INTRODUCTION
Existing literature is relatively consistent in showing that African-American and Latino youth in underserved communities are more likely to use the Emergency Department (ED) for asthma as a primary source of care.1 This episodic care impedes development of the patient self-regulation skills necessary for asthma control.2 Therefore, acute care settings potentially represent a promising means of identifying and intervening upon asthma patients with the greatest need.
Puff City is a web-based, computer-tailored intervention that targets urban teens.3, 4 The program has been evaluated in high school-based randomized trials. In these school-based trials, students with active asthma (symptoms in the last year and/or currently being treated for asthma) were invited to participate, regardless of asthma control, severity, or health care access. Study results showed positive outcomes for students in the intervention group.3, 4
There is evidence that internet access is increasing among US African-American, Latino, and low income communities. In the most recent school-based trial of Puff City, 90% of the students without computers were able to complete at least 3 of the 4 online sessions."3 Given the positive results of the Puff City school-based programs, we wished to explore the feasibility of initiating Puff City in the ED. This would consist of an online baseline questionnaire in the ED, followed by online sessions that are accessed during and after the ED visit.
The pilot study described here is the result of funding from The National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) PAR-13-002 which “encourages applications proposing pilot studies to obtain data that are critical for the design of robust clinical trials”. We present the preliminary results of a study funded through the R34 mechanism and designed to assess the feasibility of conducting a randomized controlled trial to evaluate initiation of Puff City in an urban ED setting. In this paper we describe (1) the engagement of ED staff in the implementation of a recruitment protocol in the ED; (2) recruitment and refusal rates; and (3) an evaluation of the time needed for baseline completion in the ED. Other concerns included acceptability of participation during an acute exacerbation and our ability to reach marginalized youth. This paper represents the first of a two-phase assessment, in which phase I informs recruitment strategies, and phase II evaluates retention and intervention efficacy in this subgroup of youth presenting to the ED for acute asthma.
Setting
Children’s Hospital of Michigan (CHM) of the Detroit Medical Center (academically affiliated with Wayne State University) is a tertiary care hospital in urban Detroit, shouldering the bulk of indigent care in Detroit. In 2013, a total of 403 patients aged 13-19 years made a visit to CHM Emergency Department for asthma.
Henry Ford Health System (HFHS/HFH) is an 802-bed tertiary care hospital, education, and research complex located in Detroit's New Center area. In 2013, a total of 110 patients aged 13-19 years visited the HFHS Pediatric Emergency Department for asthma.
For this study, the University of Michigan Center for Health Communications Research (UM-CHCR) provided technical expertise in web hosting, interactive web response, randomization set-up, online baseline assessment, computer randomization, control website set-up, and system updates.
Design
The Puff City intervention
The project was approved by HFHS, CHM/WSU (Wayne State University), and University of Michigan Institutional Review Boards. Parental consent and patient assent were required for participation. The intervention is described in previous publications.3,4 Briefly, Puff City is a web-based, asthma management tool that uses tailoring, defined as the “assessment and provision of feedback based on information that is known or hypothesized to be most relevant for each individual participant of a program.”4 Computer algorithms are used to assemble theory-driven feedback based on the user’s characteristics, beliefs, and attitudes, creating an extensive array of message permutations. This allows targeted delivery of health messages, and very personalized asthma education.3, 4 Puff City focuses on controller medication adherence, keeping an inhaler nearby, and smoking reduction or cessation. Also included is information on trigger avoidance, instructions on using medication delivery devices and basic asthma physiology. Behavioral theories applied in Puff City include the Transtheoretical Model, the Health Belief Model5, and aspects of Motivational Interviewing (MI). “Scores” for asthma self-regulation are derived using the Asthma Self-Regulation Development Interview6,developed by Zimmerman et al., which describes sequential "phases" of asthma self-regulation including asthma symptom avoidance, asthma acceptance, asthma compliance, and asthma self-regulation.6 Submodules for Puff City were created in order to apply a more intense tailoring strategy to “resistant adolescents,” defined as adolescents exhibiting no positive change in behavior after the first of four consecutive sessions. Values-based strategies and MI concepts are used to help teens overcome ambivalence to change through empathy and support, as opposed to directives and emotional pulls.7, 8 In addition to the four weekly sessions, a booster session at six months was created to sustain positive change and correct early stages of relapse.
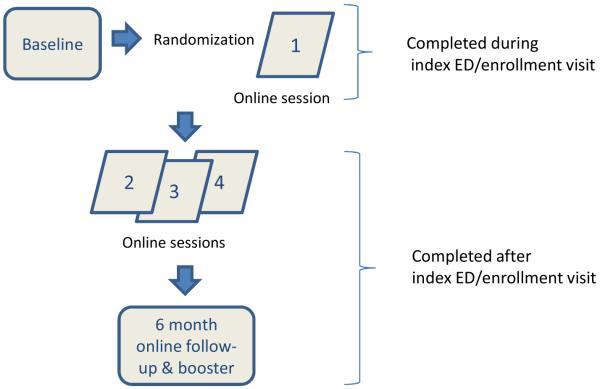
The program consists of a baseline survey, followed by 4 weekly online sessions (Fig. 1). When signing on for the first session, patients are randomized to a treatment (tailored Puff City program) or control group (access to four sessions of existing online asthma education programs).3, 4 Participants are asked to complete a 6 and 12 month follow-up survey. For the intervention group, the 6 month survey triggers the booster session for resistant or relapsed participants. Parents/guardians of participating patients are invited to complete a baseline survey and a 12 month follow-up survey. Automated email and SMS (short message service or “text message”) reminders to complete the online sessions were set-up by the UM-CHCR.
Figure 1.
Puff City ED Intervention: Feasibility Study
Inclusion and exclusion criteria
To participate, teens had to be 13-19 years of age and have a physician diagnosis of acute asthma at the ED visit. Eligibility was confirmed by ED clinical staff. Teens < 18 years had to be accompanied by a parent or legal guardian (caregiver) who would give written consent. All teens provided written assent. Teens were excluded if they had previously participated in Puff City or did not speak English.
METHODS
Engaging ED staff
During start-up, the team met with ED clinicians and research staff at HFHS and CHM to develop strategies for recruitment. IRB approval was obtained from the Henry Ford Health System IRB for HFHS and from Wayne State University IRB for CHM. ED Principal Investigators also provided input on inclusion criteria and developed awareness strategies. CHM had research staff in the ED dedicated to patient recruitment for ongoing studies. At HFHS, research staff and several existing ED clinic staff were trained to recruit and enroll for the study. Research staff at HFHS implemented a schedule to monitor the ED for eligible patients using the electronic ED surveillance system, while CHM study team members monitored the ED surveillance system when on shift.
Before study onset at HFHS, fliers were posted in the ED and an informational letter was emailed to the ED physicians describing Puff City, the eligibility criteria, and the recruitment start dates. Research staff also attended nursing “huddles” to give a brief description of the study and provided updates to physicians, fellows, and residents during monthly divisional meetings. At CHM, monthly status updates were provided during the ED divisional meetings attended by physicians, fellows, and residents, and at monthly ED RN (registered nurse) Practice council meetings attended by RNs, student nurses, and RN leadership. In addition, white boards posted in each ED pod (5 locations) and updated at the start of each RA (research assistant) shift, listed the names of ongoing studies, including basic eligibility criteria and the pager or office number of recruitment staff. Investigators and research coordinators at both sites assisted in identifying and enlisting the help and cooperation of physicians, RNs and key ED staff.
Recruitment strategies
ED Recruitment
We recruited eligible teens during presentation to participating EDs for acute asthma (index visit). Research staff conducted recruitment using remote electronic surveillance and triage systems (EMSTAT and FIRSTNET) to identify teens potentially eligible for the study. At CHM, if a potentially eligible patient presented, the RA/recruiter would flag the patient to notify the ED staff, go to the ED, review eligibility with the treating clinicians, and if eligible, a clinician would first inquire if the family wanted to hear about a research study. If yes, the RA/recruiter would complete the eligibility form and introduce the study to the teen and caregiver and begin the informed consent and assent process, including HIPPA authorization. At HFHS, research staff used EMSTAT to identify potentially eligible patients, and then approached patients after obtaining clearance from a clinician. A process similar to that described for CHM was followed. Caregivers could opt to refuse the caregiver portion of the study while providing permission for the teen to participate. Research staff requested an email address for email reminders and a phone number for SMS reminders. Owning a computer was not a requirement for participation. After receiving written assent from the patient and written consent from the caregiver, some patients chose to return to the ED at a later date and complete the enrollment process.
Recruitment from patient listings or “Mail/phone”
HFHS used the patient encounter databases to obtain listings of patients that had made recent visits to an HFHS ED with a primary or secondary ICD9 code for asthma during the recruitment period. At CHM a daily log including all presentations for age-eligible patients was screened to identify patients that presented to the CHM ED with an asthma-related presentation that had not already been approached in the ED by recruiters. At both sites, a letter was mailed to the patient’s home, followed by a phone call to explain the study. Interested caregivers and teens were asked to come to HFHS or CHM for an enrollment visit. At that time, research staff initiated the consent and enrollment process described above.
Components of study enrollment
Baseline and Session 1
After obtaining consent/assent, teens completed an online baseline questionnaire from a laptop or from a mobile in-house computer. Items on the baseline included current asthma care, current medications used, continuity of care, and patient-provider communication, as well as teen smoking behavior. Questions on asthma control and symptom frequency asked recent periods (last 2 weeks or past 30 days). For this pilot, the first of four sessions was delivered in the ED (if the patient was being enrolled there) or at the enrollment visit. Before leaving the ED patients are given an exit packet containing instructions on how to access the program from any computer with internet access, and information on computer resources in the area. Caregivers were interviewed by the recruiter while the teen was completing the web-based baseline on the computer. The caregiver baseline included questions about teen’s asthma medications, health care encounters, and functional status.
Exit Information
After the enrollment process was complete, the patient was given a packet containing information on how to access Puff City from any computer, numbers to call if there were problems, a schedule of incentives, and information on computer resources in the area.
Estimated enrollment time
The time needed to complete enrollment was taken from recruitment logs and averaged across recruiters. For this estimate, recruitment activities included monitoring electronic triage units, calling patients from patient listings, mailing introductory letters, enrollment of patients returning to the clinic specifically for the study, and actual enrollment during an ED visit.
Randomization
Randomization was stratified by gender, ED visits for asthma in last 12 months, and Asthma Control Test score (ACT). The ACT is a 5-question, patient-based tool designed to help physicians identify patients with poorly controlled asthma, assess frequency of asthma symptoms, use of rescue medications, and impact of asthma on daily functioning over the course of the previous 4 weeks.9 As this was a study conducted among persons with acute asthma exacerbations, randomization was stratified by < 15 on the ACT versus ≥ 15, instead of <19 versus ≥ 19.
Statistical analysis
Descriptive analyses were used to describe participant and non-participant characteristics and ED staff responses to a survey on the recruitment process. A two-sample t-test was used for continuous variables, and a chi-square test for categorical variables when comparing differences between two groups (e.g., enrollees versus non-participants and ED enrolled versus mail/phone).
RESULTS
Recruitment and Refusal rates
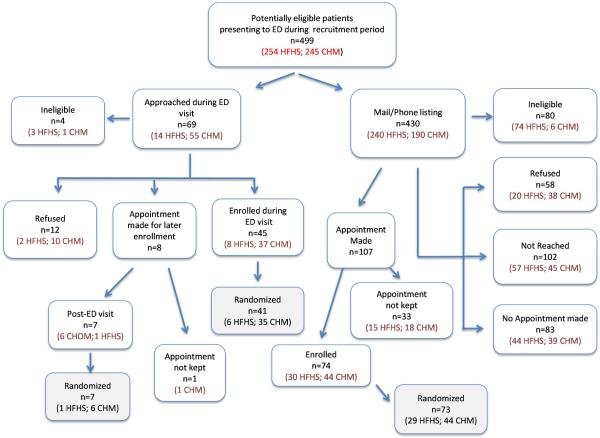
Patients were recruited at HFHS from October 2012 – October 2013 and CHM/WSU 12/03/12 – 10/01/13. Recruitment dispositions are presented in Figure 2. An estimated 499 patients meeting our eligibility criteria (ED visit for acute asthma) presented during the recruitment period. We estimate that we had actual contact in the ED or over the phone with 313 (63%) of these patients (Refused + Appointment made + Enrolled + No Appointment made. This latter group spoke with a recruiter but never committed to an appointment).
Figure 2.
Contact and enrollment disposition for the targeted population
Table 1 shows a comparison of enrolled (and randomized) to non-participants. A total of 126 patients completed a baseline, of which 121 were randomized (96%). Of these, the majority of participants were African-American (88.4%), with a mean age of 15.4 years (±1.7 years). Of the 16 teens ≥ 18 years of age, n=2 (12.5%) had a caregiver accompany them to the ED and complete the caregiver assessment. For the remaining 105 teens < 18 years of age, n=98 (93.3%) opted to complete a caregiver baseline assessment. Enrolled patients did not differ from non-participants with respect to mean age, proportion ≥ 18 years of age, race, or ethnicity. A higher percentage of African-Americans were enrolled, although this was of borderline significance (p=0.08). Patients with Medicaid insurance were more likely to enroll in the study than those with other types of insurance/un-insured (77% versus 36.5%), p<0.01.
Table 1.
Characteristics of the eligible population for combined sample (n=340)
| Factor |
Enrolled
n=121 |
Non-participant
n=219 |
p value | ||
|---|---|---|---|---|---|
| Age, mean (sd) | 15.4 | (1.7) | 15.6 | (1.9) | 0.36 |
| > 18 years, n (%) | 16 | (13.2%) | 39 | (17.8%) | 0.27 |
| Hispanic/Latino, n (%) | 8 | (6.6%) | 7 | (3.2%) | 0.15 |
| African American, n (%) | 108 | (89.3%) | 179 | (81.7%) | 0.08 |
| Medicaid, n (%) | 73 | (60.3%) | 80 | (36.5%) | <0.0001 |
| > 1 ED visit prior to index visit1, n (%) | 63 | (52.1%) | 129 | (58.9%) | 0.22 |
| Computer at home, n (%) | 86 | (71.1%) | |||
| ACT Score, mean (sd) | 15.5 | (4.8) | |||
| Provided SMS, n (%) | 104 | (86.0%) | |||
12 months prior to index visit date
Table 2 is a comparison of the study sample by recruitment strategy. Patients recruited through the mail/phone method were more likely to be Latino (p=0.05) and have Medicaid (p<0.01). Fewer enrollees recruited during an ED visit reported having a computer at home (61% ED recruitment versus 76% mail/phone recruitment; p=0.09), and these patients also had a lower mean ACT score (mean 14.3 versus 16.1; p=0.06), although these comparisons did not reach statistical significance.
Table 2.
Comparison of study sample by recruitment strategy
| Factor |
ED
n=41 |
Mail/phone
n=80 |
p value | ||
|---|---|---|---|---|---|
| Age, mean (sd) | 15.1 | (±1.5) | 15.6 | (±1.8) | 0.13 |
| Male, n (%) | 18 | (43.9%) | 36 | 45.0%) | 0.91 |
| ≥ 18 years, n (%) | 3 | (7.3%) | 13 | 16.3%) | 0.26 |
| Hispanic/Latino, n (%) | 0 | (0.0%) | 8 | 10.0%) | 0.05 |
| African American, n (%) | 38 | (92.7%) | 70 | 87.5%) | 0.54 |
| Medicaid, n (%) | 23 | (56.1%) | 50 | 62.5%) | <0.0001 |
| ≥ 1 ED visit prior to index visit1, n (%) | 20 | (48.8%) | 43 | 53.8%) | 0.61 |
| Computer at home, n (%) | 25 | (61.0%) | 61 | 76.3%) | 0.09 |
| ACT Score, mean (sd) | 14.3 | (±5.1) | 16.1 | (±4.6) | 0.06 |
| Provided SMS, n (%) | 38 | (92.7%) | 66 | 82.5%) | 0.17 |
12 months prior to index visit date
Comparing ED recruitment to mail/phone recruitment, enrollment was accomplished for 81.5% of those eligible and presenting to the ED during staffed hours (53/65), of which 90.6% were randomized. The refusal rate for ED recruitment (excluding ineligibles) was 18.5% (12/65). For mail/phone recruitment, 70.8% of persons on the listing were reached (248/350). Of those, 74 (29.8%) were enrolled, of which 98.6% were randomized. Using the denominator of 248 persons reached for mail/phone recruitment, refusals amounted to 23.4% of those reached (58/248). These figures are taken from Figure 2.
We were able to compare selected data for refusals to that of enrollees. These data was available for HFHS only, and is shown in Table 3. At HFHS, enrolled and refused were similar; however, as determined by EMR, 9% of teens who refused enrollment had Medicaid insurance, compared to 42% of those that elected to enroll in Puff City (p<0.01). Fewer refusals than enrolled had an ED visit in the past 12 months (p=0.09), (not including the index visit), and fewer enrolled patients had no insurance (2.8% versus 9.1% for enrolled and refusals, respectively, p=0.08.)
Table 3.
Comparison of enrolled and refusals for one site1
| Factor |
Enrolled
n=36 |
Refused
n=22 |
p value | ||
|---|---|---|---|---|---|
| Age, mean (sd) | 16.6 | (1.9) | 15.7 | (2.0) | 0.10 |
| Male, n (%) | 15 | (41.7%) | 11 | (50.0%) | 0.54 |
| ≥ 18 years, n (%) | 15 | (41.7%) | 5 | (22.7%) | 0.17 |
| Hispanic/Latino, n (%) | 2 | (5.6%) | 1 | (4.5%) | 0.99 |
| African American, n (%) | 32 | (88.9%) | 19 | (86.4%) | 0.99 |
| Medicaid, n (%) | 15 | (41.7%) | 2 | (9.1%) | <0. 01 |
| ≥ 1 ED visit (asthma)/12 mon2, n (%) | 34 | (94.4%) | 17 | (77.3%) | 0.09 |
| Uninsured | 1 | (2.8%) | 2 | (9.1%) | 0.08 |
HFHS only;
12 months prior to index visit date
Time needed for recruitment and enrollment
On average, the amount of time needed to enroll the patient, including completion of the baseline assessment, was 44.3 minutes at HFHS and 67.0 minutes at CHM. CHM staff spent a total of 1162.5 hours recruiting which corresponds to 13.67 hours/participant randomized. Almost half (48.2%) of the CHM participants randomized were enrolled during a non-ED scheduled research appointment (41/85). HFHS Staff spent a total of 948.72 hours recruiting which corresponds to 26.35 hours/participant randomized. At HFHS, we were able to calculate hours/participant by recruitment type. For ED enrollment (n=6 HFHS patients randomized), a total of 81.75 hours were spent in recruitment or 13.62 hours/participant randomized. For mail/phone enrollment (n=29 HFHS patients enrolled from listings + 1 patient enrolled post-ED visit), a total of 863.97 hours were spent in recruitment or 28.80 hours/participant randomized. The no-show rate for scheduled appoints was 30.8% for mail/phone versus 12.5% for ED recruitment. The approached-to-randomized ratio for mail/phone was 20.9 (i.e., of 100 persons approached, approximately 21% are eventually enrolled and randomized) versus 74.8% (48/65) for ED recruitment.
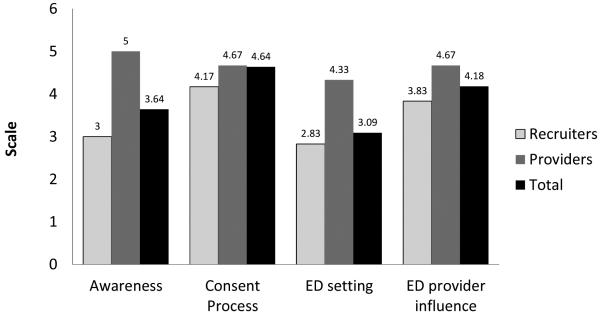
We surveyed 11 ED and research staff about the recruitment process. Providers gave relatively high marks for awareness activities (mean score of 5 out of a possible 6) and seemed to feel the interaction and activities were appropriate. Research staff, however, felt that awareness activities needed to be repeated periodically, to update new ED staff and to remind current staff of the ongoing study (mean score of 3 out of a possible 6). Scores were similar for the consent process (score of 4.67 for providers and 4.17 for research staff), but the provider score was higher than that of the staff for recruitment in the ED setting (4.33 versus 2.83, respectively).
DISCUSSION
This pilot study used the NIH R34 mechanism to assess the feasibility of implementing successful recruitment strategies for the conduct of an RCT in the ED setting. Important factors included acceptability by ED staff and patients, as well as participation and refusal rates. Several published studies, since 2000, have also explored the conduct of research in the ED. Horowitz, et al., conducted a feasibility study to screen for suicidal ideation, in which parents and youth (aged 10-21 years) were approached during an ED visit. Data collection required only 12 minutes, and was accomplished while the patient was waiting for the physician; however, the refusal rate for this study was 40%.10 Another study on prevention of alcohol misuse among youth entailed youth presenting to the ED to access an interactive program installed on a laptop.11 Refusal rate was 14.5%, and the intervention, which took 15-30 minutes, reportedly fit well into the ED operation. Mahabee-Gittens, et al. conducted a tobacco cessation intervention in an ED setting, consisting of a survey and advice on smoking cessation from trained research staff. Acceptability was high, with only 3% of staff reporting any interference with patient care.12 Zorc, et al., initiated a program in the ED that consisted of screening surveys and a 12 minute video, however, the paper did not discuss any problems associated with recruitment strategies and protocols in the ED setting.13 Our study is somewhat different from these studies, in that we are recruiting during an acute exacerbation and used web-based tools not installed on computer.
The two participating EDs had sizeable differences in terms of the number of patients presenting and used different research staffing models for recruitment; one of which was clearly more successful for recruitment in the ED. At CHM, to increase the number of staff recruitment hours in the ED, more than one recruiter per shift was avoided. A drawback to this type of shift scheduling meant that if the recruiter was with another patient or a scheduled appointment, ED coverage was sacrificed, unless the patient could be contacted via recruitment letters/calls. While we are able to reach three times as many persons using phone listings, this strategy was associated with more time spent in enrollment activities, such as arranging cab transport, no-shows and re-scheduling. As a result, only 20.9% of persons contacted were randomized using mail/phone, compared to 74.8% for ED recruitment.
In assessing feasibility, we believed that patients would be more motivated to participate during an ED visit for acute asthma, than over the phone. This was not supported by our results. Another concern was that patients would be too sick to participate in the study. Anecdotally, the staff perception was that patients may have been more amenable to participating under different circumstances.
Disruption of clinic flow was also proposed as a potential challenge. Our goal was to have enrollment take no longer than 30 minutes. According to our records, the amount of time needed to enroll the patient, including completion of the baseline assessment, ranged from 44.3 (HFHS) to 67 (CHM) minutes. ED patients were generally willing to participate, however, recruitment staff felt that the process for recruitment in the ED was “rushed”.
We have three suggestions for streamlining the conduct of this RCT in the ED. First would be to reconfigure our data collection systems. We used two separate data systems to complete enrollment. The first was the online web intervention used to collect patient-reported data necessary for enrollment and patient-reported outcomes for the study. The second was an integrated clinical data management and remote data capture system used to collect and integrate clinical data abstracted from the medical record. Jointly, this robust process created a working framework for conducting the RCT in the ED. We learned, however, that it was laborious for staff who were required to login to two systems. Second, allowing patients to return to the ED to complete enrollment can reduce the time needed in the ED. Of course, there is the potential for loss of participants between consent (in the ED) and patient completion of the first session. To alleviate this, we opted to ask consenting patients to be randomized and access session 1 while in the ED. The third suggestion would be to reduce the length of consent/assent forms and baseline and enrollment forms. These options for streamlining the process are based on conducting an RCT in the ED and must be weighed against research needs.
We had several reasons for exploring initiation of Puff City in the ED, including reaching teens with uncontrolled asthma, reaching teens with poor access to care, and the potential for increased uptake of the asthma intervention. According to ACT scores, youth that enrolled had uncontrolled asthma. ACT scores were higher for mail/phone recruitment as might be expected since this recruitment occurs days after the index acute event, however, mean scores still indicated uncontrolled asthma for mail/phone recruits. We also hoped to reach teens with limited access to care. About 11.7% of caregivers of enrolled youth reported a gap in healthcare coverage for the youth in the last 12 months. According to a 2012 report, the percentage of 6-17 year olds without health insurance in the City of Detroit was around 7-8%.14 Most youth enrolled in this study were insured through Medicaid. Medicaid insurance has been associated with poor asthma control and ED utilization.2 Although the difference in Medicaid was the only statistically significant comparison, we note that a greater percentage of refusals were > 18 years of age and were uninsured, while a smaller percentage had ED visits in the last 12 months. This may suggest that refusals were mostly older teens/young adults with milder disease. Youth this age often are uninsured (National Center for Health Statistics, 2015)15.
Compared to a school-based setting, participation was increased. In the school-based trials, we struggled to reach targeted enrollment, enrolling an estimated 30% of those eligible for the study.3, 4 Recruitment in the ED resulted in an enrollment rate of 80%. Mail/phone recruitment from patient listings was very similar to the school-based trials in which 43.1% of those contacted made appointments, while 29.8% actually enrolled. Finally, there was a concern that youth would be too sick to participate. According to our records only 1 patient (5%) gave this as a reason for refusal.
In summary, this pilot provided valuable information to inform future trials involving recruitment and initiation of an online intervention in the ED. Results suggest that we are reaching patients in need of strategies for improving their asthma management and may be reaching those that have limited access to healthcare. According to our assessment, the best method for reaching urban adolescents with uncontrolled asthma seems to be to approach them in the ED. This method is labor-intensive and enrollment activities must avoid competing with other priorities in the delivery of care. However, if research strategies and protocols can be further streamlined, ED staff seem willing to adjust. Phase II of this pilot will reveal whether the benefits of targeting youth in the ED setting are worth the hurdles encountered.
Figure 3.
Results of self-administered questionnaire on study barriers and facilitators given to participating research and Emergency Department staff
Acknowledgements
We would like to acknowledge the participating youth and their families, as well as the work of the following research staff, without whom this study would have not been possible: Dan McLaren, Brittany McKinnon, Jia Li, Dana Larkin, Michael Sheehan, Renee Bourgeois-Williams, Christina Melkonian, LaSalle McKenzie, Brianna Costello, Cyrus Farahani, Belinda Parr, and Cheryl Miree.
Financial support
The studies reported in this publication were supported by the NIH, grant R34HL109296
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions
Christine LM Joseph is PI of the project and is responsible for all aspects of the study; Mei Lu is co-investigator of the study and responsible for study design, implementation, and analysis; Stephanie Bruzzelli-Stokes is an investigator on the project, contributing to study design and intervention implementation in the HFHS emergency department; Dayna A Johnson and Elizabeth Duffy are project coordinators who contributed to study design and were responsible for coordinating all aspects of the study; Talan Zhang conducted analyses for this manuscript; Dennis R Ownby is a co-investigator and contributed to intervention development and study design; Edward Zoratti is a co-investigator and contributed to study design and implementation; Prashant Mahajan is a co-investigator contributing to study design and intervention implementation in the CHM emergency department. All of the above staff contributed to the development, content, and editing of this manuscript.
REFERENCES
- [1].Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- [2].Riera A, Walker DM. The impact of race and ethnicity on care in the pediatric emergency department. Curr Opin Pediatr. 2010;22:284–9. doi: 10.1097/MOP.0b013e32833973a5. [DOI] [PubMed] [Google Scholar]
- [3].Joseph CL, Ownby DR, Havstad SL, et al. Evaluation of a web-based asthma management intervention program for urban teenagers: reaching the hard to reach. J Adolesc Health. 2012;52:419–26. doi: 10.1016/j.jadohealth.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Joseph CL, Peterson E, Havstad S, et al. A web-based, tailored asthma management program for urban African-American high school students. Am J Respir Crit Care Med. 2007;175:888–95. doi: 10.1164/rccm.200608-1244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102–14. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- [6].Zimmerman BJ, Bonner S, Evans D, Mellins RB. Self-regulating childhood asthma: a developmental model of family change. Health Educ Behav. 1999;26:55–71. doi: 10.1177/109019819902600106. [DOI] [PubMed] [Google Scholar]
- [7].Velicer W, Prochaska J, Fava J, Norman GJ, Redding C. Smoking cessation and stress management: Applications of the Transtheoretical Model of behavior change. Homeostasis. 1998;38:216–33. [Google Scholar]
- [8].Dijkstra A, De VH. Clusters of precontemplating smokers defined by the perception of the pros, cons, and self-efficacy. AddictBehav. 2000;25:373–85. doi: 10.1016/s0306-4603(99)00073-8. [DOI] [PubMed] [Google Scholar]
- [9].Jia CE, Zhang HP, Lv Y, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. The Journal of allergy and clinical immunology. 2013;131:695–703. doi: 10.1016/j.jaci.2012.08.023. [DOI] [PubMed] [Google Scholar]
- [10].Horowitz L, Ballard E, Teach SJ, et al. Feasibility of screening patients with nonpsychiatric complaints for suicide risk in a pediatric emergency department: a good time to talk? Pediatr Emerg Care. 2010;26:787–92. doi: 10.1097/PEC.0b013e3181fa8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gregor MA, Shope JT, Blow FC, Maio RF, Weber JE, Nypaver MM. Feasibility of using an interactive laptop program in the emergency department to prevent alcohol misuse among adolescents. Annals of Emergency Medicine. 2003;42:276–84. doi: 10.1067/mem.2003.265. [DOI] [PubMed] [Google Scholar]
- [12].Mahabee-Gittens EM, Gordon J. Acceptability of Tobacco Cessation Interventions in the Pediatric Emergency Department. Pediatric Emergency Care. 2008;24:214–6. doi: 10.1097/PEC.0b013e31816a8d6f. [DOI] [PubMed] [Google Scholar]
- [13].Zorc JJ, Chew A, Allen JL, Shaw K. Beliefs and Barriers to Follow-up After an Emergency Department Asthma Visit: A Randomized Trial. Pediatrics. 2009;124:1135–42. doi: 10.1542/peds.2008-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. (D3) DDD. State of the Detroit Child. Detroit, Michigan 2012.
- [15].National Center for Health Statistics, U.S. Census Bureau Comparison of the Prevalence of Uninsured Persons from the National Health Interview Survey and the Current Population Survey, 2014 and 2015. 2015 Sep; [Google Scholar]