Abstract
Glucocorticoid and immune pathways typically interact dynamically to optimize adaptation to stressful environmental challenges. We tested the hypothesis that a dysfunctional glucocorticoid-immune relationship contributes to abnormal stress response in schizophrenia. Saliva samples from 34 individuals with schizophrenia (20 male, 14 female) and 40 healthy controls (20 male, 20 female) were collected prior to and at 3 time points following completion of a computerized psychological challenge meant to be frustrating. Salivary concentrations of cortisol and interleukin-6 (IL-6) and their response to the challenge were examined. Both cortisol and IL-6 significantly increased in response to stress in the combined sample (both p<0.05). In controls, the rise in cortisol following the challenge was negatively correlated to the subsequent changes in IL-6 (r=−.461, p=.003), such that rise of cortisol immediately after stress predicts subsequently lower IL-6 levels. In contrast, this relationship was positive in schizophrenia patients (r=.379, p=.027). The trends were significantly different (Z=3.7, p=.0002). This stress paradigm induces a rise in both cortisol and IL-6. In healthy controls, a more robust acute cortisol response was associated with a steeper decline of IL-6 levels following stress, corresponding to the expected anti-inflammatory effects of cortisol. Patients exhibited the opposite relationship, suggesting an inability to down-regulate inflammatory responses to psychological stress in schizophrenia; or even a paradoxical increase of IL-6 response. This finding may partially underlie abnormalities in inflammatory and stress pathways previously found in the illness, implicating dysregulated stress response in the chronic inflammatory state in schizophrenia.
Keywords: schizophrenia, stress, inflammation, cortisol, IL-6
1. Introduction
Stress and inflammation have been extensively studied in relationship to the disease process in schizophrenia. Stressful life experiences in prenatal development (van Os and Selten, 1998) and early childhood (Varese et al, 2012) increase the risk of developing schizophrenia in adult life; stressful life events in adolescence and adulthood may precipitate the first episode of psychosis and subsequent exacerbations of the illness (Beards et al, 2013; Docherty et al, 2009; Tessner et al, 2011). In parallel, increasing evidence indicates immune pathway dysfunction in schizophrenia: autoimmune disorders and infections are associated with increased risk of developing schizophrenia (Benros et al, 2011; Eaton et al, 2006); elevated levels of pro-inflammatory cytokines are found in high risk and first episode psychosis patients (Noto et al, 2015; Stojanovic et al, 2014) and also during symptom exacerbations and stable phases of chronic illness (Miller et al, 2011; Potvin et al, 2008); and genome-wide association studies have identified immune system gene loci as among the leading associations with schizophrenia (Andreassen et al, 2015; Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015; Schizophrenia Working Group of the Psychiatric Genomics Consortium 2014; Stefansson et al, 2009). As stress responses and immune functions are two closely interacting systems (Coutinho and Chapman 2011; Rhen & Cidlowski 2005), we hypothesized that a dysfunction in the homeostatic interactions between glucocorticoid and immune pathways could represent a pathophysiological mechanism contributing to chronic low-grade inflammation in the disorder.
To test this hypothesis, we used a psychological stress paradigm to evoke responses of both the hypothalamic-pituitary-adrenal axis (cortisol) and immune system (using interleukin-6 [IL-6] as a representative inflammatory cytokine) and studied their interactions in schizophrenia patients. Many immune markers have been associated with schizophrenia, although few have been consistent across studies. In three quantitative reviews, Potvin et al (2008) found an increase in IL-1RA, sIL-2R, and IL-6 and a decrease in IL-2 in schizophrenia; Miller et al (2011) found increased IL-1β, IL-6, TGF-β in first episode cases and IL-12, IFN-γ, TNF-α, and sIL-2R in stable, treated patients; and Upthegrove et al (2014) found that elevated IL-1β, sIL-2R, IL-6, and TNF-α were associated with treatment-naïve, first episode schizophrenia. Only two cytokines, IL-6 and sIL-2R, were consistently found to be elevated in schizophrenia across these meta-analyses. Similar to how cortisol acts as a systemic signal to coordinate stress responses, cytokines are proteins that act systemically to regulate immunologic responses (Steinke and Borish, 2006). IL-6 is a pleiotropic, pro-inflammatory cytokine that is released from several immune and non-immune cell types, and mediates acute inflammatory responses to immune challenges (Kishimoto et al 1992; Kopf et al 1994). Dysregulated IL-6 production is known to be associated with many inflammatory and autoimmune disorders (Yao et al, 2014).
Under normal physiological conditions, glucocorticoids and cytokines closely interact. Cortisol exerts anti-inflammatory effects by inhibiting transcription of pro-inflammatory cytokines, among other mechanisms (Coutinho and Chapman 2011; Rhen & Cidlowski 2005). This is why glucocorticoids are routinely used to suppress acute inflammatory reactions in many medical conditions. Therefore, one would expect that higher cortisol responses should be associated with lower pro-inflammatory cytokine responses. In schizophrenia, both elevated cortisol levels (Gunduz-Bruce et al, 2007; Mondelli et al, 2010; Ryan et al, 2004) and elevated plasma levels of IL-6 (Miller et al, 2011; Potvin et al, 2008) have been found, suggesting a potential abnormality in cortisol-immune interaction. However, we lack direct evidence of disrupted cortisol-immune interactions in schizophrenia patients. Establishing a laboratory model to test interactions between the HPA axis and immune system may provide important leads regarding the pathophysiology of stress and inflammation in schizophrenia. Clinical laboratory investigation of such interactions presents a methodological challenge in that these interactions involve multiple feedback mechanisms, which must be studied within a paradigm that incorporates a dynamic response over time. In human studies, this can be probed through the use of stress-inducing tasks. Previous studies have demonstrated that a rise in inflammatory cytokines, along with cortisol, can be detected following psychological challenges including computerized cognitive tasks, cold pain and the Trier Social Stress Test (Goodin et al, 2012; Izawa et al, 2013; Kunz-Ebrecht et al, 2003; Steptoe et al, 2007). Furthermore, these studies showed an inverse relationship between cortisol response and change in the inflammatory markers in healthy volunteers; this suggests that the anti-inflammatory effects of cortisol can be studied in vivo with brief stress challenges and noninvasive saliva sampling. Hence, our approach was to examine the relationship of salivary cortisol and IL-6 responses to a stress challenge, and to compare this relationship between schizophrenia patients and healthy controls.
2. Methods
2.1. Participants
Patients with schizophrenia spectrum disorder (schizophrenia or schizoaffective disorder) were recruited from Maryland Psychiatric Research Center outpatient clinics and nearby mental health clinics. Control participants were recruited through local media advertisements. Diagnoses in patients and lack of axis I diagnosis in controls were confirmed through use of the Structured Clinical Interview for DSM-IV. Exclusion criteria included major medical and neurological illnesses, history of head injury with cognitive sequelae, mental retardation, substance dependence within the past 6 months, and current substance abuse besides nicotine. Participants were excluded if they had an active infection, were taking an antibiotic, or took anti-inflammatory drugs on a regular basis. All study procedures were approved by the University of Maryland Baltimore IRB. Participants included 34 individuals with schizophrenia and 40 healthy controls. The 74 participants selected for this study are a subsample of the participants (n≈150) who have completed a stress challenge study that is ongoing. The current sample was selected to frequency-match patients and controls on age and gender. In addition, patients are well-known to have high rate of smoking (de Leon and Diaz, 2005) and as reported earlier, higher rate of distress intolerance (Nugent et al, 2014). Therefore, we also frequency-matched patients and controls on the proportion of distress intolerant versus non-distress intolerant individuals, and smokers versus non-smokers. This stringent matching was used to limit the impact of these variables on group comparisons, so that the focus is on comparing cortisol and immune responses to stress. However, the samples after matching do not necessarily represent the clinical characteristics of schizophrenia in the general population. The matching was ‘blinded’ in the sense that samples were selected prior to salivary IL-6 assays. Of the 34 patients, 9 were diagnosed with schizoaffective disorder and 25 with schizophrenia. Duration of illness ranged from 6 months to 39 years, with an average of 13.9 (± 12.6) years. Two of the patients were not taking medications at time of study; 4 were taking typical antipsychotics, 17 were taking atypical antipsychotics other than clozapine, 7 were taking clozapine, and 4 were taking more than one antipsychotic.
2.2. Clinical assessments
Cognitive deficits are among the most debilitating aspects of schizophrenia, with processing speed and working memory being particularly impacted (Dickinson et al., 2008; Forbes et al., 2009). To explore how cognitive deficits might be related to abnormalities in stress response, participants were tested with the Digit Symbol Coding task of the WAIS-3 (Wechsler, 1997) and the Digit Sequencing task from the Brief Assessment of Cognition in Schizophrenia (Keefe et al, 2004), to assess processing speed and working memory, respectively. Scores were calculated as t scores according to population norms for the Digit Symbol Coding task and the Digit Sequencing task (Keefe et al, 2008). Overall psychiatric symptoms were assessed with the mean of the 20 item Brief Psychiatric Rating Scale (BPRS); subscales for positive psychosis symptoms and anxiety/depression symptoms were also generated in order to test the influence of specific symptom domains (Overall and Gorham, 1962). All cognitive and clinical assessments were completed on a separate day from the stress-inducing behavioral paradigm.
2.3. Behavioral paradigm
The behavioral paradigm used to induce stress in participants involved two computerized tasks, the Paced Auditory Serial Addition Task (PASAT) (Lejuez et al, 2003) and Mirror-Tracing Persistence Task (MTPT) (Strong et al, 2003). The PASAT requires participants to add numbers presented on the screen consecutively; they must remember the previous number presented in order to make the correct calculation. The MTPT requires participants to guide a cursor along the outline of a star, with the movement of the cursor opposite to the movement of the mouse. In a practice session, the computer program recorded the participants’ performance; this performance is used to titrate the computer program in the testing session such that the tasks were arbitrarily difficult, with obnoxious noises played any time the participant made a mistake. Participants were instructed that they could earn a monetary bonus for completing the tasks, but were not told how long the tasks would last. They were also told they can end the task at any time. If a participant quit both tasks early, they were deemed distress intolerant (DI); otherwise they were deemed not to be distress intolerant (non-DI). Combining the two tasks allows for a more rigorous definition of distress intolerance by reducing the potential skill-dependent bias that would result if some participants were particularly skilled at arithmetic or eye-hand coordination. The order of tasks was randomized across participants. Further details on the stressor employed in this study have been published elsewhere (Chiappelli et al, 2014; Nugent et al, 2014).
2.4. Saliva collection and biochemical assay
Saliva was collected by passive drool at four time points – baseline, immediately following the behavioral challenge (0 minute post-task), and at 20 minutes and 40 minutes post-task. During the post-stress period the participants sat quietly and read magazines. All testing sessions were held between 1200h and 1600h. Participants were asked to refrain from eating, drinking or smoking for one hour before the testing session, and were instructed to rinse their mouths with water about 10 minutes prior to first saliva collection. Saliva samples were immediately stored at −80° Celsius until assay. Prior to assay, samples were thawed and centrifuged at 10,000 × g for 10 minutes. IL-6 was measured using an enzyme-linked immunosorbent assay (ELISA) (Salimetrics) according to protocol supplied by the manufacturer of the kit; the IL-6 assay kit has a detection limit of 0.07 pg/mL. Inter-assay coefficient of variance (CV) in our lab was 5.63 %, and intra-assay CV was 2.33 %. Cortisol was also measured using an ELISA kit (Salimetrics) with a detection limit of 0.007 μg/dl, with an intra-assay CV of 7.77% and inter-assay CV of 4.18%.
2.5. Data analysis
The null hypothesis is that IL-6 change is a function of the cortisol response: acute cortisol rise in response to stress will be inversely associated with the subsequent post-stress IL-6 response, and we will test whether this relationship is disrupted in schizophrenia. Repeated-measures ANOVA of the 4 time points of saliva collection were used to examine the cortisol and IL-6 responses to stress, with diagnosis as a between-subjects variable and sex as a covariate, given known influences of sex over glucocorticoid and immune functions (Chrousos, 2010; Da Silva, 1999; Duma et al, 2010). Greenhouse-Geisser corrected statistics were used. Post-hoc exploration of the data used log-transformed IL-6 levels, which successfully transformed the data to normal distribution. For this study, the acute cortisol response was defined as the difference between cortisol level immediately following the stressors (post-stress 0 minute time point) and baseline. Changes of IL-6 following acute stress were examined as the post-stress IL-6 response (post-stress 40 minutes minus post-stress 0 minute). All test were two-tailed with alpha set at .05.
3. Results
3.1. Cortisol response to stressor
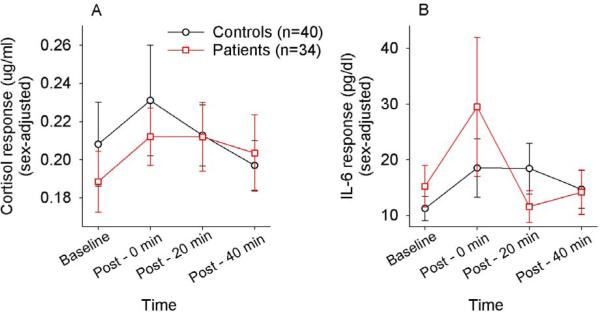
Baseline cortisol levels were not significantly different between controls and patients (t=.69, p=.50). Repeated measures ANOVA for salivary cortisol levels revealed a significant effect of time (F(2.16,153.3)=3.02, p=.048), with no significant main effect of diagnosis (p=.703) or gender (p=.592), no significant time × diagnosis interaction (p=.399) and a non-significant trend for a time × gender effect (F=2.74, p=.063). Post-hoc paired t-tests comparing baseline to post-stress time points showed that cortisol levels at the post-stress 0 minute time point (Mean ± s.d. =.222 ±.15 ug/dL) were greater than at baseline (M=.199 ±.12 ug/dL; t=2.65, p=.010). Subsequent cortisol levels at 20 or 40 minutes post-stress were not statistically significantly different from baseline (all p>.25) (Figure 1A).
Figure 1.
Time courses of salivary cortisol (A) and interleukin -6 (IL-6) (B) (sex-adjusted means) in response to the brief psychological stress. Post: minutes after the end of the stress challenge. Error bars represent standard error mean.
3.2. IL-6 response to stressor
Baseline IL-6 values were not significantly different between controls and patients (t=.96, p=.34). The repeated measures ANOVA demonstrated a significant effect of time on IL-6 levels (F(1.33,94.2)=6.80, p=.006), without a significant main effect of diagnosis (p=.892) or time × diagnosis interaction (p=.156). There was a non-significant trend towards a time × gender interaction (F=2.88, p=.081), as well as a non-significant, trend-level main effect of gender (F-3.43, p=.068). Post-hoc tests showed that, in the combined sample, average IL-6 immediately following the stressor (post0; 23.5 ± 54.8 pg/mL) was significantly greater than at baseline (13.1 ± 17.8 pg/mL; t=2.18, p=.033). Subsequent IL-6 levels at 20 or 40 minutes post-stress were not significantly different from baseline (all p>.291) (Figure 1B). These findings showed that the brief psychological stress paradigm is successful in evoking, on average, transient cortisol and IL-6 responses.
3.3. Relationship of IL-6 response and cortisol response
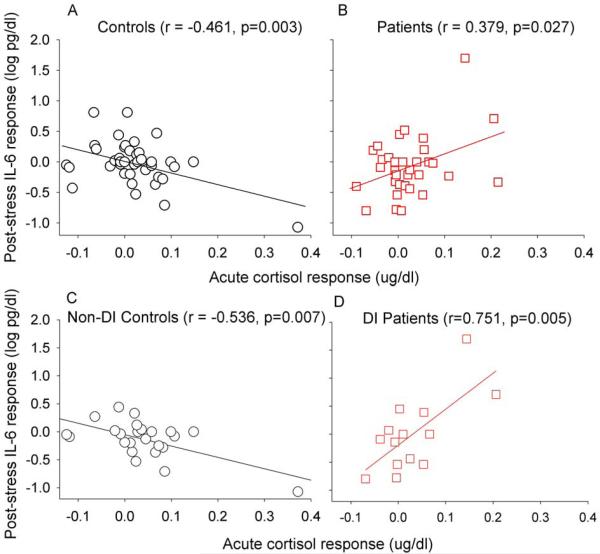
As predicted, controls showed a significant negative correlation (r=−.461, p=.003) between acute cortisol response and post-stress IL-6 change. In schizophrenia patients, an opposite, significantly positive correlation between acute cortisol response and post-stress IL-6 change was observed (r=.379, p=.027) (Figure 2A and 2B). The Fisher transformation of these correlations indicates a significant difference (Z=3.7, p=.0002). Although there appeared to be ‘outliers’ (Figure 2), Kolmogorov–Smirnov tests for normality showed that these measures followed normal distributions (all Kolmogorov-Smirnov z <0.97, all p>0.29, two-tailed). This suggests that in controls, as expected, a more robust cortisol response inhibits subsequent pro-inflammatory response. However, a relationship in the opposite direction was found in patients.
Figure 2.
Scatterplots of the relationship between acute cortisol response and post-stress IL-6 response in healthy controls (A) and schizophrenia patients (B); and in non-distress intolerant (non-DI) controls (C) and distress intolerant (DI) patients (D).
3.4. Relationship to behavior
We also examined the relationship between distress intolerance and the cortisol - IL6 responses. Among non-DI individuals, acute cortisol and post-stress IL-6 change were negatively correlated (n=44, r=−.354, p=.019), while this relationship was positive among the DI participants (n=30, r=.478, p=.025). Note that while schizophrenia patients are more likely to be DI (Chiappelli et al, 2014; Nugent et al, 2014), the proportion of DI vs. non-DI participants were arbitrarily matched in the current sample. Still, the Fisher transformation revealed that these trends were significantly different (Z=3.2, p=.0013). Further dividing the data by both diagnosis and DI phenotype, we found that the negative correlation between acute cortisol and post-stress IL-6 was driven by the non-DI controls (r=−.536, p=.007) and that the positive correlation was driven by DI patients (r=.751, p=.005) (Figure 2C and 2D); the relationship between these variables was statistically insignificant in DI controls (r=.277, p=.44) and non-DI patients (r=−.034, p=.88).
3.5. Relationship to cognition and clinical variables
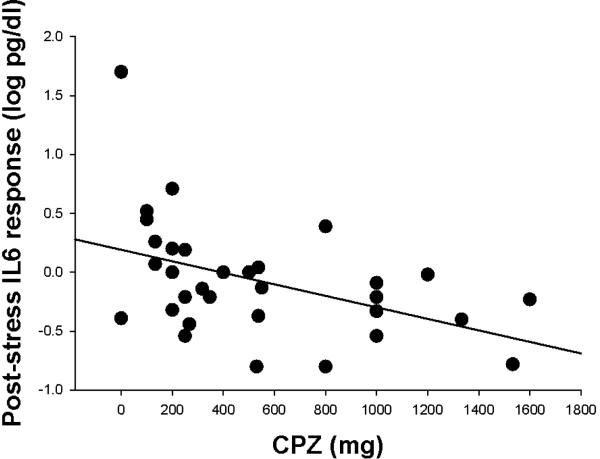
Neither age nor body-mass index (BMI) was associated with acute cortisol or post-stress IL-6 responses in either patients or controls (all p>.20). In patients, acute cortisol response was inversely correlated with processing speed (r=−.394, p=.021) but not significantly correlated with working memory (r=.020, p=.91). The post-stress IL-6 response was significantly and negatively correlated with working memory (r=−.358, p=.038) but not processing speed (r=−.276, p=.11). CPZ of antipsychotic medications was not significantly associated with acute cortisol response (r=−.011, p=.95) but interestingly, was significantly associated with post-stress IL-6 response (r=−.449, p=.008) (Figure 3). A partial correlation between acute cortisol and post-stress IL-6 changes in patients, controlling for CPZ, revealed that the correlation remained significant (r=.419, p=.015), suggesting that the correlation was not substantially influenced by current antipsychotic medication dosages. Mean BPRS scores were not associated with either the acute cortisol (r=.038) or post-stress IL-6 (r=−.183) responses in patients (both p>.05). Levels of anxiety and depression symptoms, measured with the BPRS anxiety/depression subscale, were not associated with baseline cortisol or IL-6, nor with acute cortisol or post-stress IL-6 response in patients or controls (all p>.20). There was a nominally significant relationship between BPRS psychosis subscale scores and baseline IL-6 levels in patients (r=.381, p=.031) but psychotic symptoms were not significantly related to baseline cortisol, acute cortisol response, or post-stress IL-6 response (all p>.15). Duration of illness was not significantly correlated with cortisol, acute cortisol response, or post-stress IL-6 response (all p>.17). There was no significant smoking (p=.96) or smoking × diagnosis effect (p=.57) on acute cortisol; and no significant smoking (p=.98) or smoking × diagnosis effect (p=.19) on post-stress IL-6 response. Linear regression models were used to examine the influence of CPZ, smoking, gender, BMI, and age on the relationship between acute cortisol response and post-stress IL-6 response. In controls, acute cortisol was still a negative predictor of post-stress IL-6 response even after controlling for these other variables (beta=-.563, p=.001). Similarly, in patients, acute cortisol was still a positive predictor of post-stress IL-6 response in this analysis (beta=.398, p=.014).
Figure 3.
Scatterplot of the relationship between antipsychotic medication dosage, calculated as chlorpromazine dose equivalent (CPZ), and post-stress IL-6 response in patients only.
4. Discussion
The brief stress-inducing behavioral paradigm employed in this study demonstrated a classical glucocorticoid-immune dynamic in healthy controls, such that higher acute cortisol response was associated with lower subsequent IL-6 response. This inverse relationship is consistent with the known role of glucocorticoids in suppressing pro-inflammatory cytokine production (Rhen and Cidlowski, 2005), and is also consistent with findings of previous studies examining cortisol and immune responses to stress (Goodin et al, 2012; Izawa et al, 2013; Kunz-Ebrecht et al, 2003). In individuals with schizophrenia, this relationship was disrupted such that greater acute cortisol response to stress was associated with a greater IL-6 response in the post-stress period.
The coordination of cortisol and IL-6 responses to stress is complex, and involves interactions beyond the anti-inflammatory effect of glucocorticoids. Increased cytokine production is observed in response to not only immune challenges but also physical stressors such as intense exercise and acute psychological stress (Brydon et al, 2009; LeMay et al, 1990; Steptoe et al, 2007; Zhou et al, 1993). Pro-inflammatory cytokines can act as strong stimulants of the hypothalamic-pituitary-adrenal (HPA) axis (Lyson and McCann, 1991; Mastorakos et al, 1993). In turn, glucocorticoids can suppress cytokine gene transcription via inhibition of the NF-kB pathway that normally acts positively on the IL-6 promotor (Ray and Prefontaine, 1994; Sehgal, 1992). These neuroimmunoendocrine regulatory loops may be sensitive to stress or immune challenges in early development (John and Buckingham, 2003; Johnson et al, 2002a; Johnson et al, 2002b). A poorly suppressed or exacerbated immune response to stress may lead to low-grade inflammation following everyday psychological stress or immune challenges (Rohleder, 2012; Silverman and Sternberg, 2012). Several recent large-scale genomic studies have identified an association of immune-related loci with schizophrenia (Andreassen et al, 2015; Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015; Schizophrenia Working Group of the Psychiatric Genomics Consortium 2014; Stefansson et al, 2009); the interaction of stress exposure and immune risk alleles may partially contribute to elevated levels of peripheral pro-inflammatory cytokines in schizophrenia.
Two previous studies have shown some evidence of abnormal cortisol and immune responses to stress in schizophrenia. One study used public speaking as a stressor but found that controls have higher cortisol and natural killer cell response to this stressor compared with 10 medication-naïve first episode psychosis patients (van Venrooij et al, 2012). This was interpreted as ‘blunted’ cortisol and immune response in schizophrenia, although this could be difficult to reconcile with the data of increased sensitivity to stress (Myin-Germeys et al, 2001; Myin-Germeys and van Os, 2007) or elevated pro-inflammatory status (Miller et al, 2011; Potvin et al, 2008; Upthegrove et al, 2014) in schizophrenia. In comparison, in the current study schizophrenia patients were able to mount cortisol and IL-6 responses to the brief psychological stress challenge that were statistically not different from controls, which enabled the testing of the relationship between acute cortisol and subsequent IL-6 response. The difference in response to different types of stressors is interesting and raises the issue of the optimal laboratory task(s) for modeling cortisol-immune interactions in schizophrenia. Another study found that schizophrenia patients exhibited a less robust plasma IL-6 response after gastrointestinal surgery (Kudoh et al, 2001), which also may seem contradictory to evidence of increased basal IL-6 in schizophrenia. However, stress from major abdominal surgeries does not reflect the everyday stress experienced by patients. A more modest stressor may be necessary to allow examination of the more typical temporal relationship between glucocorticoid and immune responses. This simple laboratory paradigm showed that the salivary IL-6 response is not necessarily increased or blunted in schizophrenia patients. Rather, salivary IL-6 response to stress is heterogeneous in schizophrenia patients in part depending on behavioral and initial cortisol response.
An important limitation of this study to note is that IL-6 was the only marker of inflammation examined. This limits our ability to interpret the full immune system response to stress. With a limited amount of biological sample available, we prioritized IL-6 based on the meta-analyses on peripheral inflammatory markers in schizophrenia (Miller et al, 2011; Potvin et al, 2008; Upthegrove et al, 2014) as well as previous literature suggesting that IL-6 was one of the inflammatory markers most commonly found to rise in response to psychological stress (Steptoe et al, 2007). However, further experiments incorporating additional measures of immune activation, ideally also measured in blood, are necessary to confirm a dysregulation in glucocorticoid-immune interactions in schizophrenia. An additional limitation is that the IL-6 response in some cases may be more prolonged than could be captured in the time period examined. On average, IL-6 peaked within 0 to 20 minutes after stress and largely returned to baseline by 40 minutes. However, for those individuals with low IL-6 response, we cannot rule out that they may still show an IL-6 response beyond 40 minutes. Another limitation of the study is that most of the patients were on antipsychotic medications, which may have anti-inflammatory effects (Maes et al, 1995). We found that CPZ was negatively associated with post-stress IL-6 response levels, consistent with the anti-inflammatory hypothesis. However, the partial correlation analysis showed that the relationship between acute cortisol and post-stress IL-6 change was significant even controlling for antipsychotic dose. Antipsychotic medications could potentially have masked additional findings regarding IL-6 levels in response to stress, and thus it would be valuable to examine cortisol-immune interactions in a sample of medication-free patients. Since our focus was on the response of participants to psychological stress, we used saliva samples in order to avoid additional stress induced by a blood draw, which would represent a potential confound. However, caution is necessary in interpreting how changes in salivary IL-6 reflect systemic responses. Although cortisol levels in the saliva show a tight correspondence with cortisol levels in blood (Dorn et al, 2007), correlation between concentration of IL-6 in the saliva and in blood is poor (Sjögren et al, 2006), and the degree of correlation between saliva vs. blood IL-6 responses to stress remains to be determined. Results of previous studies on cortisol levels in schizophrenia have also been more variable when salivary measures were used as opposed to blood measures (Karanikas et al, 2014). Still another factor to consider is that the oral cavity is highly exposed to microbes, and variability in oral health and hygiene can contribute to variability observed in inflammatory markers in oral fluids. In this study, we attempted to limit this variability by restricting smoking and eating in the period immediately preceding the stress challenge, and by having participants rinse their mouths with water 10 minutes before saliva collection. Additionally, since our analyses were based on the relative change in cortisol and IL-6 over time as opposed to values at a single time point, it is less likely that our conclusions are undermined by the potential confound of variable oral health.
Further investigation is needed to identify the cause and clinical consequences of dysregulated cortisol-immune interactions in stress response in schizophrenia. In this regard, the negative correlations to processing speed and working memory suggest that the extent of the post-stress IL-6 response may have clinical relevance; and is consistent with reports that levels of cytokines are linked to cognitive abnormalities in schizophrenia (Fineberg and Ellman, 2013; Frydecka et al, 2015). Together, these results support theories emphasizing stress-mediated vulnerability to schizophrenia (van Winkel et al, 2008) as well as theories that emphasize immune-mediated pathways to psychosis (Bergink et al, 2014). Disrupted interactions of HPA axis and immune signaling following psychological stress exposure may represent an important mechanism of the pathophysiological effects of stress in schizophrenia.
Highlights.
-Schizophrenia patients did not differ significantly from health controls in magnitude of salivary IL-6 response to a psychological stress task
-Acute cortisol response was inversely related to post-stress IL-6 changes in healthy controls, but the opposite pattern was found in patients
Table 1.
Demographics of the sample. Note that these data are not necessarily characteristic of either healthy controls or schizophrenia patients as these characteristics have been pre-selected to balance key variables between groups.
| Healthy Control (n=40) | Schizophrenia (n=34) | Test Statistic | p value | |
|---|---|---|---|---|
| Age [years] (SD) | 38.1 (14.1) | 39.9 (12.9) | t=.59 | .558 |
| %Male | 50.0 | 58.8 | χ2=.576 | .448 |
| %Smoker | 30.0 | 35.3 | χ2=.194 | .659 |
| % Distress intolerant | 40.0 | 41.2 | χ2=.011 | .918 |
| Body-mass index (SD) | 26.4 (4.1) | 28.8 (4.6) | t=2.35 | .021 |
| Processing Speed (SD) | 10.4 (2.8) | 7.6 (3.3) | t=3.84 | <.001 |
| Working Memory (SD) | 43.9 (13.3) | 37.2 (14.6) | t=1.99 | .050 |
| BPRS Total mean (SD) | n/a | 2.1 (.53) | n/a | |
| BPRS Anxiety/Depression subscale mean (SD) | n/a | 2.2 (.94) | n/a | |
| BPRS Psychosis subscale mean (SD) | n/a | 2.8 (1.3) | n/a |
Funding / Acknowledgments
Support was received from National Institutes of Health grants R01MH085646, R01DA027680, T32MH067533, P50MH103222, and a NARSAD Young Investigator Award from the Brain and Behavior Foundation.
Role of funding source
Funding sources had no role in collection or analysis of data, preparation of manuscript, or decision to submit manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed to the design of the experiment, acquisition and analysis of data, and preparation of the manuscript. All authors have approved the final version of the manuscript submitted.
Conflict of interest statement:
Dr. Elliot Hong receives unrestricted research funding from Mitsubishi, Your Energy Systems LLC, and Pfizer. All other authors declare no conflict of interest.
References
- Andreassen OA, Harbo HF, Wang Y, Thompson WK, Schork AJ, Mattingsdal M, Zuber V, Bettella F, Ripke S, Kelsoe JR, Kendler KS, O'Donovan MC, Sklar P, The Psychiatric Genomics Consortium (PGC) Bipolar Disorder and Schizophrenia Work Groups; The International Multiple Sclerosis Genetics Consortium (IMSGC) McEvoy LK, Desikan RS, Lie BA, Djurovic S, Dale AM, The Psychiatric Genomics Consortium PGC Bipolar Disorder and Schizophrenia Work Groups; The International Multiple Sclerosis Genetics Consortium IMSGC Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20:207–14. doi: 10.1038/mp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C. Life events and psychosis: a review and meta-analysis. Schizophr Bull. 2013;39:740–7. doi: 10.1093/schbul/sbt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry. 2014;75:324–31. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, Tsuda A, Steptoe A. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav Immun. 2009;23:217–24. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli J, Pocivavsek A, Nugent KL, Notarangelo FM, Kochunov P, Rowland LM, Schwarcz R, Hong LE. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry. 2014;71:761–8. doi: 10.1001/jamapsychiatry.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and sex versus immunity and inflammation. Sci Signal. 2010;3:pe36. doi: 10.1126/scisignal.3143pe36. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva JA. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–17. doi: 10.1111/j.1749-6632.1999.tb07628.x. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–7. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty NM, St Hilaire A, Aakre JM, Seghers JP. Life events and high-trait reactivity together predict psychotic symptom increases in schizophrenia. Schizophr Bull. 2009;35:638–645. doi: 10.1093/schbul/sbn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Ann Clin Biochem. 2007;44:281–4. doi: 10.1258/000456307780480954. [DOI] [PubMed] [Google Scholar]
- Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010;3:ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, Mortensen PB. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–8. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–66. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P, Kiejna A, Beszłej JA. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265:449–59. doi: 10.1007/s00406-014-0533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin BR, Quinn NB, King CD, Page GG, Haythornthwaite JA, Edwards RR, Stapleton L, McGuire L. Salivary cortisol and soluble tumor necrosis factor-α receptor II responses to multiple experimental modalities of acute pain. Psychophysiology. 2012;49:118–27. doi: 10.1111/j.1469-8986.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Szeszko PR, Gueorguieva R, Ashtari M, Robinson DG, Kane JM, Bilder RM. Cortisol levels in relation to hippocampal sub-regions in subjects with first episode schizophrenia. Schizophrenia Res. 2007;94:281–287. doi: 10.1016/j.schres.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Izawa S, Sugaya N, Kimura K, Ogawa N, Yamada KC, Shirotsuki K, Mikami I, Hirata K, Nagano Y, Nomura S. An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biol Psychol. 2013;94:249–54. doi: 10.1016/j.biopsycho.2013.06.006. [DOI] [PubMed] [Google Scholar]
- John CD, Buckingham JC. Cytokines: regulation of the hypothalamo-pituitary-adrenocortical axis. Curr Opin Pharmacol. 2003;3:78–84. doi: 10.1016/s1471-4892(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002a;16:461–76. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology. 2002b;27:353–65. doi: 10.1016/s0306-4530(01)00057-9. [DOI] [PubMed] [Google Scholar]
- Karanikas E, Antoniadis D, Garyfallos GD. The role of cortisol in first episode of psychosis: a systematic review. Curr Psychiatry Rep. 2014;16:503. doi: 10.1007/s11920-014-0503-7. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102:108–15. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–7. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Sakai T, Ishihara H, Matsuki A. Plasma cytokine response to surgical stress in schizophrenic patients. Clin Exp Immunol. 2001;125:89–93. doi: 10.1046/j.1365-2249.2001.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17:373–83. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, Kluger MJ. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol. Behav. 1990;47:957–961. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Lejuez C, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory based stressor. BehavTherap. 2003;26:290–293. [Google Scholar]
- Lyson K, McCann SM. The effect of interleukin-6 on pituitary hormone release in vivo and in vitro. Neuroendocrinology. 1991;54:262–266. doi: 10.1159/000125884. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995;29:141–52. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J. Clin. Endocrinol. Metab. 1993;77:1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D'Albenzio A, Di Nicola M, Fisher H, Handley R, Marques TR, Morgan C, Navari S, Taylor H, Papadopoulos A, Aitchison KJ, Murray RM, Pariante CM. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J. Stress-reactivity in psychosis : evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–24. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psych. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC); International Inflammatory Bowel Disease Genetics Consortium IIBDGC Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola LM, Honda PH, Cordeiro Q, Belangero SI, Bressan RA, Gadelha A, Maes M, Brietzke E. Effects of Risperidone on Cytokine Profile in Drug-Naïve First-Episode Psychosis. Int J Neuropsychopharmacol. 2015;18:pyu042. doi: 10.1093/ijnp/pyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent KL, Chiappelli J, Rowland LM, Daughters SB, Hong LE. Distress intolerance and clinical functioning in persons with schizophrenia. Psychiatry Res. 2014;220:31–6. doi: 10.1016/j.psychres.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE. Physical association and functional antagonism between the ~65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Anti-inflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems --2011 Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37:307–16. doi: 10.1016/j.psyneuen.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Ryan M, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary–adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB. Regulation of IL6 gene expression. 46th Forum in Immunology. Res. Immunol. 1992;143:724–734. doi: 10.1016/0923-2494(92)80011-9. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors: studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain Behav Immun. 2006;20:270–8. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Børglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Böttcher Y, Olesen J, Breuer R, Möller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Réthelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Genetic Risk and Outcome in Psychosis (GROUP) Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jönsson EG, Terenius L, Agartz I, Petursson H, Nöthen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke JW, Borish L. Cytokines and chemokines. J Allergy Clin Immunol. 2006;117:S441–S445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, Labad J. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32. doi: 10.1016/j.psyneuen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Strong D, Lejuez C, Daughters S, Marinello M, Kahler C, Brown R. The computerized mirror tracing task version 1. 2003 Unpublished manual. [Google Scholar]
- Tessner KD, Mittal V, Walker EF. Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophr Bull. 2011;37:432–441. doi: 10.1093/schbul/sbp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–8. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten J. Prenatal exposure to maternal stress and subsequent schizophrenia. the may 1940 invasion of the Netherlands. Br J Psychiatry. 1998;172:324–6. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- van Venrooij JA, Fluitman SB, Lijmer JG, Kavelaars A, Heijnen CJ, Westenberg HG, Kahn RS, Gispen-de Wied CC. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr Bull. 2012;38:272–9. doi: 10.1093/schbul/sbq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–71. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) Harcourt Assessment; San Antonio, TX: 1997. [Google Scholar]
- Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]