Abstract
Topic
To summarize the relative effects of bevacizumab (Avastin®, Genentech, Inc.) and ranibizumab (Lucentis®, Genentech, Inc.), using findings from a Cochrane Eyes and Vision Group systematic review .
Clinical relevance
Neovascular age-related macular degeneration (NVAMD) is the most common cause of uncorrectable vision loss in the elderly in developed countries. Bevacizumab and ranibizumab are the most frequently-used anti-VEGF agents injected intravitreally to treat NVAMD
Methods
We included only randomized controlled trials (RCTs) in which the two anti-VEGF agents had been compared directly. The primary outcome was 1-year gain in best-corrected visual acuity (BCVA) of 15 or more logMAR letters. We followed Cochrane methods for trial selection, data extraction, and data analyses. Relative effects of bevacizumab versus ranibizumab are presented as estimated risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs).
Results
We identified 6 eligible RCTs with 2809 participants. The proportion of eyes that gained 15 or more letters of BCVA by 1 year was similar for the two agents when the same regimens were compared: RR=0.90, 95% CI: 0.73 to 1.11. The mean change in BCVA from baseline also was similar: MD=−0.5 letter; 95% CI: −1.6 to +0.6. Other BCVA and quality-of-life outcomes were similar for the two agents. One-year treatment cost with ranibizumab was 5.1 and 25.5 times the cost for bevacizumab in the two largest trials. Ocular adverse events were uncommon (<1%); rates were similar for the two agents.
Conclusions
We found no important difference in effectiveness or safety between bevacizumab and ranibizumab for NVAMD treatment but a large cost difference.
For the past decade, intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents have been the mainstay of treatment for neovascular age-related macular degeneration (NVAMD), the leading cause of irreversible loss of vision among the elderly in developed countries. Although pegaptanib (Macugen®, Eyetech/Pfizer)was the first anti-VEGF agent demonstrated to be effective with respect to preserving or restoring visual acuity in affected eyes,1 the most frequently used anti-VEGF agents since publication of the findings from the initial trials of ranibizumab (Lucentis®, Genentech, Inc.)2,3 have been ranibizumab and bevacizumab (Avastin®, Genentech, Inc.). A Cochrane systematic review of findings from clinical trials of these three anti-VEGF agents was published in 20074 and was updated in 2014.5
Because bevacizumab has not been approved by the United States Food and Drug Administration for intraocular injection and because of the greater cost of ranibizumab compared to bevacizumab, several randomized controlled trials have been conducted to compare the effects of these two agents for the treatment of neovascular AMD. As part of the recently updated Cochrane systematic review,5 we estimated the relative effects of bevacizumab versus ranibizumab with respect to several vision-related and other outcomes. Our purpose is to summarize those findings.
METHODS
We followed the methods recommended by Cochrane.6 The protocol for the original systematic review was published in 20057; the original systematic review was published in 20084. The findings reported herein were added as part of the most recently updated version of the systematic review.5
Eligibility criteria
We included only randomized controlled trials in the review in order to minimize biased estimates of relative effects. Eligible trials directly compared intravitreal injections of bevacizumab with ranibizumab for treatment of neovascular AMD, as defined by the trial investigators, in comparable dosages and regimens. Trials of aflibercept (VEGF-Trap Eye/Eylea®, Regeneron) and trials that used bevacizumab or ranibizumab in combination with other treatments were not eligible.
Identification of eligible trials
We searched CENTRAL, which contains the Cochrane Eyes and Vision Group Trials Register (2014, Issue 3), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2014), EMBASE (January 1980 to March 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS; January 1982 to March 2014), the metaRegister of Controlled Trials (mRCT; www.controlledtrials.com), clinicaltrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en). We did not impose any date or language restriction in the electronic searches for trials. Databases were searched last on 27 March 2014.
The search strategy for the Ovid MEDLINE database is shown in the Appendix. Detailed search strategies for the other electronic databases are provided in the Cochrane systematic review.5 We reviewed the reference lists of reports from included trials and related systematic reviews to identify additional potentially relevant trials.
Trial selection
Pairs of authors independently reviewed titles and abstracts produced by the electronic searches and other search methods to identify citations that referred to trials that definitely or possibly were eligible for inclusion in the systematic review. The final eligibility decision was based on review of the full text of articles deemed definitely or potentially eligible for inclusion by either author of each pair. We documented reasons for exclusion of trials at this stage.
Outcomes of interest
The primary outcome for the review was best-corrected visual acuity (BCVA) at 1 year of follow-up. As only one eye per participant (the study eye) was randomized in all eligible trials, we defined the primary outcome for comparison of the two anti-VEGF agents to be the proportion of participants who gained 15 or more letters (3 or more lines) in the study eye from baseline to 1 year when BCVA was measured on a chart with a logMAR scale. Other visual acuity outcomes included loss of fewer than 15 letters of BCVA, avoidance of blindness, maintenance of BCVA (no change or improvement from baseline), and mean change in BCVA. All outcomes were estimated at 1 and 2 years of follow up whenever data were available. Other outcomes of interest were measures of visual function, such as contrast sensitivity and reading speed; morphological characteristics based on fluorescein angiograms or optical coherence tomography (OCT); quality of life assessed with validated instruments; cost of treatment, and ocular and systemic adverse events.
Data collection and assessment of trials for risk of bias
Pairs of review authors independently extracted data from reports and other documents from included trials regarding trial characteristics, methods, participants, interventions, outcomes, and funding resources. Data were recorded on forms developed specifically for this purpose. We contacted authors of trial reports for desired information that was not reported clearly. One review author entered the data into Review Manager8 (RevMan); a second review author verified the data entered.
Two review authors assessed sources of potential bias in each included trial in accord with Cochrane methods.6 This assessment focused on selection of trial participants and assignment to intervention arms, assessment of outcomes, completeness of follow-up of participants and compliance with assigned intervention, data analysis policy, and other methodological aspects of design and conduct of each trial. Trials were categorized as being at high, unclear, or low risk of bias for each aspect assessed. We contacted authors of trial reports to request details whenever we did not find sufficient information to assess potential biases.
Data synthesis and analysis
Data synthesis and analysis was guided by Cochrane methods.6 We analyzed BCVA as both a continuous and dichotomous outcome. We estimated risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous outcomes. For change in BCVA and other continuous outcomes from baseline to 1 and 2 years of follow-up, we estimated the mean differences (MDs) and 95% CIs. As each trial participant contributed data for only one eye (study eye), the unit of analysis for all outcomes was the individual participant. We used outcome data found in reports from individual trials or provided by trial investigators. We did not impute missing outcome data in our analyses.
We based our assessment of statistical heterogeneity of outcomes on Chi2 tests, I2 statistics, and overlap of confidence intervals for effect estimates from individual trials. We deemed a probability of less than 0.10 from a Chi2 test or an I2 statistic of 60% or greater to represent substantial statistical heterogeneity. We compared study populations, interventions, and methods of individual trials to assess clinical and methodological heterogeneity.
We used RevMan8 to perform statistical analyses. We used a random-effects model for all analyses. Although we planned to compare outcomes by type of choroidal neovascularization (CNV), we did not find outcome data reported by CNV subtype for the trials included in the systematic review.
RESULTS
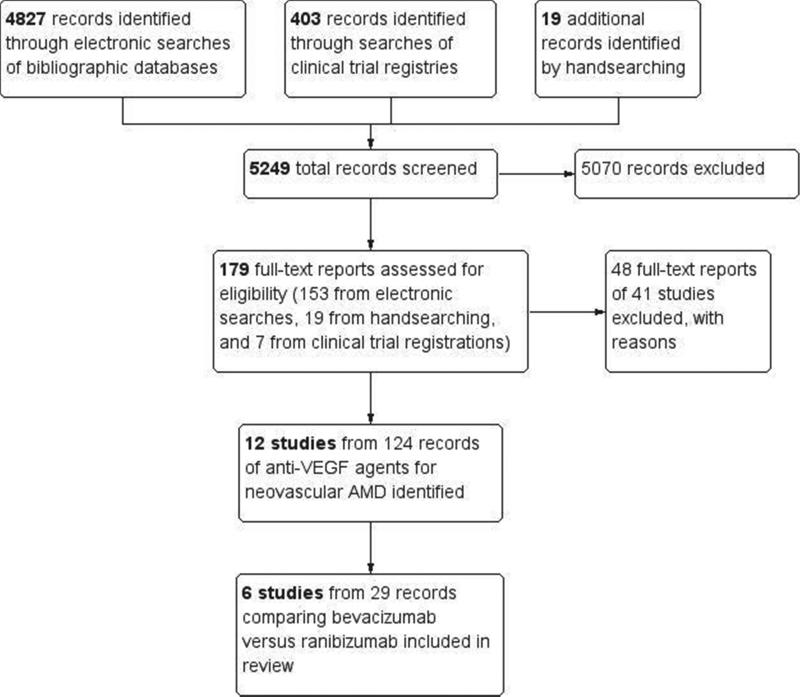
Of a total of 5249 records screened for the Cochrane systematic review, we excluded 5070 during review of titles and abstracts. We assessed 179 full-text reports and excluded 49 reports from 41 studies. We included 12 trials, with a total of 124 reports, in the complete updated Cochrane review5. Of these, 6 trials (29 records) compared intravitreal injections of bevacizumab versus ranibizumab for at least 1 year (Figure 1). We identified 3 additional potentially eligible ongoing trials in clinical trials registers,9-11 but found no published outcome data from these trials.
Figure 1.
Identification and selection of trials that had compared bevacizumab with ranibizumab for any outcome targeted for the systematic review.
Two of the 6 included trials were conducted in the United States11-13; one trial each was conducted in the United Kingdom,15,16 Austria17, France18, and India19. A total of 2809 participants enrolled in the 6 trials; the number of participants in individual trials ranged from 28 to 1208. Overall, the methodological quality of the trials was good; risk of bias was judged low for most domains assessed (Figure 2).
Figure 2.
Risk of bias for individual trials by domain assessed. Green, low risk of bias; yellow, unclear/unknown risk of bias; red, high risk of bias.
In two trials, CATT12 and IVAN15, the two anti-VEGF agents were compared directly for two regimens: monthly injections and injections as needed after 3 initial monthly injections. In CATT, participants in the monthly injection arms were re-randomized at the end of 1 year to continued monthly injections or to have injections as needed for the next year.13 In the injection-as-needed groups in IVAN, participants who required an injection during follow-up received 3 monthly injections before resuming injections as needed. In both CATT and IVAN participants were examined monthly for 2 years, regardless of the assigned agent and regimen.13,16 In the 4 remaining trials, participants were given 3 initial intravitreal injections at 1-month intervals and then re-treated as needed for either 12 months or 18 months.
Visual acuity outcomes
Gain of visual acuity
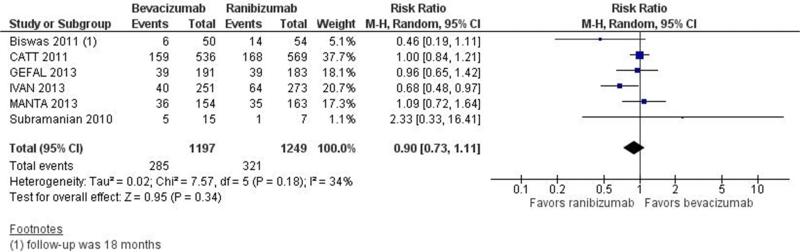
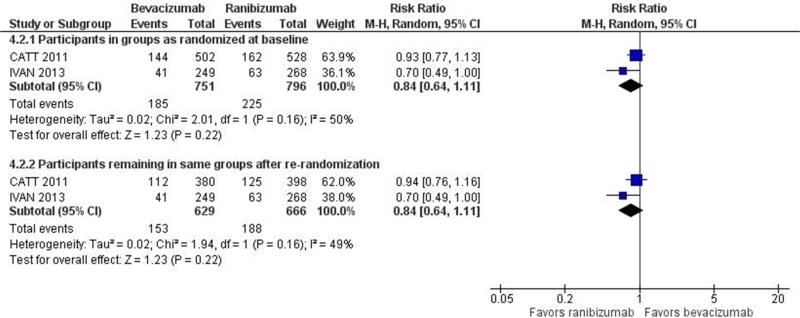
Visual acuity outcomes, based on BCVA, are summarized in Table 1. Investigators of all 6 trials reported the primary outcome for this review, i.e., 1-year gain of 15 or more letters from baseline. The proportion of participants who gained 15 or more letter from baseline was similar in the bevacizumab-treated and ranibizumab-treated groups: estimated RR=0.90, 95%CI: 0.73 to 1.11. The confidence intervals of the estimated RRs for 5 of the 6 trials included 1.00, supporting equivalence of the two agents (Figure 3a). The 2-year estimated RR=0.84 (95%CI: 0.64 to 1.11) was based on findings from the two largest trials, CATT and IVAN. The estimates were the same whether CATT participants were analyzed in their originally assigned treatment groups (Figure 3b) or as re-randomized after 1 year of treatment. The estimated treatment effect differed somewhat between CATT and IVAN, i.e., I2 statistics of 49% and 50% for this outcome. Although the 1-year and 2-year estimates of the relative effect of bevacizumab versus ranibizumab favored ranibizumab, the effect was small and not statistically significant.
Table 1.
Summary of Estimated Relative Effect of Intravitreal Bevacizumab Versus Ranibizumab on Selected Outcomes
| Outcome | No. of Trials | Bevacizumab | Ranibizumab | Estimated Relative Effect (95% CI) | ||
|---|---|---|---|---|---|---|
| No. of Events | No. at Risk | No. of Events | No. at Risk | |||
| a. After 1 year of treatment and follow up | ||||||
| Gained ≥ 15 letters of BCVA | 6 | 285 | 1197 | 321 | 1249 | RR=0.90 (0.73 to 1.11) |
| Lost < 15 letters of BCVA | 6 | 1122 | 1197 | 1176 | 1249 | RR=1.00 (0.98 to 1.02) |
| BCVA better than 20/200 | 4 | 899 | 994 | 947 | 1032 | RR=0.98 (0.96 to 1.01) |
| Mean change in BCVA, letters | 6 | — | 1197 | — | 1249 | MD=−0.5 (−1.6 to +0.6) |
| Mean reduction in CRT, μm | 4 | — | 972 | — | 1023 | MD=−14.0 (−26.5 to −1.4) |
| b. After 2 years of treatment and follow up* | ||||||
| Gained ≥ 15 letters of BCVA | 2 | 185 | 751 | 225 | 796 | RR=0.84 (0.64 to 1.11) |
| Lost < 15 letters of BCVA | 2 | 673 | 751 | 733 | 796 | RR=0.97 (0.94 to 1.00) |
| BCVA better than 20/200 | 2 | 672 | 751 | 711 | 796 | RR=1.00 (0.95 to 1.06) |
| Mean change in BCVA, letters | 2 | — | 629 | — | 666 | MD=−1.2 (−2.8 to +0.5) |
| Mean reduction in CRT, μm | 2 | — | 592 | — | 607 | MD=−12.4 (−33.8 to +9.0) |
BCVA, best-corrected visual acuity
CI, confidence interval
CRT, central retinal thickness
RR. risk ratio
Figure 3.
Forest plot showing estimated risk ratios with their 95% confidence intervals for bevacizumab versus ranibizumab for gain of 15 of more letters from baseline, for each of the 6 individual trials and combined. Part a: One-year findings. Part b. Two-year findings.
Loss of visual acuity
The investigators of each of the 6 trials reported this outcome. As shown in Table 1, at 1 year of follow-up, the estimated RR for loss of fewer than 15 letters of BCVA from baseline was 1.00 (95% CI: 0.98 to 1.02), indicating no difference between bevacizumab and ranibizumab. The estimates from the individual studies were consistent, with all confidence intervals including 1.00 (equivalence of the two agents). At 2 years, the estimated relative treatment effect for this outcome was almost identical to the effect at 1 year: RR=0.97 (95% CI: 0.94 to 1.00) when CATT participants were analyzed by the originally assigned treatment regimen and 0.98 (95% CI: −0.94 to 1.01) when they remained on the same regimen after re-randomization at the end of 1 year of treatment. The investigators of one trial14 reported that no participant had lost 30 or more letters of visual acuity during the 1-year study period; this outcome was not reported from any of the other 5 trials.
Visual acuity better than 20/200
The proportions of participants with BCVA better than 20/200 after 1 year of treatment were reported from 4 trials12,14,15,18 (Table 1). The estimated RR was 0.98 (95% CI: 0.96 to 1.01); the confidence intervals of the estimates from all 4 trials included 1.00. Only the CATT13 and IVAN16 investigators provided 2-year data for this outcome. The estimated relative risk was nearly identical to the 1-year estimate, i.e.,1.00 (95% CI: 0.95 to 1.06) or 1.01 (95% CI: 0.96 to 1.06), depending upon whether re-randomization of CATT patients was taken into account.
Maintenance of baseline visual acuity
The investigators of 2 trials14,19 assessed maintenance of visual acuity for 12 or 18 months at or near baseline BCVA or BCVA improvement. Although they reported the outcome in different ways, neither group of investigators reported an important difference in this outcome with bevacizumab relative to ranibizumab.
Mean change in visual acuity
Data for this outcome were available at 1 year from all 6 trials. The estimated mean difference (MD) in the change of BCVA from baseline to 1 year was −0.5 letter (95% CI: −1.6 to +0.6); i.e., bevaciumab-treated participants lost half a letter more, on average, than ranibizumab-treated participants. The confidence intervals on the estimates from all 6 trials included 0.00, i.e., no difference between the two agents for this outcome. The mean difference at 2 years was less than 2 letters: estimated MD=−1.2; 95% CI: −2.8 to +0.5, again indicating no clinically or statistically significant difference.
Visual function outcomes
Of the 6 trials, visual function outcomes had been reported by the investigators of only one. The IVAN investigators reported 1-year contrast sensitivity (adjusted MD=0.20 letter, 95%CI: −0.47 to +0.87) and reading index (MD=−5.5, 95% CI: −14.6 to +3.5)15; both confidence intervals include 0.0 and thus are consistent with no important difference between the two anti-VEGF agents.15 IVAN participants in the ranibizumab arms had slightly better near logMAR visual acuity compared to those in the bevacizumab arms (adjusted geometric mean ratio=0.92, 95% CI: 0.84 to 1.00; p-value=0.058). Two-year findings for these outcomes16 were nearly identical to 1-year findings.
Morphological outcomes
Change in size of lesion
The investigators of two trials, CATT and IVAN, reported 1-year12,15 and 2-year changes13,16 in the size of the neovascular lesions in study eyes. In both trials, lesion size was reported at 1 year in terms of optic disc areas (DAs). In CATT, the mean change in lesion size was similar in the bevacizumab and ranibizumab groups at 1 year: MD=0.20 DA, 95%CI: −0.09 to +0.49. Among CATT participants who remained on their original treatment regimens for 2 years, those treated with bevacizumab had larger increases in lesion size than those treated with ranibizumab: MD=1.37 mm2, 95% CI: 0.39 to 2.36 mm2.
Among IVAN participants, the median decreases in lesion size after 1 year of treatment with each of the two anti-VEGF agents were similar. For bevacizumab, the median decrease was 1.79 DAs, with an interquartile range (IQR) of 0.00 to 5.18; for ranibizumab, the median decrease was 1.92 DAs and the IQR was 0.01 to 4.81. After 2 years, bevacizumab-treated lesions had decreased in size somewhat more than ranibizumab-treated lesions: median decrease=1.86 DAs (95% CI: 5.51 to −0.16) versus 0.96 DA (95%CI: 4.29 to −0.39), respectively.
Change in size of CNV
Only one group of trial investigators reported the mean change in the size of the CNV component of the lesion from baseline. The GEFAL investigators reported an MD=0.00 DA, 95% CI: −0.32 to +0.32 for 1-year change in CNV size from baseline.18
Change in central retinal thickness
Investigators of 5 of the 6 trials reported mean 1-year change in central retinal thickness (CRT) and those of 2 trials13,16 reported mean 2-year change (Table 1). Participants treated with bevacizumab had experienced less reduction in CRT compared with participants treated with ranibizumab at both 1 and 2 year, but the differences were small (< 15 μm), well within the typical range of measurement error, and thus were judged not to be clinically meaningful. Two-year findings were similar to 1-year findings for this outcome (Table 1).
Quality-of-life outcomes
Quality-of-life outcomes had been reported from only one trial. In IVAN, quality of life was evaluated using the EQ-5D.20 After 1 year and 2 years of follow-up, the median EQ-5D summary scores were the same for the bevacizumab- and ranibizumab-treated participants: 0.85; IQR: 0.73 to 1.00. The proportions of participants who reported “no health problems” for each of the 5 subscale domains (mobility, self care, usual activities, pain or discomfort, and anxiety or depression) at both follow-up times also were similar for participants treated with each of the anti-VEGF agents.
Economic outcomes
Investigators of 2 trials, CATT and IVAN, had reported treatment costs as secondary outcomes. In the first year of follow-up in CATT, the estimated average cost of treatment per participant was US $490 for bevacizumab and US $18,590 for ranibizumab. By 2 years, these costs were US $860 and US $31,805, respectively, per participant.13 Thus, the ratio of average cost per participant, regardless of the treatment schedule, was at least 35 times as great with ranibizumab as with bevacizumab.
The IVAN investigators reported the average total cost of treatment per participant during the first year to be GB £1580 with bevacizumab and GB £8035 with ranibizumab,15 yielding a smaller first-year cost ratio for ranibizumab than for CATT participants, i.e., 5.5 in IVAN versus 37.0 in CATT. Since our systematic review was published, the IVAN investigators have reported 2-year cost data.21
Adverse events
Although adverse events were reported from all 6 trials, the types of adverse events varied among the trials. Investigators of 3 trials that followed participants for 1 year14,17,19 reported no serious ocular adverse events; types of minor ocular events were reported but without the numbers of participants who had experienced the events. No cases of endophthalmitis or retinal detachment were reported among participants in these 3 trials.
Table 2 summarizes the serious ocular adverse events reported from the remaining trials.12,13,15,16,18 The only notable difference between bevacizumab and ranibizumab was for uveitis, with a fourfold risk during the first year of follow up among bevacizumab-treated participants with 4 cases versus 1 case among ranibizumab-treated participants.
Table 2.
Summary of Estimated Relative Risk of Serious Ocular Adverse Events Following Treatment with Bevacizumab Versus Ranibizumab
| Serious Ocular Adverse Event | No. of Trials | Bevacizumab | Ranibizumab | Estimated Risk Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| No. (%) with Event | No. at Risk | No. (%) with Event | No. at Risk | |||
| a. By 1 year of treatment and follow up | ||||||
| Endophthalmitis | 2 | 4 (<1) | 832 | 3 (<1) | 838 | 1.34 (0.30 – 5.98) |
| Retinal detachment | 2 | 3 (<1) | 832 | 0 | 838 | 7.05 (0.4 - 136.3) |
| RPE tear | 2 | 3 (<1) | 882 | 3 (<1) | 913 | 1.04 (0.21 – 5.11) |
| Traumatic cataract | 3 | 1 (<1) | 1128 | 2 (<1) | 1152 | 0.51 (0.05 – 5.62) |
| Severe uveitis | 2 | 4 (<1) | 882 | 1 (<1) | 913 | 4.14 (0.46 – 37.0) |
| b. By 2 years of treatment and follow up* | ||||||
| Endophthalmitis† | 1 | 7 (1) | 586 | 4 (1) | 599 | 1.79 (0.53 – 6.08) |
| Retinal detachment | 1 | 0 | 296 | 1 (<1) | 314 | 0.35 (0.01 – 8.64) |
| RPE tear | 1 | 1 (<1) | 296 | 3 (1) | 314 | 0.35 (0.04 – 3.38) |
| Traumatic cataract | 1 | 1 (<1) | 296 | 1 (<1) | 314 | 1.06 (0.07 – 16.9) |
| Severe uveitis | 1 | 1 (<1) | 296 | 0 | 314 | 3.18 (0.13 – 77.8)) |
Table 3 summarizes the non-ocular adverse events reported from the trials. The risk of one or more serious non-ocular adverse events was greater among bevacizumab-treated participants than among ranibizumab-treated participants: estimated RR= 1.07, 95%CI: 1.06 to 1.52) after 1 year and RR=1.20, 95%CI: 1.05 to 1.37) after 2 years. The only individual non-ocular adverse events for which bevacizumab-treated participants were at excess risk were gastrointestinal disorders after both 1 year and 2 years, with estimated fourfold risk compared to ranibizumab-treated participants, and surgical or medical procedures for which bevacizumab-treated participants were estimated to have twice the risk of ranibizumab-treated participants.
Table 3.
Summary of Estimated Relative Risk of Non-Ocular Adverse Events Following Treatment with Bevacizumab Versus Ranibizumab
| Serious Non-Ocular Adverse Event | No. of Trials | Bevacizumab | Ranibizumab | Estimated Risk Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| No. (%) with Event | No. at Risk | No. (%) with Event | No. at Risk | |||
| a. By 1 year of treatment and. follow up | ||||||
| One or more event | 4 | 227 (18) | 1282 | 183 (14) | 1315 | 1.27 (1.06 –1.52) |
| Death | 4 | 25 (2) | 1282 | 20 (2) | 1315 | 1.28 (0.72 – 2.30) |
| Myocardial infarction | 4 | 8 (1) | 1282 | 10 (1) | 1315 | 0.82 (0.32 – 2.07) |
| Stroke or cerebral infarction | 4 | 5 (<1) | 1282 | 8 (1) | 1315 | 0.64 (0.21 – 1.95) |
| Transient ischemic attack | 3 | 4 (<1) | 1128 | 4 (<1) | 1152 | 1.02 (0.26 – 4.07) |
| Venous thrombotic event | 3 | 8 (1) | 1128 | 2 (<1) | 1152 | 4.09 (0.87 – 19.2) |
| Cardiac disorders | 4 | 37 (3) | 1282 | 36 (3) | 1315 | 1.05 (0.67 – 1.66) |
| Gastrointestinal disorders | 4 | 24 (2) | 1282 | 11 (1) | 1315 | 2.24 (1.10 – 4.55) |
| Infections | 4 | 42 (3) | 1282 | 27 (2) | 1315 | 1.60 (0.99 – 2.57) |
| Injuries or procedural complications | 4 | 30 (2) | 1282 | 21 (2) | 1315 | 1.47 (0.84 – 2.55) |
| Neoplasms | 4 | 20 (2) | 1282 | 21 (2) | 1315 | 0.98 (0.53 – 1.79) |
| Nervous system disorders | 4 | 25 (2) | 1282 | 24 (2) | 1315 | 1.07 (0.61 – 1.86) |
| Surgical or medical procedure | 4 | 26 (2) | 1282 | 13 (1) | 1315 | 2.05 (1.06 – 3.97) |
| b. By 2 years of treatment and follow up* | ||||||
| One or more event | 2 | 314 (36) | 882 | 271 (30) | 913 | 1.20 (1.05 – 1.37) |
| Death | 2 | 51 (6) | 882 | 47 (5) | 913 | 1.12 (0.76 – 1.65) |
| Myocardial infarction | 2 | 11 (1) | 882 | 13 (1) | 913 | 0.88 (0.39 – 1.94) |
| Stroke or cerebral infarction | 2 | 11 (1) | 882 | 14 (2) | 913 | 0.81 (0.37 – 1.78) |
| Transient ischemic attack | 1 | 1 (<1) | 296 | 1 (<1) | 314 | 1.04 (0.06 – 16.5) |
| Venous thrombotic event | 2 | 14 (2) | 882 | 6 (1) | 913 | 2.42 (0.93 – 6.26) |
| Cardiac disorders | 2 | 81 (9) | 882 | 67 (7) | 913 | 1.25 (0.92 – 1.71) |
| Gastrointestinal disorders | 2 | 37 (4) | 882 | 14 (2) | 913 | 2.74 (1.49 – 5.02) |
| Infections | 2 | 66 (7) | 882 | 50 (5) | 913 | 1.37 (0.96 – 1.95) |
| Injuries and procedural complications | 2 | 45 (5) | 882 | 35 (4) | 913 | 1.33 (0.86 – 2.05) |
| Neoplasms | 2 | 36 (4) | 882 | 38 (4) | 913 | 0.98 (0.63 – 1.53) |
| Nervous system disorders | 2 | 44 (5) | 882 | 43 (5) | 913 | 1.06 (0.70 – 1.60) |
| Surgical or medical procedure | 1 | 14 (5) | 296 | 16 (5) | 314 | 0.91 (0.44 – 1.84) |
DISCUSSION
In this summary review of findings from 6 randomized controlled trials (with 2806 participants) in which we compared the effect of intravitreal injections of bevacizumab relative to ranibizumab with respect to several different outcomes important to patients with NVAMD and their ophthalmologists, we found no important difference between the two anti-VEFG agents for clinical outcomes such as best-corrected visual acuity, visual function, and lesion morphology through 2 years of follow-up (Table 1). The investigators of only one trial had reported quality-of-life outcomes using the EQ-5D; they found no difference between bevacizumab-treated and ranibizumab-treated participants for the 5 domains assessed by that instrument. We also found no important difference with respect to most serious ocular complications (Table 2); however, rates of serious ocular adverse events were small, i.e., no more than 1%. Approximately 25% more bevacizumab-treated participants than ranibizumab-treated participants experienced one or more serious non-ocular adverse events according to the information reported from 4 trials for up to 1 year and from 2 trials for up to 2 years (Table 3). However, rates of individual non-ocular adverse events were small with both anti-VEGF agents, typically no more than 2% during the first year of treatment and no more than 5% through 2 years of treatment.
Although the overall quality of the 6 trials was good, the numbers of participants in the trials were too small to provide reliable estimates of the frequency of individual adverse events. A separate Cochrane review that focused on systemic safety of bevacizumab relative to ranibizumab20 supported our finding of no important difference between the two drugs with respect to deaths or serious adverse events over 2 years of treatment and follow-up except for somewhat more gastrointestinal disorders reported for bevacizumab-treated participants.
Since completion of our systematic review, the investigators of the Lucentis Compared to Avastin Study (LUCAS) have published findings from their 1-year RCT.25 Their conclusions regarding the comparative effectiveness of the two anti-VEGF agents mirror those of our review. The data from LUCAS and any other eligible trial published in the interim will be incorporated in the next update to this review.
A major challenge for ophthalmologists and their patients with neovascular AMD has been the choice of anti-VEGF agent. The issues have included relative effectiveness, safety, cost, availability, and quality control for preparation of bevacizumab for intravitreal injection. The findings from this review support the equivalence of bevacizumab and ranibizumab with respect to ocular outcomes. Findings from two trials documented that the cost of ranibizumab substantially exceeded the cost of bevacizumab when injected on similar schedules. Other authors have documented the lower cost of bevacizumab compared with ranibizumab to achieve the same benefits.21,22 Although the cost difference may be covered by insurance for many patients, for other patients and for insurers, both private and governmental, the excess cost represents a significant portion of total medical expenditures. Thus, long-term patient follow-up schedules that do not mandate monthly injections but achieve the same benefits have received increasing attention.
This review was conducted in accord with the methods promoted by Cochrane, with duplication of trial selection, data extraction, and interpretation of findings. Unlike the original trials that demonstrated the effectiveness of pegaptanib and ranibizumab,1-3 the 6 trials in this review that compared bevacizumab with ranibizumab were not sponsored by pharmaceutical companies. Attrition was an issue with several of the trials, as with all clinical trials in which the outcomes of interest are clinical but death is a competing outcome. The investigators of the two largest trials, CATT and IVAN, reported clinical outcomes for patients who returned for 1-year and 2-year examination and evaluation and did not impute outcomes for other participants. Ideally, outcomes of all enrollees in randomized trials are analyzed. However, despite the many imputation methods that have been proposed, no totally satisfactory method has been identified to date to incorporate unobserved outcomes into comparative analyses of treatments.
As people may live for decades after neovascular AMD is diagnosed, a major unresolved issue is the long-term effectiveness of multiple intravitreal injections of anti-VEGF agents on vision and on the risk of adverse ocular and non-ocular events. However, it may be difficult to obtain sponsorship for studies to compare long-term outcomes among patients treated with bevacizumab and ranibizumab in randomized trials. We have learned that the CATT investigators have received funding to continue follow-up of participants in that trial for up to 5 years.26 The currently available evidence indicates that patients with NVAMD and their ophthalmologists can elect to use either bevacizumab or ranibizumab without compromising vision or increasing the risk of serious adverse events within at least the first two years of intravitreal injection.
Whenever two or more similarly safe and effective treatments are available for a medical condition, treatment choice depends on other factors. These typically include physician experience and preference, patient values and preference, treatment availability, cost, and eligibility for coverage or reimbursement, and local policies. In the case of intravitreal injection of bevacizumab for treatment of neovascular AMD, both the ophthalmologist and patient must be comfortable with off-label use of this anti-VEGF agent.
ACKNOWLEDGEMENTS
We are grateful to the editorial team of the Cochrane Eyes and Vision Group (CEVG) and peer reviewers for comments and evaluation of the review from a portion of which this summary is derived. Iris Gordon and Karen Blackhall, CEVG Trials Search Coordinators, designed and conducted the electronic searches. We thank Dr. M.L. Subramanian for providing information on trial methodology and results for use in this review.
Funding: Ms. Lindsley, Dr. Vedula, and Dr. Hawkins received salary support from the Cochrane Eyes and Vision Group U.S. Project, cooperative agreement U01 EY-020522 with the National Eye Institute, the National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD. Drs. Solomon and Hawkins also received support from an unrestricted grant to the Wilmer Eye Institute from Research to Prevent Blindness, New York, New York.
Abbreviations
- AMD
age-related macular degeneration
- BCVA
best-corrected visual acuity
- CATT
Comparison of Age-related macular degeneration Treatment Trial
- CEVG
Cochrane Eyes and Vision Group
- CI
confidence interval
- CRT
central retinal thickness
- CNV
choroidal neovascularization
- DA
optic disc area
- GEFAL
Group d'Evaluation Français Avastin® versus Lucentis® [French Evaluation of Avastin® versus Lucentis®
- IQR
interquartile range
- IVAN
Inhibit VEGF in Are-related choroidal Neovascularization
- LUCAS
Lucentis Compared to Avastin Study
- MANTA
Multicenter Anti-VEGF Trial in Austria
- MD
mean difference
- RCT
randomized controlled trial
- RevMan
Cochrane's Review Manager software
- RR
risk ratio
- VEGF
vascular endothelial growth factor
APPENDIX
Details of Search Strategy for the MEDLINE Database
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1-7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp macular degeneration/
exp retinal degeneration/
exp retinal neovascularization/
exp choroidal neovascularization/
exp macula lutea/
maculopath$.tw.
((macul$ or retina$ or choroid$) adj3 degener$).tw.
((macul$ or retina$ or choroid$) adj3 neovasc$).tw.
(macula$ adj2 lutea).tw.
(AMD or ARMD or CNV).tw.
or/13-22
exp angiogenesis inhibitors/
angiogenesis inducing agents/
endothelial growth factors/
exp vascular endothelial growth factors/
(anti adj2 VEGF$).tw.
(endothelial adj2 growth adj2 factor$).tw.
(anti adj1 angiogen$).tw.
(macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibiz umab$ or bevacizumab$ or avastin$).tw.
VEGF TRAP$.tw.
or/24-32
23 and 33
12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentation: Presented in part at the Macula Society annual meeting, February 25-28, 2015, Scottsdale, Arizona.
No conflicting relationship exists for any author.
Reprints: Reprints are not available from the authors.
AUTHORS’ CONTRIBUTIONS
Conception and design of the review: MK, SSV, KL, BSH
Data acquisition for the review: MK, SSV, KL, BSH
Analysis of data: KL, BSH, SSV, MK
Interpretation of data: KL, BSH, SSV, MK, SDS
Writing the review: SDS, KL, SSV, MK, BSH
Drafting this manuscript: BSH, KL, SDS, SSV, MK
Final approval of the version to be published: SDS, MK, KL, SSV, BSH
Agreement to be accountable for aspects of the review for which responsible to ensure that questions related to the accuracy or integrity of that part of the review are investigated and resolved appropriately: BSH, KL, SSV, MK, SDS
REFERENCES
- 1.Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 4.Vedula SS, Krzystolik M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2008;(2) doi: 10.1002/14651858.CD005139.pub2. Art. No.: CD005139. DOI: 10.1002/14681858.CD005139.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon SD, Lindsley K, Vedula SS, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2014;(8) doi: 10.1002/14651858.CD005139.pub3. Art. No.:CD005139. DOI: 10.1002/14671858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S, editors. Cochrane Handbook of Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available at www.cochrane-handbook.org. [Google Scholar]
- 7.Krystolik MG, Woodcome HA, Reddy U. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for age-related macular degeneration> Cochrane Database of Systematic Reviews. 2005;(1) doi: 10.1002/14651858.CD005139.pub2. Art. No. :CD005139. DOI:10.1002/14651858.CD005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Review Manager (RevMan) [computer software]. Version 5.2. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2012. [Google Scholar]
- 9.Prevention of vision loss in patients with age-related macular degeneration (AMD) by Intravitreal Injection of Bevacizumab and Ranibizumab (VIBERA) NCT00559715. Clinicaltrials.gov/show/NCT00559715 (accessed 14 August 2013)
- 10.Lucentis compared to Avastin study [LUCAS]. A randomized, double blind, prospective multicenter study comparing the effect of Intravitreal injection of Bevacizumab (Avastin) to Ranibizumab (Lucentis) when give to patients with exudative (wet) age-related macular degeneration. NCT01127360. Clinicaltrials.gov/show/NCT01127360 (accessed 1 January 2013)
- 11.Comparison of bevacizumab (Avastin) and ranibizuma (Lucentis) in exudative age-related macular degeneration. NTR1704. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1704 (accessed 17 May 2013)
- 12.CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comparison of Age-related Macular Degeneration Treatments Trial (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian ML, Abedi G, Ness S, et al. Bevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trial. Eye. 2010;24:1708–15. doi: 10.1038/eye.2010.147. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [Erratum: Opthalmology 2012;119:1508] [DOI] [PubMed] [Google Scholar]
- 16.Chakravarthy U, Harding SP, Rogers CA, et al. on behalf of the IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–67. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 17.Krebs I, Schmetterer L, Boltz A, et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97:266–71. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 18.Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: Results from the GEFAL non-inferiority randomized trial. Ophthalmology. 2013;120:2300–09. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Biswas P, Sengupa S, Choudhary R. Comparative role of Intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration. Indian J Ophthalmol. 2011;59:191–6. doi: 10.4103/0301-4738.81023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Dakin HA, Wordsworth S, Rogers CA, et al. on behalf of the IVAN Study Investigators. Cost-effectiveness of ranibizumab for age-related macular degeneration: 2-year findings from the IVAN randomized trial. BMJ Open. 2014;4:3005094. doi: 10.1136/bmjopen-2014-005094. Doi:10.1136/bmjopen-2014-005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moja L, Lucenteforte E, Kwag KH, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews. 2014;(9) doi: 10.1002/14651858.CD011230.pub2. Art. No.: CD0112330. DOI: 10.1002/14671858.DC011230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis®) versus bevacizumab (Avastin®): modeling cost effectiveness. Br J Ophthalmol. 2007;91:1244–46. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121:936–45. doi: 10.1016/j.ophtha.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of Ranibizumab and Bevacizumab for neovascular age-related macular degeneration according to the LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–52. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services Project number 5U10Ey023530-02: Follow-up study: Comparison of AMD Treatments Trial (CATT) http://projectreporter.nih.gov/project_info_description.cfm?aid=8732657&icde=22041118&ddparam=&ddvalue=&ddsub=5&csb=default&cs=ASC.