Abstract
Objectives
More than half of all adults will be exposed to a traumatic event at some point in their lives, yet we do not yet have reliable biomarkers to help predict who experiences trauma-related symptoms in response to exposure. We tested the utility of salivary cortisol and salivary alpha amylase as markers of (1) neural reactivity to negative affective information and (2) neural hypervigilance in the absence of threat.
Participants
20 women (mean age 23.6 +/− 5.8 years) with a history of trauma exposure.
Measures
Salivary cortisol and alpha amylase reactivity were measured in response to a trauma reminder during a clinical interview. Neural reactivity to novel and familiar affective scenes was measured in a later session using functional magnetic resonance imaging.
Results
Salivary alpha amylase, but not cortisol, increased in response to the trauma reminder. Salivary alpha amylase reactivity was associated with neural reactivity in the salience network in response to novel negative scenes and neural hypervigilance as indexed by reactivity to novel neutral scenes.
Conclusions
Salivary alpha amylase might serve as a more reliable marker of trauma-related reactivity to negative affective information, and also as a marker of hypervigilance in the absence of threatening information.
Keywords: Alpha-amylase, Cortisol, Saliva, Biomarkers, Hypervigilance, fMRI
1. Introduction
More than half of all people will experience a traumatic event at some point in their lives (Kessler et al., 2005). Trauma exposure can lead not only to exaggerated physiological reactivity to trauma reminders (e.g., McTeague et al., 2010), but also to chronic elevation of basal autonomic arousal (e.g., Pole, 2007), and maladaptive and distressing hypervigilance for potential threat even in a safe environment (e.g., Dalgleish et al., 2001). Heightened reactivity to threat-relevant cues combined with generalized hypervigilance can be distracting and exhausting, as the person is constantly on alert physiologically and cognitively for potential threat. Although the identification of reliable biomarkers for trauma-related symptoms will help enhance precision of assessment and diagnosis, and non-invasive and relatively inexpensive salivary biomarkers hold particular appeal, the field has not yet identified a reliable biomarker for tonic trauma-related symptoms such as hypervigilance.
In the brain, both reactivity to threat and hypervigilance for threat are associated with heightened neural activity in the salience network: the amygdala, the dorsal anterior cingulate cortex (dACC), and the rostral middle frontal gyrus (i.e., the core areas of dorsolateral PFC and dorsomedial PFC; e.g., Bryant et al., 2005; Straube et al., 2009). The salience network is implicated in vigilance, orienting of attention, and processing of affective information (e.g., Van Marle et al., 2010). Following trauma exposure, reactivity as indexed by amygdala and dACC response is heightened to both trauma-related stimuli (e.g., Protopopescu et al., 2005; Shin et al., 2007) and trauma-unrelated, negatively-valenced stimuli (e.g., Williams et al., 2006). The neuroimaging literature on stress-related states also highlights neural reactivity to threat information in high arousal states. For example, state anxiety is associated with threat-related amygdala hyperreactivity (e.g., Bishop et al., 2004) and heightened activity in dorsal ACC and rostral middle frontal gyrus (e.g., Milad et al., 2007; Simmons et al., 2008).
In addition to reactivity to actual threat as measured by trauma-relevant or negative information, people in stress-related states show neural hypervigilance for potential threat in the salience network even in the absence of threat information. For example, people with PTSD show increased amygdala and dorsal ACC response to salient non-affective stimuli (Bryant et al., 2005), and PTSD symptoms and state anxiety also are associated with heightened amygdala response to affectively ambiguous (i.e., neutral) faces (Brunetti et al., 2010; Somerville et al., 2004). In addition, the amygdala response to novel faces is greater in people with inhibited temperament in childhood (Schwartz et al., 2003), which is linked to stress-system hyperactivity (Tyrka et al., 2006), potentially due to the additive influence of novelty beyond arousal and valence in neural responding to affective information (e.g., Weierich et al., 2010).
Trauma-related reactivity and hypervigilance are examples of overactive stress system responses, and trauma exposure is associated with alterations in the neuroendocrine response to stress, as indexed by hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system (SNS) responses. Results from investigations of HPA axis reactivity via salivary cortisol have been inconsistent, with some evidence for blunted cortisol reactivity (e.g., Elzinga et al., 2008) and some evidence for heightened cortisol reactivity (e.g., Bremner et al., 2003) in trauma-exposed people. Other studies show no relation between trauma exposure and cortisol reactivity (e.g., Simeon et al., 2007). These inconsistencies have been attributed in part to the effects of stress history profiles that reflect complex interactions between chronic stress, early life stress, and acute stressors on basal (e.g., Meewisse et al., 2007) and reactive cortisol (e.g., Suzuki et al., 2014).
More recent investigations of sympathetic reactivity using salivary alpha amylase (sAA) have been more consistent and suggest that sAA is promising as a convenient and non-invasive biomarker for SNS activity (e.g., Granger et al., 2007; Nater and Rohleder, 2009). People who have been exposed to trauma show sustained elevation of basal SNS activity (e.g., Vigil et al., 2010), and also exaggerated SNS reactivity to trauma reminders and more generally aversive stimuli (e.g., Bedi and Arora, 2007; McTeague et al., 2010). SAA is an enzyme that is synthesized and secreted from the acinar cells of the salivary glands (e.g., Baum, 1993). Under normal conditions, the acinar cells are innervated by both the sympathetic and parasympathetic branches of the autonomic nervous system. Parasympathetic impulses stimulate fluid secretion, sympathetic impulses modulate saliva composition by increasing exocytosis from the acinar cells, and in combination both branches influence the level of amylase in saliva (e.g., Proctor & Carpenter, 2007). However, during physical or psychological stress, sAA level is predominantly influenced by SNS activity in the cervical sympathetic pathway (e.g., Bosch et al., 2003; Nater et al., 2007), and sAA levels rise immediately in response to stress (e.g., Nater et al., 2007).
Further supporting the potential utility of sAA as a potential biomarker for stress-related symptoms such as hypervigilance, the salience network is extensively interconnected anatomically to the central sympathetic network, which includes the thalamus, hypothalamus, brainstem, and adrenal medulla (e.g., Westerhaus and Loewy, 2001). Through these multi-synaptic connections, amgydala-PFC circuitry modulates the downstream SNS response to stress. For example, greater amygdala and dorsal medial PFC response to affective information is associated with concurrent physiological indices of SNS activity in healthy participants (e.g., Wager et al., 2009; Yang et al., 2007).
Given the inconsistencies in cortisol reactivity data in trauma-exposed people, and the strong interconnections between the salience network and the sympathetic system, sAA reactivity might be a more reliable neuroendocrine marker for exaggerated threat sensitivity or vigilance. Our overarching aim was to test and compare two candidate analytes as potential biomarkers of excessive neural reactivity to actual threat information and vigilance for potential threat information. We assessed HPA (cortisol) and SNS (alpha amylase) reactivity to a naturalistic trauma reminder as predictive markers of hypervigilant activation patterns in the salience network (i.e., amygdala, dorsal ACC, and rostral middle frontal gyrus). We tested two specific sets of hypotheses. First, if HPA and/or SNS reactivity to trauma reminders predict neural reactivity to actual threat, we hypothesized that reactivity would be associated with activation to negatively-valenced information. Second, if HPA and/or SNS reactivity to trauma reminders predict neural hypervigilance for potential threat, we hypothesized that reactivity would be associated with activation to novel and/or neutral information.
2. Method
2.1. Participants
We recruited 20 adult women who reported exposure to potentially traumatic events in an online screening measure. Potential participants were recruited from introductory psychology subject pool at a large urban university in the northeast US and by responses to an anonymous online screen advertised on flyers. In the current analyses, we included 20 women (age M = 23.6, SD = 5.8, range 18–37 years; see Table 1) who met the trauma exposure criterion (Criterion A) of the posttraumatic stress disorder (PTSD) module of the Diagnostic and Statistical Manual of Mental Disorders IV. Additional inclusion criteria included right-handedness and eligibility for an MRI scan via a standard MRI safety screen (e.g., no metal in the body, no history of claustrophobia).
Table 1.
Participant characteristics (N = 20).
| Variable | Statistic |
|---|---|
| Age in years, M (SD) | 23.6 (5.8) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 3 (15.0) |
| Black, non-Hispanic | 4 (20.0) |
| Asian/Pacific Islander | 7 (35.0) |
| Hispanic | 1 (5.0) |
| Multiple | 2 (10.0) |
| Other | 3 (15.0) |
| Number of trauma types, M (SD) | 2.5 (0.9) |
| Trauma type, n (%) | |
| Natural disaster | 1 (2) |
| Fire/explosion | 3 (6) |
| Motor vehicle accident | 5 (10) |
| Other serious accident | 5 (10) |
| Physical assault | 10 (20) |
| Sexual assault | 5 (10) |
| Other unwanted sexual experience | 1 (2) |
| Life-threatening injury/illness | 3 (6) |
| Severe human suffering | 1 (2) |
| Witness violent death | 2 (4) |
| Sudden, unexpected death of loved one | 6 (12) |
| Caused serious injury/death of another | 1 (2) |
| Other very stressful event | 7 (14) |
| Total number of PTSD symptoms, M (SD), Range | 7.3 (5.1), 0–15 |
| Re-experiencing symptoms | 2.5 (1.7), 0–5 |
| Avoidance symptoms | 2.6 (1.9), 0–6 |
| Hyperarousal symptoms | 1.8 (1.8), 0–5 |
| Perceived Stress Scale, M (SD), Range | 23.5 (7.2), 11–38 |
| STAI-S, M (SD), Range | |
| Session 1 | 46.5 (13.3), 25–64 |
| Session 2 | 41.9 (9.7), 26–61 |
| BDI II, M (SD), Range | |
| Session 1 | 17.1 (7.1), 5–32 |
| Session 2 | 12.3 (8.2), 1–31 |
2.2. Procedure
Two study sessions were conducted on two separate days. The first session always began at 1000 h and included the Structured Clinical Interview for DSM-IV (SCID), collection of three saliva samples, and a brief set of questionnaires. Participants were fully informed regarding all study procedures and the general aims of the study prior to participation, and they were fully debriefed following the second study session. All procedures were approved by the Institutional Review Board.
2.2.1. Structured clinical interview
We conducted the full SCID for all DSM-IV Axis I disorders for the purpose of excluding participants who met criteria for major disorders with the exception of PTSD. No participant met criteria for other major diagnoses, and so none were excluded. We also asked about current medications during the interview. Two participants reported prescription medications (1 Prozac, 1 unspecified non-psychoactive medication; their data did not differ from the other participants’ data and we retained them in the analyses).
2.2.2. Saliva collection
Participants provided saliva samples before, during, and after describing their traumatic event during the SCID; the report of the traumatic event served as the trauma reminder. The first saliva sample (T1) was collected at approximately 1005 h following informed consent, the second sample (T2) was collected immediately following the participant’s description of the traumatic event, and the third sample (T3) was collected exactly 20 min after the second sample. SAA concentrations at each timepoint reflect sympathetic responses at that timepoint, whereas salivary cortisol (sCORT) concentrations at each timepoint reflect HPA-axis responses ~20 min prior to the sample collection. No participants arrived at the lab within a one-hour window since waking, therefore all samples were taken on participants’ regular diurnal curve, and none of the saliva samples captured the sCORT or sAA awakening response. We used Salimetrics Oral Swabs (Salimetrics, LLC) placed under the tongue for 2 min for saliva collection. Each sample swab was sealed in a cryogenic vial and stored in a −20C freezer until the assay procedure. Participants were asked to refrain from eating, drinking, or smoking for one hour prior to the lab session.
2.2.3. Questionnaires
Following the SCID, participants completed the questionnaires, which included the Perceived Stress Scale (PSS, Cohen et al., 1983), the State-Trait Anxiety Inventory—State Version (STAIS, Spielberger et al., 1983), and the Beck Depression Inventory II (BDI-II, Beck et al., 1996). The PSS is a 10-item scale that measures the degree to which non-specific situations in a person’s life over the past month are perceived as stressful. Item frequency is reported from 0 (“never”) to 4 (“very often”), and summed for a possible score range of 0–40. This measure is not diagnostic and therefore has no score cutoffs, however the US normative mean score for young adults is 14 (Cohen and Williamson, 1988), and scores of 20 or above are consistent with high perceived stress. The STAI-S is a 20-item scale that measures current levels of state anxiety. Item intensity is reported from 1 (“not at all”) to 4 (“very much so”), and summed for a possible score range of 20–80. This measure also is not diagnostic, however scores of 40 or above are consistent with high state anxiety. The BDI-II is a 21-item scale that measures depressed mood over the past two weeks. Item intensity is reported low to high, specific to each item, on a scale from 0 (not present) to 3 (extreme). This measure also is not diagnostic, however the following ranges are consistent with levels of generally depressed mood: 0–13 minimal, 14–19 mild, 20–28 moderate, 29–63 severe.
The second study session was scheduled within a week of the first session and included two questionnaires (STAI-S, BDI-II), and the MRI scan.
2.2.4. FMRI task
The fMRI task consisted of four event-related functional runs. These runs began approximately 20 min into the scan session, following an 18 min sequence of structural scans, field maps, and resting state scans. This timing minimized the potential for a confounding influence of scanner-related stress on the BOLD response, as the runs began after the 15 min window during which normative scanner-related stress has been shown to occur and then subside (i.e., Muehlhan et al., 2011). During each run, participants viewed 60 full-color images of randomly presented complex scenes that were positive, negative, or neutral in valence, and that were lower or higher in arousal. We selected task stimuli from a stimulus set currently being normed in our lab. The set is designed to depict scenes (rather than discrete objects or single people/animals), and allows us to balance relevant affective elements such as social vs non-social content. We selected scenes for this task based on valence and arousal ratings collected from an initial sample of 748 young adults. Valence ratings were made on a scale of 1–9, with 1 as most unpleasant and 9 as most pleasant. For the images in this study, valence ratings were: negative (M = 2.61, SD = 1.02), neutral (M = 5.59, SD = 0.84), and positive (M = 6.85, SD = 0.86). Arousal ratings also were rated on a scale from 1 to 9, with 1 for low arousal and 9 for high arousal. For the images in this study, arousal ratings were: negative (M = 5.60, SD = 1.02), neutral (M = 3.88, SD = 0.65), positive (M = 4.58, SD = 0.69). Although the arousal ratings for the negative images are slightly higher than arousal ratings for positive images, they are not significantly different. We note that our negative and positive images were less extremely valenced than images often used in imaging studies (e.g., International Affective Picture System images) in order to more closely approximate the actual valence of visual arrays encountered in daily life. Blocks 1 and 2 were novel; participants viewed each of the images in each block for the first time. Blocks 3 and 4 were familiar; images from Blocks 1 and 2 were repeated in random order in Blocks 3 and 4. We used the Opt-seq2 sequence optimization tool (https://surfer.nmr.mgh.harvard.edu/optseq/) to optimize the rapid event-related runs. Inter-trial jitter ranged from 1500 ms to 6000 ms. During each trial, participants viewed a fixation cross for 500 ms, followed by an image for 3500 ms. Each run was 332 s long. Participants were asked to press a button on the button box to indicate whether the scene was indoors or outdoors (n = 11) or to rate the arousal level for each image (n = 9).1
The task was designed and presented using E-prime experimental software (Psychology Software Tools, Pittsburgh, PA) on a PC. Images were rear-projected to a screen in the magnet bore, and participants viewed images via a mirror mounted on the head coil.
2.2.5. fMRI image acquisition
We used a Siemens Magnetom Trio Tim 3T fMRI scanner with a 32-channel gradient head coil. We conducted a localizer scan, followed by a whole brain magnetization prepared rapid gradient echo (MPRAGE) sequence to acquire high-resolution T1-weighted images (TR/TE/flip angle = 2.17s/4.33ms/7°, field of view (FOV) = 256 × 256 mm2, matrix = 256 × 256, slice thickness = 1 mm, voxel size = 1 × 1 × 1.2 mm3). Functional MRI images were acquired using a blood oxygen level dependent (BOLD) echoplanar (EPI) T2*-weighted sequence (TR/TE/flip angle = 2.0s/30ms/90°, FOV = 220 × 220 mm2, matrix = 64×64, slice thickness = 4 mm, voxel size = 3.44 × 3.44 × 4 mm3). The T1- and T2*-weighted images were collected in the same plane (30 axial slices angled perpendicular to the AC/PC line) with an interleaved excitation order and foot to head phase encoding.
2.3. Data preparation
2.3.1. Saliva assays
All assays were conducted in-house by lab personnel. We conducted alpha amylase assays using Salimetrics kinetic reaction assay kits (Salimetrics, LLC). The assay utilizes a chromagenic substrate, 2-chloro-p-nitrophenol linked to maltotriose. The amount of α-amylase present in the sample is directly proportional to the increase in absorbance measured spectrophotometrically by a standard plate reader at 405 nm. The intra- and inter-assay coefficients of variation for these kits are less than 7.5% and 6%, respectively.
We conducted cortisol assays for using Salimetrics enzyme immunoassay kits (Salimetrics, LLC). The assay utilizes a microtitre plate coated with monoclonal cortisol antibodies. The amount of cortisol present in the sample is inversely proportional to the amount of cortisol peroxidase measured spectrophotometrically by a standard plate reader at 450 nm. The intra- and inter-assay coefficients of variation for these kits are less than 5% and 10%, respectively.
2.3.2. fMRI image pre-processing
Functional and structural MRI data were analyzed using Freesurfer FS-FAST software (version 5.3; http://surfer.nmr.mgh.harvard.edu). Functional imaging data were motion corrected to the middle time point of each BOLD run using the AFNI 3dvolreg program (Cox and Jesmanowicz, 1999), and inspected for gross motion. Slices were excluded if motion was greater than 1 mm. In addition, BOLD data were intensity normalized and spatially smoothed (full-width half-maximum = 4 mm) using a 3D Gaussian filter. The first three volumes in each run were discarded to allow for T2* equilibrium effects. Following preprocessing, functional images for each participant were registered to that participant’s 3D MPRAGE image using the FreeSurfer bbregister program (Greve and Fischl, 2009).
2.4. Statistical analysis
2.4.1. Saliva data analyses
We calculated the sAA response to trauma reminders by subtracting T1 (baseline) concentrations from T2 (trauma description) concentration, as sAA reactivity is immediate. We calculated the cortisol response to trauma reminders by subtracting T2 cortisol concentration from T3 (20 min after the trauma description) cortisol concentration, due to the ~20 min lag in time-to-peak for salivary cortisol (e.g., Dickerson and Kemeny, 2004). The distribution of sAA reactivity was positively skewed, therefore we used log-transformed sAA reactivity for the analyses. We first tested the baseline to trauma reminder differences to determine reactivity by analyte. To test the predictive utility of salivary analytes on MRI data we conducted bivariate correlations between the increase in saliva analytes and the a priori brain regions of interest. We also tested the relation between increases in cortisol and alpha-amylase and PTSD symptoms.
2.4.2. fMRI image data analyses
We conducted first-level analysis using a general linear model, in which the blood oxygen level-dependent (BOLD) response for each event was modeled using a SPM canonical hemodynamic response function. We used anatomically defined region of interest (ROI) analysis for functional data from the amygdala, dorsal anterior cingulate cortex (dACC), insula, and rostral middle frontal gyrus (rMFG). The ROIs were defined a priori based on the Desikan–Killiany atlas (Desikan et al., 2006) using an automated segmentation tool in Freesurfer. BOLD percent signal change was modeled for each condition: 6 factorial combinations of valence (negative, positive, neutral) and novelty (novel, familiar) vs baseline (fixation). We set the threshold at p < .001 for the rMFG mask, and p < .05 for the amygdala, dACC, and insula masks.
3. Results
3.1. Descriptive data
Participant characteristics are presented in Table 1. At Session 1 our sample (M = 23.5, SD = 7.2) was higher than the normative young adult mean (M = 14) for the Perceived Stress Scale, and our sample mean for State Anxiety (M = 46.5, SD = 13.3) was broadly consistent with higher state anxiety normatively. The Session 1 sample mean for the Beck Depression Inventory (M = 17.1, SD = 7.1) is consistent with a mild level of depressed mood. Session 2 means for state anxiety (M= 41.9, SD = 9.7) and depressed mood (M = 12.3, SD = 8.2) were lower than Session 1 means. The Session 1 to Session 2 difference in BDI score was driven by 3 participants whose Session 2 scores were drastically lower than Session 1 scores, although the higher Session 1 scores still were only in the moderate range. Excluding these participants’ data, the Session 1 BDI mean was 16.2 and the Session 2 mean was 13.4, which is more consistent with two measurements taken several days apart. The 3 participants’ PSS and STAI scores also were higher at Session 1 compared with Session 2, but they did not differ from the other participants on Session 1 or Session 2 scores or PTSD symptoms, so we retained their data.
Participants’ sAA and cortisol levels at each timepoint are presented in Table 2. Means for the peak magnitude of the BOLD response in the regions of interest by stimulus condition are presented in Table 3.
Table 2.
Alpha amylase and cortisol by collection timepoint.
| M (SD) | |||
|---|---|---|---|
| Hormone | T1 | T2 | T3 |
| Alpha Amylase in U/ml | 46.5 (34.5) | 76.8 (56.5)** | 75.1 (76.5)* |
| Cortisol in μg/dl | 0.33 (0.31) | 0.27 (0.24) | 0.25 (0.19) |
Note: SAA reactivity is indexed by the difference from T1 to T2 samples, due to the rapid increase in sAA concentrations in response to a stressor. Cortisol reactivity is indexed by the difference from T2 to T3 samples, due to the approximately 20 min delay for the increase in cortisol concentrations in response to a stressor. There was a significant increase in sAA from baseline to the time of trauma discussion, which was maintained at T3. The maintained increase in sAA at T3 reflects the content of the interview at that point; most participants still were responding to interview questions about trauma symptoms related to the index event. There was no increase in cortisol.
p < .05.
p < .01.
Table 3.
Peak BOLD magnitude by contrast category.
| % signal change M (SE)
|
||
|---|---|---|
| Right | Left | |
| Novel negative vs Fixation | ||
| Amygdala | .17 (.10) | .26 (.08) |
| Rostral middle frontal gyrus | .22 (.09) | .11 (.08) |
| Dorsal anterior cingulate cortex | .05 (.06) | .10 (.05) |
| Insula | .14 (.07) | .23 (.05) |
| Novel neutral vs Fixation | ||
| Amygdala | .20 (.07) | .26 (.07) |
| Rostral middle frontal gyrus | .26 (.09) | .16 (.07) |
| Dorsal anterior cingulate cortex | .09 (.05) | .08 (.06) |
| Insula | .03 (.05) | .15 (.05) |
| Novel positive vs Fixation | ||
| Amygdala | .23 (.06) | .24 (.07) |
| Rostral middle frontal gyrus | .19 (.09) | .11 (.08) |
| Dorsal anterior cingulate cortex | .09 (.05) | .09 (.06) |
| Insula | .03 (.05) | .15 (.05) |
| Familiar negative vs Fixation | ||
| Amygdala | −.01 (.12) | .07 (.11) |
| Rostral middle frontal gyrus | .02 (.08) | −.08 (.08) |
| Dorsal anterior cingulate cortex | −.03 (.04) | .01 (.05) |
| Insula | −.16 (.06) | .01 (.08) |
| Familiar neutral vs Fixation | ||
| Amygdala | .07 (.07) | .10 (.09) |
| Rostral middle frontal gyrus | −.00 (.11) | −.10 (.09) |
| Dorsal anterior cingulate cortex | −.01 (.06) | −.00 (.11) |
| Insula | −.18 (.06) | −.12 (.08) |
| Familiar positive vs Fixation | ||
| Amygdala | .02 (.14) | .06 (.11) |
| Rostral middle frontal gyrus | .00 (.07) | −.09 (.06) |
| Dorsal anterior cingulate cortex | −.03 (.04) | −.07 (.04) |
| Insula | −.19 (.04) | −.19 (.07) |
3.2. SAA and cortisol reactivity
The sAA increase from baseline (T1) to the time of trauma discussion (T2) was significant, t(16) = 3.57, p = .002. There was no significant association between sAA reactivity and total PTSD symptoms, r = .222, p = .347. When examined by PTSD symptom cluster, sAA reactivity was associated with hyperarousal symptoms at a non-significant trend level (r = .387, p = .092), but was not associated with re-experiencing (r = .116, p = .627) or avoidance symptoms (r = .327, p = .159).
The increase from cortisol baseline (T2) to cortisol reactivity (T3) was not significant, t(18) = 1.01, p = .332. Due to the absence of cortisol reactivity from baseline to trauma discussion, we excluded the cortisol data from further primary analyses.
3.3. SAA reactivity and the affective brain response
3.3.1. SAA and neural reactivity
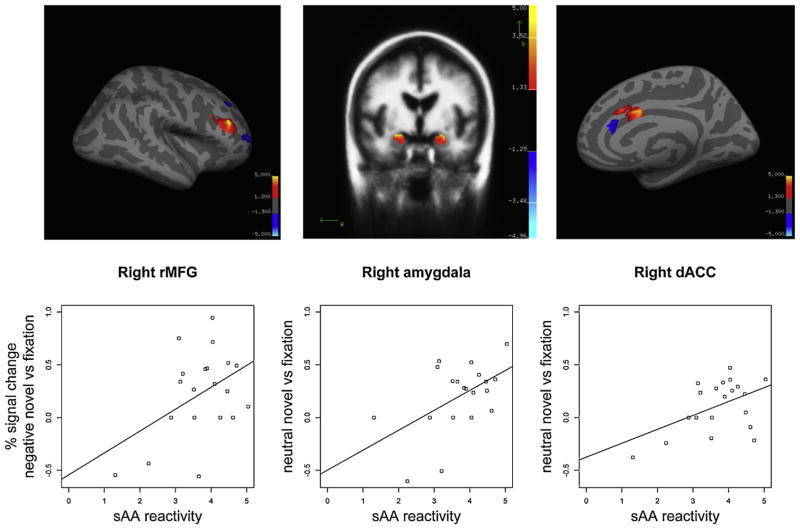
We conducted bivariate correlations to investigate relations between SAA reactivity to the trauma reminder and salience network activation to affective scenes (see Table 4). SAA reactivity was associated with the right rMFG response to novel negative images, r = .449, p = .047 (see Fig. 1). SAA reactivity was not associated with the amygdala (right: r = −.219, left: r = .042), dACC (right: r = .224, left: r = .152), or insula (right: r = .054, left: r = −.078) response to novel negative images, ps > .05.
Table 4.
Correlations between sAA reactivity and BOLD response in regions of the salience network.
| sAA reactivity
|
||
|---|---|---|
| Right | Left | |
| Novel negative vs Fixation | ||
| Amygdala | −.219 | .042 |
| Rostral middle frontal gyrus | .449* | .214 |
| Dorsal anterior cingulate cortex | .224 | .152 |
| Insula | .054 | −.078 |
| Novel neutral vs Fixation | ||
| Amygdala | .518* | .209 |
| Rostral middle frontal gyrus | .387 | .280 |
| Dorsal anterior cingulate cortex | .486* | .121 |
| Insula | .342 | .260 |
| Novel positive vs Fixation | ||
| Amygdala | .182 | .183 |
| Rostral middle frontal gyrus | .139 | .176 |
| Dorsal anterior cingulate cortex | .426 | .354 |
| Insula | .207 | .004 |
| Familiar negative vs Fixation | ||
| Amygdala | .431 | .088 |
| Rostral middle frontal gyrus | .128 | .147 |
| Dorsal anterior cingulate cortex | .153 | −.029 |
| Insula | .146 | .194 |
| Familiar neutral vs Fixation | ||
| Amygdala | .202 | .209 |
| Rostral middle frontal gyrus | .327 | .317 |
| Dorsal anterior cingulate cortex | .220 | .345 |
| Insula | .373 | .385 |
| Familiar positive vs Fixation | ||
| Amygdala | −.073 | −.066 |
| Rostral middle frontal gyrus | .120 | .120 |
| Dorsal anterior cingulate cortex | .123 | .043 |
| Insula | .271 | .184 |
p < .05.
Fig. 1.
Salivary alpha amylase reactivity to the trauma reminder during the interview was associated with reactivity to negative novel scenes in the right rMFG (left panel), and with vigilance indexed by response to neutral novel information in the right amygdala and right dACC (right two panels).
3.3.2. SAA and neural hypervigilance
In addition, sAA reactivity was associated with the right amygdala response (r = .518, p = .019) and with the right dACC response (r = .486, p = .030) to novel neutral images. In addition, an exploratory analysis revealed that sAA reactivity as measured by T3 (20 min post-stressor) minus T1 was also associated with the right amygdala response to novel neutral images (r = .509, p = .022). SAA reactivity was not associated with activation to novel neutral images in the rMFG (right: r = .387, left: r = .280), insula (right: r = .342, left: r = .260), left amgydala (r = .209), and left dACC (r = .280), ps > .05.
SAA reactivity was not related to the neural response to novel positive or familiar images in bilateral amygdala, dACC, rMFG, and insula, ps > .05.
3.4. Cortisol reactivity and the affective brain response
Although there was no increase in cortisol in response to the stressor at the group level, we conducted an exploratory analysis of the association between cortisol reactivity and BOLD responses to the affective stimuli. There was one significant correlation: lower cortisol reactivity to the trauma reminder was associated with greater activity in the right middle frontal gyrus in response to familiar negative images (r = −.458, p = .049). There were no other significant associations.
3.5. PTSD symptoms and the affective brain response
The number of PTSD symptoms was associated with the right rMFG response to novel negative images, (r = .469, p = .037). Follow-up analyses showed that the right rMFG response to novel negative images was associated with the number of re-experiencing symptoms (r = .511, p = .021) and avoidance symptoms (r = .502, p = .024), as well as hyperarousal symptoms at a trend level (r = .433, p = .056). The number of PTSD symptoms also was negatively correlated with the right amygdala response to familiar positive images, r = −.477, p = .033. There was no relation between the number of PTSD symptoms and neural response in bilateral dACC and insula, ps > .05.
4. Discussion
Consistent with prior evidence of blunted HPA-axis reactivity in people with a history of trauma exposure, we did not observe cortisol reactivity in response to our naturalistic stressor (i.e., self report of traumatic event during a clinical interview) at the group level. An exploratory analysis showed that lower cortisol reactivity was associated only with greater middle frontal activation to familiar negative images, which suggests that people with more blunted cortisol might also be more likely to continue to process negative content as salient, even when it has been seen before. On the other hand, there was marked SNS reactivity to the stressor. Taken together, these data are consistent with the evidence for the influence of trauma history profiles and also a potential differential effect of trauma on the HPA axis and the sympathetic nervous system (e.g., Gordis et al., 2008; Klaassens et al., 2009). Whereas SNS reactivity persists over time following trauma exposure, HPA activity becomes blunted to protect the body from the risk of long-term immunosuppression by excessive cortisol production (e.g., Cohen et al., 2012). HPA blunting might have the additional effect of failing to inhibit SNS reactivity; because the HPA axis normally down-regulates the SNS, less cortisol might lead to sAA hyperactivity (e.g., Fries et al., 2005). This pattern of reactivity suggests that (a) SNS reactivity is not subject to the blunting observed in the cortisol response in some trauma survivors, and (b) SNS reactivity might be a more reliable marker of trauma-related symptoms. At first glance, these results appear to be inconsistent with prior work showing no increase in sAA in response to a graphic film in a sample that included trauma-exposed adults (Chou et al., 2014). However, the unpleasant film content was by design not trauma-relevant, and one third of that sample had no trauma exposure, whereas our sample were all trauma-exposed and the trauma reminder was specific to participants’ trauma experiences. The differences in these results might reflect trauma-specificity in the magnitude of sAA reactivity, whereby self-relevant, trauma-relevant information provokes greater SNS response than unpleasant, but not personally-relevant information.
Both of our hypotheses regarding sAA as a marker for reactivity to actual threat and hypervigilance for potential threat were supported. SAA reactivity was associated with activation in the salience network and in particular in the right rostral middle frontal gyrus for novel negative scenes, supporting the potential for sAA as a marker of reactivity to actual threat. Although neural reactivity to threat-relevant or negative information is implicated in trauma (e.g., Shin et al., 2007), it is not specific to trauma, so we might expect to observe a similar relation in other stress-related conditions such as normative state anxiety. In addition, although peak magnitudes of amygdala activation across all three novel categories were higher than peak magnitudes for all three novel categories in a normative sample (e.g., Weierich et al., 2010), there was no association between sAA and amygdala activation in response to novel negative (non-trauma) information. This suggests that sAA reactivity to trauma reminders might have greater specificity as a potential marker for the hypervigilance in the absence of threat that is a signature characteristic of trauma exposure (rather than just generally unpleasant information).
Supporting our second hypothesis, sAA reactivity was also associated with activation in the right amygdala and the right dorsal anterior cingulate cortex in response to neutral novel scenes. Further, in the amygdala this association persisted twenty minutes post-stressor. Such neural hypervigilance in the absence of threat is more specific to trauma exposure, and this result highlights the promise of sAA as a potentially specific biomarker. The biology further supports the strength of this relation, as well as the dissociation between sAA and cortisol as potential markers. There are strong bidirectional projections and functional connectivity between central sympathetic areas (e.g., medulla, locus coeruleus) and the amygdala/cingulate/PFC circuit, and norepinephrine (NE) in particular operates in feed-forward projections from the former to the latter. Although the literature is far from clear, with most studies demonstrating only a strong association between NE and sAA (e.g., Ditzen et al., 2014; Thoma et al., 2012), recent evidence also shows that NE increases are capable of inducing increases in sAA secretion, even during alpha-adrenergic blockade (Kuebler et al., 2014). Future work will be necessary to determine whether the demonstrated capability is in fact the mechanism, and such data would provide additional support for potential pathways that might underlie the observed relation between sAA and neural activation in the salience network.
The lateralization of our results was unexpected. Sympathetic stress reactivity specifically predicted salience network activity in the right but not the left hemisphere. This is consistent with a growing body of evidence suggesting potential hemispheric asymmetry in sensitivity to threat (e.g., Gläscher and Adolphs, 2003). For example, the salience network in the right vs left hemisphere might be more active during rapid threat detection and processing of negative affect (e.g., Shackman et al., 2009; Yoshimura et al., 2009). In addition, stress is associated with right amygdala hyperactivity to affectively ambiguous stimuli (Somerville et al., 2004) and negative stimuli (e.g., Dannlowski et al., 2012). Consistent with these findings, our data indicate the specificity of the right-lateralized salience network overactivity to potential threat.
There are several potential limitations to our study. We did not assess waking time, so although all interview visits began at 1000 h, we were not able to control for variability in individual participants’ time since waking. This limitation is unlikely to reflect a confound in the current data for two reasons. First, we measured differences across relatively short timeframes (e.g., 20 min) along each person’s own diurnal slope. In comparing changes across these time periods with the normal diurnal patterns of cortisol and sAA (Nater et al., 2007), it is clear that the observed increases in sAA and absence of increases in cortisol were not attributable to the normative decreases in cortisol or increases in sAA across those timeframes. To the contrary, the observed sAA increase was nearly 3 times the normative increase during a 30 min period, and the slight but not significant cortisol decrease is consistent with the normative decrease. Second, given the distance of participants’ homes from the lab, and transit times in a very large city, no participant arrived at the lab within one hour of waking. It was thus unlikely that we accidentally captured any participant’s awakening response, ruling out the potential influence of the sharp increase in cortisol and decrease in sAA on waking. In addition, although we did not specifically assess smoking status, the base rate of smoking in the recruitment population is low (9% per campus public health survey in 2008). The potential effect of a cigarette on sAA reactivity (a decrease in sAA, and therefore not a confound for the current data; Nater et al., 2007) has been shown to disappear one hour after the cigarette (Zappacosta et al., 2002). Smoking has been associated with reduced cortisol reactivity during abstinent periods (e.g., Ginty et al., 2014), although the likely base rate in the sample is not large enough to confound the group results.
We also did not assess specific time since trauma, so we were not able to control for any influence of elapsed time on reactivity to the trauma reminder, or to neural reactivity or hypervigilance in the scanner. However, our data nonetheless represent the relation between current reactivity to a naturalistic reminder and measured neural hypervigilance, which is aligned with the timeframe of the potential use of sAA as a predictive biomarker in clinical settings. People seek therapy at widely varying amounts of elapsed time since the traumatic event, and our data suggest that sAA during clinical interview might provide a solid marker of current potentially maladaptive hypervigilance. In addition, our sample was comprised of trauma-exposed women who varied along the continuum of trauma-related symptoms, and only three met criteria for a diagnosis of PTSD. Our results therefore might not generalize to people with PTSD, although in accord with a dimensional perspective on psychopathology, we suggest that the pattern of results could be even more robust in a more severe sample. Further research should investigate the relation between sAA reactivity to trauma reminders and hypervigilance in a clinical sample. It is also possible that sAA responses to any personalized stressors, including non-trauma-related stressors, could predict neural hypervigilance for novel, neutral information in trauma-exposed or non-trauma exposed people, and further work should examine this possibility. Finally, although our recruitment of only women allowed us to control for potentially confounding sex differences in affective systems, it also limited the generalizability of our results. For example, the blunting of the HPA axis is more often reported in studies on trauma-exposed women than men (e.g., Meewisse et al., 2007). In addition, potential sex differences in types of trauma, perceived controllability, and coping abilities might contribute to differences in the impact of trauma on endocrine and neural systems (e.g., Olff et al., 2007). Future studies not only should test sex differences, but also control for oral contraceptive use and sex-related individual differences including menstrual phase, that could have blunted the current results. A larger sample will facilitate both replication and testing of these potential covariates.
This study has three major strengths. First, we uniquely and explicitly measured both neural reactivity to actual threat (i.e., reactivity to negative images) and neural hypervigilance for potential threat (i.e., reactivity in the absence of threat-relevant or negative information). Although both are relevant trauma-related phenomena, we suggest that the latter more closely represents the tonic heightened state of vigilance in trauma-exposed people, and also is more specific to trauma-related pathology. In turn, this heightened state of vigilance can predispose the person to excessive reactivity if and when an actual threat stimulus appears. Second, we demonstrated that salivary sAA might be a reliable marker of trauma-related hypervigilance, as indexed by brain activation in the absence of threat. On a practical level, although sAA reactivity to reporting of a traumatic event would not constitute a sufficient diagnostic tool on its own, this non-invasive and relatively inexpensive adjunct to initial assessment could enhance diagnostic precision by providing an index of the degree to which a particular person might be hypervigilant, and potentially maladaptively so, in the world. The primary motivation for this investigation was to test whether SNS reactivity to reminders of a person’s trauma, as measured with simple saliva samples taken, for example, during a standard psychological intake interview in the clinic, might provide a more objective marker of the degree to which a client is hypervigilant in other settings, such as walking down the street. Similarly, sAA might serve as a treatment outcome marker indexing improvement in hypervigilance over time. Third, we also intentionally utilized a relatively mild and yet naturalistic stressor; we used each participant’s self-report of the traumatic event during the clinical interview as a closer analogue to the trauma reminders people actually experience in the world. Whereas many prior studies of responses to trauma reminders have leveraged extreme representations of the traumatic events (e.g., detailed script-driven imagery, graphic videos or photographic images) to induce and measure reactivity, in daily life the reminders are likely to be more subtle. Thus our endocrine reactivity results, although smaller in magnitude than some studies, might more closely approximate the actual experience of reactivity in the world. Similarly, our affective scene stimuli presented during the fMRI session varied along the dimensions of arousal and valence, but did not represent valence extremes such as those of other image sets (e.g., mutilated bodies, highly erotic images). Because we were more interested in the actual daily experience of trauma-exposed people in the world, we used images that were more consistent with the valence and arousal levels of most visual arrays encountered in daily life.
The normative stress response is part of a properly functioning human system, and when experienced at moderate levels (i.e., lesser frequency and/or lower magnitude) it promotes healthy allostasis (e.g., McEwen, 2012). For example, the systemic response to moderate short-term stress can enhance immune function (e.g., Dhabhar, 2014), facilitate cognitive performance (e.g., Beste et al., 2013; Kofman et al., 2006), and promote resilience to future stressors (e.g., Seery et al., 2010). In response to a more extreme stressor such as a traumatic event, most people experience physiological arousal and perceptions of the context or environment as unsafe immediately following the event, and temporarily heightened reactivity and vigilance are part of the normative and adaptive response. The function of the stress response in such a situation is to prepare the system to respond to actual threat, and to enhance the person’s alertness for potential environmental threat. In most people this reaction subsides within a reasonable period of time. However, for some people the stress response to a traumatic event is disproportionate in magnitude, duration, and overgeneralization to other contexts and stimuli. Over time the cycle can become even more insidious, as the negative cognitive appraisals initially formed to interpret the actual threat event (a) can themselves increase the neuroendocrine stress response (e.g., Olff et al., 2005a), (b) are generalized to objectively safe situations or stimuli (Olff et al., 2005b), (c) become more automatic, and (d) eventually can contribute to impaired interpersonal functioning, such as difficulties with healthy relationship formation. The results of the current study might reflect such an overlearned, habitual hypervigilance in the absence of threat.
5. Conclusions
We tested the utility of salivary sAA and cortisol in predicting maladaptive affective processing following trauma exposure. In response to a trauma reminder, sAA increased from the baseline, whereas cortisol reactivity was blunted. SAA reactivity to the trauma reminder predicted both heightened neural reactivity to actual threat, and also neural hypervigilance in the absence of threat. Our results suggest that sAA could be an effective and cost-efficient biomarker for vigilant affective processing, which at the extreme could be maladaptive, following trauma.
Acknowledgments
Funding
This study was funded by National Institutes of Health NCRR G12RR003037-25S3 (ARRA supplement to MRW), NIMHD MD007599 (seed grant funding to MRW), and NIDA DA012136 (pilot grant funding to MRW).
The authors thank Jenna Rieder and Jojo Borja for technical assistance.
Footnotes
The task difference was due to experimenter error. Following Lieberman et al. (2007), who showed that rating affect reduces amygdala activation, we tested potential group differences. Participants who rated arousal displayed greater left dACC and insula response to positive images and right dACC response to novel negative images compared to participants who indicated indoor/outdoor, ps < .05. Participants did not differ in neural activation in amygdala and rMFG by task type, ps > 05. There were no differences in behavioral indices such as reaction time, and when we entered task as a covariate in our planned analyses, the results did not change. We therefore report analyses without task as a covariate.
The sponsor had no involvement in study design, data collection, analysis, and interpretation, article preparation, or the decision to submit the article for publication.
Conflict of interest
Authors have no conflicts of interest.
Contributors
Seungyeon A. Yoon and Mariann R. Weierich were both involved in study design, data collection, data analysis, and article preparation. Both authors reviewed and approved the final manuscript and its submission to Psychoneuroendocrinology.
References
- Baum BJ. Principles of salivary secretion. Ann N Y Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of beck depression inventories-IA and-II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007;99:642–649. [PMC free article] [PubMed] [Google Scholar]
- Beste C, Yildiz A, Meissner TW, Wolf OT. Stress improves task processing efficiency in dual-tasks. Beh Brain Res. 2013;252:260–265. doi: 10.1016/j.bbr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Veerman EC, Hoogstraten J, Nieuw Amerongen AV. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med. 2003;65:245–258. doi: 10.1097/01.psy.0000058376.50240.2d. [DOI] [PubMed] [Google Scholar]
- Bremner J, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer a, Afzal N, McGlashan T, Elzinga B, Anderson G, Heninger G, Southwick S, Charney D. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Brunetti M, Sepede G, Mingoia G, Catani C, Ferretti A, Merla A, Del Gratta C, Romani GL, Babiloni C. Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: neural correlates of generalized emotional response. Neuroscience. 2010;168:670–679. doi: 10.1016/j.neuroscience.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Bryant R, Felmingham K, Kemp A, Barton M, Peduto A, Rennie C, Gordon E, Williams LM. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Chou CY, La Marca R, Steptoe A, Brewin CR. Biological responses to trauma and the development of intrusive memories: an analog study with the trauma film paradigm. Biol Psychol. 2014;103:135–143. doi: 10.1016/j.biopsycho.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:386–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Sage; Newbury Park, CA: 1988. [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Moradi AR, Taghavi MR, Neshat-Doost HT, Yule W. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol Med. 2001;31:541–547. doi: 10.1017/s0033291701003567. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Ehlert U, Nater UM. Associations between salivary alpha-amylase and catecholamines—A multilevel modeling approach. Biol Psychol. 2014;103:15–18. doi: 10.1016/j.biopsycho.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Jones A, Carroll D, Roseboom TJ, Phillips AC, Painter R, de Rooij SR. Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology. 2014;48:87–97. doi: 10.1016/j.psyneuen.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis E, Granger D, Susman E, Trickett P. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Delmer O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:889–894. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kofman O, Meiran N, Greenberg E, Balas M, Cohen H. Enhanced performance on executive function associated with examination stress: evidence from task-switching and Stroop paradigms. Cognit Emot. 2006;20:577–595. [Google Scholar]
- Kuebler U, von Kanel R, Heimgartner N, Zuccarella-Hackl C, Stirnimann G, Ehlert U, Wirtz PH. Norepinephrine infusion with and without alpha-adrenergic blockade by phentolamine increases salivary alpha amylase in healthy men. Psychoneuroendocrinology. 2014;49:290–298. doi: 10.1016/j.psyneuen.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psych Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. PNAS. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L, Lang P, Laplante M, Cuthbert B, Shumen J, Bradley M. Aversive imagery in posttraumatic stress disorder: trauma recurrence, commorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen HU, Kirschbaum C. The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int J Psychophysiol. 2011;79:118–126. doi: 10.1016/j.ijpsycho.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BPR. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Biobehav Rev. 2005a;29:457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BPR. The psychobiology of PTSD: coping with trauma. Psychoneuroendocrinology. 2005b;30:974–982. doi: 10.1016/j.psyneuen.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Wright C, Shin L, Kagan J, Rauch S. Inhibited and uninhibited infants grown up: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Seery MD, Holman EA, Silver RC. Whatever does not kill us: cumulative lifetime adversity, vulnerability, and resilience. J Pers Soc Psychol. 2010;99:1025–1041. doi: 10.1037/a0021344. [DOI] [PubMed] [Google Scholar]
- Shackman A, McMenamin B, Maxwell JS, Greischar LL, Richard D, Davidson J. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychol Sci. 2009;20:1500–1506. doi: 10.1111/j.1467-9280.2009.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L, Bush G, Whalen P, Handwerger K, Cannistraro PA, Wright CI, Martis B, Macklin ML, Lasko NB, Orr SP, Pitman RK, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20:701–712. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, posttraumatic stress disorder, and healthy volunteers. Biol Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Feinstein JS, Hitchcock C, Paulus MP, Stein MB. Anxiety vulnerability is associated with altered anterior cingulate response to an affective appraisal task. Neuroreport. 2008;19:1033–1037. doi: 10.1097/WNR.0b013e328305b722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WHR. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Poon L, Papadopoulos AS, Kumari V, Cleare AJ. Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology. 2014;50:289–299. doi: 10.1016/j.psyneuen.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol. 2012;91:342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, Price LH, Carpenter LL. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology. 2006;31:1036–1045. doi: 10.1016/j.psyneuen.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marle HJF, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Vigil JM, Geary DC, Granger D, Flinn M. Sex differences in salivary cortisol, alpha-amylase, and psychological functioning following Hurricane Katrina. Child Dev. 2010;81:1228–1240. doi: 10.1111/j.1467-8624.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast V, Hughes BL, Davidson ML, Lindquist M, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF. Novelty as a dimension in the affective brain. Neuroimage. 2010;49:1–20. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903:117–127. doi: 10.1016/s0006-8993(01)02453-2. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant R. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Bischoff-Grethe A, Frank GKW, Arce E, Paulus MP. Increased amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neurosci Lett. 2007;428:109–114. doi: 10.1016/j.neulet.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cognit. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Zappacosta B, Persichilli S, Mordente A, Minucci A, Lazzaro D, Meucci E, Giardina B. Inhibition of salivary enzymes by cigarette smoke and the protective role of glutathione. Hum Exp Toxicol. 2002;21:7–11. doi: 10.1191/0960327102ht202oa. [DOI] [PubMed] [Google Scholar]