7 | |||||
| CYP3A4 | *2, *3, *4, *5, *6, *7, *8, *10, *11, *12, *13, *14, *15, *16, *17, *18, *19, *20, K96E, I193V, S252A, I431T, 465FS | *1B, *2, *3, *12, *17 | *2, *22 | *2, *6, *20, *22 | *1B, *2, *3, *12, *17 | ||
| CYP3A5 | *1A, *2, *3B, *3C, *3D, *3F, *3G, *3K, *3L, *4, *5, *6, *7, *8, *9, S100Y | *1D, *2, *3, *3B, *6, *7, *8, *9 | *3, *6, *7 | *3, *3K, *5, *6, *7 | *1D, *2, *3, *3B, *6, *7, *8, *9, | ||
| CYP4F2 | *2, *3, W12C, P13R, G185V, L278F | *3 | |||||
| DPYD | *2, *3, *4, *7, *8, *9A, *9B, *10, *11, *13, R21X, M166V | *2, *9 | *2, *7, *8, *9, *10 | ||||
| GSTM1 | *A, *B, *0 | *A, *B, CNV | |||||
| GSTP1 | *A, *B, *C, D147Y | A, B, C, D | |||||
| GSTT1 | *A, *B, A21T, F45C, V169I, *0 | CNV | |||||
| NAT1 | *4, *5, *11, *11C, *14, *15, *17, *19A, *19B, *22, *23, *27, *30, T207I | *4, *5, *11, *14, *15, *17, *19, *22 | |||||
| NAT2 | *4, *5, *5E, *6, *6J, *7, *7D, *10, *12D, *14, *14D, *14F, *17, *18, *19, L137F, K268R | *4, *5, *5A, *5C, *5D, *5E, *5G, *5J, *5K,*5P, *6A, *6B, *6C, *6E, *6F, *6I, *6N, *7A, *7B, *7C, *7D, *11, *12, *12B, *12C, *13, *14, *14B, *14C, *14D, *14E, *14F, *14G, *14I, *19 | |||||
| SLC15A2 | *2, *3, R57H, M704L | *2, *3 | |||||
| SLC22A2 | *2A, *2B, *3A, *6, *3D, *3E, *5, *7, *8, R463K | P54S, M165V, S270A, R400C, K432Q | |||||
| SLCO1B1 | *1a, *1b, *2, *3, *4, *5, *6, *7, *8, *9, *10, *11, *12, *13, *14, *15, *16, *17, *18, *21, P336R | *5, *17, *21 | *1A, *1B, *2, *3, *5, *9, *10, *11, *12, *13, *15 | ||||
| SLCO2B1 | *2, D215V | S464F (*3?) | |||||
| TPMT | *2, *3A, *3B, *3C, *3D, *4, *8, *24 | *3A, *3B, *3C | *2, *3A, *3B, *3C, *4, *8 | ||||
| UGT1A1 | *6, *8, *12, *14, *15, *27, *28, *43, *45, *60, *62, *80, *93, *112, *28+60+93, *28+60, *27+28+60, *27+28+60+93 | *6A, *6B, *7, *27, *29, *60 | *28, *36, *37 | ||||
| UGT2B7 | *1a, *1g, *2a, *2c, *2e, *3 | *2 | |||||
| UGT2B15 | *2, *4, *5, A500T | Y85D(*2?) | |||||
| UGT2B17 | H450Y, *2 | CNV? | |||||
| VKORC1 | H1, H2, H3, H4, H6, H7, H9, V29L, V45A, R58G, V66M, R98W, L128R | c.-1639G>A | c.-1639G>A, c.85G>T (p.V29L), c.121G>T (p.A41S), c.134T>C (p.V45A), c.172A>G (p.R58G), c.196G>A (p.V66M), c.383T>G (p.L128R) | *2 (c.-1639G>A) | *2, *3, *4 | *2/H1, *2A, *2B, *3, *3F;BHT3, *4, *7RE, BHT2RE, BHT4, H2/H5, H4, H6, H7A, H7B, H8, H9 |
Assays do not include all genes or alleles.
DNA Preparation
Approximately 2 mg of DNA was prepared from each of the selected cell lines by the Coriell Cell Repositories using Gentra/Qiagen Autopure (Valencia, CA) per manufacturer's instructions or previously described methods.13
Assays Used in the Characterization Study
Affymetrix DMET Plus Array
Following manufacturer's instructions, genomic DNA was amplified using multiplex PCR and further enriched using molecular inversion probe PCR. Amplified DNA products were purified, fragmented, labeled, and hybridized to the DMET Plus Array. Arrays were stained with a fluorescent antibody and scanned on a GeneChip Scanner 3000 7G (Affymetrix). Data were analyzed using the DMET Console Software version 1.3 (Affymetrix). The DMET array is optimized to detect nucleotide variants and homozygous deletions and does not distinguish between one or more copies of a gene. For CYP2D6, a gene with copy number changes associated with clinically relevant phenotypes, three additional TaqMan Gene Copy Number Assays (Applied Biosystems, Foster City, CA) were used to test for deletions and duplications. The samples were tested according to the manufacturer's instructions and run on the ABI 7900HT Real Time PCR System (Applied Biosystems). The copy number data were analyzed using the Applied Biosystems TaqMan Gene Copy Number Assays Macro (Applied Biosystems) and was combined with the raw DMET array results to report more conclusive result for CYP2D6.
AutoGenomics INFINITI CYP3A4-3A5, NAT2, and CYP2D6 Assays
The INFINITI CYP3A4-3A5, NAT2, and CYP2D6 assays (AutoGenomics, Inc., Vista, CA) were performed as previously described14 for CYP2C19 using multiplex PCR involving target-specific amplification of extracted DNA. Analyte-specific detection primer extension and subsequent hybridization of fluorescent-labeled primers to capture probes arrayed on the biofilm chip is automated using the INFINITI analyzer, which then scans the microarray, analyzes the data, and produces a report on the detection of single-nucleotide polymorphisms, copy number variants (deletions, duplications), insertions, and other types of variants.
GenMark Dx eSensor 2C19 Test, Warfarin Sensitivity Test, and 3A4/3A5 Assays
All genotyping was performed as per the manufacturer's instructions (GenMark Diagnostics, Carlsbad, CA). The technology and performance of GenMark genotyping have been described elsewhere.15, 16, 17 In brief, the regions surrounding the interrogated variants were amplified using multiplex PCR, incubated with allele-specific oligonucleotide signal probes labeled with a ferrocene derivative, and hybridized with capture probes bound to gold-plated electrodes through test-specific eSensor cartridges and the eSensor XT-8 System (GenMark Diagnostics). All genotypes were determined by voltammetry using the eSensor analysis software version 1.3.1 (GenMark Diagnostics).
Agena Bioscience iPLEX ADME PGx Pro Panel
Specific DNA fragments were amplified from genomic DNA in eight PCR reactions, and alleles were subsequently interrogated using single-base extension (SBE) reactions. Genotypes were detected using a MassARRAY Analyzer 4 system and haplotypes assigned using an ADME PGx Pro Reporter plugin version 1.0.2 for the Typer Analyzer software version 4.0.147 (Agena Bioscience). The UGT1A1 TA repeat assay genotypes were also incorporated into the ADME PGx Reporter version 1.0.2 output using a modified ADME PGx Pro database (http://www.biotechniques.com/protocols/2012_Protocol_Guide/Development-and-Research-Validation-of-the-iPLEX----ADME-PGx-Panel-on-the-MassARRAY-System/biotechniques-330915.html, last accessed April 17, 2015).
Agena Bioscience iPLEX ADME CYP2D6 Panel
Specific DNA fragments were amplified from genomic DNA in three PCR reactions, and alleles were subsequently amplified using SBE. Genotypes were detected using a MassARRAY Analyzer 4 system and haplotypes assigned using an ADME CYP2D6 Reporter plugin version 1.0 for the Typer Analyzer software version 4.0.147 (Agena Bioscience).
Agena Bioscience iPLEX ADME CYP2C9/VKORC1 Panel
Specific DNA fragments were amplified using genomic DNA in three PCR reactions, and alleles were subsequently interrogated using SBE. Genotypes were detected using a MassARRAY Analyzer 4 system and haplotypes assigned using an ADME CYP2C9/VKORC1 Reporter plugin version 1.0 for the Typer Analyzer software version 4.0.147 (Agena Bioscience).
Agena Bioscience iPLEX ADME CYP2C19 Panel
Specific DNA fragments were amplified using genomic DNA in two PCR reactions, and subsequently alleles were interrogated using SBE. Genotypes were detected using a MassARRAY Analyzer 4 system and haplotypes assigned using an ADME PGx Pro Reporter plugin version 1.0.2 for the Typer Analyzer software version 4.0.147 (Agena Bioscience).
Agena Bioscience iPLEX UGT1A1 TA Repeat Assay
A specific DNA fragment was generated using PCR and genomic DNA, and alleles were subsequently interrogated using a homogeneous mass extension reaction. Genotypes were detected using a MassARRAY Analyzer 4 system and assigned manually using the Typer Analyzer software version 4.0.147 (Agena Bioscience). The TA repeat genotypes were also incorporated into the ADME PGx Reporter version 1.0 output using a modified ADME PGx Pro database.
Laboratory-Developed Test for LifeTech Taqman Platform
Specimens were analyzed using the LifeTech QuantStudio 12K Flex software version 1.2.2 (LifeTech, Grand Island, NY) and subjected to Taqman allele discrimination using LifeTech reagents in a custom-designed open array. Genomic DNA was amplified and mixed with dual-labeled oligonucleotides that hybridize to a specific target sequence. Hydrolysis by the 5′-3′ exonuclease activity of Taq polymerase releases the fluorescent reporter signal, permitting quantitative measurement of the accumulation of the PCR product via the fluorophore signal.18 Software programs used were Genotyper version 1.3 (LifeTech) and Alleletyper version 1.0 (LifeTech).
For copy number analysis, CYP2D6 and a reference gene were compared using commercially available reagents from LifeTech. Individual samples were run in quadruplicate. Each replicate was normalized to the reference gene to obtain a ΔCt (FAM dye Ct, VIC dye Ct), and then a mean ΔCt for each sample (from the four replicates) was calculated. All samples were then normalized to a calibrator sample to determine ΔΔCt. Relative quantity is 2 − ΔΔCt, and copy number is 2 × relative quantity. Copy number was assigned using CopyCallerSoftware version 2.0 (LifeTech).
Luminex xTAG CYP2C19 Kit Version 3, CYP2C9+VKORC1 Kit, CYP2D6 Kit Version 3
All genotyping was performed per the manufacturer's instructions (Luminex Molecular Diagnostics, Toronto, ON, Canada). The technology and performance of Luminex genotyping have been described elsewhere.17, 19, 20 Briefly, the regions surrounding the interrogated variants were multiplex PCR amplified, subjected to allele-specific primer extension, hybridized to specific xMAP microspheres via oligonucleotide tags, and fluorescence measured on a Luminex xMAP platform (Luminex Corporation, Austin, TX). All genotypes were determined using the appropriate xTAG Data Analysis Software (Luminex Molecular Diagnostics).
Characterization Protocol
Each of the testing laboratories received one 10-μg aliquot of DNA from each of the cell lines to be tested. The samples were coded, and the expected genotypes were not revealed to the laboratories. Each laboratory tested tier 1 samples or both tier 1 and tier 2 samples using their standard assay methods. The platforms and assays used in the study, the alleles detected by each, and the sample set(s) tested with each method are indicated in Table 1. The results were submitted to the study coordinators (L.V.K., V.M.P., and R.E.E.), who examined the data for quality and discrepancies and determined the consensus genotype. If discrepancies were noted, the participating laboratory was requested to reevaluate the sample in question (without providing the expected genotype) to determine the cause of the inconsistency.
Results
Unless otherwise stated, the wild-type (*1) alleles were assigned in the absence of other detectable variant alleles. DNA samples from Coriell cell lines containing pharmacogenetic variants that are frequently included in expanded panel pharmacogenetic assays were selected for this study. A list of 221 variants in 41 genes that were included in two or more commonly used and comprehensive pharmacogenetic assays [Affymetrix DMET, Agena Bioscience (formerly Sequenom) iPLEX ADME PGx Pro, Illumina VeraCode ADME, and some laboratory-developed tests] was assembled. Sources of data, including the Coriell Repository catalog (http://www.coriell.org/research-services/cell-culture/biobank-catalog, last accessed April 20, 2015), the 1000 Genomes Project (http://www.ncbi.nlm.nih.gov/variation/tools/1000genomes, last accessed March 3, 2015), and genotypic data from collaborating laboratories were examined to identify Coriell cell lines expected to have variants in the targeted alleles. On the basis of this analysis, DNA from 137 cell lines from the Coriell Repositories were selected for further characterization and consensus verification.
Nine laboratories, using a variety of pharmacogenetic assays, volunteered to test the samples. The results from five laboratories (five platforms, seven assays) (Table 1) that reported diplotype data are described here. The results of the DNA sequence analysis and other genotyping assays will be presented separately. Consensus diplotypes were determined by examining the variant calls made by each platform. There was good concordance among the platforms; however, discrepancies in diplotype assignments related to assay design and variable nomenclature were identified. Discrepancies in diplotype calls among platforms were resolved by manually evaluating the stated haplotypes in light of the variants tested by each platform. Available databases, such as The Human Cytochrome P450 (CYP) Allele Nomenclature Database (www.cypalleles.ki.se) and PharmGKB (www.pharmgkb.org), were used as haplotype standards for the various genes (Supplemental Table S1). The consensus diplotype data for 28 genes that were tested using two to six platforms (CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, CYP4F2, DPYD, GSTM1, GSTP1, GSTT1, NAT1, NAT2, SLC15A2, SLC22A2, SLCO1B1, SLCO2B1, TPMT, UGT1A1, UGT2B7, UGT2B15, UGT2B17, and VKORC1) are given in Supplemental Table S2. Diplotype calls from all platforms used to make the consensus calls on the 28 genes are available on the GeT-RM website (http://wwwn.cdc.gov/clia/Resources/GetRM/default.aspx; all URLs last accessed March 3, 2015).
The alleles that were interrogated for each locus, as well as those identified or absent from the study samples, are listed in Table 2. The alleles detected by each assay platform varied, but most common pharmacogenetic variants were identified among the 137 DNA samples. Many alleles were included in only one assay because of differences in platform design. Some of these were identified in study samples (Table 2) and are presented in the consensus diplotype data (Supplemental Table S2) in parentheses to indicate that this allele has not been confirmed by a separate method. Overall, of the 485 unique alleles tested, we identified 108 alleles by two or more assays and an additional 73 alleles by only one assay.
Table 2.
Alleles Identified in the DNA Samples Tested
| Gene | Alleles tested in study | Alleles verified by two or more methods | Alleles found by only one laboratory | Alleles not found in study samples | Alleles tested by only one method |
|---|---|---|---|---|---|
| CYP1A1 | *2, *2C, *3, *4, *5, *6, *7, *8, *9, *10, G45D, R279W, I286T, F381L, A463G | *2, *4, *5 | *2C | *3, *6, *7, *8, *9, *10, G45D, R279W, I286T, F381L, A463G | *2C,*10, G45D, R279W, I286T, F381L, A463G |
| CYP1A2 | *1A, *1C, *1D, *1F, *1K, *1L, *2, *3, *4, *5, *6, *7, *8, *11, *15, *16 | *1A, *1C, *1F, *1K, *1L | *1D, *2, *3, *4, *5, *6, *7, *8, *11, *15, *16 | *1D, *2, *3, *4, *5, *6, *8, *11, *15, *16 | |
| CYP2A6 | *2, *4, *5, *6, *7, *8, *9, *11, *12, *13, *17, *20, *26, *28, 387FS, *1X2b | *2, *4, *9, *17, *20 | *8 | *6, *7, *11, *13, *28, 387FS, *1X2b | *5, *12, *13, *26, *28, 387FS, *1X2b |
| CYP2B6 | *2, *3, *4, *5, *6, *7, *8, *11, *12, *13, *14, *15, *16, *18, *19, *20, *21, *22, *26, *27, *28 | *2, *6, *7, *18 | *4, *5, *11, *15, *20, *22, *27 | *3, *8, *12, *13, *14,*16, *19, *21, *26, *28 | *3, *4, *5, *7, *11, *12, *14, *15, *19, *20, *21, *22, *26, *27 |
| CYP2C8 | *1A, *2, *3, *4, *5, *7, *8, *12, L390S, P404A | *2, *3, *4, | *1A | *5, *7, *8, *12, L390S, P404A | *1A, *12, L390S, P404A |
| CYP2C9 | *1A, *1B, *1C, *1D, *2, *2A, *2B, *2C, *3, *3A, *3B, *4, *5, *6, *7, *8, *9, *10, *11, *11A, *11B, *12, *13, *14, *15, *16, *17, *18, *19, *20, *21, *22, *23, *24, *25, *26, *27, *28, *29, *30, *31, *32, *33, *34, *35, Y358C | *2, *3, *5, *6, *8, *9, *10, *11, *12 | *1A, *1B, *1C, *2A, *18, | *1D, *2B, *2C, *3A, *3B, *4, *7, *11A, *11B, *13, *14, *15, *16, *17, *19, *20, *21, *22, *23, *24, *25, *26, *27, *28, *29, *30, *31, *32, *33, *34, *35, Y358C | *1A, *1B, *1C, *1D, *2A, *2B, *2C, *3A, *3B, *7, *11A, *11B, *17, *18, *19, *20, *21, *22, *23, *24, *26, *28, *29, *30, *31, *32, *33, *34, *35, Y358C |
| CYP2C19 | *1A, *1B, *1C, *2, *2A, *2B, *3, *3A, *3B (*20), *4, *4A, *4B, *5, *5A, *5B, *6, *7, *8, *9, *10, *11, *12, *13, *14, *15, *16, *17, *18, *19, *20, *21, *22, *23, *24, *25, *26, *27, *28, 439FS, 241FS, V331I | *2, *3, *4, *4B, *6, *8, *9, *10, *13, *15, *17 | *1A, *1B, *1C, *2A, *2B, *4A, *12, *27 | *3A, *3B (*20), *5, *5A, *5B, *7, *11, *14, *16, *18, *19, *20, *21, *22, *23, *24, *25, *26, *28, 439FS, 241FS, V331I | *1A, *1C, *2A, *3A, *3B (*20), *4A, *4B, *11, *16, *18, *19, *20, *21, *22, *23, *24, *25, *26, *27, *28, 439FS, 241FS, V331I |
| CYP2D6 | *2, *2A, *2D, *2L, *2M, *3, *4, *4B, *4J, *4K, *4M, *4N;P, *5, *6, *6C, *7, *8, *9, *10, *10A, *10B, *11, *12, *14, *14A, *14B, *15, *17, *18, *19, *20, *21, *21A, *21B, *27, *29, *30, *34, *35, *36, *38, *39, *40, *41, *42, *44, *45A, *56A, *56B, *57, *58, *63, *64, *65, *68, *69, *70, *71, *82, *83, *84, 1XN, 2XN, 4XN, CNV, DUP, S486T, XN | *2, *3, *4, *5, *6, *7, *9, *10, *14, *15, *17, *29, *35, *41, XN, *2XN, *4XN, *10XN, *41XN | *2A, *21, *21B, *36, *40 | *2D, *2L, *2M, *4B, *4J, *4K, *4M, *4N;P, *6C, *8, *11, *12, *14A, *14B, *18, *19, *20, *21A, *27, *30, *34, *38, *39, *42, *44, *45A, *56A, *56B, *57, *58, *63, *64, *68, *69, *70, *71, *82, *83, *84, S486T | *2D, *2M, *4B, *4J, *4K, *4N;P, *6C, *10A, *10B, *14, *21, *27, *34, *36, *39, *45A, *57, *63, *65, *68, *70, *71, *82, *83, *84, S486T |
| CYP2E1 | *2, *3, *4, *5, *7, *7A, *7B, *7C | *7 | *4, *5, *7A, *7B, *7C | *2, *3 | *3, *4, *5, *7A, *7B, *7C |
| CYP3A4 | *1B, *2, *3, *4, *5, *6, *7, *8, *10, *11, *12, *13, *14, *15, *16, *17, *18, *19, *20, *22, K96E, I193V, S252A, I431T, 465FS | *1B, *2, *3, *22 | *14, *15, *16, | *4, *5, *6, *7, *8, *10, *11, *12, *13, *17, *18, *19, *20, K96E, I193V, S252A, I431T, 465FS | *4, *5, *7, *8, *10, *11, *13, *14, *15, *16, *18, *19, K96E, I193V, S252A, I431T, 465FS |
| CYP3A5 | *1A, *1D, *2, *3, *3B, *3C, *3D, *3F, *3G, *3K, *3L, *4, *5, *6, *7, *8, *9, S100Y | *1D, *3, *6, *7, | *1A, *3C, *3G | *2, *3B, *3D, *3F, *3K, *3L, *4, *5, *8, *9, S100Y | *1A, *3C, *3D, *3F, *3G, *3L, *4, S100Y |
| CYP4F2 | *2, *3, W12C, P13R, G185V, L278F | *3 | *2 | W12C, P13R, G185V, L278F | *2, W12C, P13R, G185V, L278F |
| DPYD | *2, *2A, *3, *4, *7, *8, *9, *9A, *9B, *10, *11, *13, R21X, M166V | *2, *9 | *4, *9A | *2A, *3, *7, *8, *9B, *10, *11, *13, R21X, M166V | *2A, *3, *4, *9A, *9B, *11, *13, R21X, M166V |
| GSTM1 | *A, *B, *0 (DEL) | DEL, *A, *B | |||
| GSTP1 | *A, *B, *C, *D, D147Y | *A, *B, *C | *D, D147Y | *D, D147Y | |
| GSTT1 | *A, *B, A21T, F45C, V169I, *0 (DEL), CNV | DEL | AXN, A, B | A21T, F45C, V169I, | *A, *B, A21T, F45C, V169I |
| NAT1 | *4, *5, *11, *11C, *14, *15, *17, *19, *19A, *19B, *22, *23, *27, *30, T207I | *4, *11, *14, *17 | *5, *11C, *15, *19, *19A, *19B, *22, *23, *27, *30, T207I | *11C, *19A, *19B, *23, *27, *30, T207I | |
| NAT2 | *4, *5, *5A, *5C, *5D, *5E, *5G, *5J, *5K, *5P, *6, *6A, *6B, *6C, *6E, *6F, *6I, *6J, *6N, *7, *7A, *7B, *7C, *7D, *10, *11, *12, *12B, *12C, *12D, *13, *14, *14B, *14C, *14D, *14E, *14F, *14G, *14I, *17, *18, *19, L137F, K268R | *4, *5, *6, *7, *14 | *5A, *5C, *7B, *12, *13 | *5D, *5E, *5G, *5J, *5K, *5P, *6A, *6B, *6C, *6E, *6F, *6I, *6J, *6N, *7A, *7C, *7D, *10, *11, *12B, *12C, *12D, *14B, *14C, *14D, *14E, *14F, *14G, *14I, *17, *18, *19, L137F, K268R | *5A, *5C, *5D, *5G, *5J, *5K, *5P, *6A, *6B, *6C, *6E, *6F, *6I, *6J, *6N, *7A, *7B, *7C, *10, *11, *12, *12B, *12C, *12D, *13, *14B, *14C, *14E, *14G, *14I, *17, *18, L137F, K268R |
| SLC15A2 | *2, *3, R57H, M704L | *2 | *3, R57H, M704L | R57H, M704L | |
| SLC22A2 | *2A, *2B, *3A, *3D, *3E, *5, *6, *7, *8, P54S, M165V, S270A, R400C, K432Q, R463K | *3, *7 | *2, *2A, *2B, *3A, *3D, *3E, S270A, K432Q, R400C | *6, *5, *8, P54S, M165V, R463K | *2A, *2B, *3A, *3D, *3E, *5, *6, *7, *8, P54S, M165V, S270A, R400C, K432Q, R463K |
| SLCO1B1 | *1A, *1B, *2, *3, *4, *5, *6, *7, *8, *9, *10, *11, *12, *13, *14, *15, *16, *17, *18, *21, P336R | *1A, *1B, *5, *14, *15, *17, *21 | *2, *3, *4, *6, *7, *8, *9, *10, *11, *12, *13, *16, *18, P336R | *4, *6, *7, *8, *14, *16, *18, *21, P336R | |
| SLCO2B1 | *2, D215V, S464F (*3?) | none | S464F | *2, D215V | *2, D215V, S464F (*3?) |
| TPMT | *2, *3A, *3B, *3C, *3D, *4, *8, *24 | *3A, *3C, *8 | *2, *3B, *3D, *4, *24 | *3D, *24 | |
| UGT1A1 | *6, *6A, *6B, *7, *8, *12, *14, *15, *27, *27+28+60, *27+28+60+93, *28, *28+60+93, *28+60, *29, *36, *36B, *37, *43, *45, *60, *62, *80, *93, *112 | *6, *27, *28, *60 | *6A, *7, *28B, *36, *36B, *37 | *8, *12, *14, *15, *29, *43, *45, *62, *80, *93, *112 | *6A, *6B, *7, *8, *12, *14, *15, *27+28+60, *27+28+60+93, *28+60+93, *28+60, *29, *36, *36B, *37, *43, *45, *62, *80, *93, *112 |
| UGT2B7 | *1A, *1G, *2, *2A, *2C, *2E, *3 | *2 | *1A, *1G, *2C, *3 | *2A, *2E | *1, *1A, *1G, *2A, *2C, *2E, *3 |
| UGT2B15 | *2, *4, *5, A500T, Y85D(*2?) | *2, *5 | *4 | A500T, Y85D(*2?) | *2, *4, *5, A500T, Y85D(*2?) |
| UGT2B17 | H450Y, *2, CNV | *2, CNV | H450Y | H450Y, *2, CNV | |
| VKORC1 | H1, H2, H3, H4, H5, H6, H7, H7A, H7B, H8, H9, H2/H5, *2, *2A, *2B, *3, *3F, *4, V29L, V45A, R58G, V66M, R98W, L128R, A41S, -1639G>A | c.-1639G>A, (*2, *3, *4, H1, H2, H4, H6, H7)† | H3, H5, H7B, H9 | V29L, V45A, R58G, V66M, R98W, L128R, A41S | H3, H7A, H7B, H8, H2/H5, *2A, *2B, *3F, V29L, V45A, R58G, V66M, R98W, L128R, A41S, -1639G>A |
Additional alleles and nomenclature were reported, but only c.-1639G>A was used for consensus.
CYP1A1
Two platforms tested the tier 1 and one tested the tier 2 samples for variants in CYP1A1. Both platforms were 100% concordant in their genotype calls. Of note, one platform identified all *2 variants as *2C. Per The Human Cytochrome P450 (CYP) Allele Nomenclature Database, *2A is defined by c.3798T>C (formerly known as m1), *2B is defined as having c.3798T>C and c.2454A>G (p.I462V), and *2C is defined as having c.2454A>G (p.I462V). When *2 or *2C was reported, the consensus was called *2.
CYP1A2
Two platforms tested the tier 1 and one tested the tier 2 samples for variants in CYP1A2. Both laboratories were 100% concordant in their genotype calls. Of note, neither laboratory could distinguish *1A/*1L from *1C/*1F. Per The Human Cytochrome P450 (CYP) Allele Nomenclature Database, *1A is defined as no variant detected, *1L is defined as having c.-3860G>A and c.-163C>A, *1C is defined as having c.-3860G>A, and *1F is defined as having c.-163C>A.
CYP2A6
Two platforms tested the tier 1 and one tested the tier 2 samples for variants in CYP2A6. There was relatively good consensus among the platforms, with differences observed attributable to the assay design. The Affymetrix platform was optimized for single-nucleotide variants and homozygous deletions, but it was unable to identify whether only one copy of the CYP2A6 deletion (*4 heterozygotes) is present. The Agena Bioscience iPLEX ADME PGx Pro Panel could detect *8 (g.1454G>T; p.R485L); however, although included in the assay, this variant was not detected by the Affymetrix platform. c.-48T>G, which is present in *9, *13, and *15, was included in the Agena Bioscience iPLEX ADME PGx Pro Panel and was reported by this assay as *9;*13;*15. The Affymetrix platform detects c.-1013A>G for *9. When both c.-48T>G and c.-1013A>G were detected, the consensus genotype was called *9 (c.-48T>G, c.-1013A>G).
CYP2B6
Three platforms tested the tier 1 samples for variants in CYP2B6, and two platforms tested the tier 2 samples. There was relatively good consensus among the platforms with most differences due to platform design. Only one platform was designed to detect *4, *5, *11, *15, *20, *22, and *27; thus, these alleles could not be independently verified. One of the challenges in analyzing haplotypes in CYP2B6 is that c.785A>G (p.K262R) is found in many alleles (eg, *4, *6, *7, *14, *16, *20, *26). c.1459C>T (p.R487C) is found in both *5 and *7. c.516G>T is found in *6, *7, *9, *13, *19, *20, *26, *29, *34, *36, *37, and *38. When c.516G>T, c.785A>G, and c.1459C>T are present together, the most likely interpretation is *1/*7 with all of the variants in cis, although *5/*6 in trans cannot be ruled out (see samples NA12717, NA17235, NA17658, NA20509, NA23313, and NA23348).
CYP2C8
Three platforms tested the tier 1 samples for variants in CYP2C8, and two platforms tested the tier 2 samples. The platforms were 100% concordant in their genotype calls. Of note, one platform identified all *1 variants as *1A. The other two platforms reported their results as *1 when no variant was detected. When *1 or *1A was reported, the consensus was called *1.
CYP2C9
Five platforms (six assays) were used to test the tier 1 samples for variants in CYP2C9, and two platforms (three assays) were used for the tier 2 samples. There was relatively good consensus among laboratories, with most differences due to assay design. Of note, some platforms differentiate between *3 and *18. *3 is defined as c.1075A>C (p.I359L), whereas *18 is defined as c.1075A>C (p.I359L) and c.1190A>C (p.D397A). Only one laboratory typed the samples for the c.1190A>C variant. Although the consensus from the other laboratories was *3, the most likely genotype is *18. This affected 11 samples (NA10855, NA11839, NA12813, NA17204, NA17234, NA17290, NA17642, NA18563, NA18959, NA19917, and NA23405). One platform identified NA19226 and NA18873 as homozygous for *8, and two other platforms reported the samples as *1/*8. This may be due to an interfering variant in the samples. Additional variants were also identified by only one platform in several samples (NA15245, NA19917, and NA23275), which were not defined in The Human Cytochrome P450 (CYP) Allele Nomenclature Database.
CYP2C19
Five platforms (six assays) were used to test the tier 1 samples for variants in CYP2C19, and two platforms (three assays) were used for the tier 2 samples. We identified a problem with one of the assays used during this study. Because of the way the assay was designed, *2 and *10 can be interfering variants and can lead to genotyping errors.21 CYP2C19 *2 is defined by c.681G>A, and *10 is defined by c.680C>T (p.P227L). This does not affect the phenotypic prediction for a patient, but there could be other implications (eg, proficiency testing). The laboratory is working with the platform manufacturer to resolve this issue. Discrepant results were found in three samples (NA19789, NA19908, and NA23275). One platform reported a *9 [c.431G>A (p.R144H)], and other platforms designed to detect a *9 failed to detect it. The presence of *9 in NA23275 was confirmed by repeat testing with the same assay. In the case of NA19789, it is possible that the *9 probe failed. The platform reports these as a possible false-positive result. One sample in the study (NA23878) was found to have the CYP2C19 *4B (c.1A>G and g.-806C>T) allele.22 Although g.-806C>T is the defining variant for *17, two platforms correctly reported the sample as *1/*4B. Of note, we had a number of samples (NA07029, NA10865, NA12753, NA18484, NA18524, NA18855, NA19122, NA19178, NA23348, NA23872, and NA23873) for which variants (identified by only one platform) are not defined in The Human Cytochrome P450 (CYP) Allele Nomenclature Database.
CYP2D6
Five platforms (six assays) were used to test the tier 1 samples for variants in CYP2D6, and two platforms (three assays) were used for the tier 2 samples. The CYP2D6 *36 allele is a gene conversion from CYP2D6 to CYP2D7 in exon 9. Detection of this allele was problematic for several platforms in the study because most are not designed to detect *36 and it was reported as *10 (NA18524, NA18526, NA18563, NA18564, NA18565, NA18572, NA18617, NA18959, NA18980, NA23090, and NA23093). *36 is a gene conversion to CYP2D7 in exon 9 or hybrid that, in addition to the gene conversion, contains c.100C>T (p.P34S) and c.4180G>C (p.S486T), whereas CYP2D6*10 contains only c.100C>T (p.P34S) and c.4180G>C (p.S486T). *10 is a decreased functional allele, whereas *36 is a nonfunctional allele. One laboratory reported one of the gene conversion samples, NA18565, as having the *5 deletion allele. The most likely genotype for NA18565 is *10/*36, although the consensus is *10/*10. It is suspected that the gene conversion interferes with the primer binding sites for the copy number determination because only one copy was detected. CYP2D6*35 is defined by c.31G>A (p.V11M) but also contains c.2850C>T (p.R296C) and c.4180G>C (p.S486T), which are the defining variants in *2. Platforms not designed to directly detect *35 report samples containing all three variants as *2 (ie, NA06993, NA07000, NA07029, NA12003, NA17204, NA17641, NA17702, and NA20509). Both *2 and *35 are normal function alleles; thus, the predicted phenotype would not be affected. Sample NA19174 was problematic for several platforms, and no consensus was determined. The first platform identified NA19174 as *4/*17 (c.2850C>T, c.1846G>A, c.100C>T, c.1023C>T). The second platform did not identify the c.1023C>T and reported NA19174 as *4/*30. The third platform identified an unspecified genotype [c.2850C>T, c.4180G>C (homozygous), c.1846G>A, c.100C>T, c.1023C>T (homozygous)]. On the basis of the platform designs and variants detected, the most probable allele call for NA19174 is *4/*40 (c.100C>T, c.1846G>A, c.4180G>C/c.1023C>T, c.1661G>C, c.1863_1864insTTTCGCCCCx2, c.2850C>T, c.4180G>C). This was also observed in samples NA17102, NA19917, and NA23275. Duplications and deletions were also problematic, especially in platforms that primarily detect single-nucleotide polymorphisms or insertion/deletions (indels). This affected analysis of samples NA10856, NA12336, NA15245, NA18873, NA18945, NA19035, NA19785, NA21781, NA23296, NA23297, NA23313, and NA24027. Sample NA24217 was identified by one platform as *41/*41X3. The other laboratories reported the genotype as *2/*41XN. NA24217 appeared homozygous and not heterozygous for *41, suggesting that there are three copies of *41 (ie, *41XN). We could not determine a consensus CYP2D6 genotype for sample NA23878. One platform identified *4/*83 (c.100C>T, c.1846G>A, c. 4180G>C/c.843T>G, gene conversion to CYP2D7 in exon 9, c.4180G>C), one had an unspecified haplotype [c.4180G>C (homozygous), c.1846G>A, 100C>T, 2 copies)], and another platform identified *1/*4 (c.100C>T, c.1846G>A, c. 4180G>C). The most probable genotype for this sample is *4/*83. Two platforms identified the *17 variant in sample NA19238, and one platform failed to identify it and reported a false-negative result. Of note, additional variants were identified by one platform in two samples (NA19174, NA23878) that were not defined in the CYP database.
CYP2E1
Two platforms were used to test the tier 1 samples, and one was used to test the tier 2 samples for variants in CYP2E1. There was relatively good consensus between the platforms. The observed discrepancies were due to differences in assay design. The Agena Bioscience iPLEX ADME PGx Pro Panel was designed to detect *7, whereas the Affymetrix platform detected several other variations not included in the Agena assay, such as *4 and *5.
CYP3A4
Five platforms (five assays) were used to test the tier 1 samples for variants in CYP3A4, and two platforms were used for the tier 2 samples. There was good consensus among the platforms. Only one platform was designed to detect *14, *15, and *16 (samples NA15245, NA18966, NA19109, NA19226, and NA19908); thus, we were not able to independently confirm the presence of these variants. They are indicated in parentheses in the consensus genotypes.
CYP3A5
Five platforms were used to test the tier 1 samples for variants in CYP3A5, and two platforms were used for the tier 2 samples. There was good consensus among the platforms for all samples.
CYP4F2
Two platforms were used to test the tier 1 samples for variants in CYP4F2, and one platform was used for the tier 2 samples. There were only two CYP4F2 alleles, *2 [c.34T>G (p.12G)] and *3 [c.1297G>A (p.V433M)], detected in the study samples. One platform tested for both alleles, and the other platform tested only for the *3 haplotype. When the *2 was observed, no consensus was called because it could not be independently confirmed.
DPYD
Three platforms were used to test the tier 1 samples for variants in DPYD, and two platforms were used for the tier 2 samples. Only one platform tested for DPYD *4 [c.1601C>T (p.S534N)], so the presence of this allele could not be independently confirmed for tier 1 samples NA06991, NA12813, NA12878, and NA24217.
GSTM1, GSTP1, and SLC15A2
Two platforms were used to test the tier 1 samples for variants in GSTM1, GSTP1, and SLC15A2, respectively. There was good consensus between the platforms. One platform was used to test the tier 2 samples.
GSTT1
Two platforms were used to test the tier 1 samples for variants in GSTT1, and one was used to test the tier 2 samples. There was good consensus among the platforms. The Affymetrix platform was only able to test for the presence or absence of the gene, whereas the Agena Bioscience iPLEX ADME PGx Pro panel was able to detect whether zero, one, two, or more than two copies were present.
NAT1
Two platforms were used to test the tier 1 samples for variants in NAT1, and one was used to test the tier 2 samples. There was good consensus between the platforms. A consensus genotype could not be determined for one sample (NA17204) because of assay failure in one laboratory.
NAT2
Two platforms were used to test the tier 1 samples for variants in NAT2, and one was used to test the tier 2 samples. There was good consensus among the platforms. The major difference in haplotypes was related to the alleles that each platform was designed to detect. The platforms agreed very well on the *4, *5, *6, and *7 haplotype calls. Of interest, although the platforms were designed to detect *12 and *14, only the Agena Bioscience ADME PGx Panel observed the *12 haplotype.
SLC22A2
Two platforms were used to test the tier 1 samples for variants in SLC22A2, and one was used to test the tier 2 samples. Because of limited annotation for this gene, we were unable to easily compare haplotypes. Alleles that were assayed by the platforms revealed identical outcomes. Nomenclature issues affected the interpretation and reporting of the results. The Agena Bioscience platform describes the detected mutations as amino acid changes, whereas the Affymetrix platform reports star (*) alleles. The nomenclature of this gene is not annotated on PharmGKB.
SLCO1B1
Three platforms were used to test the tier 1 samples for variants in SLCO1B1, and two platforms were used for the tier 2 samples. The SLCO1B1 *14 haplotype is composed of two variants (c.463C>A, c.388A>G) in cis (ie, NA07019, NA07055, NA07056, NA07439, NA10831, NA10851, NA12236, NA17244, NA18861, and NA23874), although it is possible that these variants could be in trans (ie, *1B/*4). A similar issue is observed for *15 (c.388A>G, c.521T>C), where the most likely interpretation is that the variants are in cis but trans cannot be ruled out (ie, *1B/*5). This affects samples HG00276, NA06993, NA07000, NA07357, NA10859, NA12003, NA12892, NA15245, NA17642, NA18526, NA19109, NA20509, NA24008, and NA24217. For samples NA18540 and NA18544, the most likely interpretation for g.-11187G>A heterozygous, c.388A>G homozygous, c.521T>C heterozygous is *1B/*17 (c.388A>G,/c.521T>C, g.-11187G>A, c.388A>G), although *15/*21 (c.388A>G, c.521T>C/g.-11187G>A, c.388A>G, c.597C>T) cannot be ruled out when c.597C>T is not tested. Only one platform, Affymetrix, tested for SLCO1B1*21 (g.-11187G>A, c.388A>G, c.521T>C). The other two platforms tested for a combination of the variants (g.-11187G>A, c.388A>G, c.521T>C) but not in a single platform. In some samples (NA11993, NA12006, NA12156, NA12813, NA17448, NA18524, NA18540, NA18544, NA18563, NA18992, and NA23246), g.-11187G>A (reported as *21) was detected by the LifeTech platform and *14 was detected by the Agena Bioscience platform. The most likely interpretation of this genotype is *21, as reported by Affymetrix and LifeTech platforms.
SLCO2B1
Two platforms were used to test the tier 1 samples for variants in SLCO2B1, and one was used to test the tier 2 samples. We could not easily compare haplotypes called by the two assays because of differences in nomenclature (results reported as either nomenclature or amino acid change) and limited annotation for this gene. Variants that were assayed by both platforms revealed identical outcomes.
TPMT
Three platforms were used to test the tier 1 samples for variants in TPMT, and two platforms were used for the tier 2 samples. There was good consensus between platforms. When c.460G>A and c.719A>G are present together, the mostly likely interpretation is that they are in cis and reported as *3A. Note, it could also be reported as *3B (c.460G>A)/*3C (c.719A>G) if the two variants are in trans.
UGT1A1
Two platforms (four assays) were used to test the tier 1 samples for variants in UGT1A1. There was good consensus between the laboratories. The major difference was that the Agena Bioscience UGT1A1 TA repeat assay could distinguish 5, 6, 7, and 8 TA repeats and therefore could distinguish *28 (TA7), *36 (TA5), and *37 (TA8) from *1 (TA6). Data from each platform that was used to create the consensus genotype are given in the UGT1A1 data table, which is available on the Get-RM website.
UGT2B7
Two platforms tested the tier 1 samples for variants in UGT2B7, and one platform was used to test the tier 2 samples. There was good consensus between the assays. Because only one platform tested for *3 (c.211G>T), the presence of this allele could not be confirmed for tier 1 samples NA18952, NA18973, NA18980, NA18992, and NA19007.
UGT2B15
Two platforms tested the tier 1 samples for variants in UGT2B15, and one platform was used to test the tier 2 samples. There was good consensus between the assays. Because only one platform tested for *4 [c.1568A>C (p.K523T)], the presence of this allele could not be confirmed when identified.
UGT2B17
Two platforms tested the tier 1 samples for variants in UGT2B17, and one platform was used to test the tier 2 samples. The Affymetrix platform is optimized to detect nucleotide variants and homozygous deletions and does not distinguish between one or more copies of a gene. The Agena Bioscience platform detects only copy number. Therefore, both platforms presented identical data for the samples with a homozygous deletion.
VKORC1
Five platforms (six assays) were used to test the tier 1 samples for variants in VKORC1, and two platforms (three assays) were used for the tier 2 samples. There was relatively good consensus among laboratories, with most differences due to platform design. Most laboratories reported only c.-1639G>A genotype. Some laboratories reported using the star (*) allele nomenclature, and some laboratories reported using the two different haplotype nomenclatures. The c.-1639G>A genotype is the defining variant in *2 and is present in haplotypes H1, H2, and H5. We reported the consensus genotype only for c.-1639G>A in the consensus genotype table (Supplemental Table S2). Data from each platform that was used to create the consensus genotype are given using the H and star nomenclatures in the VKORC1 data table, which is available on the Get-RM website.
Other Loci
Consensus genotypes could not be determined for 26 additional pharmacogenetic loci that were only characterized by one laboratory (ABCC2, ABCC4, CDA, CYP1B1, CYP2A13, CYP2F1, CYP2J2, CYP2S1, CYP3A7, CYP3A43, CYP4B1, CYP19A1, FMO2, G6PD, IFNL3, ITPA, PTGIS, SULT1A1, TBXAS1, UGT1A3, UGT1A4, UGT1A6, UGT1A7, UGT1A8, UGT1A9, and UGT1A10). These data are presented in Supplemental Table S3 and on the GeT-RM website.
Discussion
This study describes the characterization of 137 publicly available cell line–derived genomic DNA samples for 28 loci potentially included in clinical pharmacogenetic testing. To ensure that the DNAs would be thoroughly characterized and would be commutable among a variety of assay chemistries and platforms, each sample was tested using several pharmacogenetic assays. We identified many but not all of the alleles commonly included in clinical pharmacogenetic assays for these genes. In addition, we identified many alleles that we did not detect during our previous GeT-RM pharmacogenetics study.10 Some of these alleles, including CYP2D6 *10XN, *7, *15, and *41XN, and CYP2C19 *6 and *9, were found in cell lines created at Coriell from patients known to have pharmacogenetic genotypes not identified during the first study. The GeT-RM program will continue collaboration with the pharmacogenetic testing community and the Coriell Cell Repositories to create and identify cell lines with alleles not identified during this study and undertake necessary characterization studies. We also generated haplotype data for an additional 26 pharmacogenetic loci, which will be corroborated by the results of the DNA sequencing analysis in the forthcoming publication. These publicly available DNA samples and associated data will be useful for laboratories when developing and validating new genetic tests and will also enable proficiency testing programs to provide diverse pharmacogenetic challenges.23 Use of RMs with a variety of alleles can help laboratories to characterize and refine their assays and also may enhance their ability to detect pharmacogenetic variants and improve performance on proficiency testing challenges.23
There are a considerable number of polymorphisms in many of the human genes associated with pharmacokinetics or pharmacodynamics of exogenous drugs. Sometimes a single variant but often combinations of polymorphisms as a haplotype dictate the activity of the pharmacogenetic gene product. Haplotypes usually have one or a few defining variants but may also have additional variants that can be included. Many nomenclature systems have been developed to describe pharmacogenetic haplotypes. In the most commonly used nomenclature system, combinations of pharmacogenetic sequence variants are designated by star (*) alleles, where *1 is designated as normal (commonly referred to as wild type or fully functional), and numbered star alleles are assigned as new variants are identified.24 The star nomenclature system is used for many pharmacogenetic genes and gene families, including the CYP450 gene family. Several nomenclatures, including the star system, are intermittently used to describe the haplotypes of other genes, such as VKORC1 and UGT1A1. Laboratories in our study often reported allele genotypes for the same gene using a variety of nomenclature formats. In many cases, such as CYP2C9 and CYP2C19, the star nomenclature was used to describe some alleles of a gene, and additional alleles of the gene were named by the predicted amino acid change (Table 1).
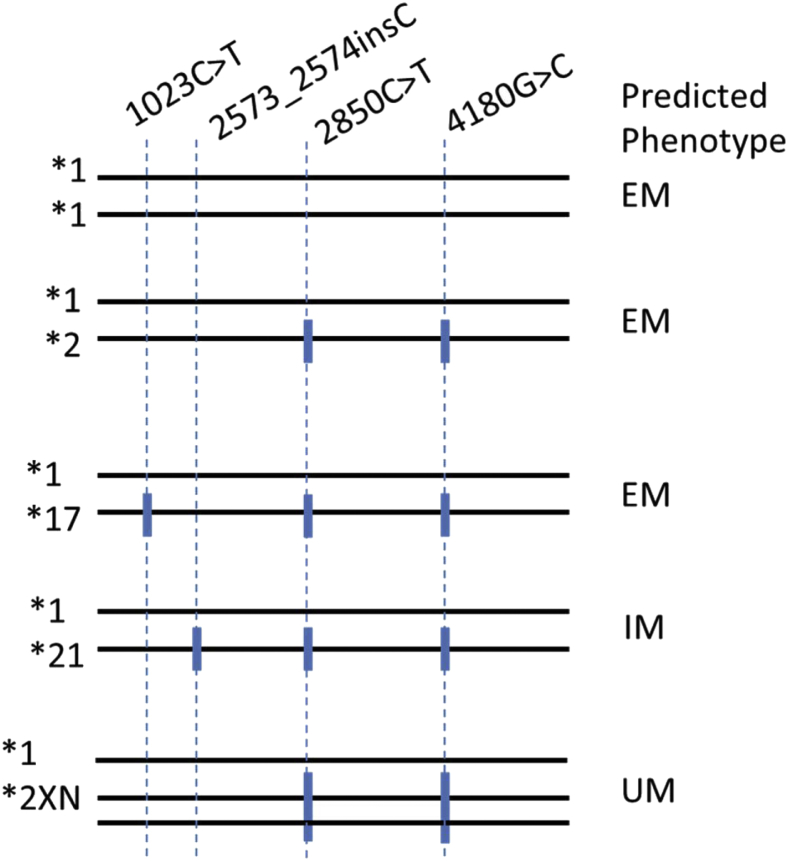
The assays used in this study varied in many ways. Without exception, no two assays that examined a particular gene were designed to detect the same set of haplotypes (Table 1). In addition, some assays used different combinations of variants to define the haplotypes that the assay detected, which ultimately can lead to discrepancies in reported star allele genotypes among platforms. For example in CYP2D6, c.2850C>T and c.4180G>C, which are the defining variants for *2 (c.2850C>T, c.4180G>C), also occur in various other haplotypes, such as *17 (c. 1023C>T, c.2850C>T, c.4180G>C) and *21 (c.2573_2574insC, c.2850C>T, c.4180G>C). *2 is a functional allele, *17 has decreased function, and *21 is nonfunctional. Determining the presence of duplications and multiplications in CYP2D6 is also important25 because CYP2D6*2XN (c.2850C>T, c.4180G>C, >2) is an increased function allele. Figure 1 shows how differences in the design of CYP2D6 assays (eg, variable inclusion of certain variants and or copy number detection) may affect interpretation of alleles. In addition, we did not assign phase to the various variants during this study, which may have implications in result interpretation and therefore deserves further review.
Figure 1.
Assay design can cause inconsistent allele calls and interpretation. A CYP2D6 assay that only detects c.2850C>T (rs16947) and c.4180G>C (rs1135840) without copy number cannot distinguish among *2 (c.2850C>T, c.4180G>C), *17 (c.1023C>T [rs28371706], c.2850C>T, c.4180G>C), *21 (c.2573_2574insT [rs72549352], c.2850C>T, c.4180G>C), or *2XN (c.2850C>T, c.4180G>C, XN), which have different predicted phenotypes. EM, extensive metabolizer; IM, intermediate metabolizer; UM, ultrarapid metabolizer.
This study highlights how the common PGx nomenclature system and variable assay designs add to the complexity of analyzing and reporting results from pharmacogenetic assays, especially when trying to compare data from the same sample analyzed on different genotyping platforms. These discrepancies could hinder patient care and adoption of pharmacogenetic assays in clinical practice. For example, physicians may have difficulty understanding laboratory reports when results from different laboratories and in the literature are described using a variety of nomenclature systems. Such inconsistencies might lead to mistakes in treatment and hinder adoption and use of pharmacogenetic tests. Regulatory agencies, proficiency testing programs and test developers similarly require standardized nomenclature so that results can be compared among platforms and also with the scientific literature. Lack of a consistent nomenclature could stifle regulatory clearance or approval of new assays and add undue confusion to analysis of proficiency testing surveys. Finally, standardization of nomenclature is critical for the accurate accumulation of data in clinical databases, such as ClinVar (http://www.ncbi.nlm.nih.gov/clinvar, last accessed March 30, 2015) and PharmGKB. Without standardization, multiple observations of the same genotype could not be related to each other, and incorrect associations between genotype and phenotype may be inferred.
To address these nomenclature issues, the GeT-RM program formed an international workgroup to review current pharmacogenetic nomenclature practices and develop a system based on the Human Genome Variation Society nomenclature to describe pharmacogenetic haplotypes and facilitate translation among different nomenclature systems. Workgroup participants include many significant stakeholders in pharmacogenetics (PharmGKB, the Pharmacogenetics Research Network, and the Clinical Pharmacogenetics Implementation Consortium), regulatory agencies (FDA), laboratory-accrediting organizations (College of American Pathologists), nomenclature committees (Human Genome Organisation and the Human Genome Variation Society), others that are responsible for a number of gene/mutation databases (National Center for Biotechnology Information, ClinVar, TPMT, CYP450 databases), pharmacogenetic assay developers, and clinical and research laboratories. The group has developed a system to standardize the way pharmacogenetic haplotypes are described and reported, as well as recommendations for ways to standardize pharmacogenetic assays.26 We are also developing graphic examples to represent pharmacogenetic haplotypes that would allow easy conversion among different nomenclatures.
In conclusion, this characterized set of 137 genomic DNA RMs are available for use in research, clinical test development, quality assurance and control, and proficiency testing to help to ensure the accuracy of clinical pharmacogenetic testing. These pharmacogenetic RMs, as well as other materials developed by GeT-RM, are publically available from the National Institute of General Medical Sciences and the National Human Genome Research Institute repositories at the Coriell Cell Repositories (https://catalog.coriell.org, last accessed September 12, 2014). Information on this and other RM characterization projects is available at the GeT-RM website.
Footnotes
Supported by the IGNITE project grant U01HG007762 and the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative (V.M.P.), and in part by NIH grant K23 GM104401 from the National Institute of General Medical Sciences (S.A.S.).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the Agency for Toxic Substances and Disease Registry.
Disclosures: R.E.E. is an employee of and owns stock in Agena Bioscience. R.E.-B.; P.H., and C.H.S. are employees of Autogenomics; L.H.T. is an employee of Coriell Cell Repository, which provided samples for this study. The Indiana University School of Medicine Pharmacogenomics Laboratory and the Mount Sinai Genetic Testing Laboratory are fee-for-service clinical laboratories that offer clinical pharmacogenetic testing. Reagents for the GenMark, Luminex, and Affymetrix assays were donated to the testing laboratories by the manufacturers.
A guest editor acted as the editor-in-chief for the manuscript. No individual at the Centers for Disease Control and Prevention was involved in the peer review process or final disposition of this article.
Current address of B.N.B., Houston Forensic Science Center, Houston, TX.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.jmoldx.2015.08.005.
Supplemental Data
References
- 1.Gladding P.A. Clinical applications of pharmacogenetics: present and near future. Cleve Clin J Med. 2013;80:477–482. doi: 10.3949/ccjm.80a.12111. [DOI] [PubMed] [Google Scholar]
- 2.Ross S., Anand S.S., Joseph P., Pare G. Promises and challenges of phamacogenetics: an overview of study design, methodological and statistical issues. JRSM Cardiovasc Dis. 2012;1 doi: 10.1258/cvd.2012.012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Organization for Standardization . International Organization for Standardization; Geneva: 2012. ISO 15189 Medical Laboratories: requirements for quality and competence. [Google Scholar]
- 4.Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Part 493—Laboratory Requirements: Clinical Laboratory Improvement Amendments of 1988
- 5.Chen B., O'Connell C.D., Boone D.J., Amos J.A., Beck J.C., Chan M.M. Developing a sustainable process to provide quality control materials for genetic testing. Genet Med. 2005;7:534–549. doi: 10.1097/01.gim.0000183043.94406.81. [DOI] [PubMed] [Google Scholar]
- 6.Association for Molecular Pathology statement: recommendations for in-house development and operation of molecular diagnostic tests. Am J Clin Pathol. 1999;111:449–463. doi: 10.1093/ajcp/111.4.449. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Good laboratory practices for molecular genetic testing for heritable diseases and conditions. MMWR Recomm Rep. 2009;58(RR-6):1–37. [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA: 2012. Molecular methods for clinical genetics and oncology testing; Approved Guideline-Third Edition MM01–A3. [Google Scholar]
- 9.MM17-A Verification and validation of multiplex nucleic acid assays-Approved Guideline. The Clinical and Laboratory Standards Institute (CLSI); Wayne, PA: 2008. [Google Scholar]
- 10.Pratt V.M., Zehnbauer B., Amos Wilson J., Baak R., Babic N., Bettinotti M., Buller A., Butz K., Campbell M., Civalier C., El-Badry A., Farkas D.H., Lyon E., Mandal S., McKinney J., Muralidharan K., Noll L., Sander T., Shabbeer J., Smith C., Telatar M., Toji L., Vairavan A., Vance C., Weck K.E., Wu A.H.B., Yeo K.-T.J., Zeller M., Kalman L. Characterization of 107 genomic DNA reference materials for CYP2D6, CYP2C19, CYP2C9, VKORC1 and UGT1A1: a GeT-RM and Association for Molecular Pathology collaborative project. J Mol Diagn. 2010;12:835–846. doi: 10.2353/jmoldx.2010.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang H., Liu X., Ramírez J., Choudhury N., Kubo M., Im H.K., Konkashbaev A., Cox N.J., Ratain M.J., Nakamura Y., O'Donnell P.H. Establishment of CYP2D6 reference samples by multiple validated genotyping platforms. Pharmacogenomics J. 2014;14:564–572. doi: 10.1038/tpj.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relling M.V., Klein T.E. CPIC: clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladding P., White H., Voss J., Ormiston J., Stewart J., Ruygrok P., Bvaldivia B., Baak R., White C., Webster M. Pharmacogenetic testing for clopidogrel using the rapid INFINITI Analyzer: a dose-escalation study. JACC Cardiovasc Interv. 2009;11:1095–1101. doi: 10.1016/j.jcin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Martis S., Peter I., Hulot J.S., Kornreich R., Desnick R.J., Scott S.A. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J. 2013;13:369–377. doi: 10.1038/tpj.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce V.M., Hodinka R.L. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol. 2012;50:3458–3465. doi: 10.1128/JCM.01384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C.C., McMillin G.A., Babic N., Melis R., Yeo K.T. Evaluation of a CYP2C19 genotype panel on the GenMark eSensor® platform and the comparison to the Autogenomics Infiniti™ and Luminex CYP2C19 panels. Clin Chim Acta. 2011;412:1133–1137. doi: 10.1016/j.cca.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Livak K.J. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 19.Melis R., Lyon E., McMillin G.A. Determination of CYP2D6, CYP2C9 and CYP2C19 genotypes with Tag-It mutation detection assays. Expert Rev Mol Diagn. 2006;6:811–820. doi: 10.1586/14737159.6.6.811. [DOI] [PubMed] [Google Scholar]
- 20.Scott S.A., Edelmann L., Kornreich R., Erazo M., Desnick R.J. CYP2C9, CYP2C19 and CYP2D6 allele frequencies in the Ashkenazi Jewish population. Pharmacogenomics. 2007;8:721–730. doi: 10.2217/14622416.8.7.721. [DOI] [PubMed] [Google Scholar]
- 21.Langaee T.Y., Zhu H.J., Wang X., El Rouby N., Markowitz J.S., Goldstein J.A., Johnson J.A. The influence of the CYP2C19*10 allele on clopidogrel activation and CYP2C19*2 genotyping. Pharmacogenet Genomics. 2014;24:381–386. doi: 10.1097/FPC.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott S.A., Martis S., Peter I., Kasai Y., Kornreich R., Desnick R.J. Identification of CYP2C19*4B: pharmacogenetic implications for drug metabolism including clopidogrel responsiveness. Pharmacogenomics J. 2012;12:297–305. doi: 10.1038/tpj.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu A.H.B. Genotype and Phenotype Concordance for Pharmacogenetic Tests Through Proficiency Survey Testing. Arch Pathol Lab Med. 2013;137:1232–1236. doi: 10.5858/arpa.2012-0261-CP. [DOI] [PubMed] [Google Scholar]
- 24.Robarge J.D., Li L., Desta Z., Nguyen A., Flockhart D.A. The star-allele nomenclature: retooling for translational genomics. Clin Pharmacol Ther. 2007;82:244–248. doi: 10.1038/sj.clpt.6100284. [DOI] [PubMed] [Google Scholar]
- 25.Gaedigk A., Ndjountché L., Divakaran K., Dianne Bradford L., Zineh I., Oberlander T.F., Brousseau D.C., McCarver D.G., Johnson J.A., Alander S.W., Wayne Riggs K., Steven Leeder J. Cytochrome P4502D6 (CYP2D6) gene locus heterogeneity: characterization of gene duplication events. Clin Pharmacol Ther. 2007;81:242–251. doi: 10.1038/sj.clpt.6100033. [DOI] [PubMed] [Google Scholar]
- 26.Kalman L., Agúndez J., Appell M.L., Black J., Bell G., Boukouvala S. Pharmacogenetic allele nomenclature: international workgroup recommendations for test result reporting. Clin Pharmacol Ther. 2015 doi: 10.1002/cpt.280. [in press] doi: 10.1002/cpt.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.