Abstract
Benzene is a known human carcinogen which must be activated to benzene oxide (BO) to exert its carcinogenic potential. BO can be detoxified in vivo by reaction with glutathione and excretion in the urine as S-phenylmercapturic acid. This process may be catalyzed by glutathione S-transferases (GSTs), but kinetic data for this reaction have not been published. Therefore, we incubated GSTA1, GSTT1, GSTM1, and GSTP1 with glutathione and BO and quantified the formation of S-phenylglutathione. Kinetic parameters were determined for GSTT1 and GSTP1. At 37 °C, the putative Km and Vmax values for GSTT1 were 420 µM and 450 fmol/s, respectively, while those for GSTP1 were 3600 µM and 3100 fmol/s. GSTA1 and GSTM1 did not exhibit sufficient activity for determination of kinetic parameters. We conclude that GSTT1 is a critical enzyme in the detoxification of BO and that GSTP1 may also play an important role, while GSTA1 and GSTM1 seem to be less important.
Keywords: benzene oxide, glutathione S-transferase, GSTT1, GSTP1, kinetics, detoxification
1. Introduction
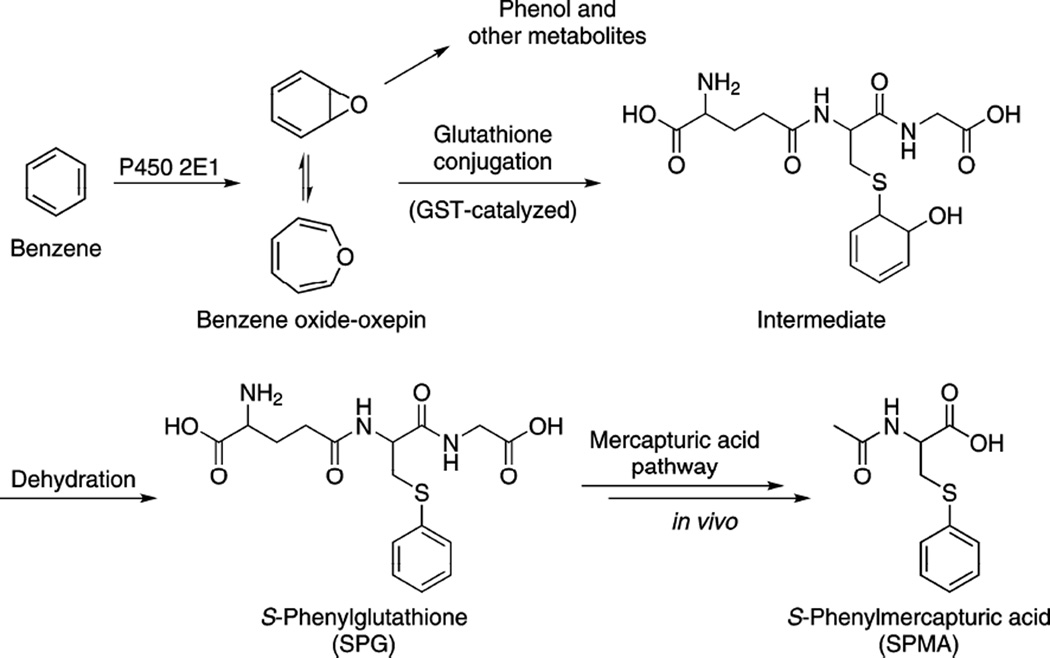
Benzene causes acute myeloid leukemia and other hematopoietic diseases [1, 2]. Although the mechanism of leukemogenesis is not fully understood, cytochrome P450-mediated metabolism of benzene is absolutely required for mutagenicity in S. typhimurium and for toxicity in mice [3–6]. Oxidation of benzene, catalyzed primarily by P450 2E1, yields benzene oxide (BO), which is in equilibrium with oxepin (Scheme 1). BO is reactive and unstable, readily rearranging to phenol. Urinary phenol and phenol conjugates account for about 40% of total benzene exposure, suggesting that activation to BO is a major metabolic pathway in humans [7]. The detoxification of BO in vivo proceeds via reaction with glutathione (GSH), resulting in excretion of S-phenylmercapturic acid (SPMA) in the urine (Scheme 1).
Scheme 1.
Metabolism of benzene in vivo resulting in the excretion of SPMA in the urine.
We have recently completed a biomarker study of urinary SPMA in more than 2200 smokers from five ethnic groups and, in a genome-wide association study (GWAS), evaluated genetic associations with more than 11 million genotyped or imputed polymorphisms in these individuals [8]. In the GWAS, we observed highly significant associations at chromosome 22q11 (P = 6.0 × 10−107) which could be explained by deletion of glutathione S-transferase theta 1 (GSTT1), with weaker associations with GSTM1 on chromosome 1p13. Differences in GSTT1 copy number explained approximately 21% of SPMA variation in this large multi-ethnic cohort. While previous studies have observed an effect of GSTT1 on SPMA levels [9], ours was by far the largest reported to date.
We are not aware of any reports in the literature on the catalysis of BO conjugation with GSH by specific glutathione S-transferases (GSTs). Formation of S-phenylglutathione (SPG) is the requisite step in the detoxification of BO and excretion of SPMA in vivo, as illustrated in Scheme 1. Thus SPMA excretion reflects exposure to benzene, its activation to BO, and the detoxification of BO. Other GSTs may also catalyze this conjugation, but the relative importance of each isozyme has not been reported. The enzymes investigated in this work are GSTA1, GSTT1, GSTM1, and GSTP1.
GSTs are highly polymorphic in the general population. GSTT1 or GSTM1 deletion is observed in 9% to 76% of the population, with differences between ethnic groups [10]. GSTA1 or GSTP1 deletion has not been reported, but polymorphisms which alter their expression or catalytic activity have been described [11]. The biological consequences of GST polymorphisms on the detoxification of BO have been of interest in occupationally-exposed workers. For workers with occupational exposure to benzene, there is a 2- to 4-fold increased risk of chronic benzene poisoning among GSTT1-null individuals [12, 13], and risk of chronic benzene poisoning is weakly correlated with GSTP1 polymorphisms [14]. These GST isozymes clearly play a role in the detoxification of benzene, but their activity toward BO has not yet been directly evaluated.
Previous reports have been mixed regarding the importance of GST isozymes in the excretion of SPMA [9]. The most consistent correlation between genotype and SPMA excretion has been observed for GSTT1. Many but not all studies have demonstrated that the GSTT1-null genotype results in lower levels of urinary SPMA, and thus less efficient detoxification of BO [15–18]. Fewer studies have shown a significant effect of the GSTM1-null genotype [19–22]. GSTA1 polymorphisms have not been studied extensively, but a mutation which lowers GSTA1 expression has been shown to affect SPMA excretion [23, 24]. The most studied GSTP1 polymorphism results in an I105V mutation in the active site, which impacts substrate specificity but in general has not affected the excretion of SPMA [25–28]. The results from our GWAS study encouraged us to directly examine the catalysis of BO detoxification by different GSTs in order to clarify their respective roles.
2. Materials and Methods
CAUTION: Benzene is a known human carcinogen, and BO is its activated form; use extreme care when handling.
2.1. Chemicals and Synthesis
Recombinant human GSTA1, GSTT1, GSTM1, and GSTP1 were purchased from MyBioSource (San Diego, CA, USA). All other reagents were purchased from Sigma-Aldrich. BO was synthesized essentially as described previously [29] in 97% purity (3% phenol).
S-Phenylglutathione was synthesized by adding BO (31 mg, 0.33 mmol) to GSH (111 mg, 0.36 mmol) in a solution of MeOH (4 mL) and 1 N NaOH (1 mL). Previous reports suggest that product yield increases under basic conditions, which should increase the nucleophilicity of the thiol and increase the stability of BO in solution [29, 30]. After 30 min, the pH was adjusted to ~2 with 1 N HCl (1.2 mL) in order to dehydrate the intermediate, yielding SPG. The product was concentrated in vacuo and purified by HPLC, resulting in 35.6 mg of SPG as a white powder (0.093 mmol, 28% yield). 1H NMR (500 MHz, D2O) δ ppm 1.97 (m, 2 H, -CH2-CH2-CO-) 2.23 – 2.37 (m, 2 H, -CH2-CH2-CO-) 3.24 (dd, J = 14.5, 8.1 Hz, 1 H, -CH2-S-) 3.42 (dd, J = 14.6, 4.9 Hz, 1 H, -CH2′-S-) 3.48 (d, J = 17.4 Hz, 1 H, -ND-CH2-COO) 3.54 (d, J = 17.4 Hz, 1 H, -ND-CH2′-COO) 3.61 (m, 1 H, D2N-CH-COO) 4.47 (dd, J = 8.1, 4.7 Hz, 1 H, -CH-CH2-S-) 7.23 – 7.29 (m, 1 H, -S-Ph) 7.32 (m, 2 H, S-Ph) 7.41 (m, 2 H, S-Ph). 13C NMR (126 MHz, D2O) δ ppm 26.3, 31.4, 34.9, 43.3, 53.3, 54.2, 127.5, 129.4 (2 C), 130.8 (2 C), 133.4, 171.4, 174.8, 176.1, 181.5. COSY and HSQC spectra were consistent with the above assignments (Supplemental Data S1).
2.2. Benzene Oxide Stability Studies
The half-life of BO in 0.1 M phosphate buffer (pH 7.4) at 37 °C was measured on a Cary 100 spectrophotometer (Agilent Technologies) based on the absorbance at 315 nm (Supplemental Data S2). Additionally, BO stability was investigated by 1H NMR on an Ascend 500 MHz spectrometer (Bruker Corporation) in various organic solvents over time under ambient light. BO was stored neat (20 °C, < 1 day), in MeOH (< 1 day, 20 °C), in Et2O (4 °C, > 60 days), in CDCl3 (−20 °C, > 75 days), or in dioxane (4 to 20 °C, > 90 days, as 1% or 10% BO solutions).
2.3. Enzyme Incubation Conditions
All buffers were prepared on the same day as the assay. The incubation buffer (pH 7.4) was prepared by combining solutions of 0.1 M K2HPO4 (80 mL) and KH2PO4 (20 mL). A stock solution of GSH (10 mM) was also prepared in phosphate buffer and adjusted to pH 7.4. The incubations were performed using a rapid quench-flow instrument (model RQF-3, KinTek Corporation). BO was stored at 4 °C as 1% or 10% solutions in dioxane, and this was added to phosphate buffer (1 mL final volume) immediately before the reaction such that the organic solvent was ≤ 1% of the volume. Final concentrations of BO were 50 µM, 100 µM, 500 µM, 1 mM, and 5 mM. Final concentration of GSH was 5 mM, and 1.7 mU of enzyme was added to each incubation. The final volume of the reaction was 34 µL. We did not have an isotopically labelled standard of SPG, so SPMA (0.4 pmol) and [D5]SPMA (3 pmol) were added as internal standards to account for variation in sample mixing, recovery, and detection. The quench-flow instrument was adjusted to 25 °C or 37 °C using a heated water recirculating bath (Isotemp 1016S, Fisher Scientific). The enzymes were pre-incubated with GSH before introducing BO. The incubation time was 7 s, and the reaction was quenched with trichloroacetic acid (TCA). The collected product mixture was desalted and purified by solid-phase extraction (Strata-X, 30 mg, Phenomenex), concentrated in vacuo to dryness, and reconstituted in 20 µL 10% MeOH in H2O. Analyses at 25 °C were performed in four replicates (Supplemental Data S3) and analyses at 37 °C were performed in triplicate.
The amount of enzyme added was based on the reported GSH conjugation activity with a model substrate. The specific activity of GSTA1 and GSTP1 with 1-chloro-2,4-dinitrobenzene (CDNB) was 6.5 U/mg, and the specific activity of GSTM1 with CDNB was 50 U/mg. GSTT1 does not catalyze GSH conjugation with CDNB, so p-nitrobenzyl chloride was used, yielding 29.4 U/mg reported specific activity. To achieve 1.7 mU activity, the masses of GSTA1, GSTT1, GSTM1, and GSTP1 added were 0.26, 0.057, 0.034, and 0.26 µg, respectively.
2.4. Analysis of S-Phenylglutathione
The samples were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). The chromatography was carried out on a Synergi Polar-RP column (150 × 0.5 mm, 4 µm, 80 Å, Phenomenex) under isocratic conditions (65% A, 35% B, 15 µL/min, 8 µL injection volume) where A was 0.1% aq. HCOOH and B was MeOH. The MS conditions were as follows: the ionization source was positive-mode electrospray ionization, the selected reaction monitoring transition (1.0 amu isolation width, 35 V collision energy) for SPG was m/z 384.1 [M + H]+ →109.0 [PhS]+, which was the major fragment observed. SPMA and [D5]SPMA fragmented similarly, with m/z transitions of 240.1 → 109.0 for SPMA and 245.1 → 114.1 for [D5]SPMA. The analyte was quantified by a linear calibration curve applying the peak area ratio of SPG to [D5]SPMA and normalizing to the amount of [D5]SPMA standard added. The limit of detection was assessed by dilution of a standard solution of SPG in water until the signal-to-noise ratio was approximately 3:1. The limit of detection for this method is approximately 2 fmol on-column. The calibration curve was linear between 2 fmol and 1040 fmol on-column.
2.5. Calculations
Nonenzymatic SPG formation was determined by incubating GSH with BO in the absence of enzyme. Enzymatic product formation was then determined by subtracting the nonenzymatic SPG formation from the total product formation. The resulting data were analyzed by nonlinear regression using Microsoft Excel [31].
3. Results
3.1 Benzene Oxide Stability Studies
BO is unstable in its pure form and in protic solvents; it rapidly rearranges to phenol and other products. Under our incubation conditions (37 °C, pH 7.4 phosphate buffer), the degradation to phenol followed first-order kinetics, with a half-life of 5.2 min (rate constant = 0.133 min−1). This is comparable to what has been reported previously in aqueous media [29]. BO also degrades rapidly in MeOH (< 1 day, 20 °C). However, BO is stable when diluted in aprotic solvents. No degradation was observed in the first 2–3 months when BO was stored in Et2O, CDCl3, or dioxane. The solution in dioxane was reevaluated by 1H NMR (500 MHz, CDCl3) again after 7 months of storage at 4 °C. The NMR spectrum showed 53% degradation to phenol and 2% degradation to another product, which was likely muconaldehyde, resulting from spontaneous oxidation of oxepin [32, 33]. Observed chemical shifts of phenol were δ ppm 7.2 (2 H), 6.9 (1 H), and 6.8 (2 H), and those of muconaldehyde were 10.3 (1 H), 9.7 (1 H), and 7.5 (2 H), with the signal from the remaining protons masked by phenol or BO; 6.3 (2 H), 5.9 (2 H), and 5.1 ppm (2 H). The observed degradation to phenol may have been catalyzed by the presence of water as the solution absorbed moisture over time, which was observed by NMR.
3.2 SPG Formation is Catalyzed by GSTs
The initial reaction product of BO with GSH is a hydroxycyclohexadiene species, which can be dehydrated to S-phenylglutathione (SPG), restoring aromaticity (Scheme 1). Previous studies have shown that this intermediate is relatively stable under neutral conditions, but readily dehydrates when exposed to acid [29, 30]. The rearrangement of BO to phenol is also catalyzed by acid. Thus the TCA quench serves three purposes: to denature the enzymes, to catalyze the dehydration of the intermediate, and to catalyze the degradation of BO to phenol.
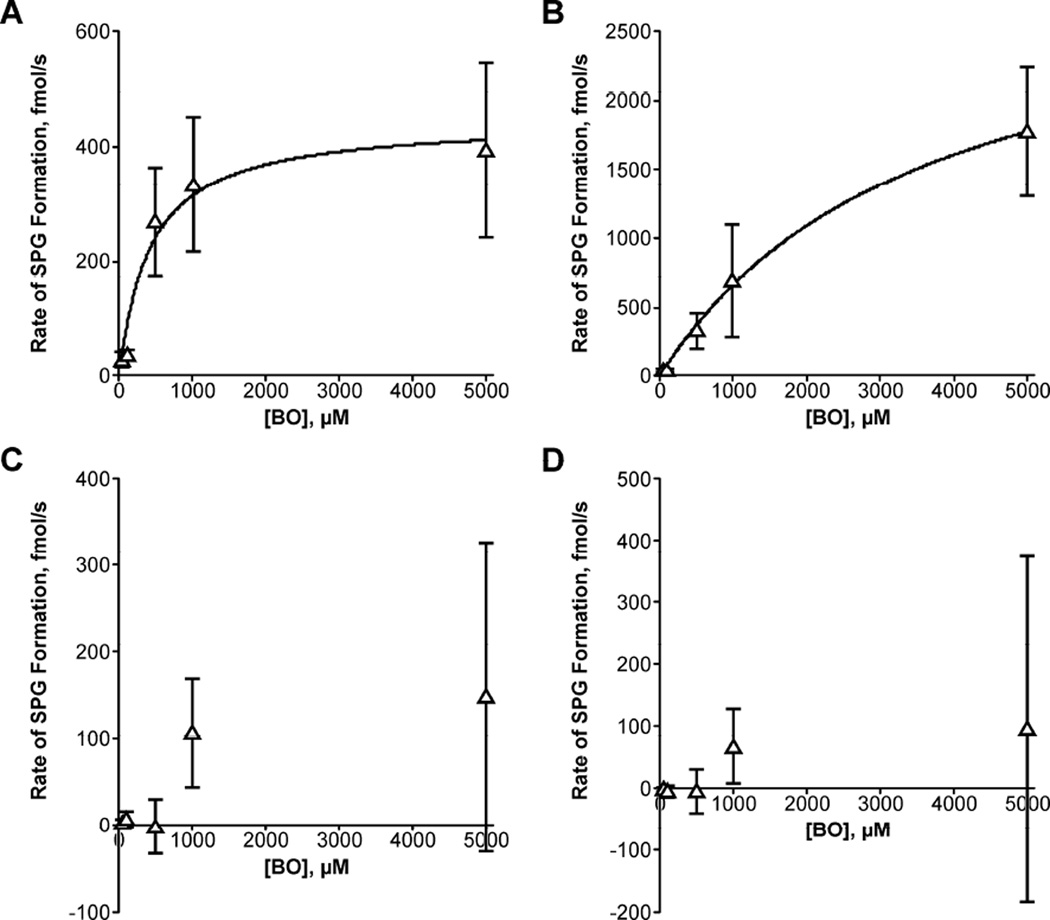
Both GSTT1 and GSTP1 catalyzed the conjugation of BO with GSH with reproducible Michaelis-Menten kinetics (Figure 1). GSTT1 had a lower putative Km than GSTP1 (Table 1), however, GSTP1 had a higher Vmax. The amount of enzyme added was determined by reported GSH conjugation activity with CDNB for GSTP1 or p-nitrobenzyl chloride for GSTT1 (1.7 mU each), not by concentration of enzyme. For this reason, direct comparison of enzyme efficiencies should be made with caution and we decided not to express activity normalized to mg protein. We also verified that SPG formation is linear with respect to time and enzyme concentration for both GSTT1 and GSTP1 under the conditions used here. The concentration of BO remained approximately constant during the 7 s reaction; we estimate that < 2% degraded to phenol and < 1% reacted to form SPG based on the half-life of BO and the product formation.
Figure 1.
Rate of SPG formation as a function of BO concentration. Experimental data are shown for five BO concentrations at 37 °C with (A) GSTT1, (B) GSTP1, (C) GSTM1, and (D) GSTA1. Data points and error bars represent the mean ± standard deviation. Calculated best-fit curves from nonlinear regression analyses for GSTT1 and GSTP1 are overlaid. Concentration of enzyme was normalized to 1.7 mU activity toward a model substrate.
Table 1.
Kinetic parameters for GSTT1 and GSTP1
| Km (µM) | Vmax (fmol/s) | |
|---|---|---|
| GSTT1 | 420 | 450 |
| GSTP1 | 3600 | 3100 |
Incubations were performed at 37 °C with 1.7 mU (0.057 µg) GSTT1 and 1.7 mU (0.26 µg) GSTP1
GSTM1 and GSTA1 showed minimal activity in conjugating BO with GSH (Figure 1). In these reactions, nonenzymatic product formation accounted for > 80% of total product formation, whereas in the GSTT1 and GSTP1 incubations, nonenzymatic product formation accounted for only 20–40% of the total. After accounting for nonenzymatic SPG formation, the remaining enzymatic formation of SPG by GSTM1 and GSTA1 was small or not detected, which made calculation of kinetic parameters impossible for these two enzymes.
The incubations were also performed at 25 °C. As expected, the enzymatic activity was lower at the lower temperature. The Km and Vmax values for GSTT1 were 1100 µM and 360 fmol/s, respectively, and those for GSTP1 were 6300 µM and 3300 fmol/s at 25 °C. Similar to the 37 °C incubations, kinetic parameters for GSTM1 and GSTA1 could not be determined at 25 °C. The spontaneous product formation was also lower at 25 °C, and it accounted for a smaller percentage of the total product formation.
4. Discussion
All human tissues express GSTs, but each tissue has a unique expression profile of GST isozymes [34]. Both the expression and the activity of each isozyme will impact the relative importance of GSTs in BO detoxification. Benzene is oxidized to BO primarily by P450 2E1 in the liver, where GSTA1 is highly expressed, GSTP1 is minimally expressed, and GSTT1 and GSTM1 expression levels are intermediate [35–38]. Benzene can also be oxidized to BO by CYP2F1 in the lung, where GSTP1 expression predominates [35, 39]. The half-life of BO is 5–6 min, which affords sufficient time for BO to leave the site of oxidation and enter the blood stream, thus GSTs that are more widely expressed may still play an important role in BO detoxification. The kidney, for example, may be an important site of metabolism because it expresses high levels of GSTT1 and plays a critical role in further metabolism of glutathione conjugates to mercapturic acids [37, 40].
The in vitro study of BO presented a unique set of challenges. The primary challenge is that BO is unstable in aqueous solution. The incubation time must then be restricted to only a few seconds, or else the concentration of BO will decrease over the course of the incubation. To perform 7-second incubations, a rapid quench flow instrument was required. However, this instrument restricted the total incubation volume to only 34 µL. Due to the short incubation time and the small reaction volume, total SPG formation was below the limit of detection (~2 fmol on-column) by LC-MS/MS when concentrations of BO were below 50 µM. Finally, the spontaneous reaction between BO and GSH must be assessed and subtracted from the total SPG formation, which introduced more variability between replicates in the calculations of enzymatic SPG formation.
The concentrations of BO used here were necessary to enable detection of SPG, but the physiological concentrations of BO are significantly lower. In a study performed in rats, a single dose of 400 mg/kg benzene resulted in only 90 nM BO circulating in the blood, although intracellular BO concentrations could not be assessed [41]. Humans are exposed to lower concentrations of benzene, so it is likely that the GSTs investigated here will not be saturated by the concentration of BO in vivo. At the lowest concentration of BO, rates of SPG formation were comparable for GSTT1 (29 ± 14 fmol/s) and GSTP1 (38 ± 9 fmol/s) at 37 °C, and rates were low for GSTA1 and GSTM1 (< 1 fmol/s). This suggests that, at physiologically relevant doses of benzene, GSTT1 and GSTP1 may both play major roles in the detoxification of BO.
Our results for GSTT1 are consistent with previous studies relating genetic polymorphisms to chronic benzene poisoning and SPMA excretion in humans exposed to benzene. GSTT1 had the lowest Km value of the isozymes tested here. The data obtained from our GWAS study show that GSTT1 deletion is strongly correlated with SPMA excretion (P = 6.0 × 10−107) and accounts for 21% of total variation in urinary SPMA. Taken together, the data strongly suggest that GSTT1 is an important enzyme in catalyzing the detoxification of BO by conjugation with GSH. We also analyzed the importance of GSTM1 copy number in our GWAS study. Our results show that deletion of GSTM1 accounted for only 1.5% of variation in urinary SPMA, which is consistent with these in vitro data that suggest a less important role for GSTM1 in the enzymatic detoxification of BO.
Polymorphism data for GSTP1 suggest a lesser impact on SPMA formation in vivo and seem to have only a weak correlation with risk of chronic benzene poisoning [14]. However, a GSTP1-null genotype has not been identified, so the majority of studies investigate the correlation between SPMA excretion and an I105V point mutation in the active site of GSTP1. It is not known if this mutation will affect the activity of GSTP1 in conjugating BO to GSH. The in vitro biochemical characterization is necessary to evaluate GSTP1 activity, as it is more difficult to observe the importance of GSTP1 in humans, where the vast majority if not all individuals express functional enzyme.
Another way to assess the importance of GSTP1 in vivo is by inducing enzyme expression. In a recent phase II clinical trial in humans, subjects were administered a beverage derived from broccoli sprouts containing a high concentration of sulforaphane [42]. Sulforaphane is a potent inducer of the Nrf2 pathway, which results in the upregulation of a number of detoxification enzymes, including the GSTP family [43]. The results of this trial demonstrated that treatment with sulforaphane significantly increased the detoxification of BO, reflected by increased excretion of SPMA. The subjects were also genotyped for GSTT1 and GSTM1 deletion, but the increase in SPMA excretion was observed in all groups receiving sulforaphane treatment, regardless of GSTT1 or GSTM1 status [42]. We believe that upregulation of GSTP1 expression was responsible for the enhanced detoxification of BO observed in these individuals.
5. Conclusion
GSTT1 and GSTP1 catalyze BO conjugation with GSH at a higher rate than either GSTM1 or GSTA1 in vitro and are likely more important in the BO detoxification process in vivo. Significant catalytic activity was not detected for GSTM1 or GSTA1 in vitro due to competing nonenzymatic product formation and lower enzymatic activity. This is the first study to determine the kinetic parameters of GST conjugation of BO, a critical intermediate in benzene carcinogenesis.
Supplementary Material
Highlights.
Benzene oxide detoxification is catalyzed by glutathione S-transferases.
GSTT1 and GSTP1 catalyzed the conjugation of benzene oxide with glutathione.
GSTM1 and GSTA1 did not catalyze the conjugation of benzene oxide with glutathione.
Acknowledgements
We thank Bob Carlson for editorial assistance. We thank the laboratories of Natalia Tretyakova and Lisa Peterson for partially furnishing equipment used in this study. This work was supported by the U.S. National Institutes of Health, National Cancer Institute [Grant CA- 138338].
Abbreviations
- BO
benzene oxide
- GSTs
glutathione S-transferases
- GSTA1
glutathione S-transferase alpha 1
- GSTM1
glutathione S-transferase mu 1
- GSTP1
glutathione S-transferase pi 1
- GSTT1
glutathione S-transferase theta 1
- SPMA
S-phenylmercapturic acid
- SPG
S-phenylglutathione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.NTP, NTP 12th Report on Carcinogens, Report on carcinogens : carcinogen profiles / U.S. Dept. of Health and Human Services, Public Health Service. National Toxicology Program. 2011;12:iii-499. [PubMed] [Google Scholar]
- 2.IARC, Chemical Agents and Related Occupations. Monographs on the Evaluation of Carcinogenic Risks to Humans. 2012:249–294. [PMC free article] [PubMed] [Google Scholar]
- 3.Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, Oesch F. Multiple activation pathways of benzene leading to products with varying genotoxic characteristics. Environ.Health Perspect. 1989;82:81–89. doi: 10.1289/ehp.898281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McHale CM, Zhang L, Smith MT. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis. 2012;33:240–252. doi: 10.1093/carcin/bgr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder R, Hedli CC. An overview of benzene metabolism. Environ.Health Perspect. 1996;104(Suppl 6):1165–1171. doi: 10.1289/ehp.961041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentine JL, Lee SS, Seaton MJ, Asgharian B, Farris G, Corton JC, Gonzalez FJ, Medinsky MA. Reduction of benzene metabolism and toxicity in mice that lack CYP2E1 expression. Toxicol.Appl.Pharmacol. 1996;141:205–213. doi: 10.1006/taap.1996.0277. [DOI] [PubMed] [Google Scholar]
- 7.Boogaard PJ, van Sittert NJ. Suitability of S-phenyl mercapturic acid and transtrans-muconic acid as biomarkers for exposure to low concentrations of benzene. Environ.Health Perspect. 1996;104(Suppl 6):1151–1157. doi: 10.1289/ehp.961041151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haiman CA, Patel YM, Stram DO, Carmella SG, Chen M, Le Marchand L, Hecht SS. Benzene exposure and genetic determinants of urinary S-phenylmercapturic acid in a multiethnic sample: important effect of glutathione-S-transferase-T1. 2015 doi: 10.1371/journal.pone.0150641. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougherty D, Garte S, Barchowsky A, Zmuda J, Taioli E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure--a literature review. Toxicol.Lett. 2008;182:7–17. doi: 10.1016/j.toxlet.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Board PG, Menon D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim.Biophys.Acta. 2013;1830:3267–3288. doi: 10.1016/j.bbagen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Harris MJ, Coggan M, Langton L, Wilson SR, Board PG. Polymorphism of the Pi class glutathione S-transferase in normal populations and cancer patients. Pharmacogenetics. 1998;8:27–31. doi: 10.1097/00008571-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Li G, Yin S, Xu J, Ji Z, Xiu X, Liu L, Ma D. Genetic polymorphisms involved in toxicant-metabolizing enzymes and the risk of chronic benzene poisoning in Chinese occupationally exposed populations. Xenobiotica. 2007;37:103–112. doi: 10.1080/00498250601001662. [DOI] [PubMed] [Google Scholar]
- 13.Wan JX, Zhang ZB, Guan JR, Cao DZ, Ye R, Jin XP, Xia ZL. Genetic polymorphism of toxicant-metabolizing enzymes and prognosis of Chinese workers with chronic benzene poisoning. Annals of the New York Academy of Sciences. 2006;1076:129–136. doi: 10.1196/annals.1371.041. [DOI] [PubMed] [Google Scholar]
- 14.Sun P, Qian J, Zhang ZB, Wan JX, Wu F, Jin XP, Fan WW, Lu DR, Zhao NQ, Christiani DC, Xia ZL. Polymorphisms in phase I and phase II metabolism genes and risk of chronic benzene poisoning in a Chinese occupational population. Carcinogenesis. 2008;29:2325–2329. doi: 10.1093/carcin/bgn208. [DOI] [PubMed] [Google Scholar]
- 15.Carrieri M, Bartolucci GB, Scapellato ML, Spatari G, Sapienza D, Soleo L, Lovreglio P, Tranfo G, Manno M, Trevisan A. Influence of glutathione S-transferases polymorphisms on biological monitoring of exposure to low doses of benzene. Toxicol.Lett. 2012;213:63–68. doi: 10.1016/j.toxlet.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Hoet P, De Smedt E, Ferrari M, Imbriani M, Maestri L, Negri S, De Wilde P, Lison D, Haufroid V. Evaluation of urinary biomarkers of exposure to benzene: correlation with blood benzene and influence of confounding factors. Int.Arch.Occup.Environ.Health. 2009;82:985–995. doi: 10.1007/s00420-008-0381-6. [DOI] [PubMed] [Google Scholar]
- 17.Qu Q, Shore R, Li G, Su L, Jin X, Melikian AA, Roy N, Chen LC, Wirgin I, Cohen B, Yin S, Li Y, Mu R. Biomarkers of benzene: urinary metabolites in relation to individual genotype and personal exposure. Chem.Biol.Interact. 2005;153–154:85–95. doi: 10.1016/j.cbi.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen M, Poole J, Autrup H, Muzyka V, Jensen A, Loft S, Knudsen LE. Benzene exposure assessed by metabolite excretion in Estonian oil shale mineworkers: influence of glutathione s-transferase polymorphisms. Cancer Epidemiol.Biomarkers Prev. 2004;13:1729–1735. [PubMed] [Google Scholar]
- 19.Mansi A, Bruni R, Capone P, Paci E, Pigini D, Simeoni C, Gnerre R, Papacchini M, Tranfo G. Low occupational exposure to benzene in a petrochemical plant: modulating effect of genetic polymorphisms and smoking habit on the urinary t,t-MA/SPMA ratio. Toxicol.Lett. 2012;213:57–62. doi: 10.1016/j.toxlet.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Angelini S, Kumar R, Bermejo JL, Maffei F, Barbieri A, Graziosi F, Carbone F, Cantelli-Forti G, Violante FS, Hemminki K, Hrelia P. Exposure to low environmental levels of benzene: evaluation of micronucleus frequencies and S-phenylmercapturic acid excretion in relation to polymorphisms in genes encoding metabolic enzymes. Mutat.Res. 2011;719:7–13. doi: 10.1016/j.mrgentox.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Lan Q, Waidyanatha S, Chanock S, Johnson BA, Vermeulen R, Smith MT, Zhang L, Li G, Shen M, Yin S, Rothman N, Rappaport SM. Genetic polymorphisms and benzene metabolism in humans exposed to a wide range of air concentrations. Pharmacogenet.Genomics. 2007;17:789–801. doi: 10.1097/FPC.0b013e3280128f77. [DOI] [PubMed] [Google Scholar]
- 22.Verdina A, Galati R, Falasca G, Ghittori S, Imbriani M, Tomei F, Marcellini L, Zijno A, Vecchio VD. Metabolic polymorphisms and urinary biomarkers in subjects with low benzene exposure. J.Toxicol.Environ.Health A. 2001;64:607–618. doi: 10.1080/152873901753246214. [DOI] [PubMed] [Google Scholar]
- 23.Manini P, De Palma G, Andreoli R, Mozzoni P, Poli D, Goldoni M, Petyx M, Apostoli P, Mutti A. Occupational exposure to low levels of benzene: Biomarkers of exposure and nucleic acid oxidation and their modulation by polymorphic xenobiotic metabolizing enzymes. Toxicol.Lett. 2010;193:229–235. doi: 10.1016/j.toxlet.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Manini P, De Palma G, Andreoli R, Poli D, Mozzoni P, Folesani G, Mutti A, Apostoli P. Environmental and biological monitoring of benzene exposure in a cohort of Italian taxi drivers. Toxicol.Lett. 2006;167:142–151. doi: 10.1016/j.toxlet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Lin LC, Chen WJ, Chiung YM, Shih TS, Liao PC. Association between GST genetic polymorphism and dose-related production of urinary benzene metabolite markers, trans, trans-muconic acid and S-phenylmercapturic acid. Cancer Epidemiol.Biomarkers Prev. 2008;17:1460–1469. doi: 10.1158/1055-9965.EPI-08-0160. [DOI] [PubMed] [Google Scholar]
- 26.Avogbe PH, Ayi-Fanou L, Autrup H, Loft S, Fayomi B, Sanni A, Vinzents P, Moller P. Ultrafine particulate matter and high-level benzene urban air pollution in relation to oxidative DNA damage. Carcinogenesis. 2005;26:613–620. doi: 10.1093/carcin/bgh353. [DOI] [PubMed] [Google Scholar]
- 27.Scheepers PT, Coggon D, Knudsen LE, Anzion R, Autrup H, Bogovski S, Bos RP, Dahmann D, Farmer P, Martin EA, Micka V, Muzyka V, Neumann HG, Poole J, Schmidt-Ott A, Seiler F, Volf J, Zwirner-Baier I. BIOMarkers for occupational diesel exhaust exposure monitoring (BIOMODEM)--a study in underground mining. Toxicol.Lett. 2002;134:305–317. doi: 10.1016/s0378-4274(02)00195-9. [DOI] [PubMed] [Google Scholar]
- 28.Rossi AM, Guarnieri C, Rovesti S, Gobba F, Ghittori S, Vivoli G, Barale R. Genetic polymorphisms influence variability in benzene metabolism in humans. Pharmacogenetics. 1999;9:445–451. [PubMed] [Google Scholar]
- 29.Henderson AP, Barnes ML, Bleasdale C, Cameron R, Clegg W, Heath SL, Lindstrom AB, Rappaport SM, Waidyanatha S, Watson WP, Golding BT. Reactions of benzene oxide with thiols including glutathione. Chem.Res.Toxicol. 2005;18:265–270. doi: 10.1021/tx049781y. [DOI] [PubMed] [Google Scholar]
- 30.Micova K, Linhart I. Reactions of benzene oxide, a reactive metabolite of benzene, with model nucleophiles and DNA. Xenobiotica. 2012;42:1028–1037. doi: 10.3109/00498254.2012.669872. [DOI] [PubMed] [Google Scholar]
- 31.Kemmer G, Keller S. Nonlinear least-squares data fitting in Excel spreadsheets. Nat.Protoc. 2010;5:267–281. doi: 10.1038/nprot.2009.182. [DOI] [PubMed] [Google Scholar]
- 32.Golding BT, Kennedy G, Watson WP. Simple Syntheses of Isomers of Muconaldehyde and 2-Methylmuconaldehyde. Tetrahedron Lett. 1988;29:5991–5994. [Google Scholar]
- 33.Golding BT, Barnes ML, Bleasdale C, Henderson AP, Jiang D, Li X, Mutlu E, Petty HJ, Sadeghi MM. Modeling the formation and reactions of benzene metabolites. Chem.Biol.Interact. 2010;184:196–200. doi: 10.1016/j.cbi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Whalen R, Boyer TD. Human glutathione S-transferases. Semin.Liver Dis. 1998;18:345–358. doi: 10.1055/s-2007-1007169. [DOI] [PubMed] [Google Scholar]
- 35.Rowe JD, Nieves E, Listowsky I. Subunit diversity and tissue distribution of human glutathione S-transferases: interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem.J. 1997;325(Pt 2):481–486. doi: 10.1042/bj3250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juronen E, Tasa G, Uuskula M, Pooga M, Mikelsaar AV. Purification, characterization and tissue distribution of human class theta glutathione Stransferase T1-1. Biochem.Mol.Biol.Int. 1996;39:21–29. doi: 10.1080/15216549600201021. [DOI] [PubMed] [Google Scholar]
- 37.Sherratt PJ, Pulford DJ, Harrison DJ, Green T, Hayes JD. Evidence that human class Theta glutathione S-transferase T1-1 can catalyse the activation of dichloromethane, a liver and lung carcinogen in the mouse. Comparison of the tissue distribution of GST T1-1 with that of classes Alpha, Mu and Pi GST in human. Biochem.J. 1997;326(Pt 3):837–846. doi: 10.1042/bj3260837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awasthi YC, Sharma R, Singhal SS. Human glutathione S-transferases. Int.J.Biochem. 1994;26:295–308. doi: 10.1016/0020-711x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 39.Powley MW, Carlson GP. Cytochromes P450 involved with benzene metabolism in hepatic and pulmonary microsomes. J Biochem Mol Toxicol. 2000;14:303–309. doi: 10.1002/1099-0461(2000)14:6<303::AID-JBT2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Anderson ME, Meister A. Inhibition of gamma-glutamyl transpeptidase and induction of glutathionuria by gamma-glutamyl amino acids. Proc.Natl.Acad.Sci.U.S.A. 1986;83:5029–5032. doi: 10.1073/pnas.83.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindstrom AB, Yeowell-O'Connell K, Waidyanatha S, Golding BT, Tornero-Velez R, Rappaport SM. Measurement of benzene oxide in the blood of rats following administration of benzene. Carcinogenesis. 1997;18:1637–1641. doi: 10.1093/carcin/18.8.1637. [DOI] [PubMed] [Google Scholar]
- 42.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer prevention research. 2014;7:813–823. doi: 10.1158/1940-6207.CAPR-14-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lii CK, Liu KL, Cheng YP, Lin AH, Chen HW, Tsai CW. Sulforaphane and alpha-lipoic acid upregulate the expression of the pi class of glutathione Stransferase through c-jun and Nrf2 activation. J.Nutr. 2010;140:885–892. doi: 10.3945/jn.110.121418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.