Abstract
Oxytocin (Oxt) is released in various hypothalamic and extrahypothalamic brain areas in response to anxiogenic stimuli to regulate aspects of emotionality and stress coping. We examined the anxiolytic action of Oxt in the hypothalamic paraventricular nucleus (PVN) while appraising if Oxt recruits GABA neurons to inhibit the behavioral, hormonal, and neuronal response to stress in female prairie voles (Microtus ochrogaster). Voles received an injection of Oxt in the PVN either before or after an elevated platform stress to determine a time-course for the effects of Oxt on the hormonal stress response. Subsequently, we evaluated if ante-stress injections of Oxt affected anxiety-like behaviors as well as neuronal activity in the PVN, using real-time in-vivo retrodialysis and immunohistochemistry with c-Fos expression as a biomarker of neural activity. In addition, we exposed voles to Oxt and a GABAA receptor antagonist, concurrently, to evaluate the impact of pharmacological blockade of GABAA receptors on the anxiolytic effects of Oxt. Elevated platform stress amplified anxiety-like behaviors and hypothalamic-pituitary-adrenal (HPA) axis activity—catalyzing corticotrophin-releasing hormone (CRH) neuronal activity and augmenting corticosterone release in circulation. Ante-stress Oxt injections in the PVN blocked these stress effects while promoting PVN GABA activity and release. Post-stress Oxt treatments were ineffective. The anxiolytic effects of Oxt were hindered by concurrent pharmacological blockade of GABAA receptors. Together, our data demonstrate ante-stress treatments of Oxt in the PVN inhibit stress activation of the HPA axis through recruitment of GABAergic neurons, providing insights to the local circuitry and potential therapeutically-relevant mechanisms.
INTRODUCTION
The neuropeptide oxytocin (Oxt) is considered a key modulator of social cognition and behaviors (Churchland and Winkielman, 2012) and exerts anxiolytic effects (Smith and Wang, 2012). Stress exposure triggers the hypothalamic-pituitary-adrenal (HPA) axis1, rousing psychological and behavioral symptomology (Armario, 2006), but also secretion of Oxt in specific regions of the brain, potentially serving as a negative feedback or inhibitory mechanism. For example, Oxt is released in the paraventricular nucleus of the hypothalamus (PVN) of female prairie voles (Smith and Wang, 2014) and rats (Neumann et al., 2000; Wotjak et al., 1998) during a stressor. However, rather than precipitating the stress response, pharmacological studies have documented that Oxt in the brain, when delivered via an intracerebroventricular infusion (Windle et al., 2004; Windle et al., 1997) or a single injection (Bülbül et al., 2011), prevents stress-induced activity in the PVN, dampening HPA axis function and the behaviors it modulates. In addition, Oxt injected directly in the PVN can also reduce anxiety-like behaviors (Blume et al., 2008; Jurek et al., 2012; Smith and Wang, 2014). Furthermore, inhibition of Oxt action in brain regions that release Oxt in response to stress (i.e., PVN (Bosch et al., 2004; Smith and Wang, 2014) and central amygdala (Ebner et al., 2005)) potentiates the behavioral and physiological stress response. Correspondingly, Oxt research in the stress field has seen a major upsurge in the past two decades, and Oxt has been targeted as a therapeutic treatment of a wide range of anxiety disorders (Lin, 2012). Yet, a concern for clinical use of Oxt as an anxiolytic is the lack of mechanistic details, particularly as it pertains to the HPA axis.
It is possible Oxt modulates this stress pathway by acting directly on and inhibiting corticotrophin-releasing hormone (CRH)-expressing neurons in the PVN. Recently, Jurek and colleagues (2015) demonstrated an intracerebroventricular injection of Oxt delays the stress-induced rise in CRH transcription in the PVN. While local Oxt release does occur in the PVN (Smith and Wang, 2014), there is limited anatomical data demonstrating Oxt receptors colocalized on CRH-expressing neurons (Dabrowska et al., 2011). One ancillary inhibitory mechanism may include gamma-aminobutyric acid (GABA) signaling. GABA is the dominant inhibitory neurotransmitter in the mammalian brain, with GABA neurons in the PVN and surrounding areas (Decavel and Van den Pol, 1990; Herman et al., 2002). Furthermore, GABA receptors are colocalized on hypophysiotropic CRH-expressing neurons in the PVN (Cullinan, 2000; Herman et al., 2002), and PVN-projecting GABAergic neurons inhibit stress-enhanced CRH expression and neuronal firing via GABAA receptors (Bali and Kovacs, 2003; Bartanusz et al., 2004). Interestingly, the suppression of stress-induced CRH expression in the PVN via global injections of Oxt is blocked by a GABAA receptor antagonist (Bülbül et al., 2011), and Oxt potentiates GABAA receptor function in vitro (Bowen et al., 2015). Thus, it could be postulated that the anxiolytic effects of Oxt occur via facilitating GABA inhibition of CRH neuronal activity.
Prairie voles (Microtus ochrogaster) while not commonly used in stress research provide some unique advantages to traditional laboratory rodents, varying from the dynamics of their social systems to their physiology. For example, female prairie voles do not display a spontaneous ovulation or estrous cycle, though ovulation can be induced 24 hr after exposure to a male conspecific (Roberts et al., 1999). This allows the use of reproductively intact females without the need for controlling the estrous cycle, which is often required given the distinct effects cycling ovarian steroids have on the stress response in females (Kajantie and Phillips, 2006). Recently, we noted Oxt release in the PVN in female prairie vole alleviates subsequent stress responses (Smith and Wang, 2014). In the current study, we investigated the local anxiolytic actions of Oxt in the PVN in female prairie voles. We established a pharmacological paradigm demonstrating intra-PVN injections of Oxt prevent the behavioral, hormonal, and neuronal response to stress while promoting GABAergic neuronal activity in the PVN. We then evaluated if the anxiolytic effects of Oxt are propagated by GABAergic excitation in the PVN.
METHODS
Subjects
Female prairie voles were descended from populations in southern Illinois and captive-bred at Florida State University. Housing conditions were previously described (Gobrogge et al., 2007). All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Florida State University.
Experimental procedure
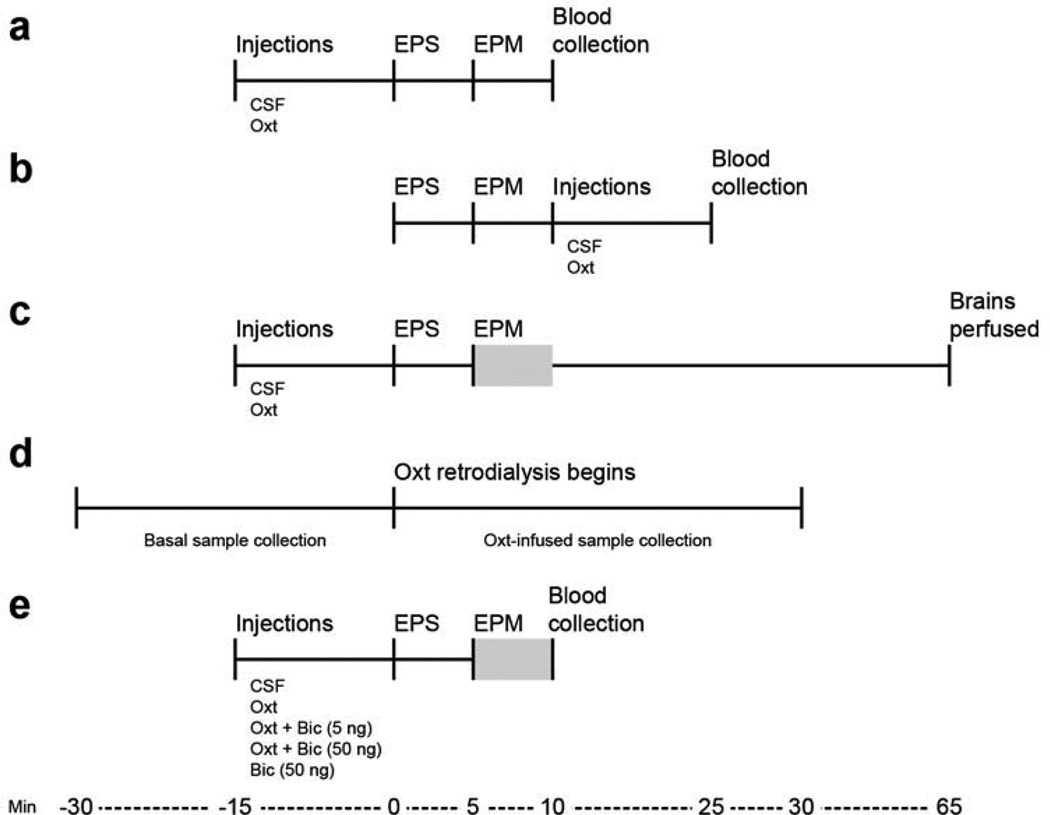
Figure 1 illustrates details for Experiments A–E. Female prairie voles underwent stereotaxic surgery to implant guide cannulae bilaterally (Experiments A–C and E) or a microdialysis probe (Experiment D) aimed at the PVN. We first determined if an intra-PVN injection of Oxt was sufficient in reducing the stress-induced rise in plasma corticosterone concentrations, considering drug treatment before (Experiment A) or after (Experiment B) stress exposure. For Experiment A, females received intra-PVN injections (200 nL/side) 15 min before the start of the 10-min stressor, which consisted of a 5-min elevated platform stress (EPS) and 5-min EPM (see description below). For Experiment B, the Oxt injection was administrated immediately after the 10-min stressor. Blood was collected 25 min post-injection for both experiments. We then employed the paradigm outlined in Experiment A to evaluate the impact that pre-stress intra-PVN injections of Oxt had on elevated plus maze (EPM) anxiety-like behaviors and neuronal activity in the PVN (Experiment C). Females received Oxt injections 15 min before the start of the 10-min stressor, and behavior was recorded during the 5-min EPM. Voles were perfused 80 min post Oxt injection to measure changes to c-Fos expression in PVN neurons with selected neurochemical phenotypes such as CRH, AVP, Oxt, and GABA. We also evaluated if infusing Oxt in the PVN for 30 min changed the local release of GABA via real-time in vivo retrodialysis (Experiment D), detailed below. Finally, we treated females 15 min before the start of the 10-min stressor with Oxt along with bicuculline, a GABAA receptor antagonist, to determine whether function of the GABAA receptor in the PVN was necessary to mediate the anxiolytic effects of Oxt on the stress-induced behavioral and hormonal response (Experiment E). Blood was collected immediately after the end of the stressor.
Figure 1.
Testing schedules of each experiment (A–E). Female prairie voles underwent stereotaxic surgery to implant guide cannulae bilaterally (A–C,E) or a microdialysis probe (D) aimed at the PVN 7 days or 24 h before behavioral testing, respectively. Females received intra-PVN injections (200 nL/side) either 15 min before (A, C, E) or immediately following (B) the start of the 10-min stressor (Min 0). Blood was collected at Min 10 (A, E) or 25 (B). Females were perfused and brains were harvested at Min 65 (C). Behaviors were recorded for five min started at Min 5 (C, E), as denoted by a grey box. Dialysate samples were collected 30 min before and during infusion of Oxt (1 µg/mL for 30 min) in probe fluid. EPS = elevated platform stress; EPM = elevated plus maze; CSF = artificial cerebrospinal fluid; Oxt = oxytocin (10 ng/200 nL/side); Bic = bicuculline (5 ng or 50 ng/200 nL/side).
Intra-PVN injections
Two 21-G guide cannulae were implanted 0.5 mm above the left and right PVN (AP, −0.74 mm; ML, ± 1.5 mm; DV, 5.0 mm; angle, ± 15°, Figure 3a) and were given at least seven days to recover. The scheduling for site-specific injections of Oxt in the PVN during an EPS paradigm was adapted from Blume et al. (2008) and included an Oxt dose demonstrated to be sufficient in affecting the stress response in female prairie voles (Smith and Wang, 2014). Drug dosages for bicuculline were based on previous site-specific pharmacological studies in prairie voles (Curtis and Wang, 2005). Oxt (Bachem, Torrance, CA) and bicuculline (Sigma, St. Louis, MO) were dissolved in artificial cerebrospinal fluid (CSF (Smith and Wang, 2014)) and delivered to voles by infusion in a 200 nL volume over 60 s, with the 200 micron microinjection needle —0.5 mm longer than the implanted guide cannulae — held in place for 30 s after injection to assure infusion. Females received either a single 200 nL injection (CSF or Oxt 10 ng per side; Experiment A–C) or two consecutive 200 nL injections (CSF/CSF; CSF/ Oxt 10 ng; bicuculline 5 ng/ Oxt 10 ng; bicuculline 50 ng/ Oxt 10 ng; or bicuculline 50 ng/ CSF; Experiment E). At the end of the experiment, brains were collected for histological verification of cannulae placement.
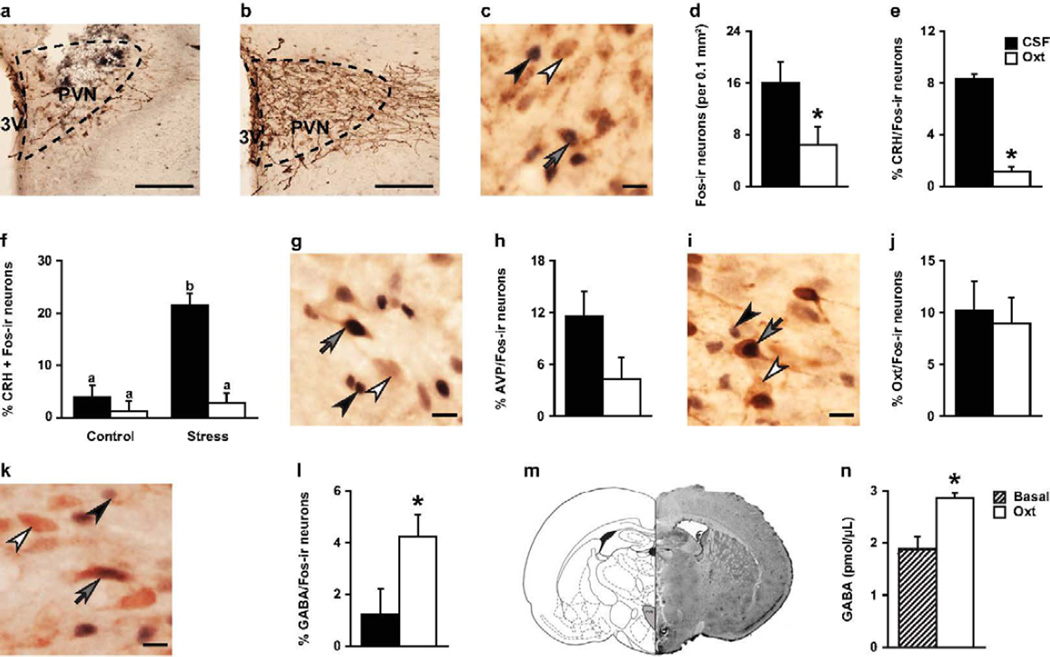
Figure 3.
Oxytocin (Oxt) treatment increased GABA release and affected basal and stress-induced c-Fos expression in specific cytoplasmic neuronal populations in the PVN. Representative photomicrograph of vole brain section illustrating the (A) location of guide cannula placement in the PVN and (B) absence of tissue disturbance in the sections selected for immunohistochemistry staining and quantification in the PVN (staining includes c-Fos and AVP-ir using DAB-nickel and DAB, respectively). (D) In general, more c-Fos-immunoreactive (-ir) neurons (per 0.1 mm2) were observed in females injected with artificial cerebrospinal fluid (CSF) with Oxt (10 ng/200 nL/side) compared to females injected with CSF only. (E) In addition, Oxt-treatment decreased the percentage of neurons double-labeled for corticotrophin-releasing hormones (CRH) and c-Fos. (F) More specifically, Oxt decreased the stress-induced rise in the percentage of CRH neurons expressing c-Fos. No effect was observed on the density (neurons per 0.1 mm2) of (H) AVP/Fos-ir neurons or (J) Oxt/Fos-ir neurons. (L) Oxt did increase the percentage of neurons double-labeled for gamma-aminobutyric acid (GABA) and c-Fos. (M) Schematic drawing (left) and representative photomicrograph of vole brain section (right) illustrate location of microdialysis probe placement in the PVN. (N) Dialysate samples collected during the 30 min infusion of Oxt (Oxt; 1 µg/mL) had significantly more GABA than samples collected during basal conditions (Basal), labeled with an asterisk and determined by Independent Sample’s T-test (p < 0.05). (C,G,I,K) Double-immunohistochemical staining of c-Fos (either (C,G,I) black or (K) blue) and (C) CRH (brown), (G) AVP (brown), (I) Oxt (brown), or (K) GABA (pink). White arrowheads indicate CRH-ir, AVP-ir, Oxt-ir, and GABA-ir neurons, black arrowheads c-Fos-ir neurons, and grey arrows both-ir neurons. (A,B) Scale = 200 µm. (C,G,I,K) Scale = 10 µm. (D,E,L,N) Bars labeled with asterisks indicate a significant difference between the CSF-treated females (CSF) and Oxt-treated females (Oxt) for a specific measure as determined by a significant main effect for the Oxt treatment in a two-way ANOVA (stress × treatment, p < 0.05). Data are expressed as mean ± SEM.
EPS and EPM
This stressor test used an EPM apparatus (Columbus Instruments, Columbus, OH) comprised of two open arms (35 L × 6.5W cm) and two closed arms (35 L × 5W × 15H cm) that cross in the middle, and is elevated 45 cm off the ground. Females were placed on and restricted to one open arm of the EPM for 5 min, creating a mild stress referred to as an EPS (Blume et al., 2008). Next, the 5-min EPM test was conducted to assess anxiety-like behaviors by placing the subject in the center of the EPM facing an open arm. In the EPM test, a conflict is established in rodents between an innate explorative drive and fear of open, exposed environments, which has been previously used and validated in voles (Hendrie et al., 1997; Smith and Wang, 2014). A trained observer blind to the treatment used J-Watcher V1.0 (Macquarie University and UCLA; http://www.jwatcher.ucla.edu/) to quantify behaviors for anxiety-like responses (latency to enter the open arm, percentage of time spent on the open arms versus total arm time, and percentage of open arm entries versus total arm entries) and locomotor activity (total arm entries).
Blood collection and corticosterone radioimmunoassay
Trunk blood (~400 µL) was collected in 20 µl EDTA and double centrifuged (6000 rpm for 15 min at 4°C) before plasma was store at −80°C. Plasma corticosterone (1:1000) was measured, in duplicates, using a commercially available kit (Diagnostic Products Corp., Los Angeles, CA) previously used and validated in prairie voles (Smith and Wang, 2014). Detecting limit for corticosterone was 7.7 ng/ mL. The intra-assay and inter-assay coefficient of variation (CV) were 2.72% and 0.46%, respectively.
Tissue preparation and double-labeled immunohistochemistry
Subjects, from Experiment C, were deeply anesthetized and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde solution. Brains were harvested, postfixed for 4 h in 4% paraformaldehyde, stored in 30% sucrose in phosphate buffer (PB) at 4°C, and then cut into 30 µm coronal sections using a sliding microtome.
A 1:4 series through the PVN of each brain was processed for double-labeling for CRH-ir/c-Fos-ir, Oxt-ir/c-Fos-ir, AVP-ir/c-Fos-ir, and GABA-ir/c-Fos-ir, respectively, using previously established protocols for light microscopy (Gobrogge et al., 2007). A two-step immunohistochemistry protocol was followed by first using nickel-enhanced diaminobenzidine (DAB-Ni) or SG to reveal black or blue/grey, respectively, nuclear staining for c-Fos and then by using DAB only to reveal brown cytoplasmic staining for CRH, Oxt, or AVP or 3-amino-9-ethylcarbazole (AEC) to reveal red cytoplasmic staining for GABA. Sections were rinsed initially in 0.1 M PB for 5 min (3×) and again after each of the following steps: incubation in 1% NaHBO4 in PB for 10 min; incubation in 3% H2O2 in PB in order to block endogenous peroxidase activity; and block in 10% normal goat serum (NGS) in 0.5% triton-PB (TPB). Each set of sections was incubated first with c-Fos antibody (1:20k; Millipore Corporation, Temecula, CA) in 2% NGS in TPB for 48 h at 4 °C. Thereafter, sections were rinsed in TPB for 5 min (3×) and incubated in a solution containing biotinylated goat anti-rabbit secondary antibody (1:3k; Vector Laboratories, Inc., Burlingame, CA) for 2 h and then in ABC complex (Vector Laboratories, Inc.) for 90 min. Lastly, sections were stained with either a DAB-Ni for sets double-labeled for CRH, Oxt, or AVP or SG for the sections double-labeled for GABA (Vector Laboratories, Inc.). The sections were rinsed in TPB for 5 min (3×), blocked in 10% NGS in 0.5% TPB, and incubated with CRH polyclonal guinea pig IgG antibody (1:8k; 48 h; Peninsula Laboratories, San Carlos, CA), Oxt polyclonal rabbit IgG antibody (1:70k; 48 h; ImmunoStar, Inc., Hudson, WI), AVP monoclonal mouse IgG antibody (1:50k; 24 h; Antibodies-online Inc., Atlanta, GA), or GABA polyclonal rabbit IgG antibody (1:10k, 72 h, Sigma) in 2% NGS in TPB 4 °C. Thereafter, sections were rinsed in TPB for 5 min (3×) and incubated in a solution containing biotinylated goat anti-guinea pig secondary antibody (for CRH), biotinylated goat anti-mouse secondary antibody (for AVP), or biotinylated goat anti-rabbit secondary antibody (for Oxt and GABA) (1:300 Vector Laboratories, Inc., Burlingame, CA) for 2 h and then in ABC complex (Vector Laboratories, Inc.) for 90 min. Lastly, sections were stained with either DAB (for sets double-labeled for CRH, Oxt, or AVP) or AEC (for the sections double-labeled for GABA) (Vector Laboratories, Inc.), mounted on slides, and cover-slipped.
As the 200 micron microinjection needle will create mechanical tissue disturbance in some the PVN, we preferentially selected sections with minimal or no tissue damage for quantification (example image is provided in Figure 3b). Densities of single- and double-labeled cells in the PVN were counted, resulting in two sections counted per stain per vole brain. The average of the counted single-labeled cells, immunoreactive to one of the neurochemical markers (i.e., CRH, AVP, Oxt, and GABA) or c-Fos, across all sections for each animal was then divided by the area of the PVN (averaging 0.225 mm2 per hemisphere) to yield the number of single-labeled cells per 0.1 mm2. Percentages of CRH-, AVP-, Oxt- and GABA-ir cells expressing c-Fos were calculated by dividing the number of these cells expressing c-Fos by the total number of single-labeled cells for the respective neurochemical marker.
Real-time in-vivo retrodialysis in the PVN and quantification of GABA by high-performance liquid chromatography (HPLC)
Microdialysis probe construction and dialysate collection were previously described (Smith and Wang, 2014). The active area of the dialysis membrane was 1.0 mm with a molecular weight cutoff of 18 kDa. Probes were perfused continuously at 1.0 µL/min with a CSF solution using a glass Hamilton syringe connected to an automatic micropump (World Precision Instruments). Voles were anesthetized and then stereotaxically implanted with a microdialysis probe aimed at the PVN (nose bar; AP, −0.7 mm; ML, 0.15 mm; DV, 6.35 mm, Figure 3m). Voles were given 24 h to recover, then dialysate samples were collected every 30 min for 2 h to establish an equilibrium between the inside and outside of the microdialysis membrane and acclimate the vole to the collection procedure. We adapted a procedure for retrodialysis of Oxt in the PVN that has been previously reported in female rats for use in prairie voles (Bosch et al., 2005). Baseline samples were collected over 30 min. The inflow tubing filled with CSF solution was then switched with an inflow tubing containing Oxt (1 µg/mL), and this Oxt-containing perfusion solution was allowed to flow through the probe for 10 min before the 30-min retrodialysis sample was collected, as this was the calculated time for the drug to reach the target site. It is expected that at least 0.16 ng of Oxt was delivered to the surrounding tissue during the retrodialysis period as estimated in vitro (Engelmann et al., 1992). Samples were collected into vials containing 5 µl 0.1 N HCl and immediately frozen on dry ice. All samples were stored at −80°C until processed, and brains were collected for histological verification of probe placement. GABA concentration was measured by the HPLC and a pre-derivatization with ortho-pthalaldehyde (OPA) at the NHLBI Biochemistry Core Facility. The Agilent 1100 HPLC (Agilent technologies) was equipped with a reverse phase column, Zorbax Eclipse Plus C18 (3.0 × 150mm, 3.5µm, Agilent Technologies) and the fluorescence detector (FLD; G1321A, Agilent Technologies) to detect the pre-derivatized GABA at Ex340/Em450. The HPLC-reverse phase column was calibrated with varying concentration of standard GABA solution. In vitro recoveries for the microdialysis membranes used in the sampling of the PVN were calculated by dipping probes (n = 3) in the CSF perfusion solution containing 1 µM of GABA measured. The CSF solution was pumped through the probes at 1.0 µL/min over 30 min and collected into vials containing 5 µl 0.1 N HCl and immediately frozen on dry ice, replicating in vivo collection conditions. The mean in vitro recovery for the microdialysis probes was 10.3% for GABA.
Data analysis
Data were analyzed using IBM SPSS Statistics 19 (SPSS, Inc.) and were expressed as mean ± SEM. Independent samples t-tests were used to analyze plasma corticosterone levels in Experiment A and B and EPM behavior in Experiment C. All neurochemical measures in Experiment C were analyzed with a two-way ANOVA (stress × drug treatment). Significant interactions (p < 0.05) were further assessed with a Student–Newman–Keuls (SNK) post-hoc test for group differences. Paired-sample t-test were used to analysis GABA concentrations in Experiment D. In Experiment E, one-way ANOVAs were used to analyze EPM behaviors and corticosterone concentrations with SNK post-hoc test for group differences. All alpha levels were set at p < 0.05.
RESULTS
Ante-stress Oxt treatment limits stress hormones and behavior
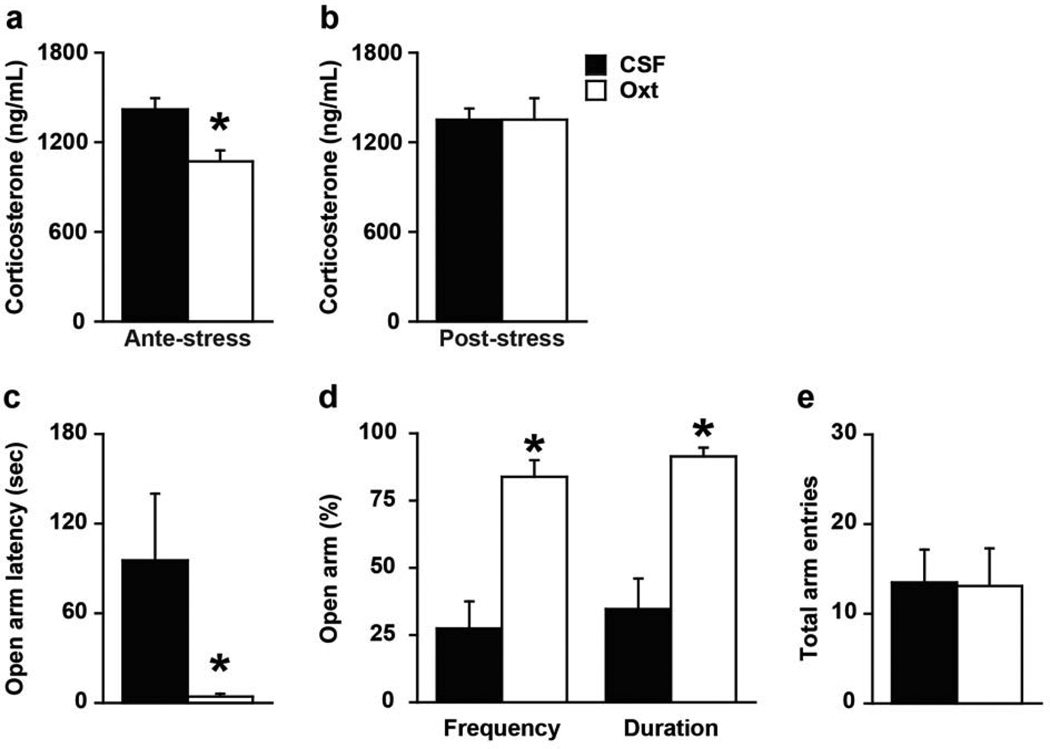
In Experiment A, females injected with Oxt in the PVN before a stressor, the EPS, had significantly lower corticosterone levels immediately following the stressor than CSF-injected females (t(20) = 3.32, p < 0.005, Figure 2a), a result that acquiesces with previous literature (Heinrichs et al., 2003). In contrast, a post-stress Oxt injection did not affect plasma corticosterone concentrations in females in Experiment B (Figure 2b).
Figure 2.
Oxytocin (Oxt) treatment prevents stress-induced rise in plasma corticosterone levels and anxiety-like behaviors. (A) Intra-PVN injections of artificial cerebrospinal fluid (CSF) with Oxt (10 ng/200 nL/side) 15 min before exposure to elevated platform stress for 10 min led to significantly lower plasma corticosterone levels in female prairie voles compared to females treated with CSF only. (B) In contrast, the same Oxt treatment immediately following the elevated platform stress exposure did not affect plasma corticosterone levels 25 min later. (C–D) Females receiving an intra-PVN injection of Oxt (10 ng/200 nL/side) 15 min prior to exposure to elevated platform stress displayed significantly less anxiety-like behavior in the EPM test. (E) No effects were observed on total arm entries in the EPM, a locomotor measurement. Bars labeled with asterisks indicate a significant difference between the CSF-treated females (CSF) and Oxt-treated females (Oxt) for a specific measure as determined by Independent Sample’s T-test (p < 0.05). A–E, Data are expressed as mean ± SEM.
Notably, in Experiment C, an ante-stress injection of Oxt in the PVN also decreased the display of anxiety-like behaviors in the EPM (Figure 2), an apparatus designed to evoke both exploratory and fear drives with higher anxiety manifesting as limited exploration of the open, exposed arms. Specifically, Oxt-treated females went into the open arms faster (open arm latency: t(17) = 2.42, p < 0.05; Figure 2c), more often (% open arm frequency: t(17) = 4.89, p < 0.0005; Figure 2d), and for a longer duration (% open arm duration: t(17) = 4.81, p < 0.005; Figure 2d). Oxt treatment did not influence locomotor behavior (i.e., total arm entries) in the EPM (Figure 2e). Thus, Oxt treatment directly affects anxiety-like behaviors in a behavior-specific manner rather than being secondary to modulated locomotion.
Ante-stress Oxt treatment modulates PVN neural activity and GABA release
Neither the stress nor Oxt treatment altered the density of AVP-immunoreactive (-ir), Oxt-ir, CRH-ir, or GABA-ir neurons in the PVN (Table 1). However, stress tended to increase the c-Fos-ir density in the PVN (F(1, 19) = 3.95, p = 0.06, control: 29.44 ± 12.92; stress: 65.14 ± 12.49) and yielded a significant increased in expression of c-Fos in CRH-ir neurons (F(1, 19) = 17.82, p < 0.0005, control: 2.52 ± 1.52; stress: 12.20 ± 1.47). This stress-induced increase in c-Fos expression was selective to CRH-ir neurons as no such effect of c-Fos expression was observed in AVP-ir (control: 5.71 ± 2.53; stress: 8.21 ± 2.45), Oxt-ir (control: 2.56 ± 2.67; stress: 7.33 ± 2.58), or GABA-ir (control: 10.83 ± 3.98; stress: 9.56 ± 3.85) neurons in the PVN.
Table 1.
Cell density in the PVN
| Cell profile | CSF-Control | CSF-Stress | Oxt-Control | Oxt-Stress | P-values |
|---|---|---|---|---|---|
| Fos-ir 3 | 7.33 ± 4.29 | 23.10 ± 4.29 | 5.92 ± 3.92 | 6.21 ± 3.63 = | 0.07 2 |
| AVP-ir 3 | 19.59 ± 3.23 | 16.83 ± 3.23 | 16.94 ±2.95 | 16.96 ± 2.73 | NS |
| Fos/AVP-ir 4 | 8.64 ± 4.05 | 14.57 ± 4.05 | 3.01 ± 3.70 | 5.59 ± 3.42 | NS |
| Oxt-ir 3 | 6.69 ± 5.75 | 11.49 ± 5.75 | 11.76 ± 5.25 | 16.28 ± 4.86 | NS |
| Fos/ Oxt-ir 4 | 6.83 ± 4.08 | 13.48 ± 4.07 | 8.47 ± 3.72 | 9.34 ± 3.45 | NS |
| CRH-ir 3 | 43.17 ± 7.25 | 33.165 ± 7.28 | 37.13 ± 6.62 | 36.48 ± 6.13 | NS |
| Fos/CRH-ir 4 | 1.95 ± 1.44a | 14.66 ± 1.44b | 3.05 ± 1.31a | 1.78 ± 1.22a | < 0.01 2 |
| GABA-ir 3 | 93.86 ± 18.68 | 64.92 ± 18.68 | 96.94 ± 17.05 | 82.63 ± 15.78 | NS |
| Fos/GABA-ir 4 | 0.57 ± 1.39 | 1.88 ± 1.39 | 4.30 ± 1.27 | 4.175 ± 1.17 | < 0.05 1 |
Note. Immunoreactive = ir; vasopressin = AVP; oxytocin = Oxt; corticotrophin-releasing hormone = CRH; gamma-aminobutyric acid = GABA;
P-values reflect significant main effect of Oxt treatment1, significant interaction between stress and Oxt treatment2, or no significant effect of either stress or Oxt treatment (NS) as determined by a two-way ANOVA. Units are reported as number of neurons (per 0.1 mm2)3 or percentage of neurons expressing c-Fos4.
Letters indicate significant group differences.
Interestingly, the Oxt treatment decreased c-Fos-ir density (F(1, 19) = 5.12, p < 0.05, Figure 3d) and c-Fos expression in CRH-ir neurons (F(1, 19) = 18.87, p < 0.0005, Figure 3c,e) in the PVN. This is consistent with the inhibition of CRH mRNA expression observed after intracerebroventricular injections of Oxt in rats (Bülbül et al., 2011). In addition, while not affecting c-Fos expression in AVP-ir (Figure 3g,h) or Oxt-ir (Figure 3i,j) neurons, Oxt treatment increased c-Fos expression in GABA-ir neurons in the PVN (F(1, 19) = 5.29, p < 0.05, Figure 3k,l). Oxt treatment did not affect the density of AVP-ir, Oxt-ir, CRH-ir, or GABA-ir neurons (Table 1). Furthermore, in Experiment D, we used real-time in-vivo retromicrodialysis with HPLC and observed a significant increase in GABA release in the PVN when Oxt was infused in our probe (t(6) = 3.56, p < 0.05, Figure 3m,n). Thus, Oxt treatment selectively inhibited CRH neuronal activity while fostering local GABA neuronal activity and release, without affecting expression of either neurochemical.
Finally, stress increased expression of c-Fos in CRH-ir neurons in the PVN, but this stress-induced increase was blocked by Oxt injections prior to the stress exposure (F(1, 19) = 26.59, p < 0.0001; Figure 3c,f). Stress and Oxt interactions were not found on the density of AVP-ir, Oxt-ir, or GABA-ir neurons in the PVN or the c-Fos expression in these cells (Table 1). Together, an intra-PVN injection of Oxt provides an inhibitory regulation of stress outputs in the PVN, as observed in the suppressed c-Fos expression in CRH-ir neurons, while promoting GABAergic activity.
Blockade of GABAA receptors abates Oxt-induced anxiolytic effects
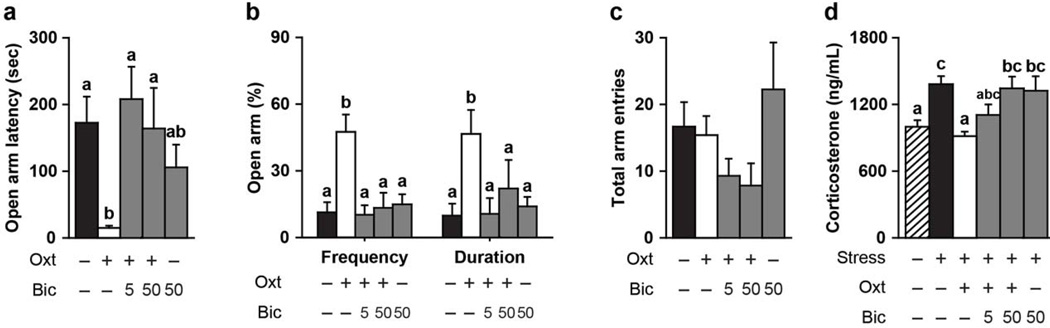
Oxt treatment significantly decreased EPM anxiety-like behavior and corticosterone levels in female prairie voles, but this anxiolytic effect was interrupted by a concurrent injection of bicuculline, a GABAA receptor antagonist (Figure 4). Oxt-treated females had a significantly reduced latency to enter the open arm (F(4, 38) = 4.12, p < 0.01, Figure 4a), entered the open arm more frequently (F(4, 38) = 7.94, p < 0.0001, Figure 4b), spend a greater percentage of time in the open arm (F(4, 38) = 3.92, p < 0.01, Figure 4b), and displayed no stress-induced rise in plasma corticosterone levels (F(5, 45) = 4.99, p < 0.001, Figure 4d) as compared to CSF-treated females. However, bicuculline injected concurrently with Oxt eliminated the Oxt-induced effect on these behaviors and corticosterone concentrations such that females who received Oxt and bicuculline prior to the EPS displayed no behavioral or hormonal differences to CSF-treated females in the EPM. Moreover, females who only received intra-PVN injections of Oxt displayed less anxiety-like behavior and changes in plasma corticosterone levels than females who received intra-PVN Oxt and bicuculline injections (i.e., reduced open arm latency and increased percentage of open arm frequency and duration). Neither Oxt nor bicuculline injections significantly affected locomotor behavior in the EPM (i.e., total arm entries, Figure 4c). High concentrations of bicuculline injections in the PVN (greater than 1.8 ug) have reported to ameliorate stress-induced HPA axis function (e.g., increasing plasma corticosterone levels) in rats. However, in the current study, injecting a lower concentration of bicuculline (50 ng) alone had no effect on any of the observed behaviors and hormones in prairie voles. Together, our data demonstrates Oxt modulates the HPA axis stress response and anxiety-like behaviors through a GABAA receptor-mediated pathway in the PVN.
Figure 4.
Oxytocin (Oxt) inhibition of stress-induced behavior and hormones requires activation of GABAA receptors in the PVN. (A–B) Females receiving an intra-PVN injection of Oxt (10 ng/200 nL/side) 15 min prior to exposure to elevated platform stress displayed significantly less elevated plus maze (EPM) anxiety-like behavior unless they also received an intra-PVN bicuculline (Bic; 5 ng or 50 ng/200 nL/side), a GABAA receptors antagonist. (C) No effects were observed on total arm entries in the EPM, a locomotor measurement. (D) Oxt treatment prevented the stress-induced rise in plasma corticosterone levels, but Bic (50 ng/200 nL/side) blocked this anxiolytic effect. (A–D) Bic treatment alone had no effect on behavior or plasma corticosterone levels. Bars labeled with different letters differ significantly by SNK's post-hoc test in which a significant main effect for the drug treatment was detected in a one-way ANOVA (p < 0.05). (A–D) Data are expressed as mean ± SEM.
DISCUSSION
Previous research has documented the anxiolytic effects of Oxt when released or injected in the PVN (Smith and Wang, 2012). The PVN is the catalyzing site for the HPA axis in the brain, so it is logical to envisage Oxt effects on HPA axis function includes modulation of this brain region. The current study reports that Oxt in the PVN limits the stress-induced rise in CRHergic neuronal activity and, subsequently reduces plasma corticosterone levels and anxiety-like behavior in female prairie voles. In addition, Oxt increased GABAergic neuronal activity and GABA release in the PVN, and when administered concurrently, a GABAA receptor antagonist, bicuculline, blocks the stress inhibition of Oxt. Together, these data suggest a potential local circuit in the PVN through which Oxt prevents the activation of the HPA axis.
The anxiolytic effects of Oxt in the PVN have been observed in the context of social buffering and as a stress-coping strategy. Recently, we reported Oxt release in the PVN is promoted through social interactions with a pair-bonded partner and serves as an anxiolytic agent in female prairie voles (Smith and Wang, 2014). Oxt release in the PVN is also observed during suckling in lactating rats (Neumann et al., 1993) and mating in male rats (Waldherr and Neumann, 2007). This has lead to the assertion that the reduced anxiety and increased calmness following such social interactions is attributable to this local neuropeptide release. Moreover, Oxt release in the PVN occurs during various stressful stimuli (e.g., immobilization (Smith and Wang, 2014); EPS (Blume et al., 2008); forced swim (Wotjak et al., 1998); shaker stress (Nishioka et al., 1998); and maternal defeat (Bosch et al., 2004)). Such a physiological response may be an adaptive stress-coping strategy, functioning as either a preventive inhibitory or negative feedback mechanism for the HPA axis response. Pharmacological studies have documented that various exogenous Oxt treatments reduce the stress response in multiple species (e.g., intranasal (Heinrichs et al., 2003); intraperitoneal (Detillion et al., 2004); subcutaneous (Petersson et al., 1999); intercerebroventricular (Bülbül et al., 2011; Windle et al., 2004; Windle et al., 1997); site-specific (Blume et al., 2008; Smith and Wang, 2014)). Our data support local anxiolytic effects of Oxt as site-specific injections blunted the stress-induced activation of CRHergic neurons in the PVN in female prairie voles, as well as downstream effects including the rise in plasma corticosterone and anxiety-like behaviors. However, the blunted stress response only occurred when the local administration of Oxt preceded the stressful event, and not afterward. This suggests Oxt action in the PVN functions in a preventive inhibitory mechanism rather than a negative feedback loop.
Autoexcitation occurs with Oxt neurons (Ludwig and Leng, 2006; Moos et al., 1984), and Oxt release may arise from neuronal somata and dendrites in Oxt synthesizing brain regions, namely the PVN and supraoptic nucleus of the hypothalamus (SON) (Ludwig and Leng, 2006; Neumann et al., 1993; Russell et al., 1992). This suggests Oxt release in the PVN could promote Oxt neuronal activity, but this did not occur in the current study. It is possible that the concentration of Oxt used in this experiment was insufficient to modulate Oxt neuronal activity, as dose-dependent effects of Oxt on neuronal activity have been documented in other brain regions. For example, a high concentration of Oxt (1 µM) disinhibits Oxt neurons in the SON via calcium-dependent depression of inhibitory inputs to those cells and reduced GABAA receptor function (Brussaard et al., 1996). However, lower concentrations of Oxt (25–100 nM) in the SON facilitates GABAA receptor mediated inhibit transmission (Israel et al., 2008). Nevertheless, in the current study, oxytocin increased GABA release and transmission in the PVN, and the anxiolytic effects of Oxt were inhibited by concomitant treatment of a GABAA receptor antagonist. We conclude Oxt release in the PVN results in local GABA release and GABAA receptor-mediated inhibition of the stress response.
Expanding on this idea, CRH neurons in the PVN are under complex afferent regulatory control from various chemical inputs. Nearly half of all synapses in the PVN are GABAergic, observed in electron microscopic (Decavel and Van den Pol, 1990; Miklos and Kovacs, 2002) and electrophysiological studies (Tasker and Dudek, 1993). In addition, over half of all CRH neurons in the PVN express GABAA receptors (Cullinan, 2000). Pharmacological studies demonstrate this neurotransmitter provides an inhibitory regulation of stress outputs, via inhibition of CRH mRNA expression (Bülbül et al., 2011) and CRH secretion (Hillhouse and Milton, 1989; Plotsky et al., 1987) in the PVN. While GABAergic neurons are located in the PVN proper, a greater population of PVN-innervating GABA neurons is found in the hypothalamic zones immediately surrounding the PVN (Tasker and Dudek, 1993). Still, our data provide evidence that the GABA neurons located in the PVN are responsive to Oxt stimulation, yielding increased GABA release. This Oxt-induced GABAergic transmission coincided with an inhibition of basal and stress-induced CRHergic activity in the PVN. CRH neuronal activity promotes an increase in the secretion of corticosterone from the adrenal cortex into circulation through increased adrenocorticotrophin hormone (ACTH) secretion from the pituitary gland (Ontjes et al., 1977; Vale et al., 1981). Thus, it is not surprising that in addition to the decreased CRHergic activity in the PVN, Oxt treatment lowered plasma corticosterone levels. Interestingly, the anxiolytic effects of Oxt were blocked by the local injection of a GABAA receptor antagonist in the PVN. Together, our data suggests exogenous Oxt in the PVN suppresses basal and stimulated HPA axis function and anxiety-like behavior through GABAA receptor-mediated inhibitory transmission. Recently, it was observed that Oxt potentiates GABAergic activity by acting directly on δ-GABAA receptors in Xenopus oocytes devoid of Oxt receptors (Bowen et al., 2015), imbuing a potential secondary mechanism for the Oxt-GABA interaction in the PVN.
Anxiety disorders are commonly treated with GABAA receptor positive allosteric modulators called benzodiazepines, such as diazepam and lorazepam. Diazepam binds allosterically to GABAA receptors to augment their function (Pritchett and Seeburg, 1990). Interestingly, Oxt and diazepam have similar inhibitory effects on anxiety-like behaviors (e.g., EPM: Oxt (Smith and Wang, 2014), diazepam (Griebel et al., 2000); light-dark box: Oxt (Waldherr and Neumann, 2007), diazepam (Griebel et al., 2000); novel-object: Oxt (Veenema et al., 2007), diazepam (Depino et al., 2008); open field: Oxt (Veenema et al., 2007), diazepam (Depino et al., 2008)). Moreover, Oxt and diazepam potentiate GABAergic transmission in the PVN through presynaptic (current study) and postsynaptic (Zahner et al., 2007) modulation, respectively. Therefore, it is possible to speculate that Oxt may succor the effects of current anti-anxiety drugs, such as diazepam, leading to greater therapeutic benefits. Further work is required to better understand the Oxt-GABAA receptor connection in the PVN and the implications as stand-alone or adjunct pharmacotherapeutic drugs. Still, GABA and Oxt act in the PVN to inhibit stress-induced corticosterone secretion and anxiety-like behavior (GABA (Marques de Souza and Franci, 2008); Oxt: current study). By demonstrating that GABAA receptor action modulates the anxiolytic effects of Oxt, our current work provides an initial evaluation of the inhibitory mechanism underlying this circuit.
Highlights.
Elevated platform stress increases HPA axis function and anxiety-like behaviors
Ante-stress injections of Oxt blocks the stress response
Oxt injected in the PVN increase GABAergic neuronal activity and local GABA release
GABAA receptor antagonism hinders anxiolytic effects of Oxt
Acknowledgements
Role of the Funding Source
This work was supported by the National Science Foundation Graduate Research Fellowship and National Institutes of Health grant NIMHF31-095464 to AS and the NIH grants NIMHR01-058616 and NIMHR01-89852 to ZW. The funding sources had no involvement in study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The HPA axis is one of the major biological stress pathways and is catalyzed through activation of CRH and vasopressin (AVP) neurons in the PVN. This stimulates a signaling cascade that increases circulating corticosterone, the end-product of the HPA axis, and rouses psychological and behavioral symptomology Armario (2006)
Contributors
AS, PE, MB, LL, and RA performed pharmacology and behavioral experiments. AS, MT, and YL performed the immunohistochemistry experiment. AS and KL performed the retrodialysis experiement. AS and ZW designed the experimental plan, supervised the students, and discussed the results. AS wrote the paper. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
REFERENCES
- Armario A. The hypothalamic-pituitary-adrenal axis: What can it tell us about stressors? CNS & Neurological Disorders - Drug Targets (Formerly Current Drug Targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Bali B, Kovacs KJ. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci. 2003;18:1518–1526. doi: 10.1046/j.1460-9568.2003.02877.x. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ. Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur J Neurosci. 2004;19:777–782. doi: 10.1111/j.1460-9568.2004.03167.x. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Luz T, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. J. Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, McGregor IS. Oxytocin prevents ethanol actions at delta subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc Natl Acad Sci U S A. 2015;112:3104–3109. doi: 10.1073/pnas.1416900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, de Vlieger TA. Postsynaptic mechanism of depression of GABAergic synapses by oxytocin in the supraoptic nucleus of immature rat. J Physiol. 1996;497:495–507. doi: 10.1113/jphysiol.1996.sp021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. Hypothalamic oxytocin attenuates CRF expression via GABAA receptors in rats. Brain Res. 2011;1387:39–45. doi: 10.1016/j.brainres.2011.02.091. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: A dual hybridization histochemical study. J Comp Neurol. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol Behav. 2005;86:338–346. doi: 10.1016/j.physbeh.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo J-D, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: A dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Depino AM, Tsetsenis T, Gross C. GABA homeostasis contributes to the developmental programming of anxiety-related behavior. Brain Res. 2008;1210:189–199. doi: 10.1016/j.brainres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behaviour and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ludwig M, Landgraf R. Microdialysis administration of vasopressin and vasopressin antagonists into the septum during pole-jumping behavior in rats. Behav. Neural Biol. 1992;58:51–57. doi: 10.1016/0163-1047(92)90907-l. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. Journal of Comparative Neurology. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hendrie CA, Eilam D, Weiss SM. Effects of diazepam and buspirone on the behaviour of wild voles (Microtus socialis) in two models of anxiety. Pharmacol Biochem Behav. 1997;58:573–576. doi: 10.1016/s0091-3057(97)00278-5. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Milton NG. Effect of noradrenaline and gamma-aminobutyric acid on the secretion of corticotrophin-releasing factor-41 and arginine vasopressin from the rat hypothalamus in vitro. J Endocrinol. 1989;122:719–723. doi: 10.1677/joe.0.1220719. [DOI] [PubMed] [Google Scholar]
- Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28:385–394. doi: 10.1523/JNEUROSCI.5198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, Neumann ID, Burg EHvd. Oxytocin regulates stress-induced Crf gene transcription through CREB-regulated transcription coactivator 3. J. Neurosci. 2015 doi: 10.1523/JNEUROSCI.1345-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Maloumby R, Hillerer K, Koszinowski S, Neumann ID, van den Burg EH. Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS One. 2012;7:e37060. doi: 10.1371/journal.pone.0037060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Lin EJ. Neuropeptides as therapeutic targets in anxiety disorders. Curr. Pharm. Des. 2012;8:5709–5727. doi: 10.2174/138161212803530871. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Marques de Souza L, Franci CR. GABAergic mediation of stress-induced secretion of corticosterone and oxytocin, but not prolactin, by the hypothalamic paraventricular nucleus. Life Sci. 2008;83:686–692. doi: 10.1016/j.lfs.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: Specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Ludwig M, Engelmann M, Pittman QJ, R L. Simultaneous microdialysis in blood and brain: Oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Ontjes DA, Ways DK, Mahaffee DD, Zimmerman CF, Gwynne JT. Part IV. ACTH action II. ACTH action on the adrenal cortex ACTH receptors and the effect of ACTH on adrenal organelles. Annals of the New York Academy of Sciences: ACTH and Related Peptides: Structure, Regulation, and Action. 1977;297:295–312. doi: 10.1111/j.1749-6632.1977.tb41862.x. [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting A-L, Uvnäs-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci. Lett. 1999;264:41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Otto S, Sutton S. Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life Sci. 1987;41:1311–1317. doi: 10.1016/0024-3205(87)90211-6. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J. Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Wolf KN, Sprangel ME, Rall WF, Wildt DE. Prolonged mating in prairie voles (Microtus ochrogaster) increases likelihood of ovulation and embryo number. Biology of Reproduction. 1999;60:756–762. doi: 10.1095/biolreprod60.3.756. [DOI] [PubMed] [Google Scholar]
- Russell JA, Neumann I, Landgraf R. Oxytocin and vasopressin release in discrete brain areas after naloxone in morphine-tolerant and -dependent anesthetized rats: push-pull perfusion study. J Neurosci. 1992;12:1024–1032. doi: 10.1523/JNEUROSCI.12-03-01024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm. Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1993;469:179–192. doi: 10.1113/jphysiol.1993.sp019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc. Natl. Acad. Sci. USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Pan HL. Benzodiazepine inhibits hypothalamic presympathetic neurons by potentiation of GABAergic synaptic input. Neuropharmacology. 2007;52:467–475. doi: 10.1016/j.neuropharm.2006.08.024. [DOI] [PubMed] [Google Scholar]