Abstract
Mutations in WNK1 and WNK4, and in components of the Cullin-Ring Ligase system, kelch-like 3 (KLHL3) and Cullin3 (CUL3), can cause the rare hereditary disease, Familial Hyperkalemic Hypertension (FHHt). The disease is characterized by overactivity of the renal sodium chloride cotransporter (NCC), which is phosphorylated and activated by the WNK-stimulated Ste20-type kinases, SPAK and OSR1. WNK kinases themselves can be targeted for ubiquitination and degradataion by the CUL3-KLHL3 E3 ubiquitin ligase complex. It is unclear, however, why there are significant differences in phenotypic severity among FHHt patients with mutations in different genes. It was reported that kelch-like 2 (KLHL2), a homolog of KLHL3, can also target WNK kinases for ubiquitation and degradation, and may play a special role in the systemic vasculature. Our recent study revealed the disease mutant CUL3 exhibits enhanced degradation of its adaptor protein KLHL3, potentially resulting in accumulation of WNK kinases secondarily. To investigate if KLHL2 plays a role in FHHt, we studied the effect of wild type and FHHt mutant CUL3 on degradation of KLHL2 and WNK kinase proteins in HEK293 cells. Although CUL3 facilitates KLHL2 degradation, the disease mutant CUL3 is more active in this regard. KLHL2 facilitated the degradation of wild type but not disease mutant WNK4 protein. These results suggest that KLHL2 likely plays a role in the pathogenesis of FHHt, and aggravates the phenotype caused by mutations in CUL3 and WNK4.
Keywords: Kelch-like protein 2, Cullin3, WNK kinase, Degradation, Familial Hyperkalemic Hypertension
Introduction
Familial Hyperkalemic Hypertension [FHHt, also known as pseudohypoaldosteronism (PHA2) or Gordon syndrome; OMIM: 145260] is a rare Mendelian disorder, featuring hypertension, hyperkalemia, and metabolic acidosis [1]. Members of With No lysine(K) kinases, WNK4 and WNK1, and components of Cullin-Ring Ligases (CRL), kelch-like 3 (KLHL3) and Cullin3 (CUL3) were identified as FHHt-causing genes [2,3]. CUL3 acts as a scaffold for ubiquitination by binding to an adaptor protein, KLHL3, via its N-terminal. WNK kinases can be recognized by KLHL3 as substrates and finally be ubiquitinated by the CUL3-KLHL3 E3 ubiquitin ligase complex [4–7]. There is a consensus that constitutive activation of NCC in distal convoluted tubule (DCT) is a major driving force behind the FHHt phenotype [8]. Since WNK kinases activate NCC through Ste20-related Proline/Alanine-rich Kinase(SPAK)/ Odd-Skipped-Related 1(OSR1) pathway [9], and both CUL3 and KLHL3 are responsible for degrading WNK kinases, it is reasonable to presume there is increased WNK activity in all 4 known FHHt-causing syndromes. Indeed, WNK1 and/or WNK4 are elevated in animal models of WNK1, WNK4 and KLHL3 disease mutations [2,4,6], and recently in CUL3-FHHt mice[10]. To date, all CUL3-FHHt mutations are heterozygous, resulting in the deletion of 57 amino acids encoded for exon 9 of the CUL3 mRNA (hereafter referred to as CUL3 Δ9) [3,11]. The phenotype of FHHt with different gene mutations varies from mild to severe, with CUL3-FHHt displaying the most severe phenotype (earliest onset age and most severe hypertension and electrolyte disturbance [3,12,13]). It has been reported that KLHL2, a homolog of KLHL3, is also expressed in the kidney and could mediate degradation of WNK kinases [14,15]. We recently reported a dominant effect of CUL3 Δ9 on KLHL3 causing WNK kinase accumulation [16]. In this study we investigated the degradation effect of CUL3 on KLHL2, and the degradation effect of CUL3-KLHL2 on WNK kinases, to explore a potential role of KLHL2 in pathogenesis of FHHt.
Materials and Methods
Antibodies and reagents: the polyclonal CUL3 antibody has previously been published [17]. Anti-myc (catalog no. M5546) and anti-flag (catalog no. F3165) antibodies used for WB were sigma mouse monoclonal. Actin polyclonal antibody (rabbit) used was from sigma. Human CUL3 WT and Δ9, and WNK kinase constructs have been reported [16]. CUL3 siRNA (catalog no. 4392420) was purchased from ThermoFisher Scientific. KLHL2 was amplified from mouse kidney cDNA, and human KLHL3 cDNA was purchased from GeneCopeia, INC (catalog no. EX-A1395-M11). Both cDNA were subcloned into the pmo-myc vector using PCR.
Western blotting: transfected HEK293 cells were harvested in 1mM EDTA in phosphate-buffered saline and sonicated for 10 seconds in radioimmunoprecipitation assay (RIPA) buffer (1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 0.01 M sodium phosphate buffer pH 7.2 and 2mM EDTA). Protein samples were separated by electrophoresis on 10% polyacrylamide gels, transferred to PVDF membranes (Bio-Rad, Hercules, CA), and incubated overnight in primary antibodies. Proteins were visualized using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence followed by digital acquisition using an Alpha Innotech (San Leandro, CA) Fluorochem SP system.
Cell culture and transfections: HEK293 cells were maintained in DMEM supplemented with 10% FBS. Cells were transiently transfected using Lipofectamine™ 2000 (Ambion, Invitrogen) as previously described.
Nephron segment PCR: mouse renal tubule segments were isolated by microdissection as described previously [18]. RNA was extracted from segments using RNA extraction kit (Invitek) according to the manufacturer’s protocol and cDNA was synthesized by reverse transcription (Tetro Reverse Transcriptase, Promega). qPCR was performed using HOT FIREPol EvaGreen qPCR Mix Plus (Solis BioDyne) and primers specific for Klhl2 (fwd: 5′-ccgccactgcctcccgcatg-3′, rev: 5′-ccacctcctttattcgaactctctttgctcggc-3′). Gene expression analysis was performed applying the ΔΔCt method and normalized against β-Actin.
Statistical analysis: unpaired t tests were used to compare 2 groups. Differences between a single control group and multiple experimental groups were first analyzed by one way ANOVA, followed by Dunnett’s Multiple Comparison Test. Time course differences were by ANOVA with repeated measures. P<0.05 was considered significant.
Results
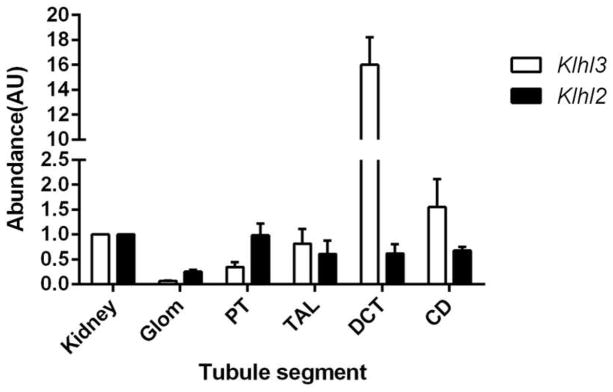
1) KLHL2 mRNA is expressed along the kidney tubules
We first examined KLHL2 expression in mouse kidney. Quantitative PCR confirmed that Klhl2 is expressed almost evenly along the tubule including thick ascending limb (TAL), DCT, and collecting duct (CD) (Fig. 1) at the mRNA level. We previously reported, using the same technique, that the pattern of Klhl3 expression was quite different, with abundant expression along the DCT and modest expression along TAL and CD [16].
Fig. 1. the expression of KLHL2 and KLHL3 along the renal tubule in mRNA level.
Klhl2 and Klhl3 expression along the nephron, analyzed by quantitative PCR. Proximal tubule (PT), TAL, DCT, CD, and glomerulus (Glom) are compared with total kidney. Values are mean ± SEM from 3 independent experiments.
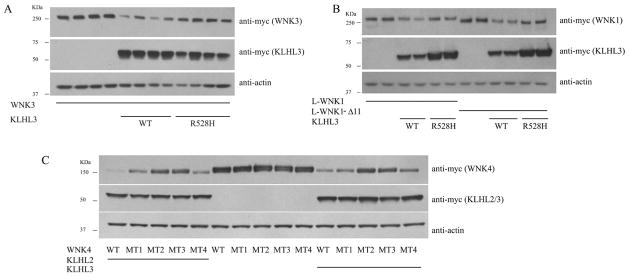
2) Both KLHL2 and KLHL3 can mediate L-WNK1-Δ11, WNK3, and WNK4 degradation
Previous studies showed KLHL3 can degrade WNK4 and L-WNK1, and that FHHt mutant KLHL3 lost the ability to degrade WNK4 and L-WNK1; this is presumed to increase NCC activity leading to FHHt [4,6]. We found that when KLHL3 and WNK3 are co-expressed in HEK293 cells, KLHL3 WT can also degrade WNK3; disease mutant KLHL3 R528H lacks this effect (Fig. 2A). As Hadchouel and colleagues reported that a form of L-WNK1 lacking exon 11 is the kidney enriched form [19], we also tested the effect of KLHL3 on L-WNK1-Δ11. Similar to L-WNK1, LWNK1-Δ11 can also be degraded by KLHL3 WT; FHHt mutant R528H lacks this effect, as well (Fig. 2B). We also found that KLHL2 can mediate degradation of all 3 WNKs (data not shown), similar to a previous report [14].
Fig. 2. both KLHL3 and KLHL2 participate in the degradation of WNK kinases, and FHHt-causing WNK4 mutant can not be degraded by KLHL2, similar to KLHL3.
A. KLHL3 WT, but not disease mutant can degrade WNK3; B. Both L-WNK1 and kidney enriched form, L-WNK1-Δ11 can be degraded by WT KLHL3, but R528H lost this effect; C. All the 4 firstly reported WNK4 disease mutant can not be degraded by KLHL2. The different WNK4 FHHt mutants marked from MT1 to MT4 denote E562K, D564A, Q565E and R1185C in order.
3) WNK4 FHHt mutants are not sensitive to degradation by either KLHL3 or KLHL2
It was reported that KLHL3 does not degrade the disease mutant WNK4 normally, leading to an increase of WNK4 and therefore to NCC activation; this is considered to be the mechanism of WNK4-FHHt [4]. In the current study, we found that KLHL2 also exhibited a partial loss of degradating capacity, with regard to WNK4 disease mutants (Fig. 2C), suggesting that KLHL2 may also play a role in WNK4 accumulation in WNK4-FHHt.
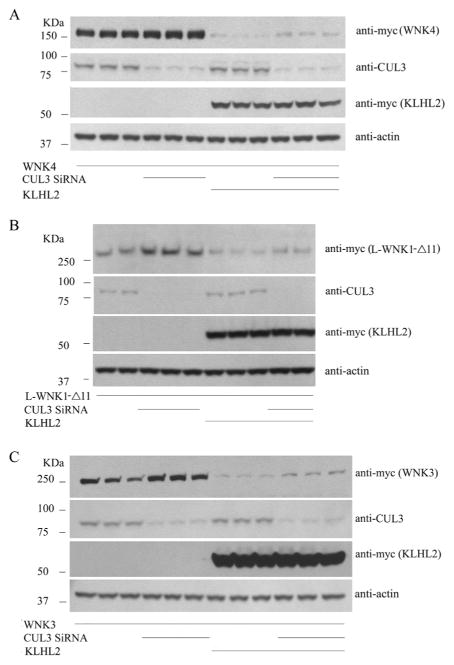
4) Endogenous cullin 3 regulates WNK kinase abundance
Exogenous CUL3 was usally used to test effects in cells, Yet HEK293 cells express CUL3 endogenously. Here, we tested the effects knocking down endogenous CUL3 on KLHL2- or KLHL3-mediated WNK degradation. The results showed that CUL3 knockdown inreases the abundance of all 3 WNK kinases (Fig. 3A–C, similar results of KLHL3 not shown), indicating that endogenous CUL3 in cells, together with its adaptors KLHL2 and KLHL3, regulates WNK kinase abundance.
Fig. 3. Endogenous cullin 3 regulates WNK kinase abundance.
Knocking down endogenous CUL3 while co-expressed with KLHL2 and WNK kinase, WNK4, L-WNK1-Δ11, and WNK3 abundance increased, as shown in Fig. 3A, B and C respectively.
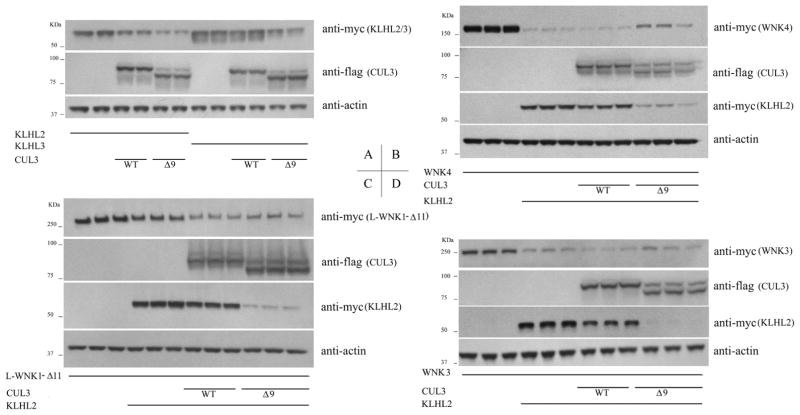
5) CUL3 Δ9 is a gain of function mutation capable of degrading both KLHL3 and KLHL2 to promote WNK signaling
CUL3 can degrade its own adaptor protein KLHL3 [7], and our recently published data showed that CUL3 Δ9 is a gain of function, instead of loss-of-function in regards to degrading KLHL3 [16]. Since KLHL2 is highly homologous to KLHL3, and KLHL2 can also serve as adaptor protein for CUL3 [14], we investigated the effect of WT and disease mutant CUL3 on KLHL2. The results show that CUL3 Δ9 degraded not only KLHL3 more than WT CUL3, but also KLHL2 (Fig. 4A).
Fig. 4. CUL3 disease mutantΔ9 degrades both KLHL3 and KLHL2 dramaticlly and causes increased WNK kinases level when co-expressed.
CUL3 Δ9 is a gain-of function on degrading both KLHL3 and KLHL2 (A); When cotransfected CUL3 WT/Δ9, KLHL2 and WNK kinase, the overdegradation of KLHL2 by CUL3 Δ9 caused a substantial increase in WNK4 (B) and WNK3 (D), similar to the existence of KLHL3 (P<0.05). The increase in L-WNK1-Δ11 in CUL3 Δ9 group showed no statistics difference compared with CUL3 WT group (C).
As shown in Fig. 4B, C and D, when co-transfected with WNK kinase, the increased degradation of KLHL2 by CUL3 Δ9 was associated with a substantial increase in WNK4 and WNK3 (P<0.05); the effect on L-WNK1-Δ11 was not significant, similar to the finding regarding KLHL3 in our previous report [16].
Discussion
It has been reported that WNK1, WNK3 and WNK4 are expressed in the distal nephron and are believed to be key regulators of NCC function through activation of SPAK and OSR1 [20]. The deletion in intron 1 of WNK1 and missense mutations of WNK4 were the first identified as causing FHHt [2]. Later, components of the Cullin-Ring-ligase complex, the scaffold protein CUL3 and the adaptor protein KLHL3, were reported to cause FHHt more commonly than WNK kinases [3]. CUL3 and KLHL3 are involved in ubiquitylation reactions and protein degradation; they ubiquitylate WNK kinases, affecting their abundance and thereby their activity. This suggests that WNKs, KLHL3, and CUL3 comprise a single regulatory network in the distal nephron.
It is widely recognized that NCC overactivation is a common feature of FHHt [8]. NCC activation in WNK1-FHHt is a result of increased expression of WNK1 due to a large deletion of intron 1, then via phosphorylation and activation of SPAK/OSR1[2,21]. Numerous molecular and biochemistry studies, as well as animal models, confirmed that SPAK/OSR1 can phosphorylate and activate NCC. Most of the WNK4 disease mutations are located in its acidic domain, which is responsible for binding to KLHL3. The disease mutant WNK4 is unable to bind to KLHL3, and be degraded by the CUL3-KLHL3 E3 ubiquitin ligase complex; this results in elevated WNK4 and NCC activation [4]. In terms of KLHL3-FHHt, KLHL3 mutants lose their ability to bind to either CUL3, or WNK kinases, thereby causing WNK kinase accumulation and NCC activation [4–7,22]. We reported that CUL3 disease mutant Δ9, is a gain-offunction mutation in regards to degradation of substrates, including its adaptor protein, KLHL3 [16]. We hypothesized that in CUL3-FHHt KLHL3 depletion by CUL3 Δ9 causes WNK kinases accumulation and finally NCC overactivation. An alternative hypothesis for the effects of CUL3 Δ9 has also been suggested in which CUL3-FHHt is caused by increased auto-ubiquitylation and degradation of CUL3 Δ9, leaving KLHL3 untouched; haploinsufficiency of CUL3 is then proposed to lead to an increase of WNK abundance. Yet, this group also confirmed our observation that mutant CUL3 Δ9 neddylation was increased in vitro, but without any specific mention of it in vivo [10].
Among the four genes known to be mutated in FHHt, CUL3-FHHt has the most severe phenotype. The reason for this difference is unknown. If CUL3 could affect NCC function only through KLHL3, it’s hard to imagine why CUL3-FHHt is more severe than KLHL3-FHHt, because both states are the disruption of CUL3-KLHL3 complex. It was reported CUL3 Δ9 mice have aortic wall thickening, and the author speculated the severe phenotype of CUL3-FHHt is partly due to increased vascular tone [10]. However, since all clinical presentations including metabolic acidosis, hyperkalemia, and hyperchloremia were also more severe in CUL3-FHHt, a principal role of the kidney may also explain the different severity between CUL3-FHHt and non-CUL3-FHHt.
We hypothesized there might be some other adaptor protein of CUL3 which serves as “secondary” adaptor of CUL3 for ubiquitinating WNK kinases in the distal nephron. There might be dozens of adaptors that can bind to CUL3, among which KLHL2 has the closest homology of KLHL3 [14]. KLHL2 was expressed in the kidney, aorta and vascular smooth muscle cells, and KLHL2 can also degrade WNK kinases, similar to KLHL3 [14,15]. Here we report the segmental expression of KLHL2 mRNA in the renal tubule, and find KLHL2 mRNA is expressed almost evenly along the tubule, which is in contrast to the high expression of KLHL3 in the DCT.
In addition to KLHL3, KLHL2 can also regulate the degradation of WNK kinases, and it can be degraded by CUL3, which helps to explain the different phenotype severity of FHHt-causing mutations. The phenotypic severity of FHHt is as follows CUL3>KLHL3>WNK4>WNK1, with CUL3 being the most severe and WNK1 being the least severe [3]. In WNK1-FHHt, only WNK1 level increases, which causes a modest NCC activation. We propose that, in WNK4-FHHt, the degradation of mutant WNK4 is hampered by decreased binding to not only KLHL3, but also KLHL2; thus, the accumulated WNK4 causes NCC activation. In KLHL3-FHHt, the degradation of both WNK1 and WNK4 are decreased because of KLHL3 mutation, and therefore NCC is activated by two WNK kinases. In CUL3-FHHt, we speculate that not only KLHL3, but also KLHL2 cannot form CRL with CUL3, so the degradation of WNK1 and WNK4 mediated by both KLHL3 and the alternative adaptor, KLHL2 is weakened; this causes the strongest NCC activation. As mentioned above, increased vascular tone is likely to be part of the reason for severe hypertension in CUL3-FHHt. Interestingly, since KLHL2 can mediate WNK3-NKCC1 signaling pathway to regulate vascular tonus induced by angiotensin II [15], we also predict that the disruption of CUL3-KLHL2 complex might be the reason for increased vascular tone in CUL3-FHHt.
WNK3 has been reported to be a strong activator of NCC [23,24], so the degradation effect of KLHL3 on WNK3 probably suggests a potential role of WNK3 in the pathogenesis of KLHL3-FHHt and CUL3-FHHt. WNK3 can also activate NKCC2[24,25], another member of Cation Chloride Cotransporter, as NCC. We cannot exclude the possibility that NKCC2, which is specifically expressed in TAL, is also activated because of WNK kinase accumulation caused by disruption of CUL3-KLHL 3 and/or CUL3-KLHL2 complexes in TAL. Obviously NKCC2 activation, if it exists, could cause increased renal absorption of sodium, chloride, and potassium, which would aggravate the FHHt phenotype. In vivo work with the CUL3-FHHt animal is important for answering this question although till now there is no good KLHL2 antibody available for detecting immunofluorescence and western-blot signal in kidney.
In summary, we found that KLHL2 was strikingly degraded by Cul3 Δ9, which in turn caused increased WNK kinase abundance. KLHL2, like KLHL3, is unable to bind and degrade mutant WNK4. Our results suggest that KLHL2 likely plays a role in the pathogenesis of FHHt, and aggravates the phenotype caused by mutation in CUL3 and WNK4.
Highlights.
The mRNA expression pattern of KLHL2 in kidney is different from KLHL3.
KLHL2 as well as KLHL3 can mediate degradation of WNK kinases.
WNK4 FHHt mutants are not sensitive to degradation by either KLHL2 or KLHL3.
CUL3 FHHt mutant exhibits enhanced activity toward KLHL2, similar to KLHL3, resulting in increased WNK kinases abundance.
KLHL2 likely plays a role in FHHt, which helps to explain the different phenotype severity of FHHt-causing mutations.
Acknowledgments
This work was supported by National Natural Science Foundation of China 81570634 and Shanghai Municipal Education Commission 15zz054 (to C. Zhang), and NIH grant R01 DK51496 (to C.-L. Yang and D.H. Ellison). A.S. Terker was supported by NIH 5T32DK067864-10.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hadchouel J, Delaloy C, Fauré S, Achard JM, Jeunemaitre X. Familial hyperkalemic hypertension. J Am Soc Nephrol. 2006;17:208–217. doi: 10.1681/ASN.2005030314. [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 3.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Välimäki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013;3:858–868. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA. 2013;110:7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23:5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 7.Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–122. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Shaughnessy KM. Gordon Syndrome: a continuing story. Pediatr Nephrol. 2015;30:1903–1908. doi: 10.1007/s00467-014-2956-7. [DOI] [PubMed] [Google Scholar]
- 9.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Yasmin, Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med. 2015;7:1285–1306. doi: 10.15252/emmm.201505444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover M, Ware JS, Henry A, Wolley M, Walsh R, Wain LV, Xu S, Van’t Hoff WG, Tobin MD, Hall IP, Cook S, Gordon RD, Stowasser M, O’Shaughnessy KM. Detection of mutations in KLHL3 and CUL3 in families with FHHt (familial hyperkalaemic hypertension or Gordon’s syndrome) Clin Sci. 2014;126:721–726. doi: 10.1042/CS20130326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osawa M, Ogura Y, Isobe K, Uchida S, Nonoyama S, Kawaguchi H. CUL3 gene analysis enables early intervention for pediatric pseudohypoaldosteronism type II in infancy. Pediatr Nephrol. 2013;28:1881–1884. doi: 10.1007/s00467-013-2496-6. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji S, Yamashita M, Unishi G, Takewa R, Kimata T, Isobe K, Chiga M, Uchida S, Kaneko K. A young child with pseudo-hypoaldosteronism type II by a mutation of Cullin 3. BMC Nephrol. 2013;14:166. doi: 10.1186/1471-2369-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi D, Mori T, Wakabayashi M, Mori Y, Susa K, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. KLHL2 interacts with and ubiquitinates WNK kinases. Biochem Biophys Res Commun. 2013;437:457–462. doi: 10.1016/j.bbrc.2013.06.104. [DOI] [PubMed] [Google Scholar]
- 15.Zeniya M, Morimoto N, Takahashi D, Mori Y, Mori T, Ando F, Araki Y, Yoshizaki Y, Inoue Y, Isobe K, Nomura N, Oi K, Nishida H, Sasaki S, Sohara E, Rai T, Uchida S. Kelch-Like Protein 2 Mediates Angiotensin II-With No Lysine 3 Signaling in the Regulation of Vascular Tonus. J Am Soc Nephrol. 2015;26:2129–2138. doi: 10.1681/ASN.2014070639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest. 2014;124:4723–4736. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer JD, Gurian-West M, Clurman B, Roberts JM. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 1999;13:2375–2387. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am J Physiol Renal Physiol. 2010;298:F216–223. doi: 10.1152/ajprenal.00295.2009. [DOI] [PubMed] [Google Scholar]
- 19.Vidal-Petiot E, Cheval L, Faugeroux J, Malard T, Doucet A, Jeunemaitre X, Hadchouel J. A new methodology for quantification of alternatively spliced exons reveals a highly tissue-specific expression pattern of WNK1 isoforms. PLoS One. 2012;7:e37751. doi: 10.1371/journal.pone.0037751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazúa-Valenti S, Gamba G. Revisiting the NaCl cotransporter regulation by with-no-lysine kinases. Am J Physiol Cell Physiol. 2015;308:C779–C791. doi: 10.1152/ajpcell.00065.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chávez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castañeda-Bueno M, Vázquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J. WNK-SPAK-NCC Cascade Revisited: WNK1 Stimulates the Activity of the Na-Cl Cotransporter via SPAK, an Effect Antagonized by WNK4. Hypertension. 2014;64:1047–1053. doi: 10.1161/HYPERTENSIONAHA.114.04036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori Y, Wakabayashi M, Mori T, Araki Y, Sohara E, Rai T, Sasaki S, Uchida S. Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms. Biochem Biophys Res Commun. 2013;439:30–34. doi: 10.1016/j.bbrc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest. 2007;117:3403–3411. doi: 10.1172/JCI32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinehart J, Kahle KT, de Los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juárez P, Muñoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]