Abstract
Introduction
Copeptin levels are elevated in severe medical conditions, an effect that is attributed to elevated arginine vasopressin (AVP) levels in response to physiological stress, resulting in activation of hypothalamus-pituitary-adrenal (HPA) axis. In the current study, we wanted to determine if copeptin is responsive to psychological stress, correlates with cortisol and adrenocorticotropin hormone (ACTH), and if associations differed by sex.
Materials and Methods
In a cross-sectional study that included 100 healthy men (41%) and women (59%) (aged 18–30 years; mean 24.6±3 years), who underwent the Trier Social Stress Test (TSST), we examined the association between percent change (peak-baseline/baseline) in copeptin levels and percent change in log ACTH and cortisol. Three baselines samples were drawn followed by blood sampling at 20, 35, 50, 65 and 85 min after TSST.
Results
There was a significant positive association between the percent change in copeptin and the percent change in log-transformed salivary cortisol (β-coefficient=0.95; p=0.02). The association between percent change in copeptin and log-transformed serum cortisol was not statistically significant in the overall population. There was a trend for a non-significant association between percent change in copeptin and percent change in log-transformed ACTH (β-coefficient=1.14; p=0.06). In males, there was a significant positive association between the percent change in copeptin levels and log-transformed salivary (β-coefficient=1.33, p=0.016) and serum cortisol (β-coefficient=0.69, p=0.01), whereas in women there was no statistically significant association.
Conclusions
We found a significant positive association between percent change in copeptin and percent change in salivary and serum cortisol among males only.
Keywords: Copeptin, Stress, Sex Differences, Trier Stress Test
Introduction
All forms of stress stimulate the autonomic (sympathetic) nervous system and the hypothalamic-pituitary-adrenal (HPA) axis (Chrousos and Gold, 1992). Activation of the HPA axis by stressors leads to secretion of corticotropin releasing hormone (CRH) and arginine vasopressin (AVP). CRH and AVP lead to the release of adrenocorticotropin hormone (ACTH) from the anterior pituitary (Chrousos, 1995; Guillemin and Rosenberg, 1955; Swanson et al., 1983). Although the primary effect of AVP is to regulate water balance through activation of the arginine vasopressin receptor 2 (AVPR2) in the kidney, AVP causes release of ACTH through activation of the AVPR1b receptors that are present on pituitary corticotrophes (Lamberts et al., 1984; Sugimoto et al., 1994).
Copeptin (also known as CT-proAVP) is the C-terminal component of the AVP precursor hormone, preprovasopressin (Holwerda, 1972). After cleavage of the signal peptide, provasopressin is formed, composed of arginine vasopressin (AVP), neurophysin II, and copeptin (Morgenthaler et al., 2008). Determining plasma AVP concentrations can be problematic, as the majority of the AVP is bound to platelets resulting in underestimation or overestimation of the AVP levels (Preibisz et al., 1983; Szinnai et al., 2007). AVP is also unstable even when stored in -20 C (Robertson et al., 1973). Measuring cortisol and ACTH levels can also be challenging due to their varying diurnal production. In addition, ACTH is unstable at room temperature, requiring immediate, special processing (Besser et al., 1971; Evans et al., 2001). Measuring copeptin, a more stable molecule, is perhaps a reasonable alternative measure of AVP activity. In fact, although copeptin does not have a definitive role in clinical practice, copeptin has been evaluated as a potential tool in the differential diagnosis of polyuria polydipsia syndrome (Fenske et al., 2011), central diabetes insipidus (Katan et al., 2007; Winzeler et al., 2015) and hyponatremic disorders (Fenske et al., 2009). Copeptin levels have also been found to be elevated in many severe medical conditions, (Morgenthaler et al., 2008), an effect that can be attributed to elevated AVP levels in response to physiological stress.
There is a growing field of research implicating chronic psychological stressors as contributors to psychiatric and cardiometabolic disorders, with dysregulation of the HPA axis as an implicated mechanism (Cohen et al., 2007). It is unclear however if copeptin is responsive to acute psychological stress and prior studies have yielded conflicting results. One study utilizing a written examination as a form of psychological stress failed to demonstrate an association between copeptin and cortisol levels prior to or after the written examination, although copeptin and cortisol levels increased following the stress of the examination (Urwyler et al., 2015). In contrast, another study (Siegenthaler et al., 2014) demonstrated that copeptin and serum cortisol levels increased significantly following Trier Social Stress Test (TSST), a standardized mental stressor, and that the association between copeptin and cortisol was stronger in women. Prior studies have included small sample sizes and racially homogeneous populations. In the current study we hypothesized that copeptin would increase when provoked by psychological stress and that changes in copeptin would be positively correlated with cortisol and ACTH, serving as another biomarker of HPA axis activity. In this observational study we measured copeptin in stored serum and saliva samples from 100 healthy adults aged 18 to 30 years, randomly selected from 400 individuals, who underwent a standardized psychosocial mental stressor (TSST), a well validated test that has been shown to reliably activate the HPA and sympathetic nervous systems, (Allen et al., 2014; Kirschbaum et al., 1993). Additionally, as previous literature suggested differences in the cortisol response to TSST in women and men (Earle et al., 1999; Kirschbaum et al., 1995; Stroud et al., 2002; Uhart et al., 2006) after HPA axis activation, we also examined whether associations differed by sex.
Materials and Methods
Subjects
Males and females from the Baltimore area with ages between 18 and 30 years were recruited by newspaper, radio, and flyer advertisements. After an initial telephone interview screening, eligible persons were invited for an initial assessment. Written informed consent was obtained from each individual in order to participate in our study. The study protocol was approved by the Johns Hopkins University School of Medicine Institutional Review Board. A physician conducted a medical history and physical exam. Each female subject also received a pregnancy test. Exclusion criteria were as follows: presence of a serious medical condition; weight greater than 250 lbs; current or lifetime history of a DSM-IV axis I psychiatric disorder (including alcohol or drug dependence), determined by a Master's level interviewer administering The Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al., 1994) nicotine dependence; drug use in the prior 90 days; use of any prescription medications within the prior 30 days ; seizure disorder; history of closed head trauma; positive urine toxicology screen; and, for females, pregnancy or lactation. All females were pre-menopausal. Females were studied during the follicular phase, which was defined as the first 12 days of the menstrual cycle documented by menstrual diary and a progesterone level less than 2 ng/mL.
Session Procedures
All study subjects were evaluated by a modified version of the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993), as previously described (Uhart et al., 2004). Prior to the TSST session, participating individuals were asked to fast overnight and to avoid any use of alcohol, illicit drugs or over the counter medications for 48 hours prior to the test. Participants presented to the study room at 1000h where a toxicology screen was initially performed; all individuals had a negative screen. From 1000h to 1015h, an intravenous catheter was placed into a forearm vein of the participants. All individuals consumed a calorie controlled breakfast and volume controlled beverage and then stayed in a quiet room until further testing was performed. Baseline serum and saliva samples were obtained prior to the TSST at 1200h (−30 minutes), 1215h (-15 minutes), and 1230h (0 minutes).
Trier Social Stress Test [TSST]
Participants were sequestered in a room and provided with audio-taped instructions for the TSST speech and mental arithmetic performance tasks. The tape informed participants that they would be taking on the role of a job applicant for the position of hospital administrator and must perform a 5-min speech that will convince interviewers that they are the ideal candidates for the proposed job. This would be followed by a 5-min oral mental arithmetic challenge. Participants were then given 10 min preparation period to mentally prepare for the session. After being escorted to a room with the two interviewer confederates, TSST testing started at 1240h. All individuals were instructed to stand at one end of a long table. The interviewers sat at the other end of the table. One interviewer video recorded the session while the other interviewer asked the participants to provide a description of his/her qualifications for the proposed job. This test was expected to be performed in 5 minutes and the participants were prompted as need by the interviewers. Following the interview process, a mental arithmetic task was performed, where participating individuals were asked to repeat a four-digit number after the interviewer, subtract 13 from it, providing their answers aloud. All participants were asked to repeat the arithmetic task if they made a mistake. After the above tests, participants were asked to return back to the study room, where they were requested to sit quietly. Saliva and blood samples were collected again at 1250h when the TSST ended (20 minutes from baseline), 1305h (35 minutes), 1320h (50 minutes), 1335h (65 minutes), and 1350h (80 minutes). Saliva and blood samples were collected at the same time points. Approximately one week before the TSST session, participants had a passive session to minimize the effects of environmental novelty and session procedures on HPA axis response (Federenko et al., 2004). In order to assess the cardiovascular response to the TSST, systolic and diastolic blood pressure as well as heart rate were measured at the same time intervals that the hormonal samples were collected. The mean increase in systolic blood pressure, diastolic blood pressure and heart rate were 24.2%, 30.5%, and 10.5%, respectively.
Laboratory values
Copeptin values were measured using an automated immunofluorescent assay (BRAHMS CT-proAVP KRYPTOR, Thermo Scientific Biomarkers, Hennigsdorf, Germany) with a 0.9 pmol/l lower detection limit and an intra-assay coefficient of variation of <15% in the 3–4 pmol/l concentration range.. Samples from 18 of our 100 participants had been thawed and re-frozen once. We compared copeptin levels in the samples that were thawed and re-frozen and in those that had not been thawed and copeptin levels did not differ. Prior studies indicate stability of copeptin under frozen conditions and on up to three freeze-thaw cycles (Morgenthaler et al., 2008; Struck et al., 2005).
Salivary and plasma cortisol and ACTH assays were performed in our laboratory by radioimmunoassay (Diagnostics Product Co., Los Angeles, CA) using a model 1470 counter (PerkinElmer, Shelton, CT). As ACTH may be unstable in room temperature (Evans et al., 2001), ACTH samples were drawn, placed on ice, and separated into plasma within 20 minutes of the blood draw. Plasma was frozen at −80 degrees C until assay. The inter- and intra-assay coefficients of variation for all assays are less than 10 %.
Psychological Stress Measures
Depressive symptoms were assessed using the Beck Depression Inventory. Beck Depression Inventory is a list of 21 symptoms of depression that are each rated in intensity along a four-point response scale from absent or mild (score of 0) to severe (score of 3). Examples include: mood, pessimism, sense of failure, lack of satisfaction, guilt feelings and self-dislike. It covers almost all aspects of DSM-IV criteria expect increased appetite, hypersomnia, and agitation and asks about symptoms within the past 2 weeks (Steer et al., 1999). Depression severity scores are created by summing the scores of the items from each item set. Severity scores are interpreted as: 0–9, minimal; 10–16, mild; 17–29, moderate; and 30–63, severe. Correlations between clinical ratings of depression and the BDI for non-psychiatric subjects range from 0.55 to 0.73 with a mean of 0.60. Perceived stress was assessed using Cohen’s Perceived Stress Scale (Cohen et al., 1983) which emphasizes the importance of the subjective interpretation of life events in a global fashion. The Perceived Stress Scale is a 14-item self-report questionnaire developed to assess the stress domains of unpredictability, lack of control, burden overload, and stressful life circumstances. All items are scored on a 5-point Likert scale ranging from 0 (never) to 4 (very often) for the preceding month. A single score is derived from the summed total of item responses with scores falling within a possible range of 0–56. Higher scores reflect greater perceived stress. Estimates of internal consistency among psychiatric outpatients (Chronbach’s α=0.80) and in the general population (Chronbach’s α=0.75) have also been within acceptable limits.
Statistical Analysis
For our analyses, we calculated percent change of copeptin, ACTH, and salivary cortisol values in response to the TSST for each participant. The percent change following the TSST was calculated as follows:
Because the distributions of the outcome hormonal measures, ACTH and salivary cortisol, were right-skewed, they were each log-transformed for analysis. The cross-sectional association of percent change in copeptin with the log percent change for ACTH and salivary cortisol was determined using linear regression models (unadjusted and adjusted for sex and body mass index [BMI]). We adjusted for BMI as both copeptin and cortisol levels have been found to be positively correlated with BMI (Champaneri et al., 2013; Saleem et al., 2009). The regression equations were:
We also performed sex-stratified analyses. Individuals with negative percent change values for ACTH and salivary cortisol were excluded from those hormone-specific linear regression analyses. We performed similar analyses substituting serum cortisol for salivary cortisol. A two-sided p-value <0.05 was used to determine statistical significance. Statistical analyses were performed using Stata version 11.1 (Cary, NC).
Results
A total of 100 healthy individuals were assessed with a mean age 24.6±3.0 years; 59% were female. While the majority of participants were White (71.2%), 7.1% were Black, 5.1% were Hispanic, 11.1% were Asian, and 5.5% self-reported other. Perceived stress and depressive symptom scores were measured prior to the TSST. The mean perceived stress score was 8.92±5.78 (8.1±5.87 for men; 9.5±5.69 for women). The median Beck Depression Inventory score was 1.5 with interquartile range [IQR] 0 to 4.5 (1.0, IQR 0 to 4 for men; 2.0, IQR 0 to 5 for women). Following TSST, participants systolic blood pressure increased a median 15.1% (IQR 9.6, 21.4) , diastolic blood pressure a median 14.1% (IQR: 9.9, 19.6) and heart rate a median 9.3% (IQR 4.0, 15.6), indicating a cardiovascular response to stress.
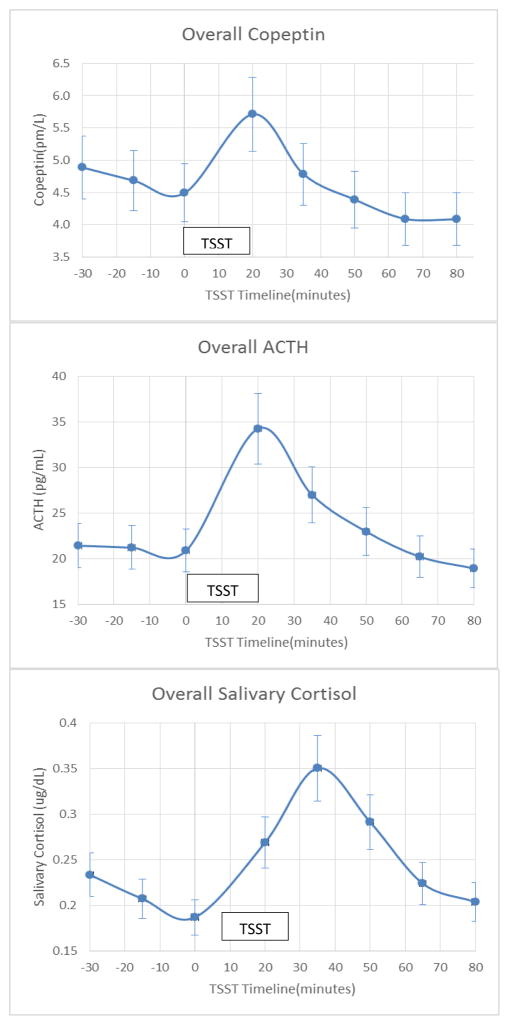
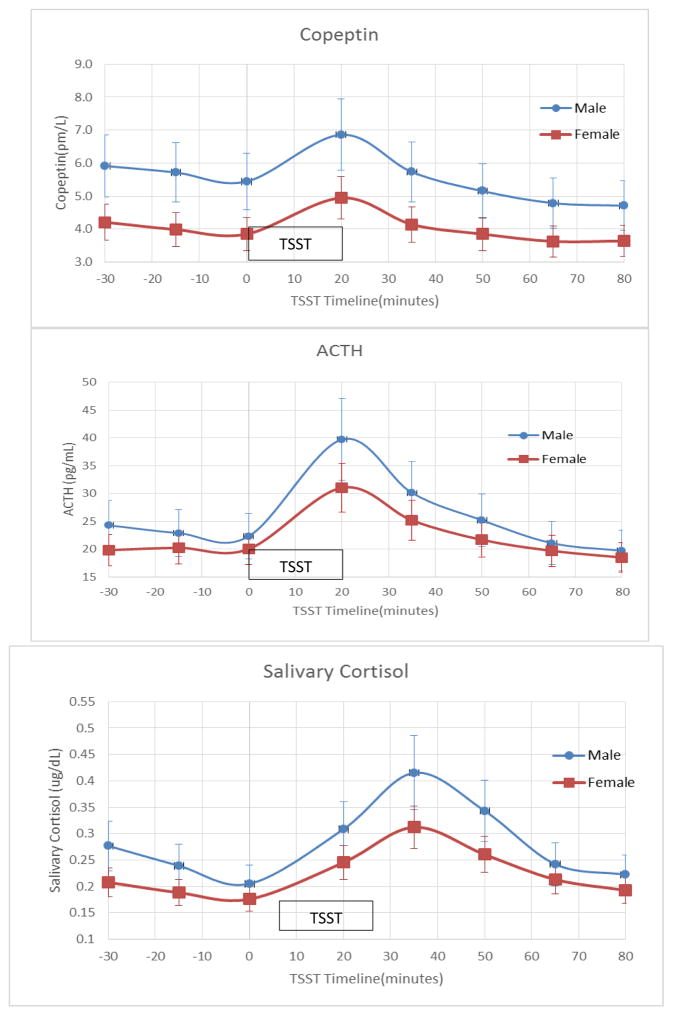
Figure 1 summarizes the changes in copeptin, ACTH and salivary cortisol levels in all participants during the TSST. Following the TSST, the levels of copeptin rose initially, followed by a rise in salivary cortisol levels. Peak copeptin and ACTH responses were seen at 20 minutes after completion of the TSST, whereas the peak salivary cortisol response was seen at 35 min respectively. Figure 2 summarizes the copeptin, ACTH, and salivary cortisol responses to the TSST stratified by sex. Although baseline copeptin levels were higher in males compared to females (5.69±2.5 pmol/L versus 4.01±2.3 pmol/L; p-value=0.015), the percent of increase in copeptin from baseline to peak levels appear to be similar (30.1±40% in males versus 31.3±25% in women; p-value=0.89).
Figure 1.
Copeptin, Adrenocorticotropin and Salivary Cortisol Responses to the Trier Social Stress Test
Figure 2.
Copeptin, Adrenocorticotropin and Salivary Cortisol Responses to the Trier Social Stress Test by Sex
Table 1 summarizes the association between the percent change in copeptin levels and the corresponding log-transformed values for ACTH and salivary cortisol. There was a significant positive association between the percent change in copeptin and the percent change in log salivary cortisol (β-coefficient=0.97; p=0.016) levels following TSST test in unadjusted analysis. The association persisted following adjustment for sex and body mass index (β-coefficient=0.95; p=0.02) (Table 1). While the direction and magnitude of the association between percent change in copeptin and percent change in log ACTH were of a similar magnitude and direction, results did not reach statistical significance in unadjusted or adjusted models (β-coefficient=1.14; p=0.06).
Table 1.
Linear Regression Coefficients for the Cross-sectional Association of Percent Change in Copeptin with Log-transformed Percent Change in ACTH and Salivary Cortisol
| Percent Change β Coefficient (95% Confidence Interval) | |
|---|---|
| ACTH (Unadjusted) n=70 |
1.06 (−0.11, 2.23) |
| ACTH (Adjusted for sex) n=70 |
1.09 (−0.09, 2.27) |
| ACTH (Adjusted for sex and body mass index) n=70 |
1.14 (−0.05, 2.33) |
| Salivary Cortisol (Unadjusted) n=74 |
0.97* (0.18, 1.76) |
| Salivary Cortisol (Adjusted for sex) n=74 |
0.95* (0.15, 1.75) |
| Salivary Cortisol (Adjusted for sex and body mass index) n=74 |
0.95* (0.15, 1.75) |
p-value<0.05 (marked with bold).
We also performed analyses stratified by sex and found that these associations between percent change in copeptin and percent change in log ACTH and salivary cortisol were driven primarily by strong associations in the men. Among men, after adjusting for BMI, there was a significant positive association between the percent change in copeptin levels and the corresponding log-transformed values for salivary cortisol (β-coefficient=1.33, p=0.016). In women the associations were of lower magnitude and not significantly different (β-coefficient=0.45 p= 0.48, adjusted for BMI) (Table 2). In both men and women, although the association between percent change in copeptin and percent change in log ACTH were of a similar magnitude and direction, results did not reach statistical significance (Table 2).
Table 2.
Linear Regression Coefficients for the Cross-sectional Association of Percent Change in Copeptin with Log-transformed Percent Change in ACTH and Salivary Cortisol Stratified by Sex and Adjusted for Body Mass Index.
| Percent Change β Coefficient (95% Confidence Interval) | |
|---|---|
| ACTH (Males) n=26 |
2.07 (−0.10, 4.25) |
| ACTH (Females) n=44 |
0.77 (−0.70, 2.23) |
| Salivary Cortisol (Males) n=28 |
1.33* (0.27, 2.38) |
| Salivary Cortisol (Females) n=46 |
0.45 (−0.83, 1.72) |
p-value<0.05 (marked with bold)
We performed similar analyses substituting serum cortisol for salivary cortisol and results were similar, although of lower magnitude. The correlation between serum and salivary cortisol was 0.72. There was a positive association between percent change in copeptin and log-transformed serum cortisol that was not statistically significant in the overall population (supplemental Table 1); however, in sex-stratified analyses, there was a significant, positive association between percent change in copeptin and log-transformed serum cortisol in men but not women, similar to our findings for salivary cortisol (supplemental Table 2).
Discussion
In the present study we investigated the role of copeptin as a biomarker of HPA activity in healthy individuals and whether there were sex differences in the association between the change in copeptin and ACTH and cortisol levels following a standardized psychological mental stress test. Our study had three main findings. First, following TSST, copeptin, ACTH and cortisol levels all increased from baseline. Second, although baseline copeptin values were higher in males than in the females, the percent increase in copeptin levels was similar in both sexes. Third, there was a significant positive association between the percent change in copeptin and the percent change of cortisol levels in men, whereas in women the associations were of lower magnitude and not significantly different.
ACTH production is mainly controlled by CRH and to a lesser degree by AVP. However, measuring AVP, CRH or ACTH in order to evaluate HPA axis activation can be challenging due to their rapid degradation and circadian variation (Besser et al., 1971; Evans et al., 2001; Orth, 1992; Preibisz et al., 1983; Robertson et al., 1973; Szinnai et al., 2007). Copeptin, a more stable molecule, has been found to be elevated with many severe and stressful medical conditions, including sepsis and septic shock, hemorrhagic shock, heart failure, myocardial infarction and acute chronic obstructive pulmonary disease exacerbation (Morgenthaler et al., 2008). Copeptin levels have also been found to be higher in surgical patients following extubation compared to a group of stable medical patients and a control group of healthy individuals. Interestingly, in that study, copeptin values were highly correlated with cortisol levels (r=0.46, p<0.001) (Katan et al., 2008). However ACTH levels were not measured in that study.
Another study evaluated the association of copeptin and HPA axis hormones, using insulin tolerance test as a stressor (Kacheva et al., 2015). Among the 118 patients that were recruited in this study, 64 of them had hypopituitarism and 12 of them had central diabetes insipidus. Plasma levels of copeptin, cortisol and ACTH increased significantly in all participants in response to the insulin tolerance test. After excluding the 12 individuals with central diabetes insipidus, a significant correlation between stress-induced copeptin and cortisol was noted (rs=0.32, p<0.001), similar to our findings. Similar to our results, this study found that the associations differed by sex; however, in the opposite direction. In sex-stratified analyses, they found a significant positive correlation between peak copeptin and cortisol levels in women (rs = 0.420; p=0.002) and a weaker but statistically significant correlation in men (rs = 0.247, p=0.045). Interestingly there was a strong positive association between stress-induced copeptin and ACTH values in women (rs = 0.471, p < 0.001) but not in men (rs = 0.039, p=0.757). These findings differ from our study in that we did not find a correlation of peak copeptin with ACTH in either men or women and we found strong and significant associations between peak copeptin and salivary cortisol in men but no association in women. One potential explanation for this discrepancy in the results is the nature of the stress test in the our study and that by Kacheva et al (Kacheva et al., 2015). The TSST is a psychosocial, mental stress test whereas ITT is a physiological stressor. Rodent studies have shown that different types of stressors can mobilize unique cocktail of ACTH secretagogues (Plotsky, 1987)—whether these effects may differ by sex is not known and represents an area of future research.
There are limited data on whether copeptin levels increase following psychological stress. Multiple studies have shown an increase in ACTH and either salivary, plasma or serum cortisol levels following TSST exposure, consistent with HPA axis activation (Gaab et al., 2002; Gaab et al., 2005; Gerra et al., 2001; Kirschbaum et al., 1999; Kirschbaum et al., 1993; Kirschbaum et al., 1994; Munro et al., 2005). To our knowledge only one prior study examined the effect of the TSST on the copeptin levels (Siegenthaler et al., 2014). In that study, copeptin and serum cortisol levels increased significantly following the TSST and there was a significant correlation between stress-induced copeptin and serum cortisol level response to the TSST. Our study expands on this prior study by including a larger number of participants (100 versus 28) which allowed us to perform sex-stratified analyses, a more multi-ethnic population, and simultaneous measurement of ACTH so that we could examine the association of copeptin with ACTH.
A prior study included 25 medical students who underwent a written examination test. Prior to examination, levels of copeptin, salivary cortisol and serum cortisol were elevated compared to levels of copeptin, salivary cortisol and serum cortisol after the examination, a result that was thought to be a consequence of the examination anticipation (Urwyler et al., 2015). There was no association, however, between copeptin and cortisol levels prior to or after the written examination. The reason for the lack of association of copeptin with cortisol levels in this study compared to ours is not clear.
The sex-specific association of percent change in copeptin with cortisol that we found adds further support to literature showing different HPA axis stress responses in men and women. Similar to our results, most studies showed higher cortisol responses in young men than in young women after exposure to laboratory stress tasks such as free speech, mental arithmetic or harassment (Earle et al., 1999; Kirschbaum et al., 1995; Stroud et al., 2002; Uhart et al., 2006).This may be related to the observation that men and women are at risk for different diseases. For example, women are more likely to develop autoimmune diseases (Kudielka and Kirschbaum, 2005), thought to be secondary to underactivity of the HPA axis (Tsigos and Chrousos, 1994), whereas men are more likely to develop cardiovascular disease, thought to be secondary to high allostatic load resulting from over activation of the stress system (McEwen, 1998). The mechanisms through which males secrete more cortisol and copeptin than females in response to the TSST are unknown. However it is known from preclinical studies that sex hormone levels can modulate the activation of the HPA axis in response to stress (Viau and Meaney, 2004).
The magnitudes of the associations of copeptin with serum cortisol were smaller than the associations with salivary cortisol. Salivary cortisol is used very commonly in the clinical and research settings for assessing HPA axis function. Salivary cortisol measures free, and not bound cortisol, and thus is not subject to binding globulin abnormalities, (Vining et al., 1983) which can perhaps explain the observed differences. The observation that the correlation between serum and salivary cortisol in our sample was high (rs=0.72) but not 1.0 probably reflects individual differences in binding globulin levels in the serum samples and underscores the reason that salivary cortisol may be a better measure of cortisol status in certain settings.
An important limitation of our study is that we did not have measures of serum osmolality or sodium levels in our cohort group of participants in response to the TSST. Changes in plasma osmolality and in sodium levels can cause alternations in copeptin levels (Balanescu et al., 2011). While we have sodium and electrolyte measurements on the participants approximately 4 weeks prior to their TSSTs, which were within the normal range, we did not measure sodium and serum osmolality during the TSST to exclude changes in copeptin secondary to osmotic changes. However, this was a healthy, community-dwelling population of young adults aged 18 to 30 years. In order to be included in the study, they had to have normal electrolyte and fluid status and individuals with hypernatremia or hyponatremia were excluded, making significant alterations in fluid status less likely.
Conclusions
In conclusion, we found a significant positive association between percent change in copeptin and percent change in cortisol following a standardized psychological stress or in healthy individuals that was stronger in men than women. Our data suggest that AVP activation may be a mechanism through which neuroendocrine activation in response to stress increases the risk for metabolic disorders, particularly in men. Our data support that copeptin may serve as a biomarker of both AVP and HPA axis activation that can be incorporated into population-based studies and clinical trials. Future studies are needed to confirm the association between copeptin and other measures of chronic HPA axis activity and to determine whether therapies targeting the AVP system may have future implications for the prevention of metabolic diseases with clinical and public health relevance. Our ability to identify populations for novel interventions in the future will be enhanced by using neuroendocrine biomarkers to identify stress-associated biological phenotypes and their clinical consequences.
Supplementary Material
Highlights.
Following TSST, copeptin, ACTH and cortisol levels all increased from baseline.
We found a positive association copeptin and cortisol levels, mainly in men.
The association of ACTH and copeptin did not reach statistical significance.
Acknowledgments
Role of the funding source
Dr. Elias Spanakis was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32 DK062707). Dr. Gary Wand was supported by NIH R01 MH76953 and K05 AA020342.
We would like to thank Nina Shah, MS for assistance in reviewing and guiding our statistical analyses. BRAHMS GmbH (part of Thermo Fisher Scientific), the CT-proAVP assay developer and manufacturer performed the copeptin testing for this study using reagents supplied gratis.
Footnotes
Conflicts of interest: None. All authors approved the final version of the manuscript.
Contributors
Elias K. Spanakis, MDa , Gary S. Wand, MDb, Nan Jib, Sherita Hill Golden, MD, MHSb,c
Affiliations: Department of Medicinea, University of Maryland School of Medicine, Baltimore, MD; Department of Medicineb, Johns Hopkins University School of Medicine and Department of Epidemiologyc, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J Clin Endocr Metab. 2011;96:1046–1052. doi: 10.1210/jc.2010-2499. [DOI] [PubMed] [Google Scholar]
- Besser GM, Orth DN, Nicholson WE, Byyny RL, Abe K, Woodham JP. Dissociation of the disappearance of bioactive and radioimmunoreactive ACTH from plasma in man. J Clin Endocr Metab. 1971;32:595–603. doi: 10.1210/jcem-32-5-595. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A, Shrager S, Golden SH. Diurnal salivary cortisol is associated with body mass index and waist circumference: the Multiethnic Study of Atherosclerosis. Obesity. 2013;21:E56–63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. The New Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Earle TL, Linden W, Weinberg J. Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. J Psychosom Res. 1999;46:125–141. doi: 10.1016/s0022-3999(98)00075-0. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 2001;34:107–112. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J Clin Endocr Metab. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- Fenske W, Quinkler M, Lorenz D, Zopf K, Haagen U, Papassotiriou J, Pfeiffer AF, Fassnacht M, Stork S, Allolio B. Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome--revisiting the direct and indirect water deprivation tests. J Clin Endocr Metab. 2011;96:1506–1515. doi: 10.1210/jc.2010-2345. [DOI] [PubMed] [Google Scholar]
- Fenske W, Stork S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocr Metab. 2009;94:123–129. doi: 10.1210/jc.2008-1426. [DOI] [PubMed] [Google Scholar]
- Gaab J, Huster D, Peisen R, Engert V, Heitz V, Schad T, Schurmeyer TH, Ehlert U. Hypothalamic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosom Med. 2002;64:951–962. doi: 10.1097/01.psy.0000038937.67401.61. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, Raggi MA, Brambilla F. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26:91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Guillemin R, Rosenberg B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955;57:599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- Holwerda DA. A glycopeptide from the posterior lobe of pig pituitaries. I. Isolation and characterization. Eur J Biochem. 1972;28:334–339. doi: 10.1111/j.1432-1033.1972.tb01918.x. [DOI] [PubMed] [Google Scholar]
- Kacheva S, Kolk K, Morgenthaler NG, Brabant G, Karges W. Gender-specific co-activation of arginine vasopressin and the hypothalamic-pituitary-adrenal axis during stress. Clin Endocrinol. 2015;82:570–576. doi: 10.1111/cen.12608. [DOI] [PubMed] [Google Scholar]
- Katan M, Morgenthaler N, Widmer I, Puder JJ, Konig C, Muller B, Christ-Crain M. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuroendocrinol Lett. 2008;29:341–346. [PubMed] [Google Scholar]
- Katan M, Morgenthaler NG, Dixit KC, Rutishauser J, Brabant GE, Muller B, Christ-Crain M. Anterior and posterior pituitary function testing with simultaneous insulin tolerance test and a novel copeptin assay. J Clin Endocr Metab. 2007;92:2640–2643. doi: 10.1210/jc.2006-2046. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Scherer G, Strasburger CJ. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin Investigator. 1994;72:804–810. doi: 10.1007/BF00180552. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Verleun T, Oosterom R, de Jong F, Hackeng WH. Corticotropin-releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J Clin Endocr Metab. 1984;58:298–303. doi: 10.1210/jcem-58-2-298. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Jochberger S, Dunser MW. Copeptin: clinical use of a new biomarker. Trends Endocrin Met. 2008;19:43–49. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Munro CA, Oswald LM, Weerts EM, McCaul ME, Wand GS. Hormone responses to social stress in abstinent alcohol-dependent subjects and social drinkers with no history of alcohol dependence. Alcohol Clinical Exp Res. 2005;29:1133–1138. doi: 10.1097/01.alc.0000172459.71517.05. [DOI] [PubMed] [Google Scholar]
- Orth DN. Corticotropin-releasing hormone in humans. Endocrine reviews. 1992;13:164–191. doi: 10.1210/edrv-13-2-164. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Regulation of hypophysiotropic factors mediating ACTH secretion. Ann NY Acad Sci. 1987;512:205–217. doi: 10.1111/j.1749-6632.1987.tb24962.x. [DOI] [PubMed] [Google Scholar]
- Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension. 1983;5:I129–138. doi: 10.1161/01.hyp.5.2_pt_2.i129. [DOI] [PubMed] [Google Scholar]
- Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973;52:2340–2352. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem U, Khaleghi M, Morgenthaler NG, Bergmann A, Struck J, Mosley TH, Jr, Kullo IJ. Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J Clin Endocr Metab. 2009;94:2558–2564. doi: 10.1210/jc.2008-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler J, Walti C, Urwyler SA, Schuetz P, Christ-Crain M. Copeptin concentrations during psychological stress: the PsyCo study. Eur J Endocrinol. 2014;171:737–742. doi: 10.1530/EJE-14-0405. [DOI] [PubMed] [Google Scholar]
- Steer RA, Cavalieri TA, Leonard DM, Beck AT. Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. Gen Hosp Psychiat. 1999;21:106–111. doi: 10.1016/s0163-8343(98)00070-x. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiat. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26:2500–2504. doi: 10.1016/j.peptides.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994;269:27088–27092. [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Szinnai G, Morgenthaler NG, Berneis K, Struck J, Muller B, Keller U, Christ-Crain M. Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocr Metab. 2007;92:3973–3978. doi: 10.1210/jc.2007-0232. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrin Metab Clin. 1994;23:451–466. [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Uhart M, McCaul ME, Oswald LM, Choi L, Wand GS. GABRA6 gene polymorphism and an attenuated stress response. Mol Psychiatr. 2004;9:998–1006. doi: 10.1038/sj.mp.4001535. [DOI] [PubMed] [Google Scholar]
- Urwyler SA, Schuetz P, Sailer C, Christ-Crain M. Copeptin as a stress marker prior and after a written examination--the CoEXAM study. Stress. 2015;18:134–137. doi: 10.3109/10253890.2014.993966. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 2004;181:223–231. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clinical Biochem. 1983;20(Pt 6):329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- Winzeler B, Zweifel C, Nigro N, Arici B, Bally M, Schuetz P, Blum CA, Kelly C, Berkmann S, Huber A, Gentili F, Zadeh G, Landolt H, Mariani L, Muller B, Christ-Crain M. Postoperative Copeptin Concentration Predicts Diabetes Insipidus After Pituitary Surgery. J Clin Endocr Metab. 2015;100:2275–2282. doi: 10.1210/jc.2014-4527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.