Abstract
Topic
Primary open angle glaucoma (POAG) is a highly prevalent condition worldwide and the most common cause of irreversible sight loss. The objective is to assess the comparative effectiveness of first line medical treatments in patients with POAG or ocular hypertension through a systematic review and network meta-analysis, and to provide relative rankings of these treatments.
Clinical Relevance
Treatment for POAG currently relies completely on lowering the intraocular pressure (IOP). While topical drops, lasers, and surgeries can be considered in the initial treatment of glaucoma, most patients elect to start treatment with eye drops.
Methods
We included randomized controlled trials that compared a single active topical medication with no treatment/placebo or another single topical medication. We searched CENTRAL, MEDLINE, EMBASE and the Food and Drug Administration's website. Two individuals independently assessed trial eligibility, abstracted data, and assessed the risk of bias. We performed Bayesian network meta-analyses.
Results
We included 114 randomized controlled trials with data from 20,275 participants. The overall risk of bias of the included trials is mixed. The mean reductions (95% credible intervals) in IOP in mmHg at 3 months, ordered from the most to least effective drugs were: bimatoprost 5·61 (4·94; 6·29), latanoprost 4·85 (4·24; 5·46), travoprost 4·83 (4·12; 5·54), levobunolol 4·51 (3·85; 5·24), tafluprost 4·37 (2·94; 5·83), timolol 3·7 (3·16; 4·24), brimonidine 3·59 (2·89; 4·29), carteolol 3·44 (2·42; 4·46), levobetaxolol 2·56 (1·52; 3·62), apraclonidine 2·52 (0·94; 4·11), dorzolamide 2·49 (1·85; 3·13), brinzolamide 2·42 (1·62; 3·23), betaxolol 2·24 (1·59; 2·88), and unoprostone 1·91 (1·15; 2·67).
Conclusions
All active first-line drugs are effective compared to placebo in reducing IOP at 3 months. Bimatoprost, latanoprost, and travoprost are among the most efficacious drugs, although the within class differences were small and may not be clinically meaningful. All factors, including adverse effects, patient preferences, and cost should be considered in selecting a drug for a given patient.
Glaucoma is an acquired disease of the optic nerve with characteristic optic nerve head changes and associated visual field defects.1–4 It is a the second leading cause of blindness worldwide.5 Nearly three quarters of all glaucoma occurs in individuals with open angles, and open angle glaucoma (OAG) is the most common form of glaucoma in nearly all countries.5 While some forms of OAG occur secondary to other phenomena, the vast majority is idiopathic and therefore is referred to as primary open angle glaucoma (POAG). US-based data suggest that POAG affects 2·3 million Americans aged 40 and older.6–8 The risk of developing POAG increases with increased intraocular pressure (IOP), age, a family history of glaucoma, use of steroids, and having ancestry of the West African diaspora (such as African Americans or African Caribbeans).1-8 Because IOP is the only known modifiable risk factor, treatment for POAG has focused on lowering IOP, which is proven to slow disease progression, decrease the rate of visual field loss, and may protect against loss of visual function and blindness.1–4
Medical treatment (e.g., topical eye drops) is considered a reasonable first line of therapy in published guidelines for the treatment of POAG.1,2 Clinicians usually prescribe a single medication chosen from one of four drug classes - beta blockers, carbonic anhydrase inhibitors, alpha-2 adrenergic agonists, and prostaglandin analogs. Among them, prostaglandin analogs have a reputation for lowering IOP more than other classes.1–4 However, existing practice guidelines and systematic reviews supporting guideline recommendations have not yet addressed the comparative effectiveness and safety of any two drugs (or any two classes of drugs), or provided a ranked order of the drugs (or classes of drugs) in terms of effectiveness and safety.1–4 This is because conventional randomized controlled trials (RCTs) and quantitative synthesis of such trials (i.e., meta-analysis) typically focus on one-at-a-time, pair-wise comparisons (e.g., active drug versus placebo). A direct comparison between two active drugs, one doctors may be most interested, is often lacking. Naïve methods of making such comparisons are common, but are often subject to bias.9,10
Network meta-analysis, an extension to standard pair-wise meta-analysis, enables simultaneous “all-way” comparisons of multiple healthcare interventions for a condition through combining direct evidence from individual trials and indirect evidence gleaned using statistical techniques across trials.10–14 Treatment effects estimated from network meta-analyses usually have improved precision compared to pair-wise meta-analyses, and inferences can be drawn even for comparisons not directly evaluated in individual trials.10–14 Network meta-analysis can also provide relative rankings for multiple competing interventions to inform decision-making.15,16 The objective of this paper is to assess the comparative effectiveness of first line medical treatments for lowering IOP in patients with POAG or ocular hypertension (OH) through a systematic review and network meta-analysis, and to provide relative rankings of these treatments.
Methods
We followed a prospective protocol in performing this systematic review. The reporting conforms to the PRISMA extension for network meta-analysis (http://www.equator-network.org/reporting-guidelines/prisma/; accessed on August 19, 2015).
Eligibility criteria for considering studies for this review
Trials were eligible for our network meta-analysis if they were reported to be randomized parallel group trials (i.e., crossover trials were not eligible), if 60% or more of randomized participants had a diagnosis of POAG and/or OH, as defined by the trialists. Trials were eligible if they evaluated first line topical medical interventions from one of four drug classes - beta blockers, carbonic anhydrase inhibitors, alpha-2 adrenergic agonists, and prostaglandin analogs - to reduce IOP or progression of visual field damage; and compared a single active treatment with no treatment/placebo or another single active topical medical treatment.
We excluded trials enrolling fewer than 10 participants in each group. We also excluded trials evaluating combination medical treatments because they are generally prescribed for patients who have failed a single first line treatment. We required no maximum or minimum duration of treatment, however, participants had to be followed for an outcome for at least 28 days after randomization.
We pre-specified difference in mean IOP measured by any method at 3 months in continuous mmHg unit as our primary outcome. If more than one IOP measure was available, we used the following order of priority in selecting IOP data for analysis: mean diurnal IOP, 24-hour mean IOP, peak IOP, morning IOP, and trough IOP. When a trial's duration was shorter or longer than 3 months, we used the IOP that was measured at the follow-up time point closest to 3 months. We pre-specified visual field as our secondary outcome. Because visual field tends to be measured and aggregated differently across trials, we included visual field outcome as defined and reported in individual trials at any follow-up time point. Only those trials providing sufficient information (i.e., measures of treatment effect as well as the associated precision) were included in our statistical analysis.
Search methods for identifying studies
We searched the Cochrane Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, and EMBASE on November 17, 2009 and updated the search on March 11, 2014. We did not impose any date or language restrictions in the electronic searches. We searched the US Food and Drug Administration's website (Drugs@FDA) in April 2014 for drugs potentially eligible for our review. The full search strategies are described in Appendix 1 (available at http://aaojournal.org).
Study selection
Two individuals independently assessed the titles and abstracts identified by the searches for potential eligibility, and the full text articles were retrieved for those that appeared relevant. Two individuals independently assessed full text articles for final eligibility. Non-English language reports were assessed by a single individual who was a native or fluent speaker of the language. We resolved discrepancies in classification of eligibility of the full text article through discussion or consultation with a third person.
Data collection and risk of bias assessment
For each included trial, two individuals independently abstracted data on the study design, participant and intervention characteristics, outcomes, risk of bias, and quantitative results for treatment effects using electronic forms developed and maintained in the Systematic Review Data Repository (http://srdr.ahrq.gov/).17,18 We graded each of the following methodological domains at “low” “high” or “unclear” risk of bias using the Cochrane Risk of Bias Tool: sequence generation and allocation sequence concealment (both items related to selection bias), masking of participants and outcome assessors (information bias), funding for the trial, and financial relationship reported by the authors.19 We compared the data extracted by two individuals and resolved discrepancies through discussion or consultation with a third person.
Data synthesis and analysis
Qualitative synthesis
We evaluated clinical and methodological heterogeneity among studies, and examined the participant characteristics and risk of bias of included trials that could affect the interpretation of cumulative evidence using qualitative synthesis.20
Quantitative synthesis
We first conducted pair-wise meta-analyses for every treatment comparison with at least two trials (i.e., direct comparisons) with an outcome measured and aggregated in a similar fashion using a random-effects model. We first assumed a comparison-specific statistical heterogeneity and then a common heterogeneity across all comparisons.21 We used STATA 13® (College Station, TX: StataCorp LP) for pair-wise meta-analyses.
We then fitted a Bayesian random-effects network meta-analysis model following Lu and Ades approach and accounted for the correlation among the multi-arm trials.22,23 We used non-informative priors and fitted the model using Markov chain Monte Carlo (MCMC) algorithms, executed using “gemtc” R package which recalls JAGS in R for MCMC sampling.24 We used 4 parallel chains and obtained 50,000 samples after a 20,000-sample burn-in in each chain. To check convergence, we used the Gelman-Rubin diagnostic and trace plots.25,26
Evaluation of the assumption for network meta-analysis
Network meta-analysis is valid when there are no important differences among the trials other than the treatments being compared.13,27 This assumption implies that participants included in the network could hypothetically be randomized to any of the treatments.13 We used our qualitative synthesis to inform our assessment of this assumption underlying network meta-analysis. We considered treatments defined in our research question to be comparable, in that any of them could, in theory, be used as a first line treatment for patients with POAG/OH.
We further assessed statistical disagreement of direct and indirect evidence, known as inconsistency, using three approaches: loop-specific approach, node-splitting approach, and a comparison of Bayesian model fit with and without assuming consistency in the network.28–32 For investigating loop-specific inconsistency, we used STATA 13® (College Station, TX: StataCorp LP).16,33 For the node-splitting approach, we used the “gemtc” package in R.29,30 For the comparison of model fit, we fitted two Bayesian network meta-analysis models with and without assuming consistency implemented in “gemtc” package in R.24 We evaluated the fit of these two models using the Deviance Information Criterion.34 We qualitatively checked the influence of selected trial characteristics (e.g., funding source, big effect size) when statistically significant inconsistency is detected and conducted sensitivity analysis by removing studies seem to introduce statistical inconsistency.27
Measures of association
We calculated the mean difference (MD) of IOP between two treatments with 95% confidence intervals or credible intervals. Because in randomized trials “mean differences based on changes from baseline can usually be assumed to be addressing the same underlying intervention effects as analyses based on final measurements,”35 change scores and final measurements were combined in the analysis. Because glaucoma drugs are expected to lower IOP, a larger negative MD means that the first drug used in the comparison reduced IOP more, and therefore is more efficacious than the comparator drug.
We combined different concentrations of the same medication in the primary analysis, and separated bimatoprost and timolol into two concentrations (bimatoprost 0·03% or bimatoprost 0·01%; timolol 0·5% or timolol < 0·5%) in an ad hoc sensitivity analysis. Bimatoprost 0·03% was the only concentration available on the market when we started this project in 2009. It was discontinued by the end of year 2012 in the United States and replaced by bimatoprost 0·01% (a concentration approved in the United States in late 2010). Bimatoprost 0·03% is still available in some countries. For timolol, where both higher and lower concentrations are available, clinicians are interested in knowing whether a lower concentration works as well as the 0·5% concentration because the side effects may be less with a lower concentration.
We also generated probabilities of each treatment taking each possible rank (e.g., the probability of timolol being ranked as the most efficacious treatment, the second efficacious treatment, so on and so forth to the worst treatment) and the cumulative ranking probabilities, known as a “SUCRA” plot.15,16 SUCRA values show the relative probability of an intervention being among the best options. Rankings based on SUCRA values account better for the uncertainly in the estimated treatment effect.15,16 All rankings used “placebo/ vehicle/no treatment” as the reference group.
Results
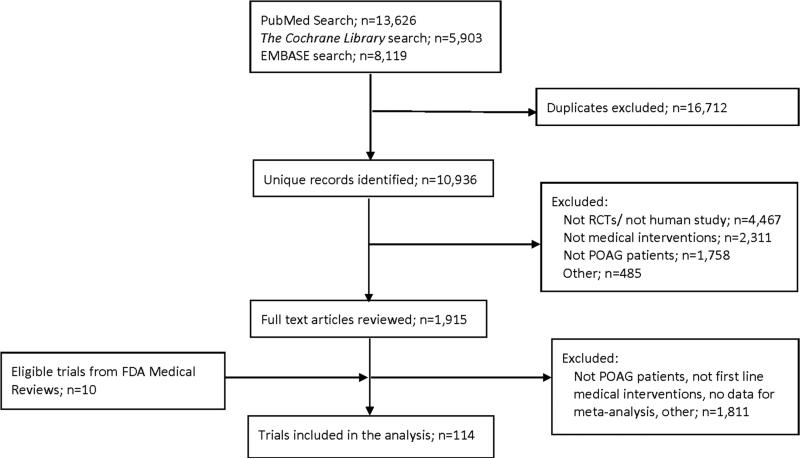
We identified 10,936 unique records from our searches, of which 114 RCTs were eligible to be included in our network meta-analysis (Figure 1; references to these RCTs are presented in Appendix 2 available at http://aaojournal.org). Included trials were published between 1983 and 2013, with more than half published after 2000 (Appendix 3. Table 1 available at http://aaojournal.org). These trials randomized a total of 20,275 participants with a sample size ranging from 17 to 976 (median=113·5; interquartile range: 50 to 260). Seventy-eight (68%) RCTs were multi-center trials. Of the 85 trials reporting the region(s) in which participants were recruited, 47 (55%) recruited participants from North America, 20 (24%) from Europe, 17 (20%) from Asia, 4 (5%) from Latin America, 4 (5%) from Oceania, and 2 (2%) from Africa (a trial could recruit participants from more than one region).
Figure 1. Selection of studies.
POAG: primary open angle glaucoma
FDA: Food and Drug Administration
RCT: Randomized controlled trial
Our search was broad and included all medical interventions for glaucoma. We reviewed a large number of full text articles because some of them will be used in different but related systematic reviews, for example, combination drug therapy for glaucoma.
Table 1.
Summary estimates for intraocular pressure at 3 months derived from pair-wise meta-analysis based on direct comparisons from 114 trials*
|
Comparison-specific heterogeneity
|
|||||||
|---|---|---|---|---|---|---|---|
| Column 1 | Column 2 | Num. of studies | Mean difference¶ | 95% CI, lower | 95% CI, upper | Tau-squared | I-squared |
| Placebo vs. | |||||||
| Brimonidine | 1 | −2.30 | −3.99 | −0.61 | NA | N A | |
| Betaxolol | 3 | −2.38 | −3.78 | −0.98 | 1.11 | 73% | |
| Levobunolol | 2 | −7.52 | −8.50 | −6.50 | NA | NA | |
| Timolol | 5 | −3.68 | −4.72 | −2.63 | 0.71 | 52% | |
| Levobetaxolol | 1 | −3.00 | −4.53 | −1.47 | NA | NA | |
| Brinzolamide | 2 | −2.17 | −3.23 | −1.10 | 0.00 | 0% | |
| Dorzolamide | 4 | −1.91 | −2.92 | −0.90 | 0.51 | 51% | |
| Bimatoprost | 1 | −4.60 | −5.60 | −3.60 | NA | NA | |
| Unoprostone | 1 | −0.20 | −1.56 | 1.16 | NA | NA | |
| Apraclonidine vs | |||||||
| Timolol | 2 | −0.84 | −3.75 | 2.08 | 3.73 | 84 % | |
| Brimonidine vs | |||||||
| Betaxolol | 1 | 1.94 | 0.84 | 3.04 | NA | N A | |
| Timolol | 4 | 0.17 | −0.70 | 1.03 | 0.55 | 81% | |
| Brinzolamide | 2 | 1.01 | 0.50 | 1.53 | 0.00 | 0% | |
| Latanoprost | 5 | −1.36 | −2.21 | −0.50 | 0.73 | 78% | |
| Travoprost | 1 | −1.20 | −3.77 | 1.37 | NA | NA | |
| Betaxolol vs | |||||||
| Levobunolol | 2 | −4.73 | −10.01 | 0.55 | 12.25 | 83% | |
| Timolol | 9 | −1.57 | −2.20 | −0.93 | 0.33 | 41% | |
| Levobetaxolol | 1 | −2.00 | −3.54 | −0.46 | NA | NA | |
| Dorzolamide | 2 | −0.30 | −0.96 | 0.36 | 0.00 | 0% | |
| Latanoprost | 2 | −1.06 | −2.62 | 0.51 | 0.33 | 25% | |
| Unoprostone | 1 | 0.60 | 0.09 | 1.11 | NA | NA | |
| Carteolol vs | |||||||
| Levobunolol | 1 | −2.90 | −4.59 | −1.22 | NA | N A | |
| Timolol | 4 | 0.03 | −0.61 | 0.68 | 0.11 | 24% | |
| Levobunolol vs | |||||||
| Timolol | 11 | −0.03 | −0.44 | 0.39 | 0.01 | 3% | |
| Timolol vs | |||||||
| Levobetaxolol | 3 | 1.25 | 0.27 | 2.23 | 0.52 | 73% | |
| Brinzolamide | 3 | 1.10 | 0.50 | 1.70 | 0.00 | 0% | |
| Dorzolamide | 7 | 0.94 | 0.41 | 1.47 | 0.22 | 43% | |
| Bimatoprost | 7 | −2.08 | −2.47 | −1.70 | 0.05 | 19% | |
| Latanoprost | 14 | −1.27 | −1.70 | −0.84 | 0.37 | 64% | |
| Travoprost | 6 | −0.90 | −1.27 | −0.52 | 0.00 | 0% | |
| Tafluprost | 1 | −0.30 | −0.72 | 0.12 | NA | NA | |
| Unoprostone | 3 | 1.35 | 0.42 | 2.27 | 0.57 | 86% | |
| Brinzolamide vs | |||||||
| Dorzolamide | 2 | −0.58 | −1.15 | 0.00 | 0.00 | 0% | |
| Dorzolamide vs | |||||||
| Latanoprost | 1 | −2.90 | −3.70 | −2.10 | NA | N A | |
| Bimatoprost vs | |||||||
| Latanoprost | 6 | 0.87 | 0.01 | 1.73 | 0.82 | 76 % | |
| Travoprost | 8 | 0.59 | −0.13 | 1.30 | 0.73 | 74% | |
| Latanoprost vs | |||||||
| Travoprost | 7 | −0.06 | −0.46 | 0.34 | 0.00 | 0% | |
| Tafluprost | 1 | −0.90 | −3.40 | 1.60 | NA | NA | |
| Unoprostone | 6 | 3.07 | 2.51 | 3.63 | 0.01 | 2% | |
There are 101 two-arm trials, 12 three-arm trials, and 1 four-arm trial (total 114 trials).
Mean difference is caculated using the intraocular pressure of the drug in column 2- coloum 1.
Ninety-one (80%) trials allowed enrolling participants if they were on ocular hypotensive medication at the time of enrollment, and of these, 75 (82%) reported using a washout period before randomization. Twelve (11%) trials reported using a run-in period before randomization, with 11/12 trials using an active drug in the run-in period. The reported median follow-up time for participants after randomization was 3 months (interquartile range: 2·8 to 6 months).
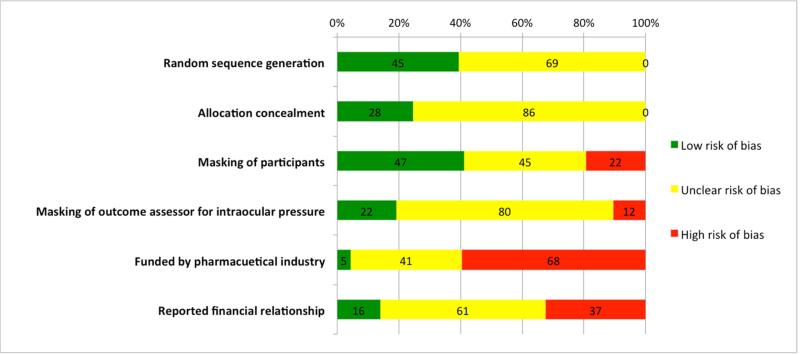
The overall risk of bias of the included RCTs is mixed at best (Figure 2; Appendix 3. Table 2 available at http://aaojournal.org). Forty-five (39%) and 28 (25%) trials were rated at low risk of bias for random sequence generation and allocation concealment, respectively; the remaining trials (69 and 86, respectively) were rated at “unclear risk of bias” on each of these two domains. Forty-seven (41%) trials reported masking of study participants (rated at “low risk of bias”); 22 (19%) trials reported that the study participants were not masked (rated at “high risk of bias”); and the remaining 45 (39%) trials were rated at “unclear risk of bias”. Twenty-two (19%) trials reported masking of outcome assessors for IOP (rated at “low risk of bias”); 12 (11%) trials reported that the outcome assessor for IOP was not masked (rated at “high risk of bias”); the remaining 80 (70%) trials were rated at “unclear risk of bias”. Seventy-one (62%) trials reported receiving funding for the research, of which 68 (96%) were funded by pharmaceutical industry and seven (10%) were funded by government (a trial could receive more than one source of funding). Thirty-seven (32%) trials reported that at least one author had financial conflict of interests.
Figure 2.
Risk of bias figure
Table 2.
Summary estimates for intraocular pressure at 3 months derived from network meta-analysis of 114 trials*
| Placebo | −2.52 (−4.11;−0.94) | −3.59 (−4.29;−2.89) | −2.24 (−2.88;−1.59) | −3.44 (−4.46;−2.42) | −4.51 (−5.24;−3.8) | −3.7 (−4.24;−3.16) | −2.56 (−3.62;−1.52) | −2.42 (−3.23;−1.62) | −2.49 (−3.13;−1.85) | −5.61 (−6.29;−4.94) | −4.85 (−5.46;−4.24) | −4.83 (−5.54;−4.12) | −4.37 (−5.83;−2.94) | −1.91 (−2.67;−1.15) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.52 (0.94;4.11) |
Apraclonidine | −1.08 (−2.65;0.52) |
0.28 (−1.29;1.87) |
−0.92 (−2.64;0.81) |
−2 (−3.58;−0.39) |
−1.18 (−2.67;0.31) |
−0.05 (−1.81;1.72) |
0.09 (−1.55;1.75) |
0.03 (−1.54;1.62) |
−3.1 (−4.65;−1.53) |
−2.33 (−3.85;−0.79) |
−2.31 (−3.88;−0.73) |
−1.85 (−3.85;0.15) |
0.61 (−0.99;2.23) |
| 3.59 (2.89;4.29) |
1.08 (−0.52;2.65) |
Brimonidine | 1.35 (0.67;2.04) |
0.15 (−0.87;1.17) |
−0.92 (−1.69;−0.16) |
−0.11 (−0.64;0.42) |
1.03 (−0.05;2.1) |
1.17 (0.4;1.92) |
1.1 (0.4;1.8) |
−2.02 (−2.69;−1.35) |
−1.25 (−1.8;−0.72) |
−1.24 (−1.92;−0.55) |
−0.78 (−2.23;0.65) |
1.69 (0.92;2.45) |
| 2.24 (1.59;2.88) |
−0.28 (−1.87;1.29) |
−1.35 (−2.04;−0.67) |
Betaxolol | −1.2 (−2.21;−0.19) |
−2.28 (−3.01;−1.55) |
−1.46 (−1.99;−0.94) |
−0.32 (−1.38;0.72) |
−0.18 (−1.03;0.65) |
−0.25 (−0.91;0.4) |
−3.37 (−4.06;−2.7) |
−2.61 (−3.2;−2.02) |
−2.59 (−3.3;−1.89) |
−2.13 (−3.58;−0.7) |
0.33 (−0.42;1.08) |
| 3.44 (2.42;4.46) |
0.92 (−0.81;2.64) |
−0.15 (−1.17;0.87) |
1.2 (0.19;2.21) |
Carteolol | −1.08 (−2.07;−0.09) |
−0.26 (−1.14;0.61) |
0.88 (−0.42;2.16) |
1.02 (−0.11;2.14) |
0.95 (−0.07;1.96) |
−2.17 (−3.16;−1.18) |
−1.41 (−2.35;−0.47) |
−1.39 (−2.4;−0.38) |
−0.93 (−2.54;0.66) |
1.53 (0.47;2.59) |
| 4.51 (3.8;5.24) |
2 (0.39;3.58) |
0.92 (0.16;1.69) |
2.28 (1.55;3.01) |
1.08 (0.09;2.07) |
Levobunolol | 0.81 (0.25;1.38) |
1.95 (0.86;3.04) |
2.09 (1.2;2.99) |
2.03 (1.28;2.77) |
−1.1 (−1.83;−0.36) |
−0.33 (−0.99;0.33) |
−0.31 (−1.06;0.44) |
0.14 (−1.32;1.6) |
2.61 (1.79;3.43) |
| 3.7 (3.16;4.24) |
1.18 (−0.31;2.67) |
0.11 (−0.42;0.64) |
1.46 (0.94;1.99) |
0.26 (−0.61;1.14) |
−0.81 (−1.38;−0.25) |
Timolol | 1.14 (0.19;2.07) |
1.28 (0.56;1.99) |
1.21 (0.69;1.73) |
−1.91 (−2.38;−1.44) |
−1.15 (−1.5;−0.79) |
−1.13 (−1.63;−0.63) |
−0.67 (−2.02;0.67) |
1.79 (1.18;2.41) |
| 2.56 (1.52;3.62) |
0.05 (−1.72;1.81) |
−1.03 (−2.1;0.05) |
0.32 (−0.72;1.38) |
−0.88 (−2.16;0.42) |
−1.95 (−3.04;−0.86) |
−1.14 (−2.07;−0.19) |
Levobetaxolol | 0.14 (−1.03;1.32) |
0.07 (−0.99;1.14) |
−3.05 (−4.09;−1.99) |
−2.28 (−3.28;−1.27) |
−2.27 (−3.32;−1.19) |
−1.81 (−3.45;−0.18) |
0.66 (−0.45;1.78) |
| 2.42 (1.62;3.23) |
−0.09 (−1.75;1.55) |
−1.17 (−1.92;−0.4) |
0.18 (−0.65;1.03) |
−1.02 (−2.14;0.11) |
−2.09 (−2.99;−1.2) |
−1.28 (−1.99;−0.56) |
−0.14 (−1.32;1.03) |
Brinzolamide | −0.07 (−0.85;0.71) |
−3.19 (−4.03;−2.36) |
−2.42 (−3.18;−1.66) |
−2.41 (−3.26;−1.55) |
−1.95 (−3.47;−0.44) |
0.52 (−0.39;1.43) |
| 2.49 (1.85;3.13) |
−0.03 (−1.62;1.54) |
−1.1 (−1.8;−0.4) |
0.25 (−0.4;0.91) |
−0.95 (−1.96;0.07) |
−2.03 (−2.77;−1.28) |
−1.21 (−1.73;−0.69) |
−0.07 (−1.14;0.99) |
0.07 (−0.71;0.85) |
Dorzolamide | −3.12 (−3.8;−2.44) |
−2.36 (−2.95;−1.76) |
−2.34 (−3.05;−1.63) |
−1.88 (−3.32;−0.45) |
0.58 (−0.19;1.36) |
| 5.61 (4.94;6.29) |
3.1 (1.53;4.65) |
2.02 (1.35;2.69) |
3.37 (2.7;4.06) |
2.17 (1.18;3.16) |
1.1 (0.36;1.83) |
1.91 (1.44;2.38) |
3.05 (1.99;4.09) |
3.19 (2.36;4.03) |
3.12 (2.44;3.8) |
Bimatoprost | 0.77 (0.27;1.26) |
0.78 (0.26;1.3) |
1.24 (−0.18;2.65) |
3.71 (2.97;4.44) |
| 4.85 (4.24;5.46) |
2.33 (0.79;3.85) |
1.25 (0.72;1.8) |
2.61 (2.02;3.2) |
1.41 (0.47;2.35) |
0.33 (−0.33;0.99) |
1.15 (0.79;1.5) |
2.28 (1.27;3.28) |
2.42 (1.66;3.18) |
2.36 (1.76;2.95) |
−0.77 (−1.26;−0.27) |
Latanoprost | 0.02 (−0.5;0.53) |
0.48 (−0.91;1.83) |
2.94 (2.33;3.55) |
| 4.83 (4.12;5.54) |
2.31 (0.73;3.88) |
1.24 (0.55;1.92) |
2.59 (1.89;3.3) |
1.39 (0.38;2.4) |
0.31 (−0.44;1.06) |
1.13 (0.63;1.63) |
2.27 (1.19;3.32) |
2.41 (1.55;3.26) |
2.34 (1.63;3.05) |
−0.78 (−1.3;−0.26) |
−0.02 (−0.53;0.5) |
Travoprost | 0.46 (−0.98;1.87) |
2.92 (2.17;3.68) |
| 4.37 (2.94;5.83) |
1.85 (−0.15;3.85) |
0.78 (−0.65;2.23) |
2.13 (0.7;3.58) |
0.93 (−0.66;2.54) |
−0.14 (−1.6;1.32) |
0.67 (−0.67;2.02) |
1.81 (0.18;3.45) |
1.95 (0.44;3.47) |
1.88 (0.45;3.32) |
−1.24 (−2.65;0.18) |
−0.48 (−1.83;0.91) |
−0.46 (−1.87;0.98) |
Tafluprost | 2.46 (1.01;3.95) |
| 1.91 (1.15;2.67) |
−0.61 (−2.23;0.99) |
−1.69 (−2.45;−0.92) |
−0.33 (−1.08;0.42) |
−1.53 (−2.59;−0.47) |
−2.61 (−3.43;−1.79) |
−1.79 (−2.41;−1.18) |
−0.66 (−1.78;0.45) |
−0.52 (−1.43;0.39) |
−0.58 (−1.36;0.19) |
−3.71 (−4.44;−2.97) |
−2.94 (−3.55;−2.33) |
−2.92 (−3.68;−2.17) |
−2.46 (−3.95;−1.01) |
Unoprostone |
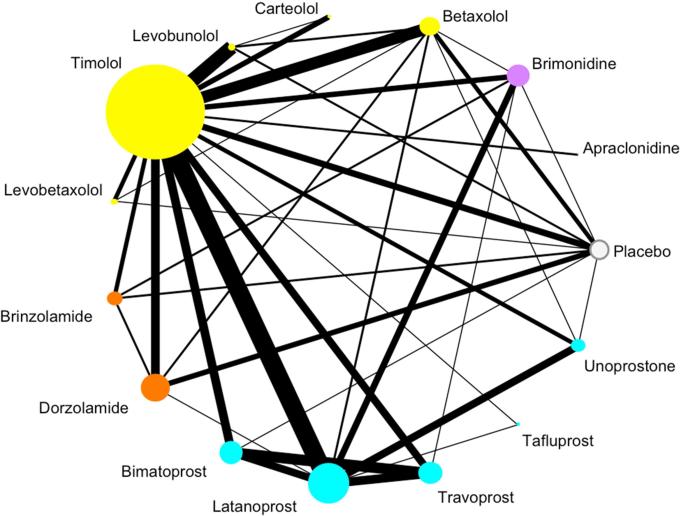
The included trials compared 15 interventions (14 active drugs and “placebo/vehicle/no treatment”) (Figure 3). Of the 39 direct comparisons, 12 (31%) are based on one trial and eight (21%) are based on two trials. The median number of trials for each direct comparison is two (interquartile range 1 to 5.5). Timolol was the most often used comparator and was studied in 70 trials (61%). One hundred-one (89%) trials had two treatment arms, 12 (11%) trials had three arms, and the remaining trial had four arms (1%).
Figure 3. Network graph.
Each node represents one drug. The drugs are color-coded by class. The size of the node is proportional to the number of participants randomized to that drug. The edges represent direct comparisons, that is, when there is a line connecting two drugs, the two drugs have been compared directly to each other in a trial. The width of the edge is proportional to the number of trials.
Total number studies =114
Number of published studies =104 (11 three-arm studies)
Number of FDA studies =10 (1 three-arm study, 1 four-arm study)
Total number of patients contributing to this network =20,275
Color coding:
White: Placebo/vehicle/no treatment
Purple: Alpha-2 adrenergic agonist
Yellow: Beta-blocker
Orange: Carbonic anhydrase inhibitor
Turquoise: Prostaglandin analog
Table 1 shows the results based on direct comparisons. Brimonidine, betaxolol levobunolol, timolol, levobetaxolol, brinzolamide, dorzolamide, bimatoprost, and unoprostone have been compared directly to placebo in 20 comparisons. When a comparison-specific heterogeneity is assumed, all drugs except unoprostone lower IOP at 3 months; the IOP reduction point estimates (using the measure under placebo arm minus the measure under the active drug) range from 1·91 to 7·52 mmHg. Levobetaxolol, brinzolamide, dorzolamide, bimatoprost, latanoprost, travoprost, tafluprost, and unoprostone have been compared directly to timolol in 44 comparisons. When a comparison-specific heterogeneity is assumed, bimatoprost, latanoprost, and travoprost lowered IOP more than timolol at 3 months; the difference in IOP compared to timolol range from 0·30 to 2·08 mmHg. The results assuming a common heterogeneity are comparable to the results assuming a comparison-specific heterogeneity (results not shown).
Table 2 shows the results based on a Bayesian network meta-analysis that combines direct and indirect comparisons. All active drugs are shown to be more effective than placebo in lowering IOP at 3 months. The mean reductions (95% credible intervals) in IOP in mmHg at 3 months, ordered from the most to least effective drugs based on the SUCRA values were: bimatoprost 5·61 (4·94; 6·29), latanoprost 4·85 (4·24; 5·46), travoprost 4·83 (4·12; 5·54), levobunolol 4·51 (3·85; 5·24), tafluprost 4·37 (2·94; 5·83), timolol 3·7 (3·16; 4·24), brimonidine 3·59 (2·89; 4·29), carteolol 3·44 (2·42; 4·46), levobetaxolol 2·56 (1·52; 3·62), apraclonidine 2·52 (0·94; 4·11), dorzolamide 2·49 (1·85; 3·13), brinzolamide 2·42 (1·62; 3·23), betaxolol 2·24 (1·59; 2·88), and unoprostone 1·91 (1·15; 2·67). Bimatoprost, when the two concentrations (0·03% and 0·01%) are not differentiated, resulted in a statistically significant lower IOP than any other active drug except tafluprost, although the pair-wise differences in lowering IOP at 3 months among bimatoprost, latanoprost, travoprost, and tafluprost are small and may not be clinically meaningful.
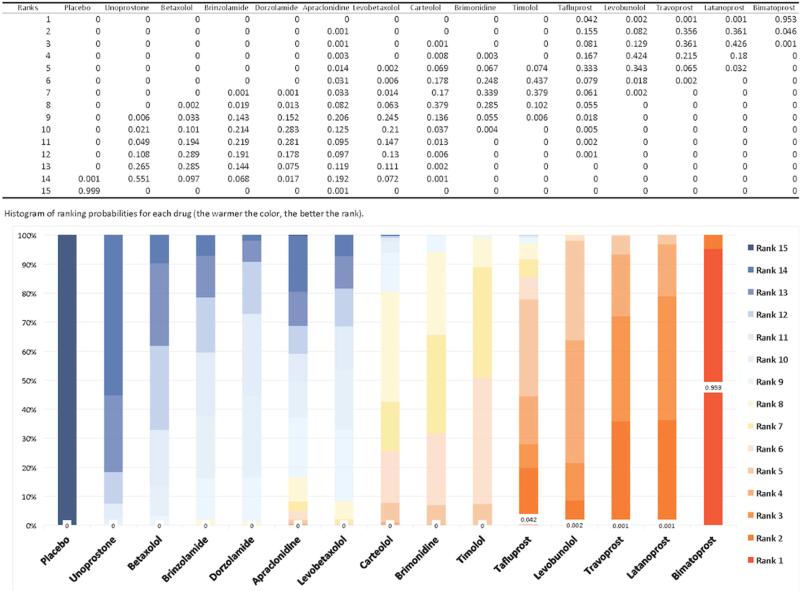
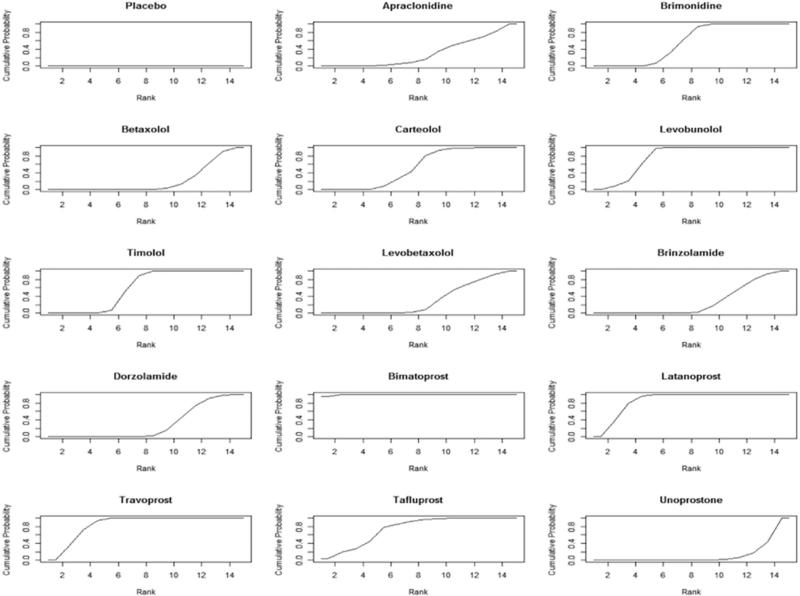
The ranking probabilities for any drug at any possible position are presented in Figure 4, ordered from the least effective drug to the most. The ranking probabilities are consistent with our estimates of treatment effect. For example, the probabilities of bimatoprost being ranked as the first, second, and third most efficacious drug for lowering IOP at 3 months is 95·3%, 4·6%, and 0·1%, respectively. The probabilities of latanoprost being ranked as the first, second, and third most efficacious drug in lowering IOP at 3 months is 0·1%, 36·1%, and 42·6%, respectively. The cumulative ranking probabilities, displayed in “SUCRA” plot, are presented in Figure 5. The larger the surface area under the curve and the faster the curve rises, the higher ranking the drug.15,16 Based on the SUCRA values derived from trials included in our network meta-analysis, bimatoprost seem to be the most efficacious drug in lowering IOP at 3 months. Of note, the two alpha agonists, apraclonidine and brimonidine, had different effectiveness profiles with brimonidine ranking close to timolol while apraclonidine was lower.
Figure 4.
Ranking probabilities for any drug at any position
Figure 5. Cumulative ranking probabilities for each drug.
The SUCRA value (SUrface under the Cumulative RAnking curve) represents the surface underneath the cumulative ranking curve and is the posterior probabilities for each drug to be among the n-best options (15, 16). The larger the SUCRA value, the higher the ranking probabilities for the drug among the drugs compared. Rankings based on SUCRA values account better for the uncertainly in the estimated treatment effects (15, 16).
SUCRA values normalized to %:
Placebo (0.01), Apraclonidine (30.55), Brimonidine (57.09), Betaxolol (21.62), Carteolol (53.44), Levobunolol (77.96), Timolol (60.39), Levobetaxolol (31.56), Brinzolamide (27.67), Dorzolamide (30.36), Bimatoprost (99.66), Latanoprost (86.56), Travoprost (85.76), Tafluprost (74.79), Unoprostone (12.44)
Sensitivity analysis
Of the 17 bimatoprost trials included in our network, 15 evaluated bimatoprost 0·03% and two evaluated bimatoprost 0·01%. Of the 70 timolol trials included in our network, 65 evaluated timolol 0·5% and five evaluated a timolol concentration lower than 0·5%.
The mean differences in intraocular pressure at 3 months comparing bimatoprost 0·03% or bimatoprost 0·01% against placebo are 5·77 (5·04; 6·50) and 4·74 (1·91; 3·19) respectively. The mean difference in IOP between bimatoprost 0·03% and bimatoprost 0·01% is small [1·04 (−0·30; 2·39)] and this difference is not statistically significant (Appendix 4 available at http://aaojournal.org). The mean differences in IOP at 3 months comparing timolol 0·5% or timolol<0·5% against placebo are similar, and are comparable to the estimate for timolol under our main analysis, in which the two concentrations are combined) (Appendix 4 available at http://aaojournal.org).
The relative rankings of the top three drugs changed in the sensitivity analysis. Using the SUCRA values, the top ranked drugs are bimatoprost 0·03%, followed by latanoprost, travoprost, and bimatoprost 0·01% (Appendix 4 available at http://aaojournal.org). Bimatoprost 0·03% is no longer manufactured or sold in the United States (although still available in some countries), and the patent expired in August 2014.
Inconsistency
We used several methods for assessing inconsistency between direct and indirect evidence. Using the loop-specific approach, we found 4/37 (11%) triangle loops (i.e., any three drugs connected in Figure 3 forms a triangle loop) that showed evidence of statistical inconsistency. Using the node-splitting approach, we found two statistically significant inconsistency nodes. We could not find any qualitative reasons to explain the inconsistency and elected to address the inconsistency by fitting an “inconsistency” model to the data and to conduct a sensitivity analysis by removing studies that seemed to introduce statistical inconsistency. The inconsistency model did not improve our model fit and did not change our conclusions. Results of the inconsistency model and sensitivity analysis are presented in Appendix 4 available at http://aaojournal.org.
Visual field
Twenty-three of the 114 trials (20%) reported visual field outcomes, of which 13 (57%) presented both a point estimate and some form of precision estimate (Appendix 5 available at http://aaojournal.org). Due to the heterogeneity in how the visual field was measured, aggregated, and reported at different follow-up times, however, no pair-wise or network meta-analysis of visual field data was possible. Not a single trial reported a difference or ratio between treatment groups with respect to visual field outcomes, although some trials provided group level data and treatment effect could be calculated based on the group level data.
Discussion
Using a systematic review and network meta-analysis, we estimated the pair-wise comparative effectiveness of 14 first-line IOP lowering drugs used in patients with POAG or OH. All drugs examined were significantly more effective than placebo in lowering IOP at 3 months. Drugs in the prostaglandin class were more efficacious than drugs in other classes, although the within class differences were generally small (bimatoprost vs. travoprost, latanoprost, or tafluprost). Bimatoprost 0.01% is no more effective than latanoprost or travoprost in lowering IOP at 3 months. Brimonidine lowered IOP more than apraclonidine; and unoprostone and betaxolol lowered IOP the least.
Our analyses confirmed existing beliefs of the comparative effectiveness of glaucoma drugs and revealed interesting new findings.1–4 Both the American Academy of Ophthalmology Preferred Practice Patterns and the UK-based National Institute for Health and Care Excellence guidelines recommend prostaglandins as the initial medical therapy for POAG and OH.1,2 Our estimate of the efficacy of latanoprost against placebo in lowering IOP, derived entirely from indirect evidence (we found no direct comparison between latanoprost and placebo; Figure 3), is consistent with a major RCT that was published after our search date.36 Unexpected results were that brimonidine, an alpha agonist, performed as well as levobetaxolol and carteolol, and better than betaxolol (three beta-blockers).
Relative IOP reduction needs to be weighed against other factors in a given decision framework. The best ranking drug in lowering IOP at 3 months (i.e., bimatoprost 0·03%) is no longer sold, being replaced on the market by a lower concentration (i.e., bimatoprost 0·01%). In some developing countries, timolol is the only accessible and affordable option among the top ranked drugs, as reflected in prescription patterns. For example, beta-blockers constitute as much as 90% of prescriptions for POAG in India;37 whereas, in the UK and US, latanoprost is the most prescribed drug.38,39 Drug safety and side effects are other important considerations. Prostaglandins have been associated with eyelash lengthening and iris color change, adverse side effects that some patients may find bothersome.3,4 Among prostaglandins, latanoprost has been reported to have a lower risk of conjunctival hyperemia (redness) than travoprost or bimatoprost.3,4 Our study ranks the relative IOP reduction for all drugs, one aspect of the decision framework, and thus does not define which medications are best for initial treatment.
Although IOP is a surrogate outcome, it is typically used in glaucoma trials, including trials submitted to regulatory agencies for market approval, as an outcome measure that quantifies intervention effectiveness.40,41 This is because IOP reduction correlates with preservation of visual field, yet definite or most meaningful outcomes such as change in visual field and optic nerve damage are not easy to quantify and effects on patient-centered outcomes such as visual function (e.g., whether a patient can drive and read) and blindness require a long follow-up time to observe.40,41 Since preservation of visual function is the primary aim of glaucoma treatment, visual field outcomes are a more meaningful gauge of the effectiveness of glaucoma treatment than IOP. Unfortunately, only 11% of trials included in our sample reported any analyzable visual field data and the data were measured and reported in many different ways, making a pair-wise meta-analysis or a network meta-analysis impossible. The short length of follow-up time of most trials (median 3 months), in contrast to what is needed – say 5-years of observing visual field change36 - also precludes meaningful assessments of visual field outcome.
Visual field was used as the primary outcome in a Cochrane systematic review of medical interventions for primary open angle glaucoma.42 Findings from this pair-wise review concluded that treating OH with any drug is more effective than placebo or no treatment in reducing the onset of visual field defects.42 When individual drugs were examined, no drug showed a significant visual field protection, although the review also found positive but weak evidence for a beneficial effect of beta-blockers as a class.
An evidence synthesis such as the network meta-analysis we have done does not overcome the underlying fact that some studies we included were at high or unclear risk of bias, had small sample size, short follow-up time and used IOP, a surrogate outcome to assess treatment effect. Others have found that high or unclear risk of bias for random sequence generation, allocation concealment, and masking of patients and outcome assessors, does not seem impact network meta-analysis results materially.43 This finding was based on an empirical meta-epidemiological study of 32 networks including over 600 RCTs.43 Some researchers have posited that the biases may mitigate through a common comparator.43–45 For example, if there exists sponsorship bias in favor of the company's own products, by connecting the trials through placebo, the indirect evidence might be less biased.44,45
The findings of this study provide critical information to answer doctors’ and patients’ question “what works best?” among all alternative topical medical treatments for POAG and OH. Using indirect evidence, we filled in gaps in the literature about the comparative effectiveness of any two glaucoma drugs. As illustrated in Table 1, direct evidence obtained from RCTs was only available for 39 of 105 (37%) comparisons that we made. Conventional pair-wise meta-analyses, summarizing these RCTs, can only answer questions where RCTs exist and can only examine pairs of interventions.9 This does not support real world clinical decision-making because each meta-analysis only takes into account one part of the overall range of choice. It is also worth noting that 43% of pair-wise meta-analyses published up to February 2012 on glaucoma medications used problematic statistical analyses, calling conclusions from these meta-analyses and systematic reviews into question.9 One common error was that the review authors pooled data by treatment group rather than analyzing the between group effect estimates, breaking the randomized nature of trials.9 We used three approaches to examine the inconsistency assumption for our network meta-analysis and found that this assumption is likely to hold in our analysis.
Network meta-analysis exploits all available direct evidence and uses statistical methods to obtain indirect evidence to form a coherent knowledge base, providing information for comparisons between pairs of drugs that may have never been evaluated in individual trials. The network meta-analysis methodology itself has been validated and matured over recent years, and its utility and added value have been demonstrated.46–51 Furthermore, we offered a comprehensive summary of a large amount of data, and in most cases, provided more precise estimates of treatment effect than the corresponding pair-wise meta-analyses (by comparing estimates in Tables 1 and 2 for the same comparison). Compared to a previous network meta-analysis on glaucoma drugs published in 2009,52 we included four times as many trials. Finally, ranking probabilities create a new metric for clinicians to use when choosing therapy. In some cases the absolute difference in IOP reduction might be small and may not be clinically meaningful; but on the other hand, ranking allows one to put options in context, for example in conjunction with considerations such as side effects, patient preference, and cost.
Conclusion
We found that all active first-line drugs are effective compared to placebo and prostaglandins were more efficacious in lowering IOP at 3 months than beta-blockers, alpha agonists, or carbonic anhydrase. Bimatoprost, latanoprost, and travoprost are among the most efficacious drugs, although the within class differences were small and may not be clinically meaningful. Most trials did not measure or report visual field or other patient-centered outcomes, such as visual function and blindness. All factors, including side effects, patient preferences, and cost should be considered in selecting a drug for a given patient.
Supplementary Material
Acknowledgements
We thank the following people who have helped with various steps of the project, including screening titles and abstraction, retrieving and managing full text articles, completing data abstraction, data management, and analysis: Dolly Chang, Su-Hsun Liu, Harini Sarathy, Rose Iyirhiaro, Lynn Huynh, Kinbo Lee, Swaroop Vedula, Xue Wang, Sueko Ng, Ian Saldanha, Cesar Ugarte, Michael Marrone, Chou-Cheng Lai, Tsung Yu, Minxuan Huang, Rashed Shah, Nan Guo, Jingwen Tan, Yichen Zhong, Vidya Venugopal, Elizabeth Ssemanda, Madhura Rane, and Manuele Michelessi.
Financial Support:
The project was funded by Grant 1 RC1 EY020140 and Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, United States (PI: Dr. Kay Dickersin). The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
DF: Consultant to Allergan, Alcon, Forsight.
None of the other authors have any commercial conflict of interest to declare.
Supplemental material is available at www.aaojournal.org.
References
- 1.American Academy of Ophthalmology . Primary Open-Angle Glaucoma Preferred Practice Patterns. American Academy of Ophthalmology; San Francisco: 2010. [August 7, 2014]. Available from: http://one.aao.org/preferred-practice-pattern/primary-openangle-glaucoma-ppp--october-2010. [Google Scholar]
- 2.National Institute for Health and Care Excellence [August 18, 2014];NICE guidelines [CG85]. Glaucoma: Dianosis and management of chronic open angle glaucoma and ocular hypertension. 2009 Available at: http://www.nice.org.uk/guidance/CG85/chapter/1-Guidance#treatment-for-people-with-coag. [PubMed]
- 3.Boland MV, Ervin AM, Friedman D, et al. Treatment for Glaucoma: Comparative Effectiveness [Internet] Agency for Healthcare Research and Quality (US); Rockville (MD): 2012. [August 7, 2014]. (Comparative Effectiveness Reviews, No. 60.) Available from: http://www.ncbi.nlm.nih.gov/books/NBK95391/ [PubMed] [Google Scholar]
- 4.Boland MV, Ervin AM, Friedman DS, et al. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(4):271–9. doi: 10.7326/0003-4819-158-4-201302190-00008. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman DS, Wolfs RC, O'Colmain BJ, et al. Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vajaranant TS, Wu S, Torres M, Varma R. The Changing Face of Primary Open-Angle Glaucoma in the United States: Demographic and Geographic Changes From 2011 to 2050. American journal of ophthalmology. 2012;154(2):303–14. e303. doi: 10.1016/j.ajo.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevent blindness America, National Eye Institute [August 7, 2014];Vision Problems in the U.S. Prevalence of Adult Vision Impairment and Age-Related Eye Disease in America. Available from: http://www.visionproblemsus.org/glaucoma.html.
- 9.Li T, Dickersin K. Citation of previous meta-analyses on the same topic: A clue to perpetuation of incorrect methods? Ophthalmology. 2013;120(6):1113–9. doi: 10.1016/j.ophtha.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenny AM, Altman DG, Song F, et al. International Stroke Trial Collaborative Group. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134, iii-iv. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Puhan M, Vedula S, et al. for the Ad Hoc Network Meta-analysis Methods Meeting Working Group. Network meta-analysis – highly attractive and more methodological research is needed. BMC Medicine. 2011;9(1):79. doi: 10.1186/1741-7015-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salanti G, Higgins JPT, Ades AE, Ioannidis JPA. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- 13.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 14.Cipriani A, Higgins JPT, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130–7. doi: 10.7326/0003-4819-159-2-201307160-00008. [DOI] [PubMed] [Google Scholar]
- 15.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):3, e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip S, Hadar N, Keefe S, et al. A Web-based archive of systematic review data. Syst Rev. 2012;1(1):15. doi: 10.1186/2046-4053-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Vedula SS, Hadar N, et al. Innovations in data collection, management, and archiving for systematic reviews. Ann Intern Med. 2015;162(4):287–94. doi: 10.7326/M14-1603. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [August 8, 2014]. Chapter 8: Assessing risk of bias in included studies. Available from www.cochrane-handbook.org. [Google Scholar]
- 20.Eden J, Levit L, Berg A, Morton S, editors. Finding What Works in Health Care: Standards for Systematic Reviews. National Academies Press; Washington, DC: 2011. Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Board on Health Care Services. Chapter 4. Standards for Synthesizing the Body of Evidence:155-77. [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 23.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association. 2006;101(474):447–459. [Google Scholar]
- 24.Valkenhoef GV, Kuiper J. [April 15, 2015];gemtc: Network Meta-Analysis Using Bayesian Methods. R package version 0.6-1. 2014 Available from http://CRAN.R-project.org/package=gemtc.
- 25.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical Science. 1992;7(4):457–72. [Google Scholar]
- 26.Gelman A, Carlin JB, Stern HS, et al. Bayesian data analysis. 3rd ed. Chapman & Hall/CRC; London: 2009. [Google Scholar]
- 27.Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013;11:159. doi: 10.1186/1741-7015-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumley T. Network meta-analysis for indirect treatment comparisons. Statistics in Medicine. 2002;21:2313–2324. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- 29.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932–44. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 30.Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Medical Decision Making. 2013;33:641–56. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research Synthesis Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analsyis: model estimation using multivariate meta-regression. Res Syn Meth. 2012;3:111–25. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White IR. Multivariate random-effects meta-regression: Updates to mvmeta. The STATA Journal. 2011;11(2):255–270. [Google Scholar]
- 34.Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc B. 2002;64(4):583–639. [Google Scholar]
- 35.Deeks JJ, Higgins JPT, Altman DG. on behalf of the Cochrane Statistical Methods Group. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [April 15, 2015]. Chapter 9.4.5.2 Meta-analysis of change scores; Available from www.cochrane-handbook.org. [Google Scholar]
- 36.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- 37.Yadav AK, Patel V. Drug use in primary open angle glaucoma: a prospective study at a tertiary care teaching hospital. Indian J Pharmacol. 2013;45(2):117–20. doi: 10.4103/0253-7613.108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmier JK, Lau EC, Covert DW. Two-year treatment patterns and costs in glaucoma patients initiating treatment with prostaglandin analogs. Clin Ophthalmol. 2010;4:1137–43. doi: 10.2147/OPTH.S13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connor AJ, Fraser SG. Glaucoma prescribing trends in England 2000 to 2012. Eye (Lond) 2014;28(7):863–9. doi: 10.1038/eye.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Glaucoma Association . In: Intraocular pressure. Consensus series 4. Weinreb RN, Brandt JD, Garway-Heath D, Medeiros FA, editors. Kugler Publications; 2007. [Google Scholar]
- 41.Weinreb RN, Kaufman PL. Glaucoma research community and FDA look to the future, II: NEI/FDA Glaucoma Clinical Trial Design and Endpoints Symposium: measures of structural change and visual function. Invest Ophthalmol Vis Sci. 2011;52(11):7842–51. doi: 10.1167/iovs.11-7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vass C, Hirn C, Sycha T, et al. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD003167.pub3. Art. No.: CD003167. DOI: 10.1002/14651858.CD003167.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaimani A, Vasiliadis HS, Pandis N, et al. Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. Int J Epidemiol. 2013;42(4):1120–31. doi: 10.1093/ije/dyt074. [DOI] [PubMed] [Google Scholar]
- 44.Salanti G, Dias S, Welton NJ, et al. Evaluating novel agent effects in multiple-treatments meta-regression. Stat Med. 2010;29(23):2369–83. doi: 10.1002/sim.4001. [DOI] [PubMed] [Google Scholar]
- 45.Alkhafaji AA, Trinquart L, Baron G, et al. Impact of evergreening on patients and health insurance: a meta analysis and reimbursement cost analysis of citalopram/escitalopram antidepressants. BMC Med. 2012;10:142. doi: 10.1186/1741-7015-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barth J, Munder T, Gerger H, Nüesch E, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta-analysis. PLoS Med. 2013;10(5):e1001454. doi: 10.1371/journal.pmed.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anothaisintawee T, Attia J, Nickel JC, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA. 2011;305(1):78–86. doi: 10.1001/jama.2010.1913. [DOI] [PubMed] [Google Scholar]
- 48.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 49.Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–15. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- 50.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62. doi: 10.1016/S0140-6736(13)60733-3. Erratum in: Lancet. 2013;382(9896):940. [DOI] [PubMed] [Google Scholar]
- 51.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015 doi: 10.1016/S0140-6736(15)60263-X. pii: S0140-6736(15)60263-X. doi: 10.1016/S0140-6736(15)60263-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.van der Valk R, Webers CA, Lumley T, et al. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol. 2009;62(12):1279–83. doi: 10.1016/j.jclinepi.2008.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.