Abstract
Objective
Collagen type 2 (Col2)-Cre or tamoxifen-inducible Col2-CreER transgenic mouse lines have been used for studies to explore the cellular and molecular pathogenesis of osteoarthritis (OA). The purpose of this study is to investigate whether the targeted cells are the same or different in the two mouse lines.
Methods
We crossed tamoxifen inducible Col2-CreER and Col2-Cre mice with Rosa tdTomato reporter mice and analyzed the labeling patterns at different time points.
Results
In the Col2-CreER mice, 90.8 [95% confidence interval (CI) (88.3, 93.2)] and 82.8 (77.4, 88.3) % of the articular surface cells are Tomato positive when tamoxifen was administered at 2 and 2.5 weeks of age and strong activity was observed even 4.5 months after injection. However, 46.0 (32.8, 59.1) and 22.2 (11.7, 32.6) % of the surface cells were Tomato positive when tamoxifen was administered at 3 and 4 weeks of age, respectively. Little to no Tomato activity in the articular surface cells was observed when tamoxifen was administered at 8 weeks of age. At any stage of tamoxifen injection, the Tomato activity was detected in growth plate and epiphyseal bone in addition to articular chondrocytes, but little in endothelium and not in the synovium and ligament. In contrast, the targeted tissues in the Col2-Cre mouse line were articular cartilage, growth plate, meniscus, endosteum, ligament, bone and synovium.
Conclusions
This study demonstrates that the pattern of targeted cells in the inducible Col2-CreER mice are partially overlapping with but different from that of targeted cells in Col2-Cre mice and the pattern varies dependent on when tamoxifen is administered.
Keywords: Osteoarthritis, Col2-Cre, Col2-CreER, tamoxifen
Introduction
Osteoarthritis (OA) is a degenerative joint disease causing dysfunction of articular cartilage. Genetic association studies suggest that genetic factors contribute significantly to the pathogenesis of OA. However, the roles of such factors in the cellular and molecular mechanisms of OA pathogenesis are largely unknown.
Collagen type II is an early marker of chondrogenesis. While it is a major product of chondrocytes, it is also synthesized by other cell types. In synovial joint regions of adult mice, the expression of the Col2a1 (Col2) gene is detected in growth plates, meniscus, articular cartilage and subchondral bone. Cells expressing cre-recombinase driven by the Col2 promoter include both chondrocytes and non-chondrocytes1, 2. Recent lineage mapping studies showed that the promoter is induced in perichondrial osteoblastic precursors and bone marrow stromal/mesenchymal stem cells in addition to chondrocytes. In addition, the skeletal tissues that are targeted in mice with tamoxifen-inducible Col2-Cre (Col2-CreER) have been shown to vary dependent on the developmental stage when tamoxifen is administrated3. Col2-Cre or tamoxifen-inducible Col2-CreER transgenic mouse lines have been used for OA studies, but whether the targeted cells are the same or different in the two mouse lines have not been investigated. In this study, we performed a lineage mapping study with Col2-Cre and Col2-CreER mice crossed with Rosa tdTomato reporter mice and analyzed the labeling patterns at different time points.
Materials and Methods
Mice
Col2-Cre4 mouse line has been described previously. Col2-Cre ERT mice5 and B6.Cg-Gt(Rosa)26Stortm14(CAG-tdTomato)Hze/J reporter mice (tdTomato mice) were purchased from The Jackson Laboratories. The Cre recombination efficiency was evaluated by Tomato fluorescence in the Col2-Cre or Col2-creER mice mated with the tdTomato mice. Mice were maintained at specific-pathogen-free conditions and housed in group of 3 to 5 mice per cage. Mice had free access to irradiated feed (Purina 5058, PicoLab Mouse Chow) and water. Genomic DNA isolated from portions of mouse tails were used for genotyping. The sequence of PCR primers for Cre were: forward 5’-GAACCTGATGGACATGTTCAGGGA-3’; reverse 5’ – CAGAGTCATCCTTAGCGCCGTAAA-3’. The sequence of PCR primers for Td Tomato were: forward 5’. All animal experiments were performed according to protocols approved by the Harvard Medical Area Standing Committee on Animals in accordance with U.S. Public Service Policy on Humane Care and Use of Laboratory Animals.
Tamoxifen injection
Tamoxifen (T5684, Sigma-Aldrich) was dissolved in corn oil at a concentration of 20mg/ml by shaking overnight at 37ºC. Animals received 75mg tamoxifen /kg body weight intraperitoneally once daily for a total of 5 consecutive days. Tamoxifen-oil mixture was stored at −20ºC until used.
Histology
Mice limbs were dissected, soft tissues were removed and fixed in 4% paraformaldehyde for 48 hours at 4ºC and then decalcified in 0.5M EDTA (PH 8.0) at 4ºC on a shaker for periods ranging from 7 to 14 days. Complete decalcification was confirmed by digital radiography (In Vivo MS FX PRO, Bruker Co). The tissues were cryoprotected in 15% sucrose/PBS for 1hr and 30% overnight at 4ºC and then placed in 30% sucrose/PBS:OCT (1:1) solution for 1hr. Samples were embedded in Tissue-Tek O.C.T. compound (Sakura, 4583) and transferred to dry ice to solidify the compound. Embedded samples were cryosectioned at 10 µm using a cryostat (OTF 5000, Bright Instrument Co Ltd.). Images were taken with Nikon 80i Upright microscope (Nikon Co.). Images of some optic fields were taken using blue and red fluorescence filters, and merged with MetaMorph Software (Molecular Devices LLC.).
Cell Count and Statistical Analysis
Tomato positive cells 20um away from the articular surface in tibia and femur of the Col2-CreER male mice were counted and were divided by the number of DAPI positive articular surface cells. Male mice were used for each experiment and statistical comparisons were analyzed by one-way ANOVA with a Bonferroni’s multiple comparison. P values <0.05 were considered statistically significant. All analyses were performed using the GraphPad Prism 6 (GraphPad Software, Inc. CA) software.
Results
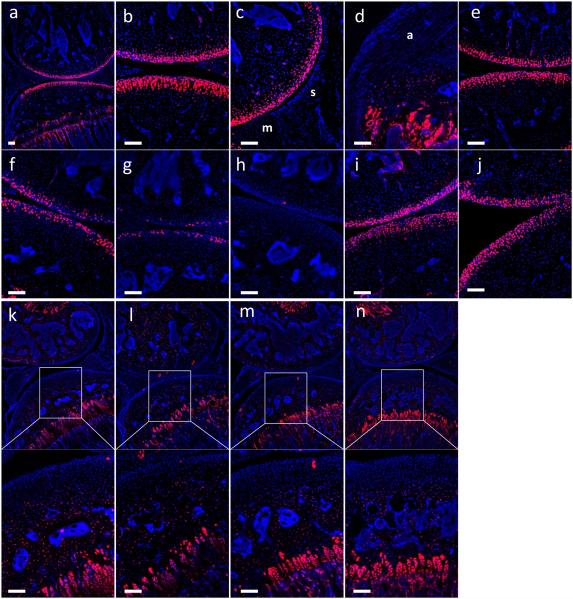
To explore patterns of targeted cells in the Col2-CreER mice when tamoxifen was administered at earlier stages, we injected tamoxifen intraperitoneally for 5 consecutive days from the first day of injection at ages of 2, 2.5, 3 and 4 weeks and sections were analyzed at 9 weeks of age. As shown in the Figures, Tomato activity was detected in articular chondrocytes, growth plate and epiphyseal bone (Fig. 1a and b), but no activity was observed in the synovium (Fig. 1c) and ligament (Fig. 1d), when tamoxifen was administered at 2 weeks of age. In the case of articular chondrocytes, Tomato activity was dependent on the age at which tamoxifen was administered. 90.8 [95% confidence interval (CI) (88.3, 93.2)] % of articular chondrocytes were Tomato positive when tamoxifen was injected at 2 weeks and 82.8 (77.4, 88.3) % when tamoxifen was injected at 2.5 weeks (Fig. 1b and e). In contrast, 46.0 (32.8, 59.1) % and 22.2 (11.7, 32.6) % of articular chondrocytes were positive when tamoxifen was injected at 3 and 4 weeks of age, respectively, in the Col2-CreER mouse line (Fig. 1f and g). Statistical analysis showed that Tomato positive cells when tamoxifen was injected at 3 or 4 weeks of age are significantly less labeled compared to the cells when tamoxifen was injected at 2 weeks of age (P<0.0001 and P<0.0001, respectively). A low level of tamoxifen-independent Tomato activity was detected in articular cartilage and subchondral bone (Fig. 1h). 86.4 (80.3, 92.5) % and 80.8 (78.6, 83.1) % of articular chondrocytes were still positive even after more than 4 months after tamoxifen was injected, when tamoxifen was injected at 2 or 2.5 weeks of age, respectively (Fig. 1i and j).
Fig. 1. Labeling pattern of the Col2-CreER mice.
Histology sections from the knee joint from 9 weeks old Col2-CreERT;tdTomato male mice (n=4) (a-g) injected intraperitoneally with tamoxifen (75mg/kg bodyweight/ day for 5days) or corn oil. Tamoxifen was administrated at 2 weeks (a-d), 2.5 weeks (e), 3 weeks (f) and 4 weeks of age (g). No-Tamoxifen control (h). Histology sections from the knee joint from 20 weeks old Col2-CreERT;tdTomato male mice (n=4) injected intraperitoneally with tamoxifen at 2 weeks (i) and 2.5 weeks of age (j). Tamoxifen was administrated at 8 weeks of age (k-n) and analyzed 1 week (k), 2 weeks (l), 3 weeks (m) or 4 weeks (n) after the first day of injection (n=3). Red represents Tomato and blue represents DAPI. s, synovium; m, meniscus; a, anterior cruciate ligament. Scale bar, 100um.
To identify which types of cells were targeted in Col2-CreER mice when tamoxifen was injected at a later stage, tamoxifen was administrated at 8 weeks of age and tissues were analyzed 1, 2, 3 and 4 weeks after the first day of injection. As shown in the figure, abundant Tomato activity was detected in growth plate chondrocytes and epiphyseal bone even 1 week after the first day of injection (Fig. 1k) and the activity was similar at every stage (Fig. 1l-n). However, little to no Tomato activity was detected in the articular cartilage, meniscus and synovium in the Col2-CreER mice (Fig. 1k-n).
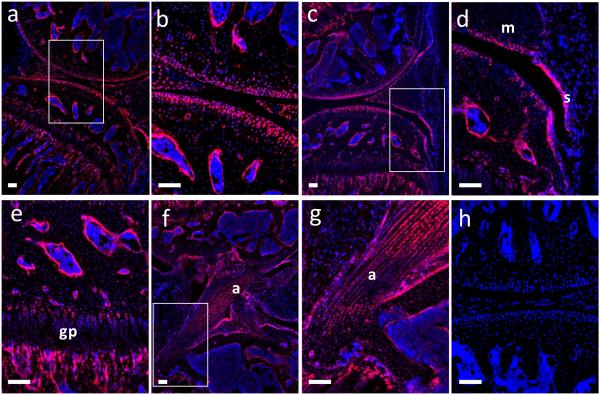
To identify the targeted cells in the Col2-Cre mouse line, we generated Col2-Cre;tdTomato transgenic mice and Tomato activity was examined in the knee joint at 8 weeks of age. As shown in figure 2, the targeted tissues were articular cartilage (Fig.2a and b), meniscus (Fig. 2a-d), growth plate (Fig. 2a and e), endosteum (Fig. 2a-e), bone (Fig 2a-g), ligaments (Fig. 2f and g) and synovium (Fig. 2c and d). The 8 weeks old Col2-Cre mice showed that almost all cells in articular cartilage were targeted (Fig. 2a and b). In contrast to the inducible Col2-CreER mouse with tamoxifen injection, no Tomato activity was detected in the knee joint of Cre-negative control mice (Fig. 2h).
Fig. 2. Labeling pattern of the Col2-Cre mice.
Histology sections from the knee joint from 9 weeks old male mice (n=4). Tomato activity in the Col2-Cre;tdTomato mice (a-g). Cre negative control (h). Red represents Tomato and blue represents DAPI. s, synovium; m, meniscus; gp, growth plate; a, anterior cruciate ligament. Scale bar, 100um
Discussion
In this study, we demonstrate that the patterns of targeted cells in inducible Col2-CreER mice when tamoxifen is administered postnatally are partially overlapping with but different from the targeted cells in Col2-Cre mice. We also demonstrate that the patterns of targeted cells in the inducible Col2-CreER mouse depend on when tamoxifen is administered.
Previous studies using the Col2 promoter showed that many types of molecules contribute to the progression of articular joint disease in osteoarthritis6. While some studies analyzed mRNA expression levels, protein levels or used reporter mice to confirm that articular cartilage was efficiently targeted, the effects on other types of tissues such as synovium, ligament, meniscus and subchondral bone were less taken into account. Our demonstration that neither Col2-Cre nor tamoxifen-inducible Col2-CreER mice target chondrocytes in a specific manner but that several kinds of mesenchymal lineage cells are targeted is consistent with previous studies in which embryonic activation of Col2-Cre promoter labels bone progenitors3, 7. The effects on several types of cells in addition to articular chondrocytes need to be considered in experimental studies using Col2 promoter-based methods to study synovial joint alterations.
The inducible Col2-CreER mice that were used in this study represents a good model for targeting specific genes in articular chondrocytes and avoid effects in the synovium and ligament, when tamoxifen is administered at 2 weeks after birth. The targeting effect lasts at least 4.5 months. However, articular chondrocytes are insufficiently targeted when tamoxifen is injected at 3 weeks of age or later. It also would be important that Col2 expression in OA is reported to be increased8, while our study showed that the expression in normal articular chondrocytes is age dependently decreased.
This study has a limitation that we did not compare male and female mice. Although our pilot study showed that initial Tomato activity was not different (data not shown), it is possible that targeting cells after tamoxifen administration in Col2-CreER mice is not the same in the two genders.
In previous OA studies, 3 different tamoxifen-inducible Col2-CreER mouse lines have been used4, 5, 9, 10. In these studies, tamoxifen was administered at various time points, ranging from 2 to 8 weeks after birth to evaluate genetic effects in the development of OA11-13. Although these Col2 transgenic lines are almost identical, the specificity and efficiency of Cre activity may differ. Taking into account the specific mouse line used and the timing of tamoxifen administration is clearly important when interpreting the data.
Acknowledgement
This work was supported by National Institutes of Health Grant NIH-AR 36819 to Bjorn Reino Olsen and by Japan Society for the Promotion of Science (JSPS) to Masashi Nagao. We thank the Nikon Imaging Center at Harvard Medical School for help with light microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement
The authors declare that they have no conflict of interest.
Author Contributions
M.N. and C.W.C. performed experiments. M.N. and B.R.O. designed the project and interpreted the results. M.N., C.W.C. and B.R.O. wrote the manuscript, and all authors revised the final version.
References
- 1.Sakai K, Hiripi L, Glumoff V, Brandau O, Eerola R, Vuorio E, et al. Stage-and tissue-specific expression of a Col2a1-Cre fusion gene in transgenic mice. Matrix Biol. 2001;19:761–7. doi: 10.1016/s0945-053x(00)00122-0. doi: S0945053X00001220 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Fosang AJ, Golub SB, East CJ, Rogerson FM. Abundant LacZ activity in the absence of Cre expression in the normal and inflamed synovium of adult Col2a1-Cre; ROSA26RLacZ reporter mice. Osteoarthritis Cartilage. 2013;21:401–4. doi: 10.1016/j.joca.2012.11.013. doi: 10.1016/j.joca.2012.11.013 [doi] [DOI] [PubMed] [Google Scholar]
- 3.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat.Cell Biol. 2014;16:1157–67. doi: 10.1038/ncb3067. doi: 10.1038/ncb3067 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev.Dyn. 2006;235:2603–12. doi: 10.1002/dvdy.20892. doi: 10.1002/dvdy.20892 [doi] [DOI] [PubMed] [Google Scholar]
- 6.Little CB, Hunter DJ. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat.Rev.Rheumatol. 2013;9:485–97. doi: 10.1038/nrrheum.2013.72. doi: 10.1038/nrrheum.2013.72 [doi] [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc.Natl.Acad.Sci.U.S.A. 2014;111:12097–102. doi: 10.1073/pnas.1302703111. doi: 10.1073/pnas.1302703111 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimmer C, Balbus N, Lang U, Aigner T, Cramer T, Muller L, et al. Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen levels. Am.J.Pathol. 2006;169:491–502. doi: 10.2353/ajpath.2006.050738. doi: S0002-9440(10)62732-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O'Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. doi: 10.1002/dvg.20261 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton MJ, Tu X, Long F. Tamoxifen-inducible gene deletion reveals a distinct cell type associated with trabecular bone, and direct regulation of PTHrP expression and chondrocyte morphology by Ihh in growth region cartilage. Dev.Biol. 2007;308:93–105. doi: 10.1016/j.ydbio.2007.05.011. doi: S0012- 1606(07)00905-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res.Ther. 2013;15:R5. doi: 10.1186/ar4133. doi: 10.1186/ar4133 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugita S, Hosaka Y, Okada K, Mori D, Yano F, Kobayashi H, et al. Transcription factor Hes1 modulates osteoarthritis development in cooperation with calcium/calmodulin-dependent protein kinase 2. Proc.Natl.Acad.Sci.U.S.A. 2015;112:3080–5. doi: 10.1073/pnas.1419699112. doi: 10.1073/pnas.1419699112 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng T, Yi L, Huang J, Luo F, Wen X, Du X, et al. Genetic inhibition of fibroblast growth factor receptor 1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice. Arthritis Rheum. 2012;64:3982–92. doi: 10.1002/art.34645. doi: 10.1002/art.34645 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]