Abstract
Dietary intake is one of the major exposure pathways of polycyclic aromatic hydrocarbons (PAHs), especially in Chinese people because foods are often prepared with grilling and/or frying that would produce high levels of PAHs. In this paper, we assessed daily dietary intakes (DDI) of PAHs, using a “duplicate plate method”, among 100 Chinese urban residents. The DDI of bento(a)pyrene ranged from 0.06 μg per day to 13.5 μg per day with a median of 0.69 μg per day, varying largely across subjects. The median Incremental Lifetime Cancer Risk (ILCR) attributable to PAH dietary intake was 6.65×10−5 (4.41×10−5 to 1.02×10−4 as inter-quartile range). The contribution of several high-PAH containing foods like barbecued, smoked or deep-fried meats to the overall DDIs was about 13%. The use of raw foods may underestimate dietary intake of PAHs and associated exposure risk considerably. Results from foods sampled in different seasons suggested that seasonal variability within an individual may contribute notably to overall variability measured in a population and more future studies with longer-term investigation on food ingestion and pollutant exposure are needed. The study indicate that measuring actually consumed foods is more appropriate for dietary intake exposure assessment, and intra-individual variance should be taken into account during study design and data analysis.
Keywords: Polycyclic aromatic hydrocarbons, cancer risk, daily dietary intake, duplicated food method, inter- and intra-variability
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are of widespread concern due to their carcinogenic and mutagenic potentials (Boström et al., 2002). They are usually produced from natural and/or anthropogenic sources during the incomplete combustion process (Shen et al., 2011, 2013), and ubiquitous in environment. Inhalation, ingestion and dermal exposure are main pathways of PAHs exposure, and for non-occupational population, the contribution of dermal contact exposure might be minimal (Li, 2007). The relative contributions of ingestion and inhalation exposure often vary in studied population, depending on habits, activity patterns, sampling time and so on. In areas with severe air pollution, the impact of inhalation exposure often attracts high public concerns, but it is commonly accepted that food ingestion contributes largely to overall PAH intake among non-smokers, not subjected to occupational exposure (Yu et al., 2011; Suzuki and Yoshinaga, 2007). For example, it was reported that daily intake of BaPeq (benzo(a)pyrene equivalent concentration) among residents in Beijing was 1.93 ng kg−1 per day, while the inhalation intake was 0.087 ng kg−1 per day (Yu et al., 2011), suggesting a larger contribution of food ingestion to the total exposure of PAHs through ingestion and inhalation. Another study in rural Jiangsu located in eastern China found that the dietary intake of PAHs contributed over 70% of the total for the subjects who did not conduct daily cook activity, and regarding the BaPeq exposure, the relative contribution from dietary exposure could be as high as 90% (Shen et al., 2014a).
PAHs in raw foods may come from the deposition of ambient particles, contaminated soils and water. The PAH contents vary across food types (Xia et al. 2010; Yu et al., 2011), and could increase significantly during the cooking process, especially grilling and deep-frying process due to the pyrolysis of fat at higher temperature and adsorption of PAHs emitted from combustion process (Alomirah et al., 2011; Chen et al., 1997; Li et al., 2003; Zhang et al., 2014). This is of particular importance in the evaluation of dietary exposure of PAHs in Chinese people as Chinese style meals can be quite different from Western-style meals. The preparation of Chinese meals typically involves stir-frying and/or deep-frying with certain types of cooking oil (e.g., rapeseed oil, corn oil, vegetable oil, peanut oil), although there appear to be major differences in food flavor across different regions of China.
Some previous studies measured PAHs levels in foods and estimated dietary exposure risk (Xia et al., 2010; Yu et al., 2011, 2015; Yang and Liu, 2000; Zhong and Wang, 2002) for Chinese population, but most of these studies were based on the data in raw foods, instead of actually ingested foods after cooking. The results from these studies, without considering cooking process, may under- or over-estimate dietary PAH intakes. Few studies estimated daily dietary intake of PAHs by using a duplicate plate method and/or a collection of actually consumed foods in households. The study by Nie et al., (2014) found that most PAHs differed notably between the raw and cooked foods, and for BaP as an example, the concentrations in cooked and raw foods were 1.30 and 0.12 ng g−1, respectively. The use of raw or cooked foods would also yield consequent difference in the estimated contributions through ingestion and inhalation pathways. For example, the study by Yu et al., (2011) based on measurements of PAHs in collected raw foods and literature-reported food consumption amounts from the National Nutrition Survey reported that diet accounted largely for low molecular weight PAHs while inhalation contributed highly to the overall exposure to high molecular weight PAHs in Beijing residents. However, the study by Shen et al., (2014a) showed that dietary intake contributed largely to the total exposure of most PAHs, and only for low molecular weight PAHs, the inhalation exposure had relatively higher contributions. The discrepancy might partly result from the difference in contamination levels and profiles of PAHs in raw and cooked foods, pointing to the importance of measuring PAHs in foods that actually consumed.
In this study, we collected all foods identical to those actually consumed during a 24-hour period in 100 urban adult residents by using a duplicate-plate method. PAHs levels in 24-hr composite food samples and daily dietary intake were estimated. A repeat sampling campaign in a second time period was conducted to examine the inter- and intra-individual variances. The corresponding cancer risks due to dietary intake were also estimated and compared to that due to the inhalation exposure.
Methods
Food sample collection using “duplicate plate method”
The study was carried out in 100 adult subjects residing in Liaoning Province's Anshan City. All the food consumed within a 24-hour period was collected using the “duplicate plate” method that is the subjects were asked to prepare two identical plates of meal (including snacks and excluding drinks), one for actual consumption, and the other for sample collection. Each subject was provided with 4 Tupperware containers and 1 cooler with ice packs for the collection of meal samples. These samples were placed inside a cooler with icepacks until returned to the laboratory. After each meal/snack sample had been weighed, all the samples within the 24-hour period were combined and blended homogeneously to form a composite sample for each subject. This was done for all of the 100 subjects in the winter time. A repeated sample collection was done in 61 out of the 100 subjects (due to unavailable of some subjects) in the next autumn, so as to evaluate the inter-individual variance in daily dietary intake.
Questionnaire and selected local food items
To help the interpretation of results, we administered a questionnaire in which each subject provided information on their dietary habits (e.g., type, frequency, and amount of food consumed on a weekly basis). In addition, we analyzed several cooked food items that were expected to have high PAH contents. Samples of these food items (N=3) were collected in a local food court, including barbecued pork, smoked sausages, smoked pork, deep-fried fish, deep-fried dolls, fried dumpling, and fried eggs.
Laboratory analysis of PAHs and quality control
Samples were extracted, purified, and analyzed using a HPLC system (Zhang et al., 2008). An aliquot (100 g) of each composite sample was placed into a 350-mL Erlenmeyer flask containing 12.5 μg of KOH in 200 mL of 95% ethanol and a mixing stirrer. After the flask was placed on a hot plate at 60°C for 4 hours, the entire content of the flask was filtered through a filter paper (0.45 μm), and the filtrate was transferred into a 2-L funnel. The Liquid-liquid extraction was performed by using 60 ml of isooctane for 10 minutes and then repeated to separate PAHs from ethanol. The isooctane phase was transferred to a 350 mL amber round flask and concentrated to 5 mL using a vacuum rotary evaporator at 60°C. If visible solid phase still occurred in the condensed extract, centrifugation was used to remove the solid residues.
Sample enrichment and purification were completed using a Sep-Pak Florisil cartridge (Waters051960, Waters Corp., MA). The cartridge was first primed with 5mL isooctane. The condensed sample extract passed through the cartridge at a flow rate of 1 mL min−1, and then 10 mL benzene was introduced into the cartridge to extract PAH from the cartridge coating material. The benzene solution in the eluent was evaporated to 0.1 mL using a nitrogen gas flow. Acetonitrile was added to make a 1 mL final solution that was further filtered through a 0.2 μm PVDF Liquid Filter into an auto-sampler amber vial sealed with a Teflon septum. The vial was wrapped with aluminum foil and stored in a freezer at ≤ −7°C before analysis. All samples were protected from direct exposure to light during sample preparation.
PAHs in extracted solution was analyzed using an HPLC system (Waters 600E, MA) equipped with a programmable fluorescence detector (LDC 4100) and an LC-PAH column (250×4.6 mm, 5 μm, Supelco Co., PA) at 30°C. A mobile phase gradient program was used. Flow rate was sustained at 1.0 mL/min. Helium (UHP/Zero-Grade) was used for solvent degassing. The fluorescence wavelength program used in the analysis was 270ex/350em nm in 0~32.8 min; 250ex/400em nm in 32.8~47 min; and 280ex/425em nm in 47~70 min. A PAH Calibration Mix (standard of 16 PAH in acetonitrile, Supelco Corp., PA) containing naphthalene (NAP), acenaphthene (ACP), fluorene (FLR), acenaphthylene, phenanthrene (PHE), anthracene (ANT), floranthene (FLT), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CHR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenzo(123-cd)pyrene (DahA), and benzo(ghi)perylene (BghiP), and was used for generating calibration curves. Acenaphthylene and indeno (1, 2, 3-cd) pyrene that had weak fluorescence responses were not targeted.
Quality control and data analysis
Reagent blanks, spiked samples, duplicate samples were analyzed as quality control measures. The method detection limits determined as 3 times of standard deviations of PAH response in blanks ranged from 0.02 (DahA) to 5.46 (FLT) ug kg−1 fresh food. Recoveries of spiked PAHs ranged from 60% (anthracene) to 170% (phenanthrene). Note that recoveries for some PAHs like naphthalene, phenanthrene and fluoranthene were substantially larger than 100% indicating the presence of artifact during the sample preparation process. In contrast, recoveries for anthracene and benzo(k)fluoranthene were substantially lower than 100% indicating losses of these compounds during the sample preparation. All these recoveries varied considerably among tests for determining recovery (n=4). Coefficients of variance (COVs) were from 2.4% (benzo(a)anthracene) to 20% (naphthalene). Precisions based on the measurements with 2 repeated injections were in 20% for all targets. The results present were not corrected by recovery which could contribute to variances in the results and thus more cautions need to be exercised when presenting results for these compounds.
Median, mean, standard derivation, and range of measured PAHs concentrations and estimated daily intakes were calculated and reported. The Daily Dietary Intakes of PAHs from all ingested foods (DDIs, μg day−1 per person) were calculated by multiplying PAHs concentrations in 24-hr composite food (μg/kg composite food sample) with daily Food Consumptions (DFCs, kg day−1 per person). The DFC was defined as the weight (mass) sum of all the fresh meals collected during each 24-hr period. For selected food items, the DDI (DDI-selected foods) is calculated from the PAH concentration in the food (μg/kg food) and estimated daily consumption (kg day−1 per person). The latter was derived from questionnaires in which monthly consumption was asked and data divided 30 days were used in the daily consumption calculation. The contributions from selected food items are then estimated from DDI-selected foods divided by the DDI from all ingested foods.
A significance level of 0.05 was adopted in data statistical analysis. The between- and within-subject variances were estimated by linear mixed-effect models (R, the ‘lme’ function in the ‘nlme’ package). The test time, gender and the job site were controlled as fixed effects, and the intercept of subject was controlled as a random effect. The “REML” method was used to do the model estimation.
The incremental lifetime cancer risk (ILCR) of the studied population attributable to PAH dietary ingestion was estimated following the equation: ILCR=ED×EF× ED×SF×CF/(BW×AT), where ED is the daily PAH intake (ng per day), EF is exposure frequency (365 days year−1), ED is exposure duration, SF is the oral cancer slop factor of benzo(a)pyrene (7.3 /(mg kg−1*d)) (Xia et al., 2010; US EPA, 2001), CF is conversion factor (10−6 mg ng−1), BW is body weight (kg), and AT is average lifespan for carcinogens (70 years or 25550 days). ED is calculated from ∑(DDIi×TEFi), where the DDIi and TEFi are daily dietary intake of PAH individual i and its corresponding toxic equivalency factor (TEF) (Nisbet and LaGoy, 1992; Petry et al., 1996).
Results and Discussion
Daily Food Consumption and PAHs concentrations in the 24-hr composite food
Based on questionnaire responses and meal records, subjects’ daily meals generally included about 1/2 amount of grain foods (mainly steamed white rice, occasionally steamed buns, stewed noodles or dumplings) and 1/2 amount of non-grain foods (usually stir-fried, deep-fried or stewed vegetables, meats, or fish). The majority of the subjects had 3 meals (breakfast, lunch and supper) a day. Four percent of subjects had additional snacks. Daily average food consumption constituted, on average, 21%, 36%, 34% and 9% of breakfast, lunch, dinner and snacks, respectively. The male subjects consumed about 1.36±0.36 kg per day foods during the winter measurement session and 1.20±0.41 kg per day during the autumn session on average. The female subjects consumed about 0.94±0.25 kg per day in the winter and 0.92±0.20 kg per day in the autumn. An analysis of ANOVA found a significant difference in DFC between genders for both seasons. The male subjects consumed about 0.3 kg more than the female subjects. In both genders, DFC was higher in the winter than in the autumn, but not significantly different (p=0.846 in males and p=0.065 in females).
Table 1 presents the concentrations of PAHs in the 24-hr composite food samples collected in two seasons of winter and autumn. The median of total 14 PAHs (∑PAHs), BaP and BaPeq (BaP equivalent concentration, calculated as sums of PAH individual concentrations times the corresponding TEF values) contents in the 24-hr composite food were 73.2, 0.56, and 0.88 ng g−1 in the winter, and 34.2, 0.38 and 0.82 ng g−1 in the autumn, respectively. The overall median concentration of ∑PAHs, BaP and BaPeq, regardless of sampling season, were53.2, 0.56 and 0.851 ng g−1, respectively. The concentrations were generally comparable to previously reported data in cooked Chinese foods, like 48.0 and 1.30 ng g−1 for total PAHs and BaP in Taiyuan (Nie et al., 2014), 47 and 0.82 ng g−1 in cooked food collected from Heshun (Chen et al., 2015), 16-74 ng g−1 for total 16 priority PAHs measured in cooked foods collected from a campus cafeteria (Zhang et al., 2014), and 15.5-411 and 2.68-30.4 ng g−1 of total PAHs and BaPeq in cooked foods collected in a rural household (Shen et al., 2014). Different food types and cooking methods may contribute to variances in reported concentrations in cooked foods. However, the level was apparently higher than those in raw foods (Xia et al., 2010; Yu et al., 2010; Nie et al., 2014 and references therein). For example, the BaP concentration in raw food in Taiyuan was only 0.12 ng g−1, and at 0.08-0.29 ng g−1 in raw foods reported for developed countries (Nie et al., 2014 and references therein).
Table 1.
Concentrations of PAHs in 24-hr composite food samples
| PAHs | MDL, μg/kg food | Concentration in 24h composite food samples (μg kg−1) |
||||||
|---|---|---|---|---|---|---|---|---|
| The Winter (n=100) | The Autumn (n=61) | |||||||
| Median | Mean±SD | (Mini~Max) | Median | Mean±SD | (Mini~Max) | |||
| 1 | NAP | 2.22 | 14.7 | 30.7±39.5 | (ND~277) | 3.69 | 8.96±14.69 | (ND ~75.5) |
| 2 | ACP | 1.54 | 1.59 | 3.11±6.35 | (ND~44.5) | 0.36 | 1.00±1.66 | (ND~11.0) |
| 3 | FLR | 0.35 | 4.23 | 5.43±4.09 | (1.10~27.5) | 1.92 | 2.24±1.66 | (ND ~9.31) |
| 4 | PHE | 4.05 | 11.5 | 14.3±12.9 | (ND~101) | 5.26 | 6.67±6.51 | (ND ~43.9) |
| 5 | ANT | 0.60 | 2.28 | 2.69±2.53 | (ND~21.1) | 0.92 | 1.07±0.74 | (ND ~3.93) |
| 6 | FLT | 5.46 | 10.1 | 23.5±56.5 | (ND~500) | 8.41 | 19.3±40.5 | (ND ~291) |
| 7 | PYR | 0.99 | 3.90 | 5.76±7.83 | (ND~57.7) | 2.36 | 2.51±1.59 | (ND ~11.1) |
| 8 | BaA | 0.22 | 0.92 | 1.32±1.83 | (ND~14.3) | 0.65 | 0.74±0.49 | (ND ~2.42) |
| 9 | CHR | 1.99 | 0.79 | 1.88±3.20 | (ND~20.5) | 0.001 | 0.04±0.14 | (ND ~0.94) |
| 10 | BbF | 0.10 | 0.53 | 0.80±1.04 | (0.13~8.50) | 0.41 | 2.65±8.32 | (ND ~41.4) |
| 11 | BkF | 0.09 | 0.23 | 0.36±0.56 | (ND~5.09) | 0.19 | 0.21±0.11 | (ND ~0.61) |
| 12 | BaP | 0.04 | 0.56 | 0.85±1.35 | (0.09~11.7) | 0.38 | 1.94±5.26 | (ND ~24.4) |
| 13 | DahA | 0.02 | 0.10 | 0.25±0.57 | (ND~1.97) | 0.019 | 0.24±0.64 | (0.02~3.91) |
| 14 | BghiP | 0.04 | 0.58 | 0.92 ±1.25 | (ND~8.11) | 0.51 | 0.57±0.41 | (ND ~3.42) |
Notes: ND- “Not detected”, below MDL (Method Detection Limit)
The PAH concentrations appeared to be higher in the winter samples than that in the autumn samples (Table 1). The differences for PAHs between the winter and autumn samples were 2.25 for benzo(g,h,i)perylene to 18.3 times for fluorene on average, except for chrysene which was not detected in most food samples in the autumn, while abundant in the winter samples. The meal record indicated that in the winter, residents consumed more roasted, barbecued or deep-fried foods. These foods were thought to have higher contamination levels of PAHs produced through the processing and cooking, as shown in the next section, and hence, resulting in high levels of PAHs in the 24-hr composite food in the winter. It is necessary to note that since the results are not corrected for recovery during the laboratory analysis and recoveries varied between sample batches, this could contribute to the observed difference between the winter and autumn food samples. However, as mentioned above the COVs in preliminary recovery experiments were in 20%, therefore, influence due to errors in the analytical method might be much smaller compared to other reasons like more digestion of high-PAH containing foods. No quantitative records of food ingredients or contents were available in the present study, which also contributed to the different PAHs in foods digested in two seasons. The results of spearman correlation analysis showed that most individual PAHs were significantly correlated among each other, indicating that profiles of PAHs in the 24-hr composite food samples were likely to have same patterns. Usually the elevation of one particular PAH was accompanied with the elevation of other PAHs. NAP, PHE, FLT and PYR were major individuals, contributing 26%, 18%, 26% and 7% of the total. For high molecular weight (MW) PAHs with MW larger than 228 (from BaA to BghiP), the total mass contribution was about 10%.
DDI of PAHs and seasonal variation
Table 2 presents results of calculated DDIs for 14 PAHs in 100 subjects in winter, and repeated measurements in autumn in 61 of the 100 subjects. Daily intake of PAHs from drinking water was assumed to be negligible compared to intakes from solid foods and this was not accounted in the present study. The DDIs of PAHs had a very large range across the subjects. The BaP intake ranged from 0.06 μg per day to 13.5 μg per day. The median value of BaP intake was 0.69 μg per day. About 75% of subjects had BaP intakes < 1.0 μg per day and ~ 95% of the subjects had BaP intakes <2.5 μg per day. The median intake of BaPeq was 1.01 μg per day based on the winter samples. When adjusted by body weight (BW), the DDI of BaP and BaPeq were 10.5 and 15.9 ng kg−1 BW per day, respectively.
Table 2.
Daily dietary intakes of PAHs in studied population
| Daily dietary intakes of PAHs (μg per day per person) |
|||||||
|---|---|---|---|---|---|---|---|
| PAHs | The Winter (n=100) | The Autumn (n=61) | |||||
| Median | Mean±SD | (Mini~Max) | Median | Mean±SD | (Mini~Max) | ||
| 1 | NAP | 19.6 | 39.1±55.4 | (0.45*~370) | 3.75 | 10.2±17.2 | (0.28*~78.9) |
| 2 | ACP | 1.97 | 3.63±6.78 | (0.04*~48.4) | 0.47 | 1.02±1.60 | (0.03*~8.52) |
| 3 | FLR | 4.86 | 6.57±5.51 | (0.89~38.9) | 1.99 | 2.41±2.01 | (0.01* ~8.91) |
| 4 | PHE | 13.0 | 16.8±16.7 | (0.01*~143) | 5.70 | 6.63±5.15 | (0.27*~30.0) |
| 5 | ANT | 2.53 | 3.20±3.39 | (0.04*~29.9) | 0.96 | 1.13±0.84 | (0.00*~4.27) |
| 6 | FLT | 11.5 | 25.4±49.9 | (0.02*~438) | 8.36 | 20.4±40.8 | (0.25* ~279) |
| 7 | PYR | 4.05 | 6.44±8.05 | (0.01*~49.8) | 2.33 | 2.60±1.63 | (0.15* ~9.72) |
| 8 | BaA | 1.03 | 1.60±2.32 | (0.01*~16.4) | 0.67 | 0.78±0.57 | (0.03*~2.57) |
| 9 | CHR | 0.91 | 2.27±3.86 | (0.002*~23.5) | 0.001 | 0.03±0.11 | (0.0004* ~0.64) |
| 10 | BbF | 0.69 | 0.95±1.29 | (0.10~9.78) | 0.43 | 2.72±9.62 | (0.01*~59.0) |
| 11 | BkF | 0.28 | 0.43±0.67 | (0.001*~5.85) | 0.20 | 0.22±0.13 | (0.01* ~0.67) |
| 12 | BaP | 0.69 | 1.00±1.62 | (0.06~13.5) | 0.41 | 2.22±6.43 | (0.01*~34.7) |
| 13 | DahA | 0.063 | 0.35±0.82 | (0.004*~2.79) | 0.030 | 0.23±0.56 | (0.01~3.49) |
| 14 | BghiP | 0.75 | 1.07 ±1.55 | (0.01*~11.3) | 0.53 | 0.53±0.42 | (0.03* ~3.17) |
Notes:
though calculated for DDI, however, the results are associated with high uncertainties as the concentrations are below the Method Detection Limit (Table 1)
It was previously reported that the DDI of total 16 PAHs and BaP were 60.8 and 1.56 μg per day for residents in Taiyuan, Shanxi province (Nie et al., 2014). The intake of BaP estimated for residents living in rural Heshun, Shanxi province was 1.0±0.38 μg per day, or 15.5±6.26 ng kg−1 per day (Chen et al., 2015). In a study in eastern China, the intake of BaPeq ranged from 33.8 to 99.6 ng kg−1 per day, with a mean and standard derivation of 56.6±18.0 ng kg−1 per day. Our results here generally are within the reported range in these studies in which duplicated plate method or a collection of real ingested cooked food was done. But, in comparison to an intake of BaPeq at 1.93 ng kg−1 per day (Yu et al., 2011), 0.08 μg per day BaP estimated by raw food in Taiyuan, and 0.05-0.42 μg per day BaP estimated from raw food in developed countries like UK AND Netherlands (Nie et al., 2014 and references therein). Our high dietary intake in the present study is thought to be mainly due to the use of cooked food instead of raw foods, as the cooking process may produce a large amount of PAHs, especially Chinese cooking practice. Therefore, the use of raw foods may underestimate PAH exposure considerably. Other reasons like differences in types and amounts of food consumed, contamination levels of raw food and food prepare way, as well as variability in laboratory analysis methods can also lead to differences in the measured PAHs concentrations and estimated intake amounts among different studies.
As expected, the DDIs of most PAHs were higher in the winter than in the autumn. The seasonal difference in DDI was thought to be mainly resulted from seasonal difference in dietary habits and in variety of food supplies-higher PAH levels in food and relatively higher DFC in the winter. As mentioned above, examination of meal records during the sampling period suggested there were more consumptions of roasted, barbecued or deep-fried foods that usually had higher PAHs contamination levels in the winter than in the autumn. The calculated within-subject variances were greater than the between-subject variances for all compounds. About 83%-99%, varying among PAH individuals, of the total variances were due to within-subject variation. It is important to note that the present study only made two repeated measurements for each individual. The quantitative percents should be not generalized. The seasonal variability in DDI observed here indicated that intra-individual variability could contribute significantly to overall variability in exposure risk assessment measured in a population. A future study with longer-term investigation on food ingestion and pollutant exposure over each month might provide more accurate information on the intra-individual variances that are valuable for capturing individual's DDI representative to his/her “average” exposure or exposure distribution..
Contributions from selected food items to the DDI of PAHs
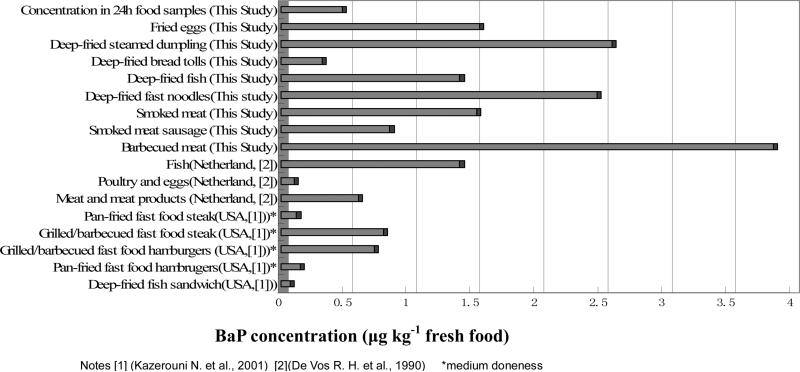
Several typical local food items, which were suspected to contain high levels of PAHs due to their processing and cooking methods, were collected in a local food court. The concentrations of BaP in these food items are listed in table 3. The daily consumptions of these food items based on questionnaires of the 100 subjects as well as estimated daily intake of BaP are also present at the same table. Figure 1 shows the comparison of BaP concentrations of these selected food items in this study with some food types in other research reports.
Table 3.
Concentrations and estimated daily intake of BaP in selected local food items
| No. | Food Items | Concentration of BaP (μg kg−1 fresh food) | Daily consumption (kg per day) | Estimated daily intake of BaP (μg per day per person) | ||
|---|---|---|---|---|---|---|
| Mean | Max | Mean | Max | |||
| 1 | Barbecued meat | 3.85 | 0.01 | 0.17 | 0.039 | 0.655 |
| 2 | Smoked meat sausage | 0.85 | 0.001 | 0.05 | 0.001 | 0.043 |
| 3 | Smoked meat | 1.53 | 0.001 | 0.02 | 0.002 | 0.031 |
| 4 | Deep-fried fish | 1.4 | 0.001 | 0.02 | 0.001 | 0.028 |
| 5 | Deep-fried fast noodles | 2.47 | 0.013 | 0.12 | 0.03 | 0.3 |
| 6 | Deep-fried bread tolls | 0.32 | 0.002 | 0.34 | 0.007 | 1.16 |
| 7 | Deep-fried steamed dumpling | 2.59 | ||||
| 8 | Fried eggs | 1.55 | ||||
| Sum of the 8 food items | 0.033 | 0.72 | 0.08 | 2.21 | ||
Figure 1.
Comparison of BaP concentration in different food items
The BaP concentrations of barbecued meat, smoked meat sausage, smoke meat and deep-fried fish were 3.85, 0.85, 1.53, 1.40 μg kg−1 respectively. These levels appear to be in compliance with Chinese national standard Tolerance Limit of Benzo(a)pyrene in Food (GB 7104-1994) in which up to 5.0 gkg−1 is permitted. The BaP concentrations of these selected food items were greater than those measured in food items reported in USA and Netherlands. Barbecued meat in this study contained the highest concentration of BaP, whereas BaP concentration in deep fried tolls was 0.32 μgkg−1, about half of grilled/barbecued food steaks or hamburgers measured in US.
As shown in Table 3, average daily consumption of these high PAH-containing food items was ~ 0.001 to 0.01 kg per day, contributing 0.033 μg per day or < 3% of the total daily food consumption. Average BaP intake from all these selected food items was 0.08 μg per day, although for some subjects, it could reach > 2 μg per day. The average contribution of 8 selected food items (in Table 3) to daily intake of PAHs was ~13%. For the majority of the subjects, DDIs of PAHs were mainly contributed by more commonly eaten foods.
Cancer risks due to dietary food ingestion among adults
The median value of estimated ILCR due to PAH intake exposure for the studied adult was 6.65×10−5, with the interquartile range of 4.41×10−5 to 1.02×10−4. The Relatively Variation Index (RVI, calculated as interquartile range divided the median) in ILCR was 97%, mainly attributable to different DDIs among the residents. Moreover, the use of different DDI data for a specific subject in exposure assessment contributed notably to the uncertainty associated with the estimation. The difference could be as high as 10-20 times for some subjects.
A previous study in Taiyuan reported that ILCR due to the dietary intake of PAHs were 4.04×10−5 for the male and 3.87×10−5 for the female (Xia et al., 2010), which were lower than the results in the present study. Recently, another study in Beijing reported the median of dietary ILCR for adults in Beijing were 1.26×10−5 and 1.24×10−5 for the male and the female, respectively, which also obviously lower than our present result. It is noted that in these two studies, dietary exposure was estimated based on the measured PAH concentrations in raw food and literature-reported food consumption amounts, which can lead to considerably underestimation of PAH contamination levels in foods, and probably bias in food consumption amounts that vary among different regions. The study by Nie et al., (2015) estimated the ILCR based on cooked food from the duplicated plate method at 9.07×10−4, which was nearly 8.3 times of 1.11×10−4 estimated based on raw food. Therefore, it appears that the “duplicated plate” method is more appropriate to estimate food intake exposure.
It is interesting to note that globally, the ILCR due to the inhalation exposure of ambient PAHs was estimated at 3.1×10−5 (Shen et al., 2014), and in some cities with severe ambient pollution reported ILCR due to the PAH inhalation exposure were 3.36×10−5 and 2.39×10−5 for the rural and the urban residents in Taiyuan, Shanxi province (Duan et al., 2014). The ILCR due to the PAH inhalation exposure in Beijing was 1.83×10−5 (1.30×10−5 −4.48×10−5 as interquartile range) and 2.40 ×10−5 (9.92×10−6−3.46×10−5) for the male and the female, respectively (Yu et al., 2015). These risk levels were comparable to that estimated in the present study for food ingestion exposure. Recently, a study in rural Heshun, Shanxi province simultaneously measured personal inhalation and food ingestion exposure and estimated the ILCR due to PAH inhalation and ingestion intake were 1.1×10−5 and 1.0×10−5, respectively (Chen et al., 2015), indicating comparable risks through food ingestion and the inhalation exposure, even in regions with severe air pollution. Therefore, in addition to efforts on the control of air pollution in China, high attentions and control strategies should be also conducted to improve food safety and protect human health.
Conclusions
In a cohort of 100 Chinese urban adult residents, a comprehensive study using the ‘duplicate diet” method, show a large range of daily dietary intakes across the two sampling seasons. A few specific foods (e.g., barbecued or smoked meats) had high PAH contents but contributed, on average, <13% of total daily PAH intakes due to their small consumption rates. The daily intake values in the present study were comparable to those measured based actually consumed food samples, but higher than those estimated based on the PAH contents in raw food ingredients in a few previous studies conducted in China. We found that the daily intake values were generally higher than those reported in developed countries like UK, USA, and the Netherlands. Results indicate that using PAH contents of raw food ingredients may underestimate cancer risks attributable to PAHs dietary exposure and that significant seasonal variability in PAH daily dietary intake needs to be considered for assessing cancer risks. The incremental lifetime cancer risk due to PAHs in digested foods was comparable to the risk due to the inhalation exposure, calling for attentions and actions on food safety control.
HIGHLIGHTS.
➢ Duplicated plate method to estimate dietary intake exposure of priority PAHs
➢ Serious ingestion exposure risk associated with cooked food intake among Chinese residents
➢ Use of raw foods underestimates dietary intake dose and consequent exposure risk
➢ Within-subject variation contributes notably to the overall variation in dietary intake
➢ Risk due to dietary PAH intake is comparable to that from the inhalation exposure
Acknowledgements
We thank all study participants for their dedication of time and food samples to this study. We appreciate the assistance of Ms. Wei Wang from Anshan Environmental Monitoring Center, Ms. Lihong Xu from Anshan Iron and Steal Company, Dr. Jun Xu from University of Science and Technology of Beijing, and Dr. In-Kyu Han from University of Medicine and Dentistry of New Jersey (UMDNJ) during the sample collection, preparation and data analysis. This study was funded by grants from the NIH-NCI (PI: JZ, Grant No: 1R21-CA94743 -01) and the Ministry of Environmental Protection of China (Grants 200809101) and State Key Laboratory of Environmental Criteria and Risk Assessment (SKLECRA2013OFP005). The views expressed in this manuscript are solely of the authors and do not necessarily reflect those of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alomirah H, Al-zenki S, Al-hooti S, Zaghloul S, Sawaya W, Ahmed N, Kannan K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food control. 2011;22:2028–2035. [Google Scholar]
- Boström C, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Torngvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Lin Y. Formation of polycyclic aromatic hydrocarbons during processing of duck meat. J. Agr. Food Chem. 1997;45:1394–1403. [Google Scholar]
- Chen Y, Shen G, Huang Y, Zhang Y, Han Y, Wang R, Shen H, Su S, Lin N, Zhu D, Pei l., Zheng X, Wu J, Wang X, Liu W, Wong M, Tao S. Household air pollution and personal exposure risk of polycyclic aromatic hydrocarbons among rural residents in Shanxi, China. Indoor Air. 2015 doi: 10.1111/ina.12204. Doi:10.1111/ina.12204. [DOI] [PubMed] [Google Scholar]
- De Vos RH, Vandokkum W, Schouten A, De Jong-berkhout P. Polycyclic aromatic hydrocarbons in Dutch Total Diet Samples (1984-1986). Food Chem. Toxicol. 1990;28:263–268. doi: 10.1016/0278-6915(90)90038-o. [DOI] [PubMed] [Google Scholar]
- Duan X, Wang B, Zhao X, Shen G, Xia Z, Huang N, Jiang Q, Lu B, Xu D, Fang J, Tao S. Personal inhalation exposure to polycyclic aromatic hydrocarbons in urban and rural residents in a typical northern city in China. Indoor air. 2014;24:464–473. doi: 10.1111/ina.12099. [DOI] [PubMed] [Google Scholar]
- Kazerouni N, Sinha R, Hsu C, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001;39:423–436. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Li C, Lin Y, Lee W, Tsai P. Emission of polycyclic aromatic hydrocarbons and their carcinogenic potencies from cooking sources to the urban atmosphere. Environ. Health Perspect. 2003;111:483–487. doi: 10.1289/ehp.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XR. PhD Dissertation. Peking University; 2007. Spatial distribution pattern of emission, dispersion and exposure of polycyclic aromatic hydrocarbons in Tianjin, China. [Google Scholar]
- Nie J, Shi J, Duan X, Wang B, Huang N, Zhao X. Health risk assessment of dietary exposure to polycyclic aromatic hydrocarbons in Taiyuan, China. J. Environ. Sci. 2014;26:432–439. doi: 10.1016/s1001-0742(13)60424-6. [DOI] [PubMed] [Google Scholar]
- Nisbet C, LaGoy P. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharm. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]
- Petry T, Schmid P, Schlatter C. The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixture of polycyclic aromatic hydrocarbons (PAHs). Chemosphere. 1996;32:639–648. doi: 10.1016/0045-6535(95)00348-7. [DOI] [PubMed] [Google Scholar]
- Shen G, Wang W, Yang Y, Ding J, Xue M, Min Y, Zhu C, Shen H, Li W, Wang B, Wang R, Wang X, Tao S, Russell A. Emissions of PAHs from indoor crop residue burning in a typical rural stove: emission factors, size distribution and gas-particle partitioning. Environ. Sci. Technol. 2011;45:1206–1212. doi: 10.1021/es102151w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Huang Y, Wang R, Zhu D, Li W, Shen G, Wang B, Zhang Y, Chen Y, Lu Y, Chen H, Li T, Sun K, Li B, Liu W, Liu J, Tao S. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future prediction. Environ. Sci. Technol. 2013;47:6414–6424. doi: 10.1021/es400857z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Zhang Y, Wei S, Chen Y, Yang C, Lin P, Xie H, Xue M, Tao S. Indoor/outdoor pollution level and personal inhalation exposure of polycyclic aromatic hydrocarbons through biomass fuelled cooking. Air Qual. Atmos. Health. 2014a;7:449–458. [Google Scholar]
- Shen H, Tao S, Liu J, Huang Y, Chen H, Li W, Zhang Y, Chen Y, Su S, Lin M, Xu Y, Li B, Wang X, Liu W. Global lung cancer risk from PAH exposure highly depends on emission sources and individual susceptibility. Sci. Rep. 2014b;4:6561. doi: 10.1038/srep06561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int. Arch. Occ. Env. Hea. 2007;81:115–121. doi: 10.1007/s00420-007-0188-x. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Integrated Risk Information System (IRIS): benzo[a]pyrene (BaP); CASRN 50-32-8. 2001 [Google Scholar]
- Xia Z, Duan X, Qiu W, Liu D, Wang B, Tao S, Jiang Q, Lu B, Song Y, Hu X. Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci. Total Environ. 2010;408:5331–5337. doi: 10.1016/j.scitotenv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu Q. Determination of Benzo(a)Pyrene in Rice Plant. Food Sci. (in Chinese) 2000;21:45–47. [Google Scholar]
- Yu Y, Wang X, Wang B, Tao S, Liu W, Wang X, Cao J, Li B, Lu X, Wong M. Polycyclic aromatic hydrocarbon residues in human milk, placenta and umbilical cord blood in Beijing, China. Environ. Sci. Technol. 2011;45:10235–10242. doi: 10.1021/es202827g. [DOI] [PubMed] [Google Scholar]
- Yu Y, Li W, Wang H, Wang B, Wang X, Ren A, Tao S. Risk of human exposure to polycyclic aromatic hydrocarbons: a case study in Beijing, China. Environ. Pollut. 2015;205:70–77. doi: 10.1016/j.envpol.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ding J, Shen G, Zhong J, Wang C, Wei S, Chen C, Chen Y, Lu Y, Shen H, Li W, Huang Y, Chen H, Su S, Lin N, Wang X, Liu W, Tao S. Dietary and inhalation exposure to polycyclic aromatic hydrocarbons and urinary excretion of monohydroxy metabolites - a controlled case study in Beijing, China. Environ. Pollut. 2014;184:515–522. doi: 10.1016/j.envpol.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Han I, Zhang L, Crain W. Hazardous chemicals in synthetic turf materials and their bioaccessibility in digestive fluids. J. Expo. Sci. Environ. Epidemiol. 2008;18:600–607. doi: 10.1038/jes.2008.55. [DOI] [PubMed] [Google Scholar]
- Zhong WK, Wang MJ. Some polycyclic aromatic hydrocarbons in vegetables from northern China. J. Environ. Sci. Healh A. 2002;37:287–296. doi: 10.1081/ese-120002588. [DOI] [PubMed] [Google Scholar]