Abstract
Oral colon-specific drug delivery is of great interest for ulcerative colitis (UC) therapy. Here, an emulsion-solvent evaporation method was used to fabricate microparticles (MPs) with pH-sensitive Eudragit S100 (ERS100) and poly(lactide-co-glycolide) (PLGA), and the MPs were loaded with curcumin (an efficient anti-inflammatory agent). The resultant spherical MPs had a desirable particle size ranging from 1.52 to 1.91 μm. Their loading efficiency could be regulated by changing the weight ratios of ERS100 and PLGA, with some MPs exhibiting loading efficiencies over 80%. It was observed that the fast release of curcumin from MPs in buffers (pH 1.2 and 6.8) could be significantly decreased by increasing the PLGA content. ERS100/PLGA MPs with a weight ratio of 1:2 (MPs-4) were able to maintain sustained release of curcumin, releasing ~ 48% of the initial drug load at pH 7.2–7.4 during a 20 h-incubation. Most importantly, in vivo experiments revealed that orally administered MPs-4 had a superior therapeutic efficiency in alleviating colitis in a UC mouse model, compared to curcumin. Collectively, our one-stepfabricated curcumin-loaded MPs have the properties of pH-sensitivity, controlled drug release and colon targeting, and thus may hold promise as a readily scalable drug carrier for the efficient clinical treatment of UC.
Keywords: Oral administration, pH sensitivity, curcumin, microparticle, ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a chronic relapsing disorder associated with uncontrolled inflammation in the gastrointestinal tract (GIT) [1]. It often affects the innermost mucosa, and the affected intestinal areas run continuously from the rectum up to the colon, with occasional involvement of the ileum [2]. The main goal of UC therapy is to control inflammation, achieve mucosal healing, and reduce surgeries and hospitalizations [3, 4]. Over the past four decades, the traditional treatments have been limited to the use of anti-inflammatory drugs and immune-suppressive medications [5, 6]. Although some of these agents have proven effective in controlling inflammation, their further clinical application has been seriously restricted by issues with poor long-term efficacy and adverse effects [7].
Curcumin, a hydrophobic polyphenol derivative extracted from a natural herbal source, has recently received increasing attention for UC therapy because it can efficiently downregulate inflammatory cytokines, scavenge free radicals, and promote mucosal healing [8, 9]. Numerous studies have demonstrated that curcumin can attenuate inflammation in colitis animal models and reduce the relapse rate of UC in clinic [10, 11]. Importantly, clinical trials have shown that curcumin is relatively safe for humans, even when given at a high dose (12 g/day) for 3 months [12]. As a result, curcumin has been recognized as a “generally regarded as safe” (GRAS) compound by the U.S. Food and Drug Administration (FDA) [13]. However, curcumin has several major drawbacks, including an extremely low solubility in aqueous solutions (0.456 μg/mL), resulting in low bioavailability [14], and high rate of metabolism that results in a short half-life [15, 16]. To address these problems, researchers have sought to deliver curcumin directly to colitis tissues using various systems, such as pellets, micelles and nanoparticles [10, 17, 18].
Orally administered microparticles (MPs) may offer an efficient drug delivery system, as they are characterized by a high drug loading capacity and may target colitis tissues based on epithelial enhanced permeation and retention effect (eEPR) [2]. This effect has been mainly ascribed to the histopathological abnormalities of colitic tissues, such as disrupted intestinal barrier function, increased epithelial permeability, and significant infiltration of inflammatory cells into the mucosa [2, 19]. Consequently, drug-loaded particles with diameters less than 10 μm can potentially accumulate in gaps between cells, increasing the local drug concentration and exerting their therapeutic effect there. In contrary, larger particles may be easily excreted with diarrhea (a very common symptom of UC seen in 66–92% of cases) [10].
With respect to colitis-targeting drug release, pH-sensitive systems represent a leading approach because the pH differs along the GIT [20]. Eudragit S100 (ERS100), which is the most commonly investigated biocompatible polymer for colon-specific drug delivery, has been accepted for oral administration by the regulatory agencies of the U.S., Europe and Japan [21]. It selectively dissolves in aqueous media of pH 6–7, releasing any loaded drug to the colon. Makhlof et al. reported that ERS100 MPs release their loaded drugs specifically to the upper GIT, and found that most drugs are released immediately when the pH shifts to neutral [22]. To obtain a more sustained drug release, poly(lactide-co-glycolide) (PLGA) can be introduced into the system. This hydrophobic polyester polymer has been approved by FDA for biomedical application and shows good biocompatibility, biodegradability and permeability [23]. Here, we hypothesized that ERS100/PLGA MPs might enable the specific and sustained release of curcumin to colitis tissues. To the best of our knowledge, this is the first study examining the therapeutic efficacy of curcumin-loaded ERS100/PLGA MPs against an experimental mice model of UC.
2. Materials and methods
2.1. Materials
PLGA (Mw = 38–54 kg/mol), poly(vinyl alcohol) (PVA, 86–89% hydrolyzed, low molecular weight) and curcumin were purchased from Sigma-Aldrich (MO, USA). The pH-sensitive ERS100 was received as a gift from Evonik (Darmstadt, DE). Dextran sulfate sodium (DSS, 36–50 kDa) was obtained from MP Biomedicals (OH, USA). Buffered formalin (10%) was supplied by EMD Millipore (MA, USA). Hematoxylin and eosin were from Richard-Allan Scientific (MI, USA). All other chemicals were obtained from Sigma-Aldrich (MO, USA).
2.2. Fabrication of MPs
Curcumin-loaded MPs were fabricated by a modified emulsion-solvent evaporation technique described previous by our laboratory [23, 24]. 100 mg of ERS100, PLGA, or polymeric mixtures of ERS100 and PLGA with different weight ratios (2:1, 1:1 and 1:2), and curcumin (6 mg) were dissolved in a solvent system consisting of dichloromethane (DCM)/methanol. The obtained polymeric drug solution was added drop-wise to 4 mL of 3% (w/v) acidic PVA solution (pH 2.0) under vortex. This emulsion was immediately poured into 100 mL of 0.3% (w/v) acidic PVA solution (pH 2.0). After that, the organic solvent was evaporated under low vacuum conditions (Rotary evaporator, Yamato RE200, CA, USA). The MPs formed by this method were collected by centrifugation at 4,500 g for 10 min, washed twice with acidic solution (pH 2.0), and dried in a lyophilizer. The resultant MPs were stored at −20 °C in airtight container. Blank MPs with ERS100/PLGA weight ratio of 1:2 were prepared as a control.
2.3. Physicochemical characterization of MPs
2.3.1 Particle size and polydispersity index (PDI) analysis
Particle size (μm) and PDI of MPs were measured by dynamic light scattering (DLS) using 90 Plus/BI-MAS (Multi angle particle sizing). The average and standard deviations of the diameters or PDI were calculated using 3 runs. Each run is an average of 10 measurements.
2.3.2 Drug loading and encapsulation efficiency
The entrapped drug in various MPs was determined by measuring the intrinsic fluorescence of curcumin on a PerkineElmer EnSpire multimode plate reader (Perkin Elmer, Boston, MA, USA). In a typical example, MPs (3 mg) were dissolved in dimethyl sulfoxide. Then the solution (100 μL) was transferred to a black 96-well plate. The fluorescence intensity of curcumin was measured at 528 nm emission wavelength (485 nm excitation wavelength). The encapsulation efficiency was calculated as the percentage of actual drug loading to the theoretical drug loading.
2.3.3 Fluorescence microscopy
A drop of MPs suspension was mounted on a freshly cleaned glass slide and dried at room temperature. Images were acquired using the FITC channel on an Olympus fluorescence microscopy equipped with a Hamamatsu Digital Camera ORCA-03G.
2.3.4 Release profiles of curcumin from MPs
The in vitro release profiles of curcumin from various MPs were evaluated in gradually pH changing buffers: hydrochloric acid/potassium chloride (pH 1.2), acetic acid/sodium acetate (pH 6.8) and phosphate buffered saline (pH 7.2–7.4). These buffers were selected based on the normal variations of the pH along the GIT from the stomach (pH ~1.5) and the proximal small intestine (pH 6.0–6.8) to the ileocecal region (pH ~7.3). 5 mg of MPs were suspended in buffer (pH 1.2). The suspension was transferred into a regenerated Cellulose Dialysis tube (molecular weight cut-off = 10,000 Da) and the sample-filled tube was closed tightly at both ends to keep each tube surface area equivalent. Then, the closed bag was put into a centrifuge tube and immersed in 20 mL release medium (pH 1.2, 6.8 and 7.2–7.4). The tube was put in a water bath shaking at 37 °C with 100 rpm stir. At appropriate time points, outer solution was taken for measurement and fresh release medium was added. The amount of curcumin in the outer solution was measured according to the method described in section 2.2.3.
2.3.5 Scanning electron microscopy (SEM)
Various MPs were suspended in buffers (pH 6.8) and incubated in a water bath shaking at 100 rpm for 4 h at 37 °C. After that, a drop of MPs suspension without treatment or MPs treated with buffer (pH 6.8) were mounted on a freshly cleaved glass slide using carbon adhesive tape and sputter-coated with a mixture of gold and palladium (60:40) in an argon atmosphere under low pressure. The morphology of MPs was observed with a SEM (LEO 1450VP, Zeiss, Germany).
2.4 In vivo experiments
2.4.1 Experimental animals
FVB male mice (8 weeks of age, The Jackson Laboratory) were used in the animal experiments. Mice were group housed (25 °C), photoperiod (12:12-h light-dark cycle), and allowed unrestricted access to potables and standard mouse chow. All the animal experiments were approved by Georgia State University Institutional Animal Care and Use Committee.
2.4.2 DSS-induced colitis and treatments
Colitis was induced by replacing their drinking water with a DSS solution (3%, w/v), and colonic inflammation was assessed 6 days after the beginning of DSS treatment. The animals were divided into 5 groups (5 mice per group): healthy control group, DSS control group, empty MPs-treated DSS group, curcumin-treated DSS group and MPs-4-treated DSS group. Curcumin was formulated in saline solution containing Tween-80 (10%, v/v) for curcumin-treated DSS group. Empty MPs and MPs-4 were suspended in acidic solution (pH 2.0) for empty MPs-treated DSS group and MPs-4-treated DSS group, respectively. A 50 mg/kg dose of curcumin was chosen for curcumin-treated DSS group and MPs-4-treated DSS group. Each formulation (0.3 mL) was administered daily by oral gavage for 5 consecutive days. During the DSS-treated period, mice were weighted every day. After 6 days, mice were sacrificed by CO2 euthanasia. Spleen weight and colon length were measured. A piece of distal colon was taken for MPO analysis.
2.4.3. Myeloperoxidase (MPO) activity
Neutrophil infiltration into the colon was quantified by measuring MPO activity. Briefly, a portion of colon was homogenized in 1:20 (w/v) of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethyl ammonium bromide on ice using a homogenizer (Luzern, Switzerland). The homogenate was then sonicated for 10s, freeze-thawed three times, and centrifuged at 16,000 g for 15 min. The supernatant (14 μL) was then added to O-dianisidine hydrochloride (1 mg/mL) and 0.0005% hydrogen peroxide, and the change in absorbance at 460 nm was measured. MPO activity was expressed as units per mg of protein, where one unit was defined as the amount that degrades 1 μmol of hydrogen peroxide per min at 25 °C.
2.4.4. Haematoxylin and eosin (H&E) staining
Tissue samples were evaluated for mucosal architecture change, cellular infiltration, inflammation, goblet cell depletion, surface epithelial cell hyperplasia, and signs of epithelial regeneration using H&E staining. Colon tissues from different mice groups were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections with a thickness of 5 μm were stained with heomatoxylin and eosin. Images were taken using an Olympus microscope and D-26 color camera.
2.5. Statistical analysis
Statistical analysis was performed using ANOVA test followed by a Bonferroni post-hoc test (GraphPad Prism) or Student’s t-test. Data were expressed as mean ± standard error of mean (S.E.M.). Statistical significance was represented by *P<0.05 and **P<0.01.
3. Results and discussion
3.1. Fabrication of curcumin-loaded MPs
Many methods have been employed to fabricate polymeric microspheres, including atomization, emulsification and coacervation. In this study, we prepared curcumin-loaded MPs using the emulsion-solvent evaporation technique, which is a well-established method for fabricating active-substance-loaded MPs [25]. In this method, the rapid addition of the organic phase (containing the polymers and curcumin) to the aqueous phase together with an emulsifier (PVA) immediately forms an oil/water emulsion, theoretically via the Gibbs-Marangoni effect and a capillary break-up mechanism [26]. The evaporation of the organic solvents (DCM/methanol) under reduced pressure transfers curcumin to the polymer-based hydrophobic core through hydrophobic interactions (under the “like dissolves like” principle), and then further solidifies the particles to form compacted MPs [27]. The presence of an emulsifier at the interface serves to separate the oil and water phases, and is necessary to prevent aggregation of the MPs [28]. PVA has been extensively used as an emulsifier for the fabrication of MPs [29]. During MP formation, the hydrophobic segments of PVA penetrate into the organic phase and remain entrapped in the polymeric matrices (ERS100 and PLGA) of the MPs. The hydrophilic segments of PVA then surround the MPs, stabilizing them through steric hindrance.
3.2. Physicochemical characterization of MPs
3.2.1 Particle size and PDI
Particle size and size distribution are critical for MPs, as these parameters directly impact the stability, biodistribution and muco-adhesive properties of MPs [30, 31]. As summarized in Table 1, DLS measurements indicated that the average hydrodynamic diameter and PDI of MPs-1 were 2.14 μm and 0.47, respectively. With the introduction of PLGA to MPs, the diameter and PDI tended to decrease, perhaps reflecting the formation of compact hydrophobic interactions between the mixed polymers and curcumin. The MP diameters and size distribution obtained herein would be considered favorable for colitis targeting because of the eEPR effect and their ability to resist clearance via diarrhea.
Table 1.
Characteristics of the curcumin-loaded microparticles (mean ± S.E.M.; n=3).
| Microparticles | ERS100/PLGA Ratioa | Particle Size (μm) | Polydispersity Index | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| MPs-1 | 1:0 | 2.14 ± 0.68 | 0.47 ± 0.12 | 5.21 ± 0.19 | 74.39 ± 5.42 |
| MPs-2 | 2:1 | 1.91 ± 0.17 | 0.35 ± 0.21 | 5.44 ± 0.22 | 79.13 ± 4.33 |
| MPs-3 | 1:1 | 1.61 ± 0.24 | 0.32 ± 0.12 | 5.83 ± 0.15 | 83.91 ± 2.16 |
| MPs-4 | 1:2 | 1.52 ± 0.12 | 0.35 ± 0.14 | 5.81 ± 0.13 | 87.63 ± 3.55 |
| MPs-5 | 0:1 | 1.1 ± 0.05 | 0.28± 0.11 | 6.27 ± 0.09 | 95.35 ± 2.01 |
ERS100/PLGA Ratio= weight of ERS100/weight of PLGA.
3.2.2 Drug loading and encapsulation efficiency
As shown in Table 1, the drug loading and encapsulation efficiencies were highest (6.3% and 95.4%, respectively) when PLGA was used as the drug carrier. These parameters decreased slightly as the ERS100 content increased, which is consistent with a previous report [32]. This suggests that PLGA tends to form stable curcumin-loaded MPs based on hydrophobic interactions, whereas some of the drug diffused from ERS100/PLGA MPs into outer medium due to swelling of the ERS100 matrix.
3.2.3 Fluorescence microscopy
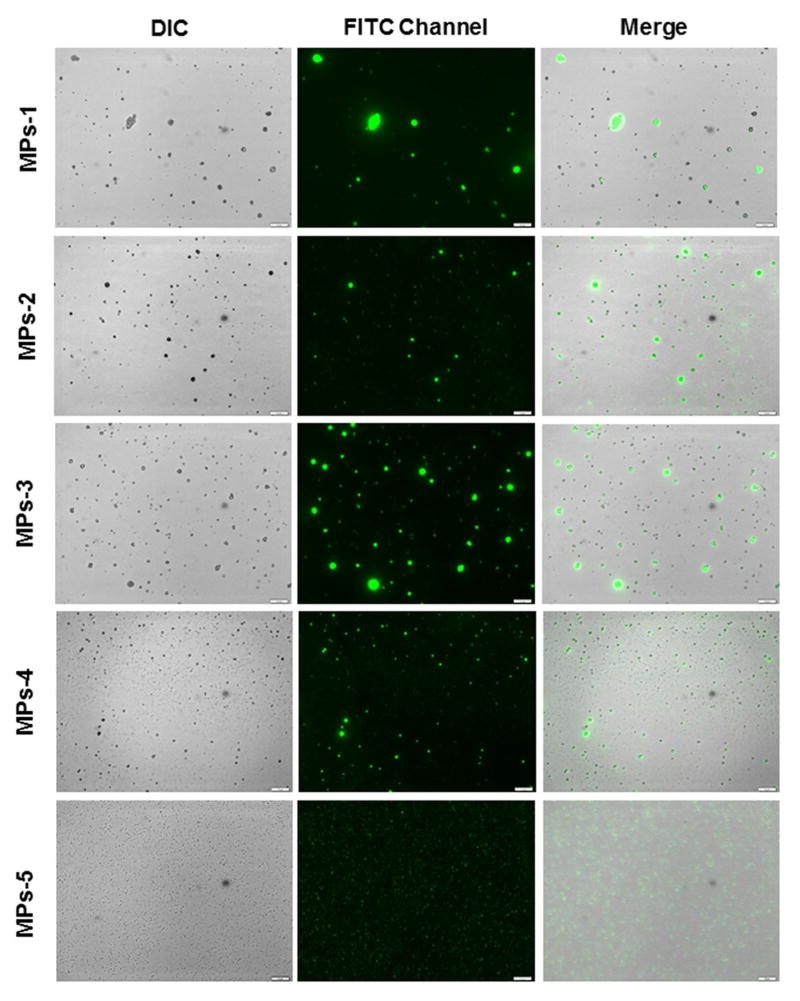
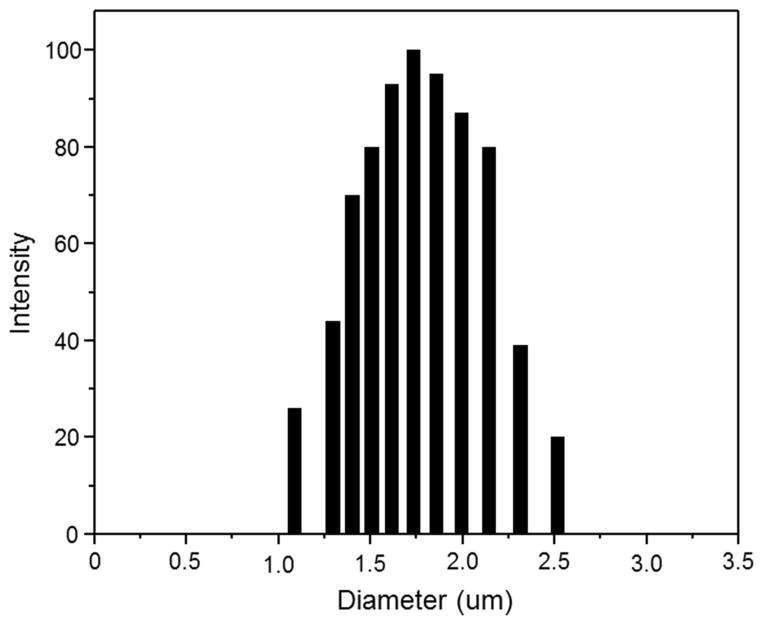
Fig. 1 shows representative fluorescence microscopic images of various MPs. All the MPs appeared as bright green dots, indicating that the optical property of curcumin has been retained. MP-1, MP-2 and MP-3 were characterized by wide and asymmetrical size distributions. However, MP-4 showed a relatively symmetrical size distribution and significantly smaller sizes in comparison to other ERS100-contained MPs. This result was confirmed by their size distribution profile, as shown in Fig. 2. Notably, curcumin fluorescence can enlarge the apparent size of individual MPs owing to the point-spread function. Thus, the observed fluorescent dots were much larger than the hydrodynamic sizes presented in Table 1 and Fig. 2.
Fig. 1.
Representative fluorescence images of curcumin-loaded MPs, detected using the FITC channel of fluorescence microscopy. The scale bar represents 10 μm.
Fig. 2.
Representative size distribution of MPs-4.
3.2.4 pH-dependent morphological changes
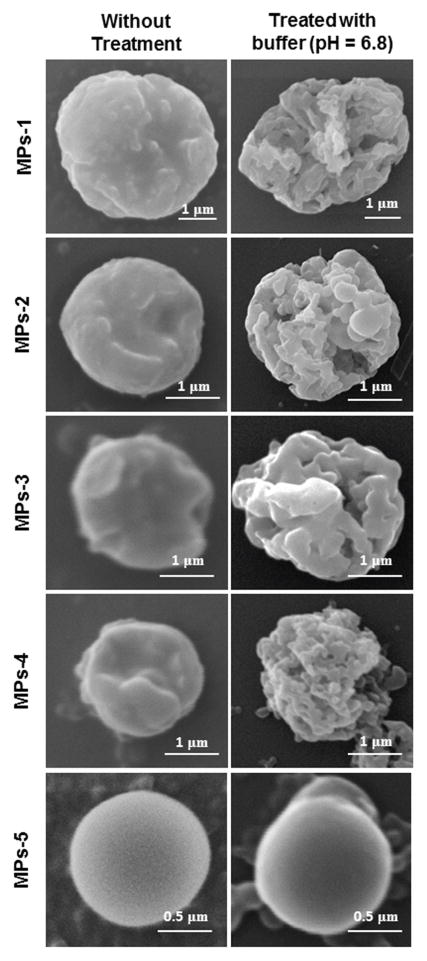
The morphological stability and pH-dependent degradation of MPs were evaluated by SEM. Figure 3 shows the representative SEM images of MPs without treatment and MPs treated with buffer (pH 6.8). PLGA particles displayed a well-defined spherical shape with smooth surface morphology even after 4 h of incubation in buffer (pH 6.8). Prior to incubation, all ERS100-contained MPs had relatively rough surfaces with no significant difference in morphology. After incubation in buffer, these MPs exhibited numerous small surface pores, indicating dissolution of the ERS100.
Fig. 3.
Representative SEM images of MPs as a function of the ERS100/PLGA weight ratio (w/w) with or without incubation in buffer (pH 6.8) for 4 h.
3.2.5 Drug release profile
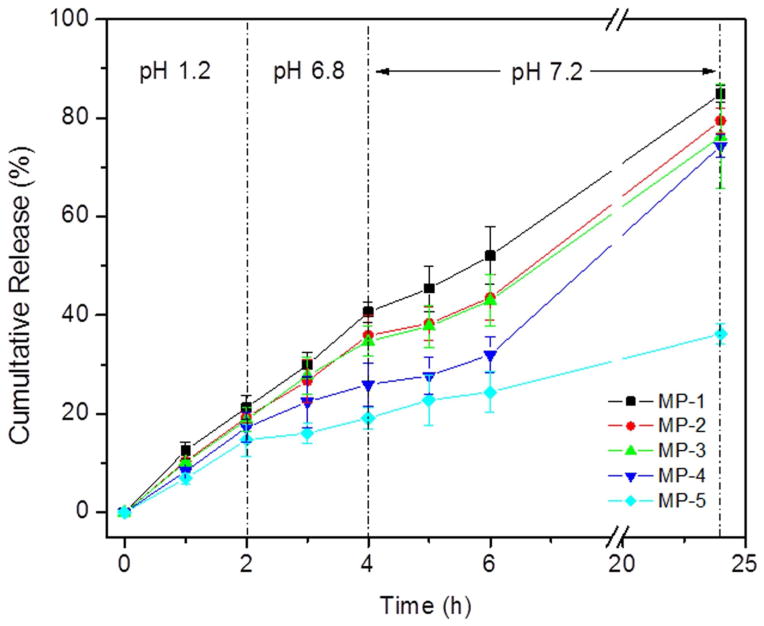
Although curcumin could be an important anti-inflammatory agent for UC treatment, its oral administration yields relatively low therapeutic efficiency. To overcome this issue, researchers have sought to develop strategies for colitis tissue-targeted curcumin delivery and sustained drug release [10]. Here, the in vitro release profiles of curcumin from MPs were studied in a buffer that underwent gradual changes in pH, as shown in Figure 4. ERS100 and ERS100/PLGA MPs displayed somewhat similar releases of curcumin, but an increase in the ERS100 content was associated with a considerable increase in the burst release of curcumin. This is likely to reflect that the swelling of ERS100 content increases the permeability of the MPs, thereby increasing the diffusion-based loss of curcumin. Among the ERS100-contained MPs, MPs-4 exhibited the lowest curcumin burst release at pH 1.2 and 6.8. Most importantly, MPs-4 released more curcumin to buffer (pH 7.2–7.4) than other MPs. It was obvious that MP-4 was capable of well-controlled, timed release of entrapped curcumin for more than 24 h, and thus used this MP for our in vivo studies.
Fig. 4.
Release profiles of curcumin from different MPs incubated in a buffer that gradually changed pH over 24 h (n=3).
3.3 In vivo therapeutic activity
3.3.1 Body weight loss and MPO
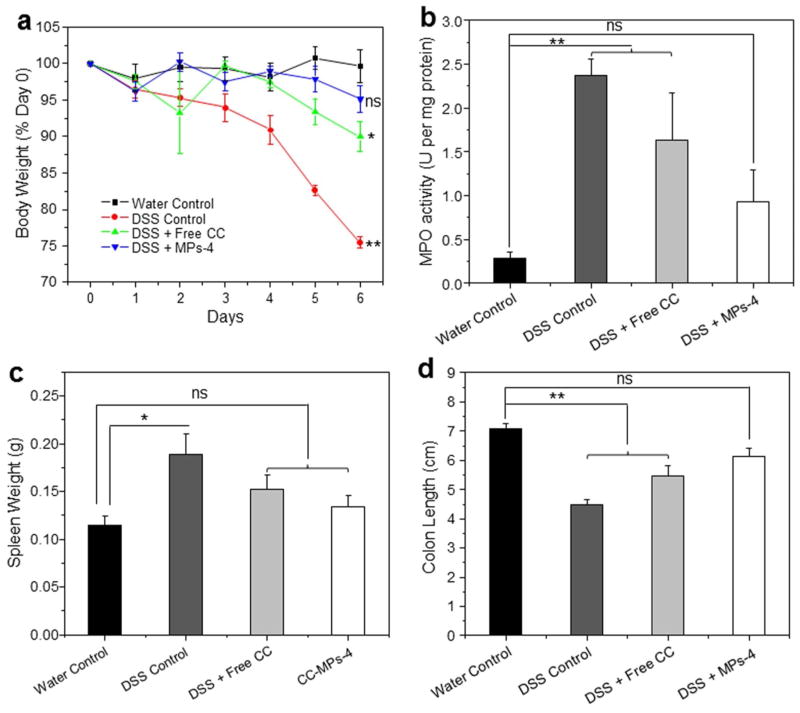
The DSS-induced mouse model of colitis has histological characteristics similar to those of human UC, including a marked body weight loss, rectal bleeding, reduction in colon length, destruction of the intestinal epithelium, and mucosal or transmural in ammatory cell in ltration. This model has a number of advantages, including its simplicity and reproducibility, and the persistence of symptoms after DSS treatment is discontinued [33, 34]. Therefore, we believe that this widely used DSS-induced mice colitis should be a useful model for evaluating the therapeutic efficiency of MP-4. As seen in Figure 5a, the body weight in the healthy control group remained relatively stable throughout the study. Mice in the DSS control group decreased their body weight by approximately 24.5% after the 6-day DSS treatment, which was ~ 2.5-fold greater than the weight loss seen in the curcumin-treated DSS group. Of all the DSS-treated groups, the smallest weight loss (~ 4.5%) was observed in the MPs-4-treated DSS group. The observed decreases in body weight were correlated with substantial increases in MPO activity (Fig. 5b), which is a hydrogen peroxide oxidoreductase that is specifically found in mammalian granulocytic leukocytes, including polymorphonuclear leukocytes, monocytes and basophils. The MPO activity level, which was the lowest in the colonic tissues from healthy control mice, was markedly higher in mice from DSS control group. Notably, there was no significant difference in MPO activity between healthy control group and MPs-4-treated DSS group. Additionally, no markedly variation in body weight and MPO activity between DSS control group and empty MPs-treated DSS group has been detected (see Supplementary Material Fig. S1), indicating no therapeutic effect of empty MPs.
Fig. 5.
Effects of orally administered MPs-4 on DSS-induced colitis in mice. (a) Mouse body weight over time, normalized as a percentage of the day-0 body weight and given as the mean for each treatment group, (b) colonic MPO activity, (c) spleen weight and (d) colon length were measured. The results are expressed as units of MPO activity per mg of protein. Each point represents the mean ± S.E.M. (n = 5). Statistical significance was assessed using the ANOVA test followed by a Bonferroni Post-test (*P < 0.05; **P < 0.01; ns, not significant).
3.3.2 Spleen weight and colon length
As shown in Figure 5c, the healthy control group had a spleen weight of 0.11 g. A significant increase in spleen weight (to 0.19 g) was observed in the DSS control group. As to MPs-4-treated DSS group, a slight increase in spleen weight to almost 0.13 g was observed, which was much closer to the healthy control group in comparison to the curcumin-treated group. Moreover, the colon lengths in the different groups exhibited a similar pattern (Fig. 5d).
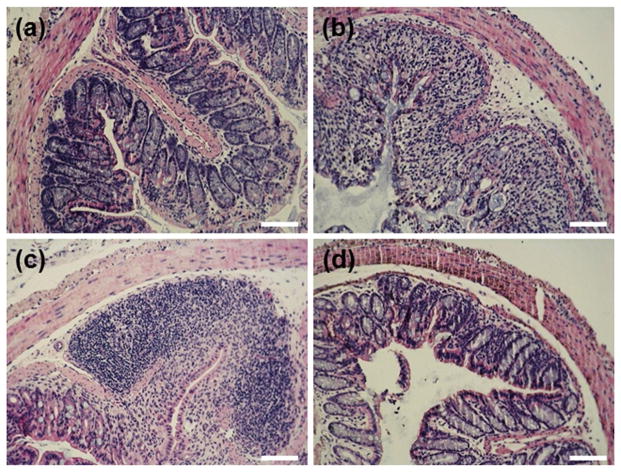
3.3.3 H&E staining
The beneficial effect of MPs-4 on DSS-induced colitis was also demonstrated by histological examinations of mouse colon tissues. As shown in Figure 6a, tissues from the healthy control group had a normal colon histology that showed no sign of inflammation or disruption of healthy tissue morphology. Consistent with the literature [35], colon tissues from the DSS control group exhibited clear signs of inflammation, including cell infiltration, goblet cell depletion, and an irregular mucosal structure (Figure 6b). Tissues from the curcumin-treated DSS group showed a slight decrease in the level of inflammation (Figure 6c). However, tissues from the MPs-4-treated DSS group had no obvious inflammation, and the tissue morphology was much closer to that of the healthy baseline tissue, with no severe symptoms of colitis (Figure 6d).
Fig. 6.
Representative H&E-stained colon sections from the (a) healthy control group, (b) DSS control group, (c) curcumin-treated DSS group and (d) MPs-4-treated DSS group. Scale bars = 100 μm.
4. Conclusion
In the present study, pH-sensitive Eudragit S100 (ERS100)/PLGA microparticles (MPs) were fabricated for colitis-specific curcumin delivery. These spherical MPs had a particle size in the range of 1.5 to 1.9 μm, and their drug loading and encapsulation efficiencies showed slight increases as the PLGA content increased. We found that ERS100/PLGA MPs with a weight ratio of 1:2 (MPs-4) could function as a promising carrier, showing high initial loading and controlled delivery of curcumin to colitic tissues. In vivo experiments demonstrated that MPs-4 exhibited a much better therapeutic efficacy against experimental ulcerative colitis (UC) compared with curcumin. Collectively, our findings indicate that MP-4 could represent an effective strategy for the UC tissue-targeted curcumin delivery with greatly improved bioavailability.
Supplementary Material
Highlights.
Eudragit S100 (ERS100)/poly(lactide-co-glycolide) (PLGA) microparticles (MPs) were fabricated as curcumin carriers for ulcerative colitis (UC) therapy.
ERS100/PLGA MPs with weight ratio of 1:2 (MPs-4) have desired sustained release profile of curcumin.
MPs-4 exhibited much better therapeutic efficacy for UC treatment compared with free curcumin.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (Merit Award to D.M., BX002526), the National Institutes of Health of Diabetes and Digestive and Kidney by the grants RO1-DK-071594 (to D.M.), the American Heart Association Postdoctoral Fellowship Grant 13POST16400004 (to B.X.), and Fundamental Research Funds for the Central Universities (SWU114086 and XDJK2015C067). Dr. Merlin is a recipient of a Career Scientist Award from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xiao B, Laroui H, Ayyadurai S, Viennois E, Charania MA, Zhang Y, Merlin D. Biomaterials. 2013;34:7471. doi: 10.1016/j.biomaterials.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao B, Merlin D. Expert Opin Drug Deliv. 2012;9:1393. doi: 10.1517/17425247.2012.730517. [DOI] [PubMed] [Google Scholar]
- 3.Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Nat Rev Gastroenterol Hepatol. 2010;7:15. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 4.Iacucci M, de Silva S, Ghosh S. Can J Gastroenterol. 2010;24:127. doi: 10.1155/2010/586092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laroui H, Theiss AL, Yan Y, Dalmasso G, Nguyen HT, Sitaraman SV, Merlin D. Biomaterials. 2011;32:1218. doi: 10.1016/j.biomaterials.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriegel C, Amiji M. J Control Rel. 2011;150:77. doi: 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimpen F, Pavli P. Intern Med J. 20102;40:58. doi: 10.1111/j.1445-5994.2010.02163.x. [DOI] [PubMed] [Google Scholar]
- 8.Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemone V, Fayad R. Food Funct. 2012;3:1109. doi: 10.1039/c2fo30097d. [DOI] [PubMed] [Google Scholar]
- 9.Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, Yu XP, Peng DD, Su L, Xiao B, Zhang ZS. PLoS One. 2010;5:e12969. doi: 10.1371/journal.pone.0012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beloqui A, Coco R, Memvanga PB, Ucakar B, des Rieux A, Preat V. Int J Pharm. 2014;473:203. doi: 10.1016/j.ijpharm.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Lahiff C, Moss AC. Inflamm Bowel Dis. 2011;17:E66. doi: 10.1002/ibd.21710. [DOI] [PubMed] [Google Scholar]
- 12.Gupta SC, Patchva S, Aggarwal BB. AAPS J. 2013;15:195. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adapala N, Chan MM. Lab Invest. 2008;88:1329. doi: 10.1038/labinvest.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arya PP, Pathak K. Int J Pharm. 2014;460:1. doi: 10.1016/j.ijpharm.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Dulbecco P, Savarino V. World J Gastroentero. 2013;19:9256. doi: 10.3748/wjg.v19.i48.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irving GR, Karmokar A, Berry DP, Brown K, Steward WP. Best Pract Res Clin Gastroenterol. 2011;25:519. doi: 10.1016/j.bpg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Song Z, Feng RL, Sun M, Guo C, Gao Y, Li L, Zhai G. J Colloid Interface Sci. 2011;354:116. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Eur J Pharm Biopharm. 2010;76:475. doi: 10.1016/j.ejpb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Lautenschlager C, Schmidt C, Lehr CM, Fischer D, Stallmach A. Eur J Pharm Biopharm. 2013;85:578. doi: 10.1016/j.ejpb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Mura C, Nacher A, Merino V, Merino-Sanjuan M, Manconi M, Loy G, Fadda AM, Siez-Sales O. Colloids Surf B Biointerfaces. 2012;94:199. doi: 10.1016/j.colsurfb.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Seremeta KP, Chiappetta DA, Sosnik A. Colloids Surf B Biointerfaces. 2013;102:441. doi: 10.1016/j.colsurfb.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Makhlof A, Tozuka Y, Takeuchi H. Eur J Pharm Biopharm. 2009;72:1. doi: 10.1016/j.ejpb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Xiao B, Zhang M, Viennois E, Zhang Y, Wei N, Baker MT, Jung Y, Merlin D. Biomaterials. 2015;48:147. doi: 10.1016/j.biomaterials.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao B, Yang Y, Viennois E, Zhang Y, Ayyadurai S, Baker M, Laroui H, Merlin D. J Mater Chem B. 2014;2:1499. doi: 10.1039/C3TB21564D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. J Control Release. 2009;133:90. doi: 10.1016/j.jconrel.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 26.Mora-Huertas CE, Fessi H, Elaissari A. Adv Colloid Interface Sci. 2011;163:90. doi: 10.1016/j.cis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Chen YF, Rosenzweig Z. Nano Lett. 2002;2:1299. [Google Scholar]
- 28.Mu L, Feng SS. Pharm Res. 2003;20:1864. doi: 10.1023/b:pham.0000003387.15428.42. [DOI] [PubMed] [Google Scholar]
- 29.de Jalon EG, Blanco-Prieto MJ, Ygartua P, Santoyo S. Int J Pharm. 2001;226:181. doi: 10.1016/s0378-5173(01)00811-0. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Byrne JD, Napier ME, DeSimone JM. Small. 2011;7:1919. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawar D, Goyal AK, Mangal S, Mishra N, Vaidya B, Tiwari S, Jain AK, Vyas SP. AAPS J. 2010;12:130. doi: 10.1208/s12248-009-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KS, Park SJ. Colloids Surf B Biointerfaces. 2010;76:404. doi: 10.1016/j.colsurfb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Perse M, Cerar A. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grisham MB. Inflamm Bowel Dis. 2008;14(Suppl 2):S132. doi: 10.1002/ibd.20682. [DOI] [PubMed] [Google Scholar]
- 35.Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, Zhang Z, Baker MT, Zhang B, Gewirtz AT, Merlin D. Gastroenterology. 2014;146:1289. doi: 10.1053/j.gastro.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.