Abstract
Impulsivity, which can be subdivided into impulsive action and impulsive choice, is implicated as a factor underlying drug abuse vulnerability. Although previous research has shown that dopamine (DA) systems in prefrontal cortex are involved in impulsivity and substance abuse, it is not known if inherent variation in DA transporter (DAT) function contributes to impulsivity. The current study determined if individual differences in either impulsive action or impulsive choice are related to DAT function in orbitofrontal (OFC) and/or medial prefrontal cortex (mPFC). Rats were first tested both for impulsive action in a cued go/no-go task and for impulsive choice in a delay discounting task. Following behavioral evaluation, in vitro [3H]DA uptake assays were performed in OFC and mPFC isolated from individual rats. Vmax in OFC, but not mPFC, was correlated with performance in the cued go/no-go task, with decreased OFC DAT function being associated with high impulsive action. In contrast, Vmax in OFC and mPFC was not correlated with performance in the delay discounting task. The current results demonstrate that impulsive behavior in cued go/no-go performance is associated with decreased DAT function in OFC, suggesting that hyperdopaminergic tone in this prefrontal subregion mediates, at least in part, increased impulsive action.
Keywords: dopamine transporter, cued go/no-go, impulsive action, delay discounting, impulsive choice, rat
1. Introduction
Impulsivity is a construct that encompasses various behaviors and is typically subdivided into two broad categories: impulsive action and impulsive choice (Winstanley et al., 2010). Impulsive action is conceptualized as motor impulsivity (e.g., the inability to inhibit a prepotent response), and impulsive choice is considered to reflect cognitive impulsivity (e.g., consistently choosing a small, immediate reward over a large, delayed reward). Impulsive action and impulsive choice can be measured in human and laboratory animals with the cued go/no-go task and the delay discounting task, respectively (Mahrer, 1956; Gross and Weiskrantz, 1962; Rachlin and Green, 1972; Hogg and Evans, 1975; Mazur et al., 1985; Evenden and Ryan, 1996; Harrison et al., 1999). Because impulsive responding in each task has been associated with increased substance abuse liability in humans (Kollins et al., 2005; Weafer et al., 2011), determining the shared neurobiology between impulsive behavior and substance use disorders may lead to improved treatment outcomes for individuals with comorbid impulse-control and substance use disorders.
The role of dopamine (DA) in substance abuse and impulsivity is of particular interest because drugs of abuse, as well as medications used to treat impulse-control disorders (Biederman and Faraone, 2005), increase extracellular DA (Creese and Iversen, 1975; Moghaddam and Bunney, 1989; Kuczenski and Segal, 1997; Jones et al., 1998; Volkow et al., 2002; Caillé and Parsons, 2003). Furthermore, DA systems in various subregions of the prefrontal cortex (PFC) are implicated in substance abuse and impulsivity. Specifically, decreased DA transmission is observed in alcoholics (Narendran et al., 2014) and smokers (Luijten et al., 2013). Within PFC, animals exhibiting low levels of either impulsive action or impulsive choice have higher DA D2 receptor mRNA levels in the prelimbic portion of medial prefrontal cortex (mPFC; Simon et al., 2013). Although mRNA levels do not necessarily reflect differences in receptor protein (Tian et al., 2004), other research has shown that overexpression of DA D1 receptors in prelimbic mPFC is associated with increased impulsive choice in a delay discounting paradigm (Sonntag et al., 2014). Despite these DA receptor mRNA and protein differences, little is known about the potential role of presynaptic mechanisms of DA signaling within impulsivity-relevant prefrontal cortical regions.
Medications that are efficacious in treating impulse-control disorders, such as methylphenidate and amphetamine, target the DA transporter (DAT; Ritz and Kuhar, 1989; Volkow et al., 1998), indicating that DAT is likely an important mediator of impulsivity. Additionally, polymorphisms in the DAT1 gene are associated with increased impulsivity (Waldman et al., 1998; Paloyelis et al., 2010). Furthermore, GBR12909, a selective DAT inhibitor, reduces impulsive choice in the delay discounting task in rats (Evenden and Ryan, 1996; van Gaalen et al., 2006; Baarendse and Vanderschuren, 2012), but increases impulsive action in the five-choice serial reaction time task (5-CSRT; Baarendse and Vanderschuren, 2012), suggesting that DAT may be differentially involved in impulsive choice and impulsive action. Although DAT has been implicated in impulsivity, it is unknown if individual differences in DAT function mediate distinct facets of impulsive behavior. Thus, the purpose of the current study was to determine if inherent variation in DAT function in orbitofrontal cortex (OFC) and mPFC is associated with impulsive action and/or impulsive choice.
2. Experimental Procedures
2.1. Subjects
Eighteen male, experimentally naïve Sprague-Dawley rats (250–275 g; Harlan Laboratories, Indianapolis, IN) were housed individually in a temperature-and humidity-controlled colony with a 12/12 h light/dark cycle. Following 5 days of acclimation, rats were food restricted (85% of free feeding body weight), and had free access to water in their home cages. Experiments were conducted during the light phase. Rats were cared for in accordance with the 2011 edition of the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 2010) and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.2. Behavioral apparatus
Operant chambers (28×21×21 cm; ENV-008; MED Associates, St. Albans, VT) with aluminum front and back walls and Plexiglas sides were located inside sound-attenuating chambers (ENV-018M; MED Associates, St Albans, VT). A recessed food tray (5 × 4.2 cm) was located 2 cm above the floor in the bottom-center of the front wall. Retractable levers (4.5 cm) were mounted 6 cm above the floor on each side of the food tray. A 28-V white cue light was located 6 cm above each lever. A white house light was mounted in the center of the back wall. All responses and scheduled consequences were recorded and controlled by a computer interface using Med-IV software.
2.3. Experimental Design
Each rat was tested in both the cued go/no-go and delay discounting tasks, with order of testing counterbalanced. Following the last behavioral test day, rats were killed by rapid decapitation and both OFC and mPFC were obtained from each rat to determine the kinetic parameters of DAT function.
2.3.1. Cued go/no-go task
Previously described procedures were used (Marusich et al., 2011). Training began with 3 days of autoshaping (Brown and Jenkins, 1968), in which both levers were extended and the house light was illuminated. During 60-min autoshaping sessions, rats received one sucrose-based pellet (45 mg; F0021 dustless precision pellet, Bio-Serve, Frenchtown, NJ) on a continuous schedule of reinforcement following responses on the active lever. The position of the active lever was counterbalanced across sessions for each rat. To facilitate the acquisition of lever responding, sucrose pellets were delivered non-contingently on a variable time (VT) 100 sec schedule of reinforcement. Responses on the inactive lever were recorded, but had no programmed consequence. Following either contingent or non-contingent delivery of a sucrose pellet, both levers were retracted for 2 sec. Autoshaping sessions ended after delivery of 60 reinforcers or after 60 min elapsed. Following autoshaping, training continued for 4 consecutive daily 20-min sessions employing a variable interval (VI) schedule (VI-4, VI-8, VI-14, and VI-20 sec) of sucrose pellet reinforcement.
The cued go/no-go task was employed for 14 consecutive daily 40-min sessions. Sessions consisted of 2-min “go” components in which reinforcers were available, alternated with 2-min “no-go” components in which reinforcers were not available (extinction). Go components were signaled by cue light illumination. Active lever responses on a VI-20 sec schedule resulted in sucrose pellet reinforcement. No-go components were signaled by the absence of cue light illumination; responses on the previously active lever were recorded, but had no programmed consequence. During both go and no-go components, responses on the inactive lever were recorded, but had no programmed consequence. The cues signaling go and no-go components were not counterbalanced across rats. However, it is important to note that previous work has shown that counterbalancing the cue used to signal each component does not alter performance in this task (Hellemans et al., 2005). The primary dependent measure from the cued go/no-go task was calculated as the number of responses during go trials divided by the number of responses during no-go trials (i.e., VI/EXT ratio) averaged across the last 7 sessions, when stable performance was achieved.
2.3.2. Delay discounting task
The delay discounting task was conducted for 21 days using previously described procedures (Perry et al., 2005). Sessions began with house light illumination and ended following completion of 60 trials or when 2 hr elapsed. Each session included 15 blocks of 4 trials. For each block, the first 2 trials were forced-choice trials, and the last 2 trials were free-choice trials. During forced-choice trials, only one lever (left or right; counterbalanced across trials) was extended, and the cue light above the extended lever was illuminated. During free-choice trials, both levers were extended, and cue lights above both levers were illuminated. A response on one lever (fixed ratio [FR] 1 schedule of reinforcement) resulted in immediate delivery of one sucrose pellet, and a response on the other lever (FR 1) resulted in delivery of 3 sucrose pellets after a delay. Location of the levers delivering 1 or 3 pellets alternated across sessions. Delay to the larger reinforcer (initially set at 0 sec) was adjusted according to responses during the free-choice trials in each block. Responding on the lever delivering 3 pellets increased the delay to the larger, delayed reinforcer by 1 sec. Responding on the lever associated with the small, immediate reinforcer decreased the delay to the larger reinforcer by 1 sec. Delay was adjusted following each individual free-choice trial (i.e., the delay to the larger, delayed reward was adjusted twice during each block of trials). A minimum delay of 0 sec and a maximum delay of 45 sec for the 3-pellet reinforcer were imposed. Delay on the final free-choice trial during each session was used as the initial delay for the first free-choice trial of the next session. During the delay, cue lights were turned off, although the house light remained illuminated until delivery of the 3 pellets. Following a response on either lever, an adjusting inter-trial interval (ITI) was imposed, such that the ITI lasted 60 sec. During the ITI, both cue lights and house light were extinguished, and lever presses had no programmed consequences. The main outcome measure, mean adjusted delay (MAD) score, was calculated by averaging all the adjusting delays on the free-choice trials during the session. MAD scores for the last 10 sessions were averaged, when stable performance was achieved.
2.3.3. [3H]DA uptake assays
Within 60 min following the final behavioral test session, DAT function was determined using synaptosomes obtained from both OFC and mPFC from each animal. Kinetic parameters of transporter function (i.e., Vmax [pmol/min/mg] and Km [μM]) for [3H]DA uptake were determined using a previously published method (Darna et al., 2015). Each rat brain was dissected on an ice-cold plate to obtain OFC and mPFC. Regions were identified according to the atlas of Paxinos and Watson (1998) and obtained by making a coronal cut in the frontal cortex just anterior to the olfactory tubercles. From the anterior portion, the olfactory bulbs were removed and two sagittal cuts were made ~1.2 mm on either side of the midline; the mPFC was obtained as the segment between the two cuts. The remaining lateral sections of cortical tissue were then placed together and a cut in the horizontal plane was made to divide the dorsal and ventral portions in half; the OFC was obtained as the ventral segment.
Each brain region was homogenized in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer (clearance, ~0.003 inch). Homogenates were centrifuged at 2000g for 10 min at 4°C, and resulting supernatants were centrifuged at 20,000g for 17 min at 4°C. Resulting pellets were resuspended in 2.2 ml of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4) to obtain synaptosomal suspensions. Nonspecific [3H]DA uptake was determined in the presence of 10 μM nomifensine. Also, [3H]DA uptake was assessed in the presence of desipramine (5 nM) and paroxetine (5 nM) to inhibit the norepinephrine transporter (NET) and serotonin transporter (SERT), respectively, isolating uptake of DA by DAT (Zhu et al., 2004).
OFC and mPFC synaptosomes (50 and 40 μg protein/100 μl, respectively) were incubated in buffer containing desipramine and paroxetine (125 μl) in a metabolic shaker for 5 min at 34°C. Then, 1 of 7 [3H]DA concentrations (0.01–1 μM, in 25 μl) was added for a total incubation volume of 250 μl. Incubations continued for 5 min at 34°C and were terminated by addition of 3 ml of ice-cold assay buffer. Samples were filtered immediately through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 hr) using a Brandel cell harvester (model MP-43RS; Brandel Inc., Gaithersburg, MD). Filters were washed 3 times with 3 ml of ice-cold buffer containing 1 mM pyrocatechol. Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences, Boston, MA). Protein concentrations were determined using bovine serum albumin as the standard (Bradford, 1976). Specific [3H]DA uptake was obtained by subtracting nonspecific uptake from total uptake, and kinetic parameters (Vmax and Km) were determined using GraphPad Prism.
2.3.4. Statistical analyses
Analyses of behavioral data were conducted using Graph-Pad Prism 4.0. To determine stability in the cued go/no-go task, linear trend analyses were performed on VI responses/EXT responses (VI/EXT) averaged across the last 7 sessions. To determine stability in the delay discounting task, MAD scores were averaged across the final 10 sessions, with a non-significant slope indicating stability. Pearson’s correlation analyses determined relationships of VI responses/EXT responses (VI/EXT) with either Vmax or Km for [3H]DA uptake in OFC and mPFC. Pearson’s correlation analyses also determined relationships of MAD scores with either Vmax or Km for [3H]DA uptake in OFC and mPFC. Additionally, because there was a relatively narrow spread of VI/EXT ratios and MAD scores, Spearman correlation analyses were conducted to determine if the non-significant Pearson correlations obtained may have resulted from this narrow spread. Separate Pearson’s correlations determined relationships of reinforcers earned in each behavioral task with either Vmax or Km for [3H]DA uptake in OFC and mPFC. For the [3H]DA uptake assay, kinetic parameters Vmax and Km indicate maximal transport velocity and affinity of DA for DAT, respectively. Log-transformed Km values were used for statistical analyses. Statistical significance was declared at p < 0.05 in all cases. For OFC, two rats were excluded from data analyses due to the lack of saturation in the [3H]DA uptake assay, which did not allow for determination of Vmax.
3. Results
3.1. Cued go/no-go and delay discounting tasks
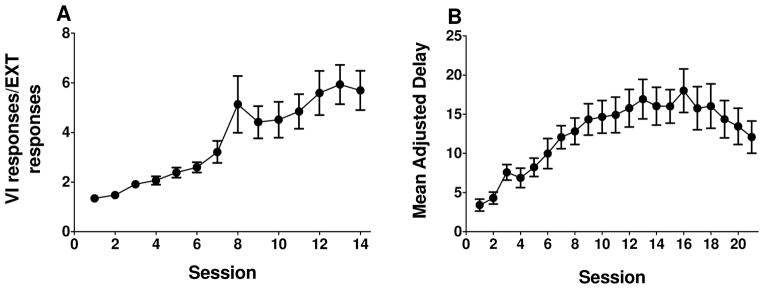
Linear trend analyses showed that rats exhibited stable VI/EXT responding across the final 7 sessions of the cued go/no-go task (t(124) = 1.340, p = 0.183; Fig. 1A), as well as stable MAD scores across the final 10 sessions of the delay discounting task (t(178) = 1.543, p = 0.125; Fig. 1B). Performance in the cued go/no-go task was not correlated with performance in the delay discounting task (Pearson’s r = −.231, p = .356; data not shown). Number of reinforcers earned in each task was not correlated with either VI/EXT responses (Pearson’s r = −.293, p = 0.238) or MAD scores (Pearson’s r = −0.074, p = 0.772; data not shown).
Figure 1.
Acquisition of the cued go/no-go and delay discounting tasks in rats evaluated subsequently for DAT function (n = 18). A: Mean (± SEM) ratio of variable interval responses and extinction responses (VI/EXT responses) plotted as a function of session for 14 days of the cued go/no-go task. B: Mean (± SEM) adjusted delay (MAD) plotted as a function of session for 21 days of the delay discounting task. Note that task order was counterbalanced across rats.
3.2. Correlation analyses of kinetic parameters for [3H]DA uptake in mPFC and OFC with behavioral outcome measures
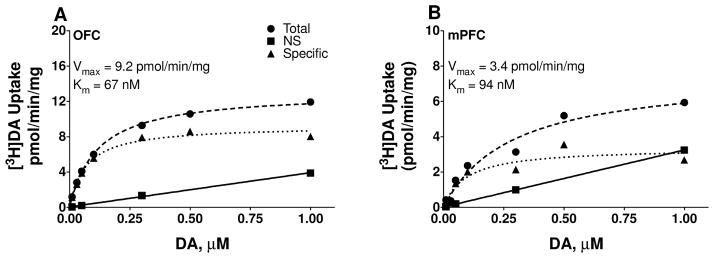
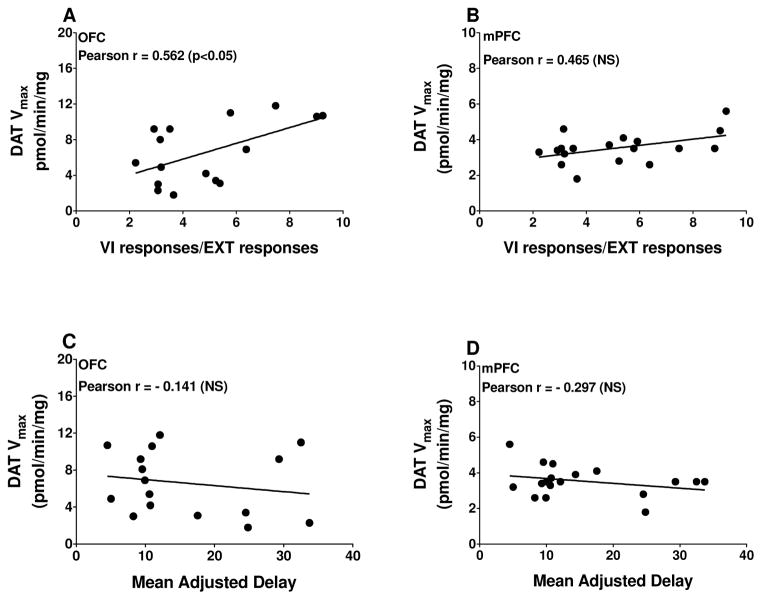
As illustrated in Fig. 2, specific [3H]DA uptake was saturable in both OFC and mPFC when assessed in tissues from an individual rat. Kinetic parameters (Vmax and Km) for [3H]DA uptake were obtained from saturation curves, and these values were used in correlational analyses with behavioral outcome measures. Vmax for [3H]DA uptake from individual rats was positively correlated with VI/EXT ratios in OFC (Pearson’s r = 0.562, p = 0.024; Fig. 3A), but not in mPFC (Pearson’s r = 0.465, p = .052, Fig. 3B). Spearman rho value was 0.404 for mPFC, suggesting that Pearson’s correlation between DAT function and VI/EXT responses was not masked by a narrow range of VI/EXT scores. For the delay discounting task, MAD scores were not correlated with Vmax in either OFC (Pearson’s r = −0.185, p = 0.494; Fig. 3C) or mPFC (Pearson’s r = −0.298, p = 0.231; Fig. 3D). Spearman rho values of −0.156 and −0.025 indicate that the non-significant Pearson correlations were not due to a narrow range of MAD scores. No significant correlations were obtained between Km for OFC or mPFC [3H]DA uptake with either behavioral task (OFC VI/EXT: Pearson’s r = −0.014, p = 0.960; OFC MAD: Pearson’s r = 0.093, p = 0.733; mPFC VI/EXT: Pearson’s r = −0.100, p = 0.693; mPFC MAD: Pearson’s r = 0.064, p = 0.801; data not shown).
Figure 2.
Saturation curves for [3H]DA uptake at DAT in OFC (A) and mPFC (B) in synaptosomes from a representative rat. Nonspecific [3H]DA uptake was determined in the presence of 10 μM nomifensine. Specific uptake (▲) was obtained by subtracting nonspecific uptake (■) from total uptake (●). Vmax represents the maximal velocity of [3H]DA uptake and Km represents affinity of DAT for DA.
Figure 3.
Pearson’s correlation of Vmax for [3H]DA uptake in OFC and mPFC synaptosomes with VI/EXT responses (A and B) and mean adjusted delay (C and D). Note that lower VI/EXT responses indicate increased impulsive action and lower MAD scores indicate increased impulsive choice. Data points represent responses from individual rats (n = 16 for OFC and n = 18 for mPFC).
Pearson’s correlation analyses were conducted to determine if the number of reinforcers earned in either behavioral task was associated with DAT kinetic parameters from OFC and mPFC. No significant correlations were obtained between Vmax and either behavioral task (OFC VI/EXT: Pearson’s r = −0.412, p = 0.113; OFC MAD: Pearson’s r = −0.221, p = 0.411; mPFC VI/EXT: Pearson’s r = −0.015, p = 0.954; mPFC MAD: Pearson’s r = −0.046, p = 0.857; data not shown). Similarly, no significant correlations were obtained between Km and either behavioral task (OFC VI/EXT: Pearson’s r = −0.185, p = 0.493; mPFC VI/EXT: Pearson’s r = −0.451, p = 0.060; OFC MAD: Pearson’s r = −0.077, p = 0.778; mPFC MAD: Pearson’s r = 0.131, p = 0.604; data not shown).
4. Discussion
The goal of the current study was to determine if individual differences in DAT function within either OFC or mPFC are associated with either impulsive action or impulsive choice as measured by cued go/no-go and delay discounting tasks, respectively. The major finding of the current study is that rats exhibiting higher levels of impulsive action (higher VI/EXT responses) had lower maximal velocity of [3H]DA uptake in OFC, suggesting hyperdopaminergic tone in this prefrontal subregion. However, because we did not measure directly DA release, we cannot rule out the possibility that decreased DAT function in high impulsive action is a compensatory response to lower amounts of DA release in OFC. Despite this limitation, the current results demonstrate that OFC DAT function is linked to individual differences in impulsive action.
In the cued go/no-go task, a higher VI/EXT ratio score indicates decreased impulsive action (i.e., greater responding during the go component relative to the no-go component). Results showed that the maximal velocity of DA uptake at DAT in OFC, but not in mPFC, was correlated with this measure of impulsive action, with greater impulsive action being associated with reduced DA uptake in OFC. Previous research has shown that lesions to mPFC, but not OFC, increase impulsive action in the five-choice serial reaction time task (5-CSRT; Chudasama et al., 2003; Muir et al., 1996). Conversely, lesions to OFC primarily affect reversal, but not acquisition, of a go/no go discrimination (Chudasama and Robbins, 2003; Schoenbaum et al., 2002, 2003; Izquierdo et al., 2004; but see Eichenbaum et al., 1983). The cued go/no-go task used in the current experiment and reversal learning paradigms used in previous studies both can be considered forms of discrimination learning that are dependent on action cancellation. DA is known to play a critical role in discrimination learning, as amphetamine impairs reversal learning in rats (Connolly and Gomez-Serrano, 2014; Fletcher et al., 2005; Idris et al., 2005) and increases impulsive action in cued go/no-go task performance in impulsive individuals (Fillmore et al., 2003; but see de Wit et al., 2002). The current results extend these previous studies by providing evidence that inherent variation in DAT function specifically in OFC may underlie individual differences in discrimination learning.
In addition to impulsive action, OFC is implicated in impulsive choice, as increases in impulsivity are observed following excitotoxic lesions of this region (Kheramin et al., 2002, 2004; Mobini et al., 2002; Rudebeck et al., 2006). Other studies have reported either a decrease or no change in impulsive choice following lesions to OFC (Abela and Chudasama, 2013; Jo et al., 2013; Mar et al., 2011; Mariano et al., 2009; Winstanley et al., 2004). These discrepancies may result from differential destruction of subregions of OFC, as lesions to medial OFC increase sensitivity to delayed reinforcement, whereas lesions to lateral OFC decrease impulsive choice (Mar et al., 2011). Additional methodological factors that may explain the discrepancies observed across studies include baseline levels of impulsive choice and the use of cues to signal the delay to the larger reinforcer. For example, inactivation of OFC increases impulsive choice in low impulsive rats when the delay is signaled, but decreases impulsive choice in high impulsive rats when the delay is not signaled (Zeeb et al., 2010).
Despite the discrepancies in preclinical studies regarding the role of OFC in impulsive choice, clinical studies have demonstrated that DA contributes to impulsive choice, as acute methylphenidate or amphetamine decreases delay discounting (de Wit et al., 2002; Pietras et al., 2003). Consistent with the human literature, preclinical studies show that systemic amphetamine or GBR-12909 decrease impulsive choice in delay discounting tasks (van Gaalen et al., 2006; Baarendse and Vanderschuren, 2012; but see Cardinal et al., 2000; Evenden and Ryan, 1996). In the current study, DAT function in OFC was not correlated with impulsive choice expressed as MAD scores from the delay discounting task. Although DA release was not measured directly, an increase in extracellular levels of the DA metabolite dihydroxyphenylacetic acid (DOPAC) in OFC has been observed during performance of a delay discounting task in rats (Winstanley et al., 2006), suggesting a role of intra-OFC DA utilization in impulsive choice. However, consistent with the current results, Winstanley et al. (2006) did not observe a correlation between extracellular DA in OFC and impulsive choice. One important consideration is that DA systems outside of PFC subregions also have been implicated in impulsive choice. For example, high impulsive rats show reduced DA release in nucleus accumbens (Diergaarde et al., 2008), and overexpression of the DAT gene in this region increases delay discounting in rats (Adriani et al., 2009). Thus, nucleus accumbens DAT function may contribute to individual differences in impulsive choice.
Although OFC DAT function was related to performance in the cued go/no-go task, DAT function in mPFC was not associated with performance in either the cued go/no-go or delay discounting tasks. In humans, mPFC thickness and activation are negatively correlated with impulsive choice (Antonelli et al., 2014; Bernhardt et al., 2014). Preclinical research has shown that mPFC is involved in impulsive behavior, as temporary inactivation or lesions to this region increase impulsive action (Chudasama et al., 2003; Pezze et al., 2009; Feja and Koch, 2014) and impulsive choice (Churchwell et al., 2009; Gill et al., 2010; but see Cardinal et al., 2001; Feja and Koch, 2014; Stopper et al., 2014). It is important to note that the rat infralimbic subregion of mPFC is proposed to be homologous to human subgenual cingulate, whereas rat prelimbic subregion is proposed to be homologous to human dorsal anterior cingulate (Ongür and Price, 2000). There are a few limitations to the current study that may have precluded significant correlations between mPFC DAT function and impulsive behaviors. First, the sample size (n = 18) in the current study was relatively small, which can decrease statistical power. Second, mPFC is composed of several subregions, including anterior cingulate, infralimbic, and prelimbic cortices (ACC, ILC, and PLC, respectively), and previous studies have shown dissociable effects following lesions to specific mPFC subregions. For example, lesions to ACC reduce accuracy in the 5-CSRT task, whereas lesions to PLC/ILC increase perseverative responding without altering accuracy (Chudasama et al., 2003; Passetti et al., 2002). Future work is needed to determine if DAT function in mPFC subregions differentially mediate individual differences in cued go/no-go performance. Third, DAT function was lower in mPFC (3.53 ± 0.20) relative to OFC (6.59 ± 0.87); furthermore, rats that completed the delay discounting evaluation first had higher DAT function (3.88 ± 0.28 pmol/min/mg) relative to rats that completed cued go/no-go task first (3.10 ± 0.21 pmol/min/mg). The lower DAT function observed in mPFC may reflect a floor effect, which may have narrowed the variability in Vmax values across rats. However, this explanation does not seem likely, as we have previously shown differences in mPFC DAT function in differentially-housed animals (Yates et al., 2012; Zhu et al., 2004), as well as following pharmacological treatments of methylphenidate (Somkuwar et al., 2013; Yates et al., 2012), and the range of Vmax scores in the current study (1.80–5.60 pmol/min/mg) was similar to the range in a previous report (1.26–7.30 pmol/min/mg; Yates et al. 2012). Also, Spearman correlation analyses indicated that the non-significant Pearson correlations were not due to a narrow spread of Vmax values. Overall, the current results, in conjunction with our previous work (Darna et al., 2015; Yates et al., 2015), indicate that inherent variation of monoamine transporter function in mPFC may not underlie individual differences in distinct facets of impulsivity.
Highlights.
OFC DAT function is negatively correlated with cued go/no-go task performance.
Individual differences in delay discounting performance were not correlated with OFC DAT function.
Inherent variation in mPFC DAT function does not underlie individual differences in impulsive action or impulsive choice.
Acknowledgments
Funding and Disclosure
This work was supported by National Institutes of Health grant P50 DA05312 (Center for Drug Abuse Research Translation) and T32 DA16176. The authors would like to thank Kate Fisher and Emily Denehy for technical assistance.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci. 2013;37:640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- Adriani W, Boyer F, Gioiosa L, Marcì S, Dreyer J-L, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neurosci. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Antonelli F, Ko JH, Miyasaki J, Lang AE, Houle S, Valzania F, Ray NJ, Strafella AP. Dopamine-agonists and impulsivity in Parkinson’s disease: impulsive choices vs. impulsive actions. Hum Brain Mapp. 2014;35:2499–2506. doi: 10.1002/hbm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJ, Vanderschuren LJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology. 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Smallwood J, Tusche A, Ruby FJ, Engen HG, Steinbeis N, Singer T. Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self-generated thought and temporal discounting. Neuroimage. 2014;90:290–297. doi: 10.1016/j.neuroimage.2013.12.040. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:258–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé S, Parson LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–3149. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly NP, Gomez-Serrano M. D4 dopamine receptor-specific antagonist improves reversal learning impairment in amphetamine-treated male rats. Exp Clin Psychopham. 2014;22:557–564. doi: 10.1037/a0038216. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Darna M, Chow JJ, Yates JR, Charnigo RJ, Beckmann JS, Bardo MT, Dwoskin LP. Role of serotonin transporter function in rat orbitofrontal cortex in impulsive choice. Behav Brain Res. 2015;293:134–142. doi: 10.1016/j.bbr.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79:434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology. 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- Gill TM, Castaneda PJ, Janak PH. Dissociable roles of the medial prefrontal cortex and nucleus accumbens core in goal-directed actions for differential reward magnitude. Cereb Cortex. 2010;20:2884–2899. doi: 10.1093/cercor/bhq036. [DOI] [PubMed] [Google Scholar]
- Gross CG, Weiskrantz L. Evidence for dissociation of impairment on auditory discrimination and delayed response following lateral frontal lesions in monkeys. Exp Neurol. 1962;5:453–476. doi: 10.1016/0014-4886(62)90057-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res. 1999;100:99–112. doi: 10.1016/s0166-4328(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–220. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Hogg J, Evans PL. Stimulus generalization following extra-dimensional training in educationally subnormal (severely) children. Br J Psychol. 1975;66:211–224. doi: 10.1111/j.2044-8295.1975.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology. 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Kim KU, Lee D, Jung MW. Effect of orbitofrontal cortex lesions on temporal discounting in rats. Behav Brain Res. 2013;245:22–28. doi: 10.1016/j.bbr.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2004;175:206–214. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison to amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, Hester R, Smits M, Nijs IM, Pepplinkhuizen L, Franken IH. The role of dopamine in inhibitory control in smokers and non-smokers: a pharmacological fMRI study. Eur Neuropsychopharmacol. 2013;23:1247–1256. doi: 10.1016/j.euroneuro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Mahrer AR. The role of expectancy in delayed reinforcement. J Exp Psychol. 1956;52:101–105. doi: 10.1037/h0040837. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JN, Walton ME, Rushworth MF, Baxter MG, Campbell TG. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30:472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. A multivariate assessment of individual differences in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Exp Clin Psychopharmacol. 2011;19:275–284. doi: 10.1037/a0023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Snyderman M, Coe D. Influences of delay and rate of reinforcement on discrete-trial choice. J Exp Psychol Anim Behav Process. 1985;11:565–575. [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse. 1989;4:156–161. doi: 10.1002/syn.890040209. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG. Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry. 2014;171:881–888. doi: 10.1176/appi.ajp.2014.13121581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8. Washington: National Academy Press; 2010. [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attention dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology. 2009;202:307–313. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology. 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37:1779–1788. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacol. 2013;86:309–316. doi: 10.1016/j.bcp.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology. 2014;231:1615–1626. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB. Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb Cortex. 2014;24:154–162. doi: 10.1093/cercor/bhs297. [DOI] [PubMed] [Google Scholar]
- Tian Q, Stepaniants SB, Mao M, Weng L, Feetham MC, Doyle MJ, Yi EC, Dai H, Thorsson V, Eng J, Goodlett D, Berger JP, Gunter B, Linseley PS, Stoughton RB, Aebersold R, Collins SJ, Hanlon WA, Hood LE. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol Cell Proteomics. 2004;3:960–969. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, Cleveland HH, Sanders ML, Gard JM, Stever C. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–1776. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Milich R, Fillmore MT. Behavioral components of impulsivity predict alcohol consumption in adults with ADHD and healthy controls. Drug Alcohol Depend. 2011;113:139–146. doi: 10.1016/j.drugalcdep.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Yates JR, Darna M, Gipson CD, Dwoskin LP, Bardo MT. Dissociable roles of dopamine and serotonin transporter function in a rat model of negative urgency. Behav Brain Res. 2015;291:201–208. doi: 10.1016/j.bbr.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Darna M, Gipson CD, Dwoskin LP, Bardo MT. Isolation rearing as a preclinical model of attention/deficit-hyperactivity disorder. Behav Brain Res. 2012;234:292–298. doi: 10.1016/j.bbr.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology. 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]