SUMMARY
Objective
To determine whether mandibular condylar cartilage degradation induced by experimentally abnormal occlusion could be ameliorated via systemic administration of strontium or NBD peptide.
Methods
Six-week-old female C57BL/6J mice were used. From the seventh day after mock operation or unilateral anterior crossbite (UAC) treatment, the control and UAC mice were further respectively pharmacologically treated for 2 weeks or 4 weeks of saline (CON + Saline and UAC + Saline groups), SrCl2 (CON + SrCl2 and UAC + SrCl2 groups) or NBD peptide (CON + NBD peptide and UAC + NBD peptide groups). Changes in condylar cartilage and subchondral bone were assessed 21 and 35 days after mock operation or UAC procedure by histology and micro-CT. Real-time PCR and/or immunohistochemistry (IHC) were performed to evaluate changes in expression levels of col2a1, aggrecan, ADAMTS-5, tnf-α, il-1β, nfkbia, nuclear factor-kappaB phospho-p65 in condylar cartilage, and rankl/rank/opg in both condylar cartilage and subchondral bone.
Results
Cartilage degradation with decreased col2a1 and aggrecan expression, and increased ADAMTS-5, tnf-α/il1-β, nfkbia and NF-κB phospho-p65 was observed in UAC + Saline groups. Subchondral bone loss with increased osteoclast numbers and decreased opg/rankl ratio was found in UAC + Saline groups compared to age-match CON + Saline groups. Cartilage degradation and subchondral bone loss were reversed by treatment of SrCl2 or NBD peptide while the same dosage in control mice induced few changes in condylar cartilage and subchondral bone.
Conclusions
The results demonstrate reverse effect of systemic administration of strontium or NBD peptide on UAC-induced condylar cartilage degradation and subchondral bone loss.
Keywords: Temporomandibular joint, Dental occlusion, NBD (NEMO-binding domain) peptide, Strontium, Osteoarthritis, Cartilage, Subchondral bone
Introduction
Osteoarthritis (OA) is histologically characterized by cartilage degradation and abnormal subchondral bone remodeling1,2. It is generally accepted that appropriate joint biomechanical loading is essential for cartilage anabolic metabolism3, while abnormal joint loading results in cartilage metabolism imbalance favoring catabolic metabolism and OA changes4–8. Abnormal joint loading could downregulate expression of proteoglycans and Collagen II and upregulate expression of inflammatory factors, such as interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α), and cartilage extracellular matrix degrading enzymes, such as a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) and matrix metalloproteinases (MMPs)9. Several recent reports indicate that nuclear factor-kappaB p65 (NF-κB p65) could directly bind to the promoters of targeting genes encoding for cartilage degrading factors10, such as ADAMTS-511, so NF-κB p65 is considered to play a vital role as a pro-catabolic and pro-apoptotic factor of chondrocyte during OA development12.
Subchondral bone provides the mechanical support for the overlying articular cartilage. In the early stage of knee OA there was a significant decrease in bone volume fraction and changes in subchondral bone architecture parameters, such as trabeculae thickness and connectivity13, and increased levels of serum and urinary bone remodeling markers, such as bone sialoprotein14, N-terminal type I collagen telopeptides (NTx) and C-terminal type I collagen telopeptides (CTX-1)15. In addition, low bone mineral density and increased subchondral bone resorption were observed in the early stage of OA animal models1,4,5,16. Inhibition of bone resorption by pharmacologic inhibitors, such as bisphosphonates17, strontium ranelate18,19 or OPG20 could effectively prevent surgically induced-OA progression in rodents although contradictory results were obtained from clinical studies aimed to prevent the cartilage damage of knee OA by delivering bisphosphonates to OA patients16. It seems that cartilage homeostasis and metabolism is mechanically and biochemically coupled with the subchondral bone16.
The bone remodeling process is initiated by bone resorption coupled by the subsequent bone formation. Bone mass is lost when the enhanced osteoclast activity surpasses the osteoblastic bone formation. Using genetically-modified mouse models it has been reported that the canonical and non-canonical NF-κB pathways play important roles in promoting osteoclast differentiation and maturation21. One of the NF-κB pathway activators is receptor activator for nuclear factor-kappaB ligand (RANKL)/receptor activator for nuclear factor-kappaB (RANK) system. Although previous studies have considered that RANKL in bone tissues mainly come from the osteoblastic lineage cells22, recent studies from our group and others have clearly shown that chondrocytes could also secret RANKL which regulates postnatal bone remodeling through modulation of osteoclast formation4,23,24. One of the canonical NF-κB pathway inhibitors is the cell-permeable NF-κB essential modulator (NEMO) binding domain (NBD) peptide which could specifically bind to the NEMO, the inhibitor of nuclear factor kappa-B kinase subunit gamma (IKKγ), and interfere with the formation of IkB kinase (IKK) complex formation. Previous reports showed that the NBD peptide could efficiently inhibit osteoclast formation, and then decreased the pathological bone resorption in inflammatory arthritis25,26. Recently, a clinically available drug, strontium ranelate, has been proved to improve bone structures and to decrease the risk of fracture of menopause osteoporotic patients27 and was also reported to have both structure- and symptom-modifying activity in patients with knee OA28. The possible mechanism is that the strontium inhibits osteoclast maturation and promotes osteoclast apoptosis, both of which have been reported to be regulated by the NF-κB pathway29,30.
The temporomandibular joint (TMJ) is one of the most common sites of OA31. We previously reported that the aberrant biomechanical loading resulting from disorder dental occlusion, termed unilateral anterior crossbite (UAC), could induce obvious subchondral bone loss and increased osteoclast activity in companion with the cartilage degradation in mouse mandibular condyles6,8 while both the subchondral loss and cartilage degradation were attenuated by reducing functional dietary loading8. Whether systemic administration of NBD peptide or strontium could ameliorate mandibular condyle cartilage degradation and subchondral bone loss induced by UAC requires further investigation. In the present study, the histomorphological and micro-CT analysis of mouse mandibular condylar cartilage and/or subchondral bone was performed. Real-time PCR and/or immunohistochemistry (IHC) were carried out to evaluate the expression levels of cartilage catabolic and anabolic factors including Collagen II and aggrecan, the aggrecan degrading enzyme ADAMTS-5, the inflammation factors tnf-α and il-1β, nfkbia (nuclear factor kappa-B inhibitor, alpha) and NF-κB phospho-p65 in condylar cartilage. The mRNA expression levels of rank/rankl/osteoprotegerin (opg) in both condylar cartilage and subchondral bone were also determined by Real-time PCR. We hypothesized that systemic administration of NBD peptide or strontium reverses the UAC-induced mandibular condylar cartilage degradation and subchondral bone loss.
Material and methods
Mice and sample preparation
One hundred and eight 6-week-old C57BL/6J female mice (weight about 16 g) were provided by the Animal Center of Fourth Military Medical University. All procedures and animal care were approved by the University Ethics Committee and performed according to institutional guidelines. In the UAC groups, UAC was applied to the mice as we previously described7,8. In the non-UAC groups, mice underwent a mock operation procedure with no abnormal biomechanical stimulation. At the seventh day after mock operation or UAC treatment, the control and UAC mice were further respectively pharmacologically treated for 2 weeks and 4 weeks as follows (n = 9): intragastric administration of saline (CON + Saline and UAC + Saline groups), intragastric administration of 4 mmol/kg SrCl2 (43966-5, Sigma–Aldrich Co. LLC, USA) in saline (CON + SrCl2 and UAC + SrCl2 groups) and i.p. injection of 5 mg/kg NBD peptide (IMG-2000-5, IMGENEX, USA) (CON + NBD peptide and UAC + NBD peptide groups) and therefore three non-UAC control groups (CON + Saline, CON + SrCl2 and CON + NBD peptide) and three UAC groups (UAC + Saline, UAC + SrCl2 and UAC + NBD peptide) were formed for the two time point. The intraperitoneal injections were performed once every two days, while the intragastric administrations were performed once per day. All mice were sacrificed at the end of the third or fifth week after the mock operation or the installing procedure of UAC. No mice showed any sign of disability, and they all received the same standardized hard diet throughout the experiment8.
Because no differences in degrading changes were found between the left side and right side of the TMJs in the UAC mice in our previous report6, left side TMJ tissue blocks from six mice of each group at the two time point were fixed, decalcified and embedded in paraffin. The right side condyles from the six mice of each group at the two time point (n = 6) used for micro-CT scanning (Inveon, Siemens, MUC, Bavaria, Germany) were separated from the mandibular skulls4, and were immediately fixed with 3% glutaral-dehyde in 0.1 M sodium cacodylate buffer. For each group at the two time point, the condylar cartilages and subchondral bones of the 6 TMJs from 3 mice were respectively carefully separated and preserved at −80°C for RNA extraction. Condylar subchondral bone 3 mm beneath the cartilage-bone junction was cross-sectioned and collected for RNA extraction as we reported6. Two condyles from a mouse were pooled to create a single sample of the cartilage or subchondral bone respectively for RNA extraction and 3 independent samples were formed (n = 3).
Micro-CT
The micro-CT scanning was reconstructed with an isotropic voxel size of 10 μm and the three-dimensional images acquired from microtomographic slices were utilized for quantitative evaluation. For subchondral bone histomorphometry, two cubes (each 0.25 × 0.25 × 0.25 mm) were selected at the middle of the center and posterior of condylar subchondral bone. Within the selected regions, bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) were measured by Health Care MicroView ABA 2.1.2 software as described previously4,6,8.
Histological staining and histomorphometric analysis
Fifteen central and para-central 5 μm thick sagittal sections were prepared consecutively, and sections were randomly selected for H&E staining, Safranin O staining, tartrate-resistant acid phosphatase (TRAP) staining and IHC staining of Collagen II, ADAMTS-5 and NF-κB phospho-p65 (Ser536). H&E and Safranin O staining was used for histological assessment8, and TRAP staining was used for the identification of osteoclasts following the manufacturer’s instructions (Sigma 387-A, St Louis, USA). Condyle cartilage thickness, condylar cartilage cellular density, the percentages of degraded cartilage areas, the Collagen II-positive areas and the number of subchondral bone TRAP-positive osteoclasts were measured as we reported previously4,6,8. Briefly, the condylar cartilage was evenly divided into anterior, central and posterior thirds and the cartilage thickness in the central or posterior third was measured and averaged. The condylar cartilage cellular density and the percentages of degraded cartilage areas were calculated by the value of the total cell number or the value of the total degraded cartilage areas in the central and posterior thirds divided by the value of the total area of the central and posterior thirds, respectively. TRAP-positive osteoclasts were counted in five randomly selected high-power (400×) fields under a microscope (Leica DM 2500, Wetzlar, Germany) and the averaged value was used as the value for this section8. All the measurements were carried out from three sections per joint and the averaged value was used to represent this joint for statistical analysis (n = 6).
RNA extraction and real-time PCR
Total RNA was extracted by using Trizol (Invitrogen Life Technologies, CA, USA). The primers for target genes were listed in Table I. Gene expression was analyzed with the Applied Biosystems 7500 Real-time PCR machine (Applied Biosystems, CA, USA) with GAPDH as the internal control. The results were calculated as the relative quantification compared to the 2-week CON + Saline group, which was set at 1. Data were collected from 3 independent pooled samples (n = 3).
Table I.
Gene primers
| Genes | Forward primer | Reverse primer |
|---|---|---|
| col2a1 | CATCCAGGGCTCCAATGATGTA | ATGTCCATGGGTGCGATGTC |
| aggrecan | TTCCACCAGTGCGATGCAG | TGGTGTCCCGGATTCCGTA |
| ADAMTS-5 | AAGGGCACAGGCTACTATGTGGTC | CAATAATGCCGTCACATCCAGTTC |
| tnf-α | GACTAGCCAGGAGGGAGAACAGA | CCTGGTTGGCTGCTTGCTT |
| il-1β | TCCAGGATGAGGACATGAGCAC | GAACGTCACACACCAGCAGGTTA |
| nfkbia | GAAGCCGCTGACCATGGAA | GATCACAGCCAAGTGGAGTGGA |
| rank | GGCTTACCTGCCCAGTCTCATC | AAGCATCATTGACCCAATTCCAC |
| opg | TTACCTGGAGATCGAATTCTGCTTG | GTGCTTTCGATGAAGTCTCACCTG |
| rankl | GCAGCATCGCTCTGTTCCTGTA | CCTGCAGGAGTCAGGTAGTGTGTC |
| gapdh | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
Immunohistochemical staining
A standard, three-step, avidin-biotin complex IHC staining protocol was carried out8. The primary antibodies were goat anti-Collagen II (sc-7763, 1:50, Santa Cruz), rabbit anti-ADAMTS-5 (ab41037, 1:50, Abcam) and rabbit anti-NF-κB phospho-p65 (Ser536, ab131109, 1:50, Abcam). In negative control slides, non-immune rabbit or goat serum was substituted for the primary antibody5. The percentage of ADAMTS-5-positive or phospho-p65-positive chondrocytes were calculated by the number of immunepositive cell divided by total cell number from the six square frames (each 0.08 mm × 0.08 mm) in the center and posterior condylar cartilage thirds as reported previously8. The averaged value of the percentages from three sections of each joint was calculated to represent the sample for further statistical analysis (n = 3).
Statistical analysis
Data in the figures are expressed as means and 95% confidence intervals (CIs), and “n” represents the number of independent observations from different mice per group. Normality of data distribution was tested by Shapiro–Wilk test with 95% confidence and Levene’s test was used to assess homogeneity of variance. The percentages of degraded cartilage areas were compared by using the non-parametric Kruskal–Wallis test and Mann–Whitney U test. For the other data, the assumptions of parametric tests were fulfilled and two factors, group and time, were taken into consideration when two-way ANOVA was applied for statistical analysis. When significant differences were found, Tukey test was used for post hoc comparison between groups. SPSS 16.0 (SPSS Inc, IL, USA) was used for the statistical analysis and P-values less than 0.05 were considered statistically significant for all statistical tests.
Results
All mice are healthy and no significant body weight changes were noticed among the six groups at the two sampling times (The body weights of the control and UAC mice were shown in Supplemental Fig. 1).
Cartilage histomorphology and expression of proteoglycan, Collagen II, aggrecan and ADAMTS-5
In the control groups of mandibular condylar cartilage, the chondrocytes are distributed in zones. There is abundant extracellular matrix proteoglycan and Collagen II in the hypertrophic layer as revealed by H&E, Safranin O staining and Collagen II IHC staining (Figs. 1 and 2). The CON + Saline, CON + SrCl2 and CON + NBD peptide groups showed no obvious histomorphological differences. The cartilage thickness, condylar chondrocyte density and the mRNA expressing levels of col2a1 and aggrecan by chondrocytes were similar among the three control groups both for 2-week and 4-week time point (Figs. 1 and 2, P > 0.05).
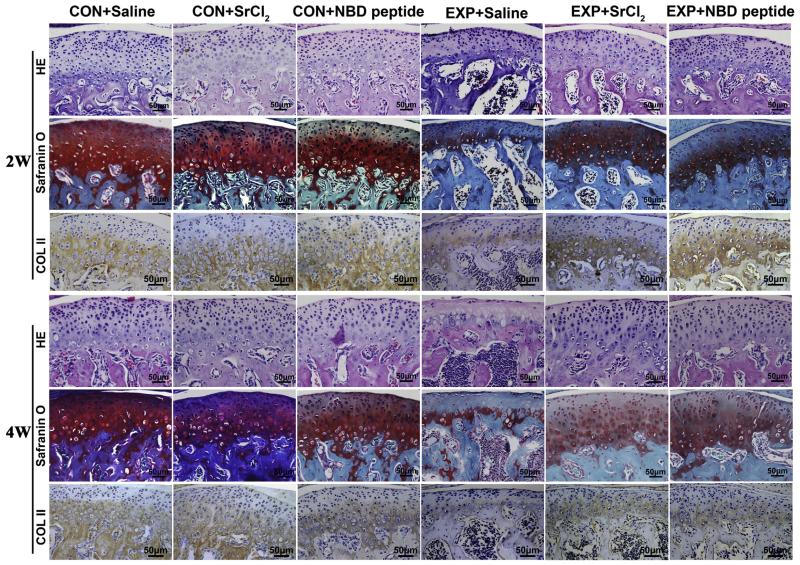
Fig. 1.
Histomorphological observation on mandibular condyles from the control and UAC mice. Representative sections of Hematoxylin & Eosin (HE) staining, safranin O staining and Collagen II IHC staining were shown. Bars = 50 μm. CON + Saline = non-UAC (unilateral anterior cross-bite) control mice with 2-week or 4-week intragastric gavage of Saline; CON + SrCl2 = the control mice with 2-week or 4-week intragastric gavage of SrCl2 (4 mg/Kg, 1 time/1d); CON + NBD peptide = the control mice with 2-week or 4-week intraperitoneal injection of NBD peptide (5 mg/Kg, 1 time/2d); UAC + Saline = the UAC mice with 2-week or 4-week intragastric gavage of Saline; UAC + SrCl2 = the UAC mice with 2-week or 4-week intragastric gavage of SrCl2 (4 mg/Kg, 1 time/1d); UAC + NBD peptide = the UAC mice with 2-week or 4-week intraperitoneal injection of NBD peptide (5 mg/Kg, 1 time/2d). The mice were undergoing mock procedure or UAC treatment. From the seventh day after of a mock operation (control) or UAC treatment (UAC), the control and UAC mice were further pharmacologically treated for 2 weeks (2w) or 4 weeks (4w) and sampled. The same group designations are also used in the other figures.
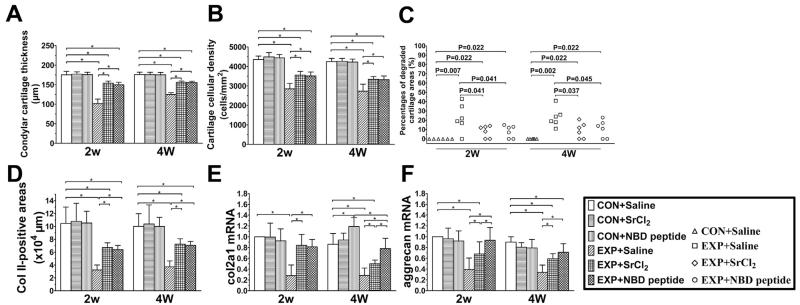
Fig. 2.
Changes of mandibular condylar cartilage thickness (A), condylar cartilage cellular density (B), percentages of degraded cartilage areas (C), Collagen II-positive areas (D) and cartilage mRNA expressing levels of col2a1 (E) and aggrecan (F) from the control and UAC mice. n = 6 in (A–C) and n = 3 in (D–F). For the percentages of degraded cartilage areas in (C), every point represents the measurement outcome of an independent sample while the other data are expressed as means and 95% confidence intervals (CIs) and * indicates statistically significant differences between groups. (A) P-values between groups with * are less than 0.001. (B) P-values between groups with * are less than 0.001. (D) P-values: 2w: P-values between groups with * are less than 0.001; 4w: CON + Saline vs UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P = 0.003 and P = 0.002; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P < 0.001 and P = 0.001. (E) P-values: 2w: P-values between groups with * are less than 0.001; 4w: CON + Saline vs CON + NBD peptide, UAC + Saline and UAC + SrCl2: all P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.011 and P < 0.001; UAC + SrCl2 vs UAC + NBD peptide: P = 0.001. (F) P values: 2w: non-UAC control (CON + Saline) vs UAC + Saline and UAC + SrCl2: P < 0.001 and P = 0.003; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.007 and P < 0.001; UAC + SrCl2 vs UAC + NBD peptide: P = 0.017; 4w: CON + Saline vs UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P < 0.001 and P = 0.007; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.001 and P < 0.001.
In the UAC + Saline groups, condylar cartilage degradation was observed although the fibrocartilage on the surface of the condylar cartilage was intact [Figs. 1 and 2, (A)–(F)]. There were degraded areas in the condylar cartilage with reduced cell number and the loss of proteoglycan. The histological analysis demonstrated a lower chondrocyte density, thinner mandibular condylar cartilage thickness and decreased Collagen II-positive areas in 2-week and 4-week UAC + Saline groups compared to the age matched CON + Saline groups [all P < 0.001, Figs. 1 and 2, (A)–(B), (D)]. Quantitative realtime-PCR assay showed that the expressions of col2a1 and aggrecan mRNA in UAC + Saline groups were significantly decreased compared to the Con + Saline groups and that the expressions of ADAMTS-5 in mandibular condylar condylar cartilage from 2-week and 4-week UAC + Saline groups were significantly elevated compared to the CON + Saline groups [detailed P values are shown in the legend, Fig. 2, (E)–(F) and Fig. 3].
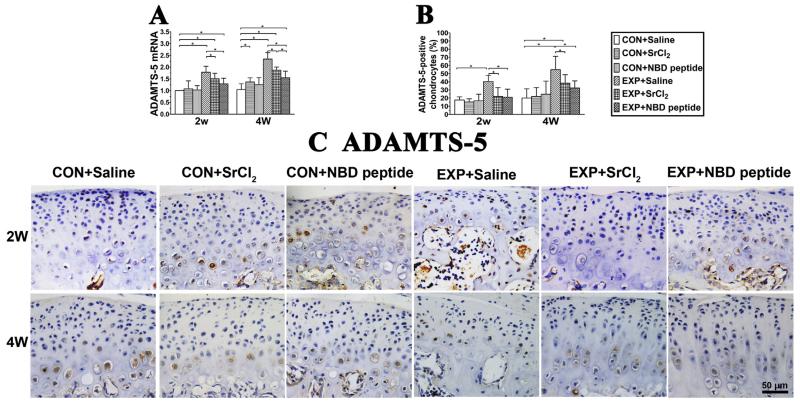
Fig. 3.
Expression changes of ADAMTS-5 in mandibular condylar cartilage from the control and UAC mice. (A) ADAMTS-5 mRNA. P values: 2w: CON + Saline vs UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P < 0.001 and P = 0.023; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.03 and P < 0.001; 4w: CON + Saline vs CON + SrCl2, UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P = 0.015, P < 0.001, P < 0.001 and P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.001 and P < 0.001; UAC + SrCl2 vs UAC + NBD peptide: P = 0.018. (B) Percentages of ADAMTS-5-positive chondrocyte. P values: 2w: P-values between groups with * are less than 0.001; 4w: CON + Saline vs UAC + Saline and UAC + SrCl2: P < 0.001 and P = 0.008; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.017 and P = 0.018. (C) Representative sections of IHC staining of ADAMTS-5 in condylar cartilage from the 2-week and 4-week groups were shown. Bar = 50 μm n = 3; data are expressed as means and 95% confidence intervals (CIs); * indicates statistically significant differences between groups.
It was further found in UAC + SrCl2 and UAC + NBD peptide groups, the cartilage degradation was less severe than the UAC + Saline groups, as shown by increased cartilage thickness (all P < 0.001) and cellular density (all P < 0.001), decreased percentages of degraded cartilage areas [detailed P values are provided in Fig. 2(C)], and larger Collagen II-positive areas [detailed P values are shown in the legend, Fig. 2(D)]. The mandibular condylar cartilage in UAC + SrCl2 and UAC + NBD peptide groups exhibited higher mRNA expression levels of col2a1 and aggrecan, but lower ADAMTS-5 level than the UAC + Saline groups [detailed P values are shown in the legend, Fig. 2, (E)–(F) and Fig. 3]. Compared with 4-week UAC + SrCl2 group, the 4-week UAC + NBD peptide group showed relatively higher mRNA expression level of col2a1, and lower mRNA expression level of ADAMTS-5 mRNA [detailed P values are shown in the legend, Fig. 2, (E)–(F) and Fig. 3].
Cartilage expression of tnf-α, il1-β, nfkbia and NF-κB phospho-p65
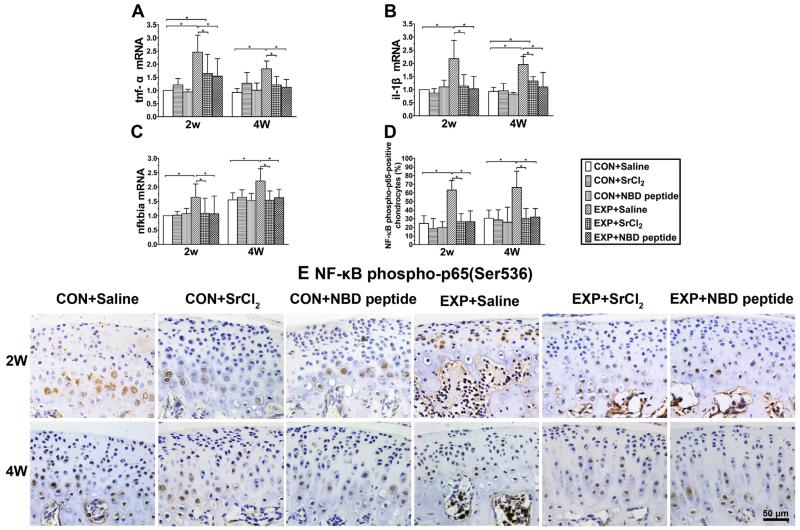
The mRNA expression levels of tnf-α/il1-β and nfkbia were increased in the UAC + Saline groups compared to their age-matched CON + Saline groups, whereas the 2 or 4 weeks treatment of UAC mice by SrCl2 or NBD peptide decreased the expression levels of tnf-α/il1-β and nfkbia [Fig. 4(A)–(C)], causing their expression to return to the control levels [detailed P values are shown in the legend, Fig. 4(A)–(C)]. The result of NF-κB phospho-p65 IHC staining showed that percentages of phosphorylated NF-κB p65-positive chondrocytes in the UAC + Saline groups were remarkably higher than those in the CON + Saline groups (all P < 0.001), while no such change was noticed in the age-matched UAC + SrCl2 and UAC + NBD peptide groups [detailed P values are shown in the legend, Fig. 4(D)–(E)].
Fig. 4.
Expression changes of tnf-α, il-1β, nfkbia and NF-κB phospho-p65 (Ser536) in mandibular condylar cartilage from the control and UAC mice. (A) tnf-α mRNA. P values: 2w: CON + Saline vs UAC + Saline and UAC + SrCl2: P < 0.001 and P = 0.017; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.03 and P = 0.001; 4w: CON + Saline vs UAC + Saline: P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.001 and P < 0.001. (B) il-1β mRNA. P values: 2w: P-values between groups with * are less than 0.001; 4w: CON + Saline vs UAC + Saline and UAC + SrCl2: P < 0.001 and P = 0.016; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: both P < 0.001. (C) nfkbia mRNA. P values: 2w: CON + Saline vs UAC + Saline: P = 0.003; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.009 and P = 0.008; 4w: CON + Saline vs UAC + Saline: P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P < 0.001 and P = 0.001. (D) Percentages of NF-κB phospho-p65 (Ser536)-positive chondrocyte were calculated from the selected cartilage frames. P-values between groups with * are less than 0.001. (E) Representative sections of IHC staining of NF-κB phospho-p65 in condylar cartilage from the 2-week and 4-week groups were shown, Bar = 50 μm. n = 3, data are expressed as means and 95% confidence intervals (CIs); * indicates statistically significant differences between groups.
Subchondral bone histomorphometry
In controls, the 2-week or 4-week treatment of SrCl2 and NBD groups displayed slightly but not significantly higher BV/TV than the CON + Saline groups. There were no significant subchondral bone histomorphological differences among these three control groups for the time points (Fig. 5, P > 0.05).
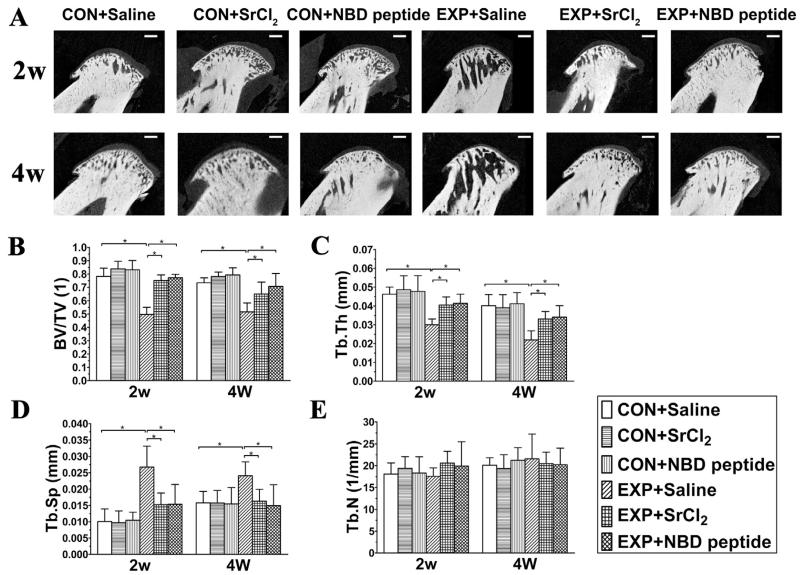
Fig. 5.
Micro-CT images of the mandibular condylar head (A) and histomorphometry of mandibular condylar subchondral bone in the control and UAC mice. (B) Bone volume fraction (BV/TV), (C) trabecular thickness (Tb.Th), (D) trabecular separation (Tb.Sp) and (E) trabecular number (Tb.N) were calculated from the selected subchondral cubes. n = 6; data are expressed as means and 95% confidence intervals (CIs); * indicates statistically significant differences between groups. Bars = 200 μm. (B) P values: 2w: P-values between groups with * are less than 0.001; 4w: CON + Saline vs UAC + Saline: P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.013 and P < 0.001. (C) P values: 2w: CON + Saline vs UAC + Saline: P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.022 and P = 0.011; 4w: CON + Saline vs UAC + Saline: P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.015 and P = 0.006. (D) P values: 2w: CON + Saline vs UAC + Saline: P < 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.0012 and P = 0.001; 4w: CON + Saline vs UAC + Saline: P = 0.027; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.045 and P = 0.012.
In the UAC + Saline groups, large marrow cavities were observed from the histological and micro-CT images [Figs. 1 and 5(A)]. Bone histomorphometric analysis revealed significant reductions in BV/TV and Tb.Th, and increases in Tb.Sp when compared to their age-matched CON + Saline mice [all P < 0.001, Fig. 5(B)–(D)]. However, in UAC + SrCl2 and UAC + NBD peptide groups, there was an attenuated subchondral bone loss to a similar extent, displaying as higher values of BV/TV and Tb.Th, and lower values of Tb.Sp than their age-matched UAC + Saline groups [detailed P values are shown in the legend, Fig. 1 and Fig. 5(A)–(D)]. The values of Tb.N showed no difference among all the groups [Fig. 5(E)].
TRAP positive cells
In the mouse mandibular condyle subchondral bone, TRAP-positive cell numbers were similar in the three control groups [Fig. 6(A) and (B), P > 0.05].
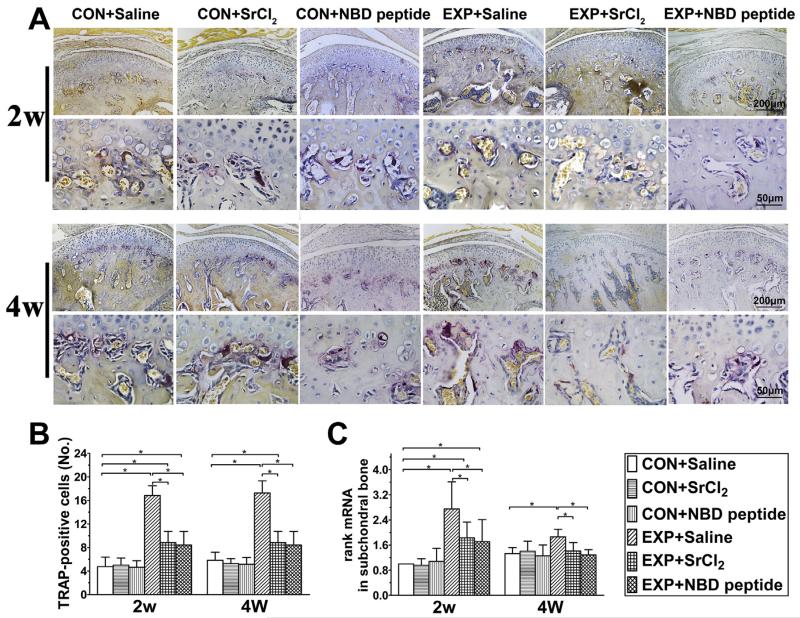
Fig. 6.
The osteoclast numbers in the mandibular condylar subchondral bone in the control and UAC mice. (A) TRAP staining in the mouse mandibular condylar subchondral bone. Bars = 200 μm in the low-magnification images and Bars = 50 μm in the high-magnification images. (B) Quantitative analysis of TRAP-positive osteoclasts (n = 6). P values: 2w: P-values between groups with * are less than 0.001; 4w: CON + Saline vs UAC + Saline and UAC + SrCl2: P < 0.001 and P = 0.032; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: both P < 0.001. (C) mRNA expressing levels of rank in condylar subchondral bone were shown (n = 3). P values: 2w: CON + Saline vs UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P = 0.005 and P = 0.015; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.002 and P = 0.001; 4w: CON + Saline vs UAC + Saline: P = 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.002 and P < 0.001. Data are expressed as means and 95% confidence intervals (CIs); * indicates statistically significant differences between groups.
In the UAC + Saline groups, TRAP-positive cells was significantly increased compared to the age-matched control groups [both P < 0.001, Fig. 6(A) and (B)]. In UAC + SrCl2 and UAC + NBD peptide groups, however, the numbers of TRAP-positive cells were reduced compared to their age-matched UAC + Saline groups [detailed P values are shown in the legend, Fig. 6(A) and (B)].
Expression of rank/rankl/opg
The rank mRNA levels in subchondral bone of 2-week and 4-week UAC + Saline groups were significantly increased compared to their age-matched control groups but in 2-week UAC + SrCl2 and UAC + NBD peptide groups, the rank mRNA levels were decreased compared to the 2-week UAC + Saline group. In 4-week CON + SrCl2 and 4-week CON + NBD peptide groups, the mRNA levels were decreased rank to the 4-week CON + Saline group level [detailed P values are shown in the legend, Fig. 6(C)].
The mRNA expression levels of opg and rankl in cartilage showed no differences in the three non-UAC control groups, while the 4-week CON + SrCl2 and CON + NBD peptide groups showed an increased opg/rankl mRNA ratio in condylar subchondral bone as a result of increased expression of opg in 4-week CON + SrCl2 group and slight decrease of rankl in 4-week CON + NBD peptide group compared to the 4-week CON + Saline group [detailed P values are shown in the legend, Fig. 7(A)–(F)].
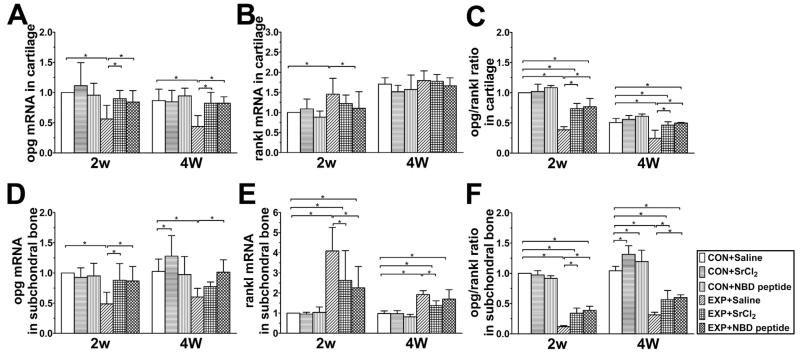
Fig. 7.
Expression of opg and rankl in mandibular condylar cartilage and subchondral bone of the control and UAC mice (n = 3). Data are expressed as means and 95% confidence intervals (CIs); * indicates statistically significant differences between groups. (A) P values: 2w: CON + Saline vs UAC + Saline: P = 0.001; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.006 and P = 0.021; 4w: P-values between groups with * are less than 0.001. (B) P values: 2w: CON + Saline vs UAC + Saline: P = 0.003; UAC + Saline vs UAC + NBD peptide: P = 0.022. (C) P-values between groups with * are less than 0.001. (D) P values: 2w: UAC + Saline vs CON + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P = 0.001 and P = 0.001; 4w: UAC + Saline vs CON + Saline and UAC + NBD peptide: P = 0.001 and P = 0.001; CON + Saline vs CON + SrCl2: P = 0.047. (E) P values: 2w: CON + Saline vs UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P = 0.001 and P = 0.010; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: P = 0.003 and P < 0.001; 4w: CON + Saline vs UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P = 0.004 and P < 0.010; UAC + Saline vs UAC + SrCl2: P < 0.001. (F) P values: 2w: P values between groups with * are less than 0.001; 4w: CON + Saline vs CON + SrCl2, CON + NBD peptide, UAC + Saline, UAC + SrCl2 and UAC + NBD peptide: P < 0.001, P = 0.023, P < 0.001, P < 0.001 and P < 0.010; UAC + Saline vs UAC + SrCl2 and UAC + NBD peptide: both P < 0.001.
In the UAC + Saline groups, mRNA levels of opg in cartilage or subchondral bone were reduced while those of rankl expression were significantly or slightly increased, resulting in decreases of the ratio of opg/rankl in both cartilage and subchondral bone compared to their age-matched CON + Saline groups [Fig. 7(A)–(F)].
In the UAC + Saline groups, mRNA levels of opg in cartilage or subchondral bone were reduced while those of rankl expression were significantly or slightly increased, resulting in decreases of the ratio of opg/rankl in both cartilage and subchondral bone compared to their age-matched CON + Saline groups [Fig. 7(A)–(F)]. In UAC + SrCl2 and UAC + NBD peptide groups, however, there were consistent increases in the opg mRNA expression and the ratio of opg/rankl in both the mandibular condyle cartilage and subchondral bone compared to their age-matched UAC + Saline groups, although the ratio of opg/rankl in cartilage of the UAC + SrCl2 and UAC + NBD peptide groups were still lower than those of the age-matched non-UAC + CON Saline groups [detailed P values are shown in the legend, Fig. 7(A)–(F)].
Discussion
The current data demonstrate that systemic administration of either strontium or NBD peptide reverses not only the subchondral bone loss but also the mandibular condylar cartilage degradation induced by UAC as evidenced by increased cartilage thickness and cell density, increased expression of cartilage matrix molecules such as Collagen II and aggrecan, and decreased expression of cartilage catabolic factors, such as ADAMTS-5, il-1β and tnf-α. In contrast, systemic administration of either strontium or NBD peptide induces no significant changes in non-UAC control condylar cartilage, and slightly increases the values of BV/TV and opg/rankl mRNA ratio in the subchondral bone. These findings are supported by the recent finding that abnormal remodeling of subchondral bone caused by activation of TGF-β signaling leads to OA-like pathological changes in cartilage of both knee and TMJs32,33, and inhibition of the TGF-β signaling could largely attenuate cartilage degeneration32. What deserves to be mentioned is that there were indeed profound cell losses in the fibrous layer, as well as in the proliferative layer of the articular cartilage in UAC groups. However the surfaces of the condylar cartilage were intact. This phenomenon was noticed in all of our previous reports using the same methods6–8. It seems different from knee joint17. Based on our findings in this report and previous reports6–8, the observed degenerative alterations are unique to mandibular condyles and are quite different from those displayed in other joints. The fibrous layer is much thicker in TMJ condylar cartilage than that in knee cartilage, which might be a contribution to the phenomenon, yet needs a future clarification.
The subchondral bone provides the mechanical support for the overlying articular cartilage and undergoes constant modeling or remodeling in response to changes of the joint loading. Loss of subchondral bone will trigger degradation of the overlying cartilage through aggravating their biomechanical environment16,34–36. The improved subchondral bone architectures via systemic administration of strontium and NBD peptide may then take a role in protecting cartilage from degradation considering the biomechanical effect of subchondral bone on the overlied cartilage as Radin et al.34 proposed. Further, NF-κB signal has been shown to have multiple effects on chondrocytes metabolism, promoting the expression of pro-inflammatory cytokines, such as il-1β and tnf-α and matrix degrading enzymes, such as ADAMTS-510–12. Intraarticular injection of adenoviral vector-expressing NF-κB p65-specific shRNA to inhibit the NF-κB signal has been shown to alleviate cartilage degradation and synovium inflammation in the rat experimental knee OA model37. There is a possibility that the systemically delivered drugs may pass through the osteochondral junction and act directly on articular cartilage via osteochondral vascular tissues as it was suggested38, because the tide mark or boundary between cartilage and bone in TMJs of young mice like the currently used is not obvious as displayed in our results. That means the strontium or NBD peptide may be able to penetrate into cartilage tissue and acts directly on chondrocytes and inhibits the NF-κB signal evoked by the abnormal loading in UAC mice. Further studies on the mechanism of strontium or NBD peptide on articular cartilage and subchondral bone will help us elucidate the direct effect of strontium on NF-κB signal under normal or OA situation.
Previous studies have revealed that targeting subchondral bone with the agents regulating bone remodeling, such as bisphosphonates, estrogens, calcitonin, OPG and PTH (1–34)16–20 has protective effects on both subchondral bone and articular cartilage in OA. Strontium has been proposed as an anti-osteoporotic drug capable of rebalancing bone turnover, improving bone morphology, preserving bone matrix mineralization, and restoring bone biomechanical properties in in vitro and in vivo studies and clinical studies39–41. The current results demonstrate that oral SrCl2 treatment at 4 mmol/kg/d was able to effectively preserve the normal properties of subchondral bone in mandibular condyles of UAC mouse, as demonstrated by the improvement in morphology, microarchitecture indices, osteoclast number and opg/rankl ratio. Although the mechanisms by which the strontium exerts its effects remains relatively unclear, the findings of present studies implied that SrCl2 exerts its effects by mediating RANKL/RANK/OPG pathway because treatment with SrCl2 inhibits expression of rankl in subchondral bone of UAC mice, but stimulates their expression of opg, hence increased opg/rankl ratio in mandibular condyle subchondral bone, a result that is in line with previous study showing that SrCl2 could up-regulate OPG expression and increase opg/rankl ratio in osteoblasts41, and inhibit osteoclast activity29, and that the NBD peptide could effectively inhibit the increased NF kappaB signaling in active osteoclasts but spare the normal NF kappaB signaling during osteoclast activity25.
In conclusion, the present studies indicate a rescue effect of the systemic administration of strontium or NBD peptide on the subchondral bone loss and cartilage degeneration in the early stage OA induced by UAC. This would be of clinical significance considering that a pharmacological administration of strontium or NBD peptide might be helpful to promote mandibular condyle recovering from degradation caused by abnormal occlusions.
Supplementary Material
Acknowledgments
We thank Shu-Jing Cai for assistance in tissue section preparation and Professor Chang-Sheng Chen (Department of Health Statistics, School of Preventive Medicine, Fourth Military Medical University) for assistance with the statistical analysis.
Role of the funding source
This work was supported by the National Natural Science Foundation of China (No. 81271169, 81300898).
Footnotes
Author contributions
Yun-Dong Liu, Kai Jiao and Mei-Qing Wang contributed to the conception and design of the study. Yun-Dong Liu, Hong-Xu Yang, Kai Jiao, Li-Fan Liao and Hong-Yun Zhang contributed to the acquisition, collection and assembly of data. Yun-Dong Liu and Li-Fan Liao contributed to the statistical analysis. Yun-Dong Liu, Kai Jiao, Lei Lu, Di Chen and Mei-Qing Wang contributed to the analysis and interpretation of the data. All authors contributed in revising the manuscript critically. All authors approved the final version to be submitted.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2015.07.022.
References
- 1.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, et al. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Polur I, Lim C, Servais JM, Dobeck J, Li Y, et al. Early-onset osteoarthritis of mouse temporomandibular joint induced by partial discectomy. Osteoarthritis Cartilage. 2009;17:917–22. doi: 10.1016/j.joca.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Sorensen KP, Gupta T, Kilts T, Young M, Wadhwa S. Altered functional loading causes differential effects in the subchondral bone and condylar cartilage in the temporomandibular joint from young mice. Osteoarthritis Cartilage. 2009;17:354–61. doi: 10.1016/j.joca.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao K, Niu LN, Wang MQ, Dai J, Yu SB, Liu XD, et al. Subchondral bone loss following orthodontically induced cartilage degradation in the mandibular condyles of rats. Bone. 2011;48:362–71. doi: 10.1016/j.bone.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Jiao K, Zhang M, Zhou T, Liu XD, Yu SB, et al. Occlusal effects on longitudinal bone alterations of the temporomandibular joint. J Dent Res. 2013;92:253–9. doi: 10.1177/0022034512473482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu L, Huang J, Zhang X, Zhang J, Zhang M, Jing L, et al. Changes of temporomandibular joint and semaphorin 4D/plexin-B1 expression in a mouse model of incisor malocclusion. J Oral Facial Pain Headache. 2014;28:68–79. doi: 10.11607/jop.1082. [DOI] [PubMed] [Google Scholar]
- 7.Wang YL, Zhang J, Zhang M, Lu L, Wang X, Guo M, et al. Cartilage degradation in temporomandibular joint induced by unilateral anterior crossbite prosthesis. Oral Dis. 2014;20:301–6. doi: 10.1111/odi.12112. [DOI] [PubMed] [Google Scholar]
- 8.Liu YD, Liao LF, Zhang HY, Lu L, Jiao K, Zhang M, et al. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage. 2014;22:302–12. doi: 10.1016/j.joca.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su SC, Tanimoto K, Tanne Y, Kunimatsu R, Hirose N, Mitsuyoshi T, et al. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22:845–51. doi: 10.1016/j.joca.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–48. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Hirata M, Saito T, Itoh S, Chung UI, Kawaguchi H. Transcriptional induction of ADAMTS-5 protein by nuclear factor-kappaB (NF-kappaB) family member RelA/p65 in chondrocytes during osteoarthritis development. J Biol Chem. 2013;288:28620–9. doi: 10.1074/jbc.M113.452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolbos RI, Zuo J, Banerjee S, Link TM, Ma CB, Li X, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage. 2008;16:1150–9. doi: 10.1016/j.joca.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersson IF, Boegard T, Dahlstrom J, Svensson B, Heinegard D, Saxne T. Bone scan and serum markers of bone and cartilage in patients with knee pain and osteoarthritis. Osteoarthritis Cartilage. 1998;6:33–9. doi: 10.1053/joca.1997.0090. [DOI] [PubMed] [Google Scholar]
- 15.Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 2002;46:3178–84. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- 16.Karsdal MA, Bay-Jensen AC, Lories RJ, Abramson S, Spector T, Pastoureau P, et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann Rheum Dis. 2014;73:336–48. doi: 10.1136/annrheumdis-2013-204111. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S, Chen K, Lan Y, Zhang N, Jiang R, Hu J. Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone. 2013;53:340–9. doi: 10.1016/j.bone.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 18.Yu DG, Ding HF, Mao YQ, Liu M, Yu B, Zhao X, et al. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol Sin. 2013;34:393–402. doi: 10.1038/aps.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelletier JP, Kapoor M, Fahmi H, Lajeunesse D, Blesius A, Maillet J, et al. Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1beta in the synovium. Ann Rheum Dis. 2013;72:250–7. doi: 10.1136/annrheumdis-2012-201710. [DOI] [PubMed] [Google Scholar]
- 20.Kadri A, Ea HK, Bazille C, Hannouche D, Liote F, Cohen-Solal ME. Osteoprotegerin inhibits cartilage degradation through an effect on trabecular bone in murine experimental osteoarthritis. Arthritis Rheum. 2008;58:2379–86. doi: 10.1002/art.23638. [DOI] [PubMed] [Google Scholar]
- 21.Soysa NS, Alles N. NF-kappaB functions in osteoclasts. Biochem Biophys Res Commun. 2009;378:1–5. doi: 10.1016/j.bbrc.2008.10.146. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien CA. Control of RANKL gene expression. Bone. 2010;46:911–9. doi: 10.1016/j.bone.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usui M, Xing L, Drissi H, Zuscik M, O’Keefe R, Chen D, et al. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2008;23:314–25. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Jin H, Zhu M, Li J, Zhao L, Zhang Y, et al. Chondrocyte beta-catenin signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model. Arthritis Rheumatol. 2014;66:107–20. doi: 10.1002/art.38195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–24. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 26.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–22. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 27.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–68. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 28.Reginster JY, Badurski J, Bellamy N, Bensen W, Chapurlat R, Chevalier X, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis. 2013;72:179–86. doi: 10.1136/annrheumdis-2012-202231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurtel-Lemaire AS, Mentaverri R, Caudrillier A, Cournarie F, Wattel A, Kamel S, et al. The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J Biol Chem. 2009;284:575–84. doi: 10.1074/jbc.M801668200. [DOI] [PubMed] [Google Scholar]
- 30.Caudrillier A, Hurtel-Lemaire AS, Wattel A, Cournarie F, Godin C, Petit L, et al. Strontium ranelate decreases receptor activator of nuclear factor-KappaB ligand-induced osteoclastic differentiation in vitro: involvement of the calcium-sensing receptor. Mol Pharmacol. 2010;78:569–76. doi: 10.1124/mol.109.063347. [DOI] [PubMed] [Google Scholar]
- 31.Schmitter M, Essig M, Seneadza V, Balke Z, Schroder J, Rammelsberg P. Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofac Radiol. 2010;39:231–4. doi: 10.1259/dmfr/16270943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao K, Zhang M, Niu L, Yu S, Zhen G, Xian L, et al. Overexpressed TGF-beta in subchondral bone leads to mandibular condyle degradation. J Dent Res. 2014;93:140–7. doi: 10.1177/0022034513513034. [DOI] [PubMed] [Google Scholar]
- 34.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986:34–40. [PubMed] [Google Scholar]
- 35.Day JS, Ding M, van der Linden JC, Hvid I, Sumner DR, Weinans H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res. 2001;19:914–8. doi: 10.1016/S0736-0266(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 36.Boyd SK, Muller R, Zernicke RF. Mechanical and architectural bone adaptation in early stage experimental osteoarthritis. J Bone Miner Res. 2002;17:687–94. doi: 10.1359/jbmr.2002.17.4.687. [DOI] [PubMed] [Google Scholar]
- 37.Chen LX, Lin L, Wang HJ, Wei XL, Fu X, Zhang JY, et al. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-kappaBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16:174–84. doi: 10.1016/j.joca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, Mc Williams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15:743–51. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Saidak Z, Marie PJ. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136:216–26. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Tat SK, Pelletier JP, Mineau F, Caron J, Martel-Pelletier J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone. 2011;49:559–67. doi: 10.1016/j.bone.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Peng S, Liu XS, Zhou G, Li Z, Luk KD, Guo XE, et al. Osteoprotegerin deficiency attenuates strontium-mediated inhibition of osteoclastogenesis and bone resorption. J Bone Miner Res. 2011;26:1272–82. doi: 10.1002/jbmr.325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.