Abstract
Background
β-trace protein (BTP) and β2-microglobulin (B2M) are novel glomerular filtration markers that have stronger associations with adverse outcomes than creatinine. Comparisons of BTP and B2M to creatinine and cystatin C are limited by the absence of rigorously developed GFR estimating equations for the novel markers.
Study Design
Study of diagnostic test accuracy.
Setting & Participants
Pooled database of three populations with chronic kidney disease (CKD) with mean measured GFR of 48 ml/min/1.73m2 (N=3551; MDRD [Modification of Diet in Renal Disease] Study, AASK [African American Study of Kidney Disease and Hypertension], and CRIC [Chronic Renal Insufficiency Cohort] Study).
Index Tests
GFR estimated using creatinine, cystatin C, BTP or B2M
Reference Test
GFR measured as the urinary clearance of iothalamate.
Results
For BTP and B2M, coefficients for age, sex and race were smaller than for creatinine, and were similar or smaller than for cystatin C. For B2M, coefficients for sex, age and race were smaller than for creatinine, and were similar (age and race) or smaller (sex) than for cystatin C. The final equations with BTP (BTP, age and sex) or B2M (B2M alone) were less accurate than either the CKD-EPI (CKD Epidemiology Collaboration) creatinine or cystatin C equations. The combined BTP-B2M equation (BTP and B2M alone) had similar accuracy to the CKD-EPI creatinine or cystatin C equation. The average of the BTP-B2M equation and the CKD-EPI creatinine-cystatin C equation was not more accurate than the CKD-EPI creatinine-cystatin C equation.
Limitations
No external validation population, study population was restricted to CKD, few participants older than 65 years or non-black, non-white race.
Conclusions
BTP and B2M are less influenced by age, sex and race than creatinine and less influenced by race than cystatin C, but provide less accurate GFR estimates than the CKD-EPI creatinine and cystatin C equations. The CKD-EPI BTP and B2M equation provides a methodological advance for their study as filtration markers and in their associations with risk and adverse outcomes, but further study is required before clinical use.
Keywords: Beta-trace protein (BTP), beta-2-microglobulin (B2M), filtration marker, chronic kidney disease (CKD), estimated glomerular filtration rate (eGFR), measured GFR, estimating equation, kidney function, diagnostic accuracy
In adult medical care, clinical assessment of kidney function is routine.1 Clinical laboratories now usually report an estimated glomerular filtration rate (eGFR) when serum creatinine is measured.2 Estimates of glomerular filtration rate (GFR) are more accurate and more useful than the serum concentrations of filtration markers alone because they take into account clinical and demographic factors that are associated with their non-GFR determinants and are expressed on the “GFR scale.” Adding cystatin C to creatinine improves the accuracy of eGFR for assessment of GFR compared to eGFR using either marker alone3–5. Use of cystatin C in combination with creatinine strengthens the association of decreased eGFR with subsequent risk of cardiovascular disease, death and other outcomes6. However, even with the use of both of these established markers, the accuracy of eGFR vs measured GFR (mGFR) and its use for clinical decision making such as drug dosing and for prediction of adverse outcomes remain suboptimal. A current area of emphasis is the evaluation of novel filtration markers to improve estimation of mGFR and prognosis.
β-trace protein (BTP) and β2-microglobulin (B2M) are novel endogenous filtration markers. A 168 amino acid glycoprotein enzyme produced in the central nervous system, BTP promotes the conversion of prostaglandin H2 to prostaglandin D2 7,8; B2M is a 100 amino acid protein component of class I major histocompatibility molecules and is found on the surface of nucleated cells9. Similar to cystatin C, both are low molecular weight serum proteins that are filtered by the glomerulus and retained in the blood as GFR declines. Each has been shown to have stronger associations with death, cardiovascular disease or kidney disease outcomes compared to eGFR from creatinine10–18. However, comparisons of the utility of BTP and B2M to serum creatinine and cystatin C as measures of kidney function and as prognostic factors for adverse outcomes and complications of chronic kidney disease (CKD) are limited by the absence of rigorously developed GFR estimating equations using these novel markers.
We developed GFR estimating equations using BTP and B2M, alone and in combination, from participants of two clinical trials (the MDRD [Modification of Diet in Renal Disease] Study and AASK [African American Study of Kidney Disease and Hypertension]) and an observational study of CKD (the CRIC [Chronic Renal Insufficiency Cohort] Study)4,19,20. We compared these equations with the CKD-EPI creatinine and cystatin C-based equations in these study participants for bias, precision and accuracy.
Methods
Data Sources
The CKD-EPI (CKD Epidemiology Collaboration) is a research group formed to develop and validate improved estimating equations for GFR by pooling data from research studies and clinical populations. We combined individual-level patient data from the MDRD Study, AASK, and the CRIC Study4,19,20. The GFR was measured in each of these studies using urinary clearance of iothalamate4,19,20. The institutional review boards of all participating institutions approved the original studies.
Laboratory Methods
All measurements were performed in serum samples thawed after being stored at −80°C. We measured serum cystatin C by the Siemens particle-enhanced immunonephelometric assay (PENIA) and a BN II nephelometer at the Cleveland Clinic Research Laboratory for the MDRD Study and AASK (coefficient of variation [CV], 3.2%)21 and at the University of Pennsylvania for the CRIC study (CV, 4.9%)4. Cystatin C values were adjusted so that they could be traceable to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group for the Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements (IRMM) certified reference materials3,22,23. Creatinine, BTP and B2M were measured at the University of Minnesota in 2012–2013. We measured serum creatinine by the Roche enzymatic method (Roche-Hitachi P-Module instrument with Roche Creatininase Plus assay, Hoffman-La Roche, Ltd., Basel, Switzerland), traceable to the National Institute of Standards and Technology (NIST) creatinine standard reference material 967 (CV, 1.71%)24. We measured BTP on the Siemens Dade Behring Nephelometer (CV, 5.36%). B2M was measured on the Siemens Dade Behring Nephelometer (CV, 3.09%) for CRIC and on the Roche Modular P (CV, 3.2%) for the MDRD Study and AASK. We performed comparison studies to show the equivalence of the two assays and their stability over time.
Metrics for Equation Performance
We assessed bias as the median difference between mGFR and eGFR, and precision as the interquartile range (IQR) for the differences24,25. We assessed accuracy as root mean squared error and as the percentage of estimates greater than 30% different from mGFR (1 − P30). Confidence intervals were calculated by bootstrap methods (2000 bootstraps)26. Significance of the differences among equations was determined using McNemar’s test for 1 − P30. We also compared the newly developed equations for their ability to predict mGFR thresholds of < 60, <45 and < 30 ml/min/1.73 m2. The 95% confidence intervals for the area under the receiver operating characteristic curve and comparison between the receiver operating characteristic curves were performed using the method of DeLong, DeLong, and Clarke-Pearson27
Development and Validation of Equations
We developed estimation equations for GFR based on BTP and B2M, alone and in combination with each other. We limited our study variables to age, sex and race (black vs. non-black) because they are included in the existing CKD-EPI equations. We did not develop new equations using creatinine and cystatin C because the existing CKD-EPI equations were developed in these studies and perform well3,24. If GFR estimates from BTP or B2M prove to be useful, they could be averaged with estimates from the existing CKD-EPI equations3,24. However, for comparison of the magnitude of coefficients for filtration markers, age, sex and race across all of the markers, we included equations with these filtration markers. In preliminary analyses we verified that the existing CKD-EPI equations performed similarly to equations using the same variables developed in this population (Table S1, available as online supplementary material).
We pre-specified a process we used previously to develop and validate equations to estimate GFR3,24. Briefly, we randomly divided the study population in each study into two subpopulations: one for equation development (two-thirds of the participants) and the other for internal validation (the remaining one-third of the participants), and then pooled the data from each study. We first assessed correlation coefficients of the log of each marker to each other and to the log of measured GFR. In the development dataset, we used least squares linear regression to relate log-transformed mGFR to log BTP and/or log B2M, age, sex and race. We tested for nonlinearity with the use of nonparametric smoothing splines to characterize the shape of the relationship of log mGFR with log of the markers, and none was seen. Age, sex or race were included if they were significant at a p-value of <0.01 in the overall development dataset or within each study. We compared the magnitude and direction of the coefficients across studies. We evaluated the performance in the overall development population and in subgroups defined by age, sex, race and study and compared models with age, sex or race to models that included only the filtration markers. We brought forward models into internal validation for verification of the statistical significance of predictor variables if more complicated models demonstrated lower absolute bias by greater than 2 ml/min/1.73 m2 and reduced root mean squared error by greater than 3% in either the total population or in the subgroups. In other studies, we had used larger changes to select models for internal validation. Here, we elected to use less stringent criteria in order to avoid overlooking small but potentially important improvements due to these novel markers3,24.
In the internal validation dataset, we compared the new equations to each other, the existing CKD-EPI creatinine and cystatin C equations, and the average of the combined BTP-B2M equation with the CKD-EPI creatinine-cystatin C equation. We also compared performance of equations in the overall dataset and in the subgroups defined by eGFR (<30, 30–59, ≥60 mL/min/1.73 m2), age (<40, 40–65, and >65 years), sex (male vs. female), diabetes status (yes vs. no), body mass index (<20, 20–24, 25–29 and ≥30 kg/m2), and in the three studies. Diabetes was defined as either fasting serum glucose >126 mg/dL or hemoglobin A1c greater than 6.5, diagnosis of diabetes by self- report, taking insulin injections or tablets for diabetes, or following a special diet for diabetes in past five years. Finally, the development and internal validation datasets were combined into one dataset to derive final regression coefficients which are presented in the paper.
Performance in subgroups was compared using median difference, since bias within subgroups is a cause of imprecision and inaccuracy overall. Analyses were performed using R (Version 3.1.0)28 and SAS (Version 9.4, SAS Institute Inc, Cary, NC).
Results
Clinical Characteristics
In the development population, mean ±standard deviation of mGFR was 47.7 ± 21.8 (range, 6–168) mL/min/1.73 m2 (Table 1). The mean age was 54.0 ±11.7 (range, 19–75) years and 54.5% were Black. Clinical characteristics were similar in the development and internal validation datasets (Table 1). Clinical characteristics of the participants in each study are shown in Table S2.
Table 1.
Clinical characteristics of development and internal validation study population
| Total (N = 3551) |
Development (n = 2380; 67.0%) |
Internal Validation (n = 1171; 33.0%) |
p | |
|---|---|---|---|---|
| Participants | ||||
| MDRD | 800 (23%) | 536 (23%) | 264 (23%) | 0.9 |
| AASK | 1364 (38%) | 914 (39%) | 450 (39%) | |
| CRIC | 1387 (39%) | 930 (39%) | 457 (39%) | |
| Age, y | 54.0 ± 11.7 | 54.3 ± 11.7 | 53.4 ± 11.8 | 0.03 |
| Male sex | 2146 (60%) | 1427 (60%) | 719 (61%) | 0.4 |
| Black race | 1936 (55%) | 1308 (55%) | 628 (54%) | 0.5 |
| Nonwhite, nonblack race | 294 (8%) | 198 (8%) | 96 (8%) | 0.7 |
| Body mass index, kg/m2 | 30.1 ± 6.5 | 30.1 ± 6.4 | 30.2 ± 6.7 | 0.6 |
| Diabetes | 784 (22%) | 511 (21%) | 273 (23%) | 0.2 |
| Current smoking | 618 (17 %) | 412 (17 %) | 206 (18%) | 0.8 |
| mGFR, ml/min/1.73 m2 | 47.7 ± 21.8 | 47.5 ± 21.7 | 48.1 ± 21.9 | 0.4 |
| Creatinine, mg/dl | 1.9 ± 0.9 | 1.9 ± 0.9 | 1.9 ± 0.9 | 0.9 |
| Cystatin C, mg/L | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.6 ± 0.6 | 0.4 |
| BTP, mg/L | 1.3 ± 0.8 | 1.3 ± 0.7 | 1.3 ± 1.0 | 0.8 |
| B2M, mg/L | 4.3 ± 2.5 | 4.3 ± 2.4 | 4.3 ± 2.5 | 0.9 |
B2M, β2-microgobulin; BTP, β-trace protein; MDRD, Modification of Diet in Renal Diseases. AASK, African American Study of Kidney Disease and Hypertension, CRIC, Chronic Renal Insufficiency Cohort; mGFR, measured glomerular filtration rate
Note: Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation. Conversion factor creatinine in mg/dl to µmol/L, × 88.4
Equation Development
All filtration markers were negatively correlated with mGFR, with Pearson correlations (ρ) ranging from −0.804 to −0.878, and positively correlated with each other, with ρ ranging from 0.749 to 0.904, with the highest correlation between cystatin C and B2M (ρ =0.904) (Table S3). For the purposes of comparing the magnitude of the age, sex and race coefficients across markers, we developed equations for each marker and both markers combined and with and without age, sex and race. In general, the coefficients for BTP and B2M in the equations were smaller (closer to zero) than the coefficients for creatinine and cystatin C (Table S4). For BTP equations, coefficients for age and sex were all significant, but were smaller than in equations with creatinine, and were similar (age and sex) or smaller (race) than in equations with cystatin C (Box 1). However, equations that included age and sex reduced bias in men and in people younger than 40 years, and reduced root mean squared error in women and people older than 65 years (Table S5). Including race did not improve performance. Thus the final equation included BTP, age and sex. For B2M equations, coefficients for sex, age and race were significant but were but were smaller than in equations with creatinine, and were similar (age and race) or smaller (sex) than in equations with cystatin C (Box 1). However B2M equations that included age, sex or race did not substantially improve performance overall or in subgroups compared to equations that used the marker alone (Table S5), and thus the final equation included just B2M alone (Box 1). For equations that used both BTP and B2M, age and sex coefficients were significant but did not substantially improve performance overall or in subgroups, and thus the combined BTP-B2M equation included just the filtration markers (Box 1).
Box 1. CKD-EPI equations for GFR estimation from BTP, B2M and the combination.
| BTP | GFR = 55 × BTP−0.695 × 0.998age × 0.899 if female |
| B2M | GFR = 133 × B2M−0.852 |
| BTP-B2M | GFR = 96 × BTP−0.278 × B2M−0.588 |
Note: Coefficients are derived from the combined development and internal validation datasets B2M, β2-microgobulin; BTP, β-trace protein; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate
Internal Validation
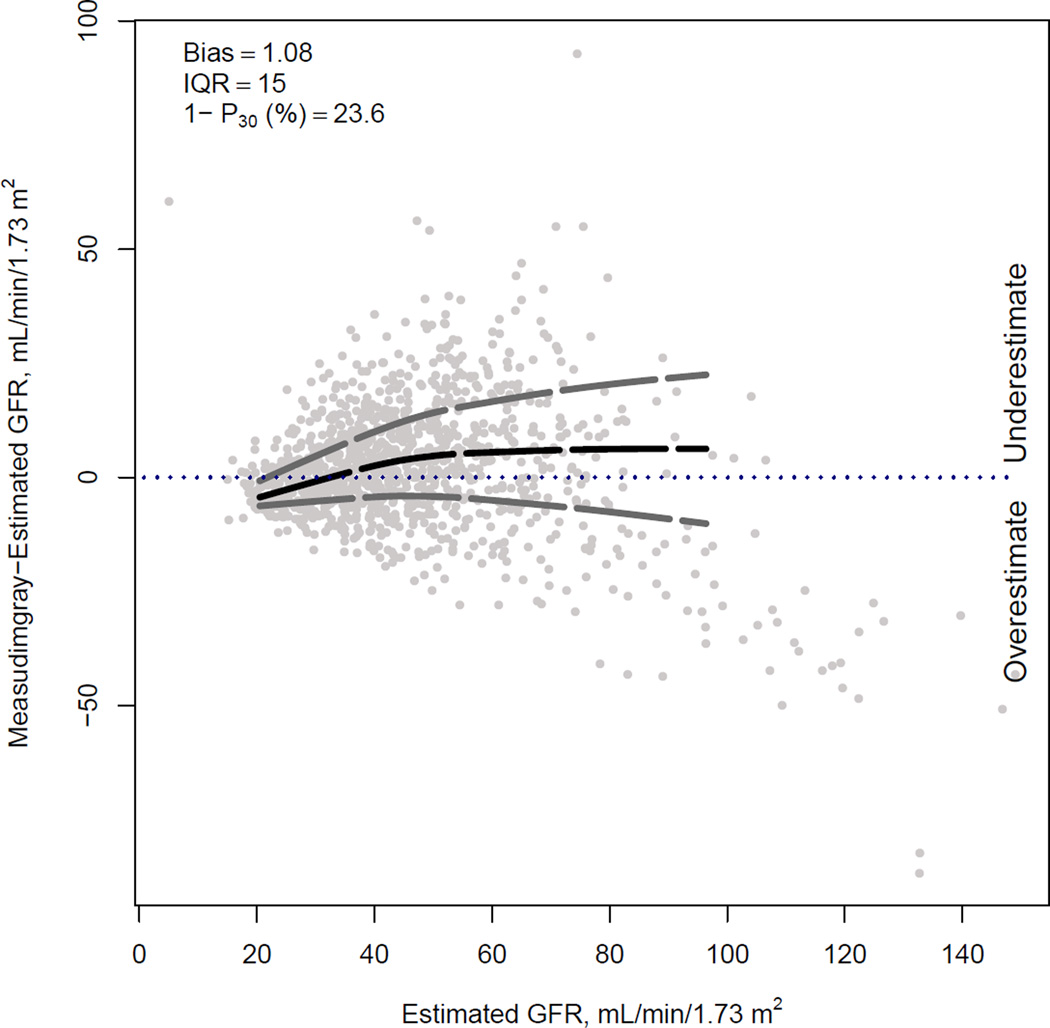
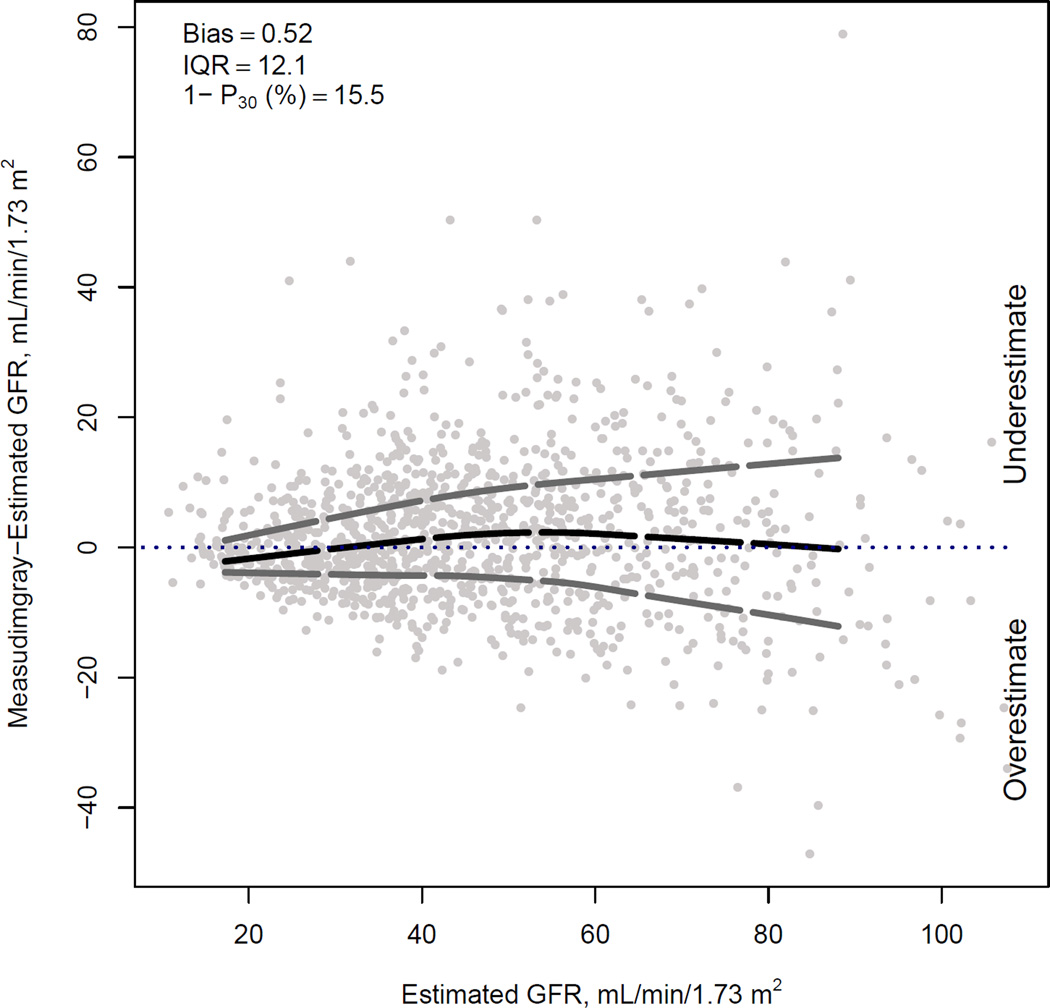
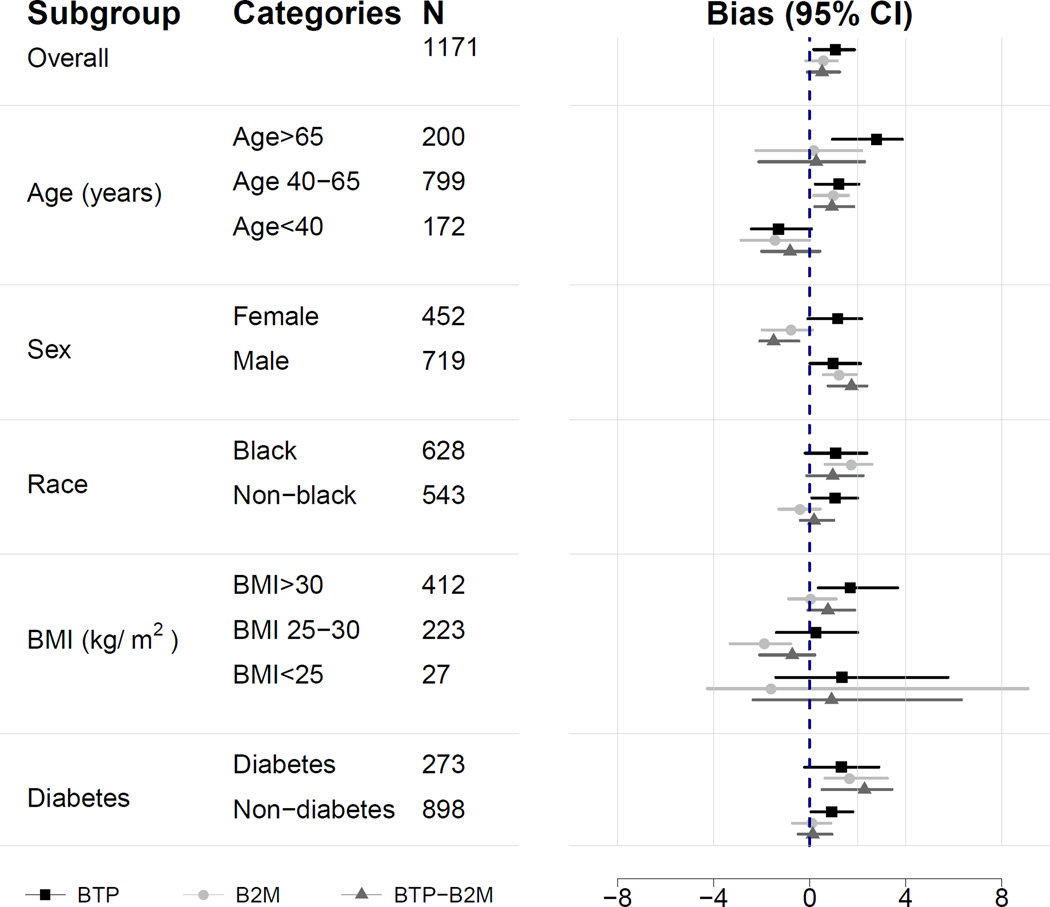
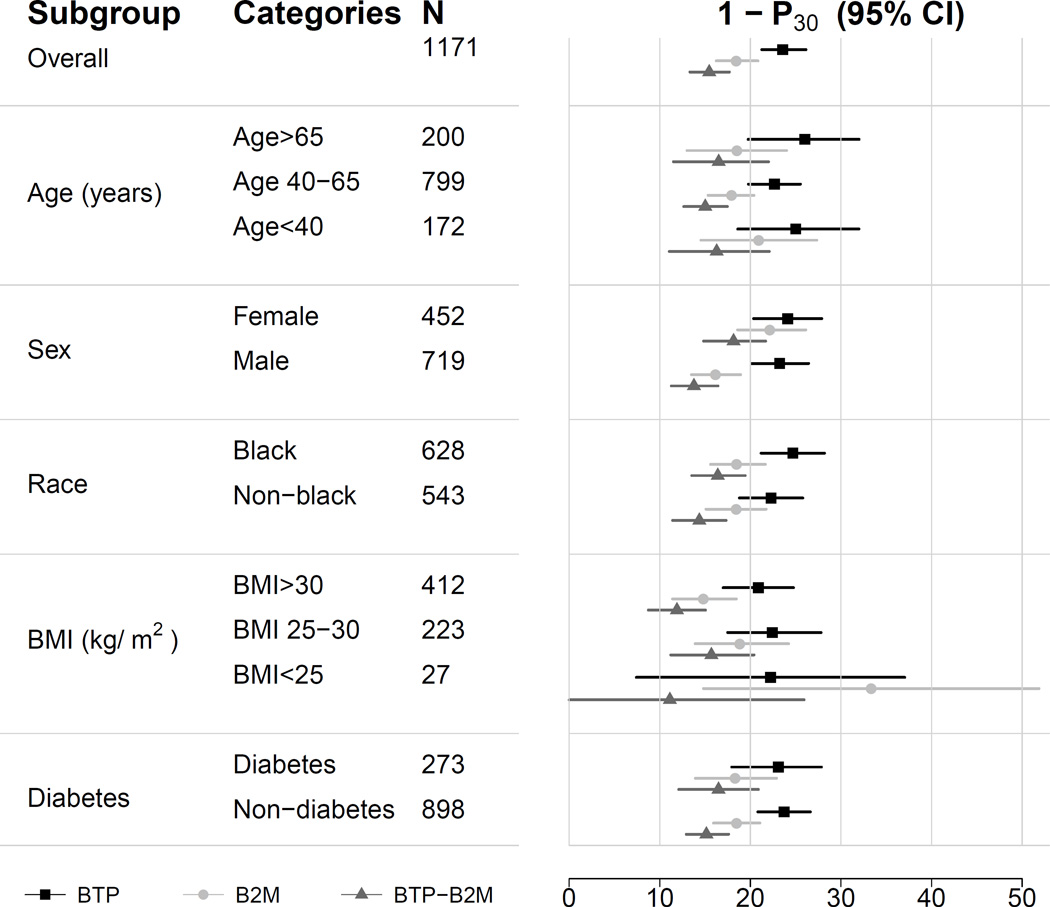
Figure 1 compares the median difference of mGFR vs eGFR by level of eGFR for the three new equations in the internal validation dataset. For BTP and B2M equations, there is a small underestimation of mGFR at the higher eGFR range; this underestimation is less with the use of both BTP and B2M. Among the three equations, there is minimal variation in the median difference between mGFR and eGFR across subgroups of age, sex, race, body mass index and diabetes (Figure 2).
Figure 1. Comparison of performance of BTP, B2M and BTP-B2M equations in the internal validation dataset.
Difference between measured and estimated vs. estimated GFR. Shown are smoothed regression line and 95% CI (computed using the Lowess smoothing function in R), using quantile regression, excluding lowest and highest 2.5% of estimated GFR values.
Figure 2. Performance of estimating equations by subgroups.
Shown is the median difference between measured and estimated GFR.
Table 2 compares the performance of new to existing equations in the internal validation dataset. Improved precision was noted with using two vs one filtration markers, regardless of whether BTP was combined with B2M or creatinine was combined with cystatin. The BTP and B2M equations are less accurate than the CKD-EPI creatinine or CKD-EPI cystatin C equations, whereas the combined BTP-B2M equation has similar accuracy to the CKD-EPI creatinine and CKD-EPI cystatin C equations. The combined CKD-EPI creatinine-cystatin C equation is more accurate than the CKD-EPI creatinine and CKD-EPI cystatin C equations, as has been previously shown3, and is also more accurate than the combined BTP-B2M equation. The average of the combined BTP-B2M equation with CKD-EPI creatinine-cystatin C equation had similar accuracy as the CKD-EPI creatinine-cystatin C equation. Of the newly developed equations, the combined BTP-B2M equation was better able to predict mGFR < 30, < 45 and < 60 ml/min/1.73 m2 than equations with either marker alone (Figure S1).
Table 2.
Performance of GFR estimating equations in internal validation dataset
| Description | IQR (95% CI) | 1−P30 (%)(95% CI) | 1−P20 (95% CI) | RMSE (95% CI) |
|---|---|---|---|---|
| BTP* | 15.0 (14.1, 15.9) | 23.6 (21.3, 26.1) | 43.6% (40.8%, 46.5%) | 0.274 (0.255, 0.299) |
| B2M* | 12.9 (12.2, 13.8) | 18.4 (16.2, 20.8) | 37.2% (34.6%, 40.1%) | 0.243 (0.231, 0.257) |
| BTP-B2M* | 12.1 (11.4, 13.0) | 15.5 (13,3, 17.7) | 35.4% (32.5%, 38.1%) | 0.224 (0.213, 0.235) |
| CKD-EPI Creatinine* | 11.6 (10.9, 12.4) | 16.4 (14.2, 18.6) | 34.5% (31.7%, 37.3%) | 0.224 (0.213, 0.236) |
| CKD-EPI Cystatin C* | 11.4 (10.6, 12.4) | 16.9 (14.9, 18.6) | 34.8% (32.1%, 37.6%) | 0.228 (0.217, 0.239) |
| CKD-EPI Creatinine-Cystatin C | 9.3 (8.7, 10.1) | 11.3 (9.5, 13.2) | 25.5% (23.1%, 28.0%) | 0.189 (0.180, 0.199) |
| Average of CKD-EPI Creatinine-Cystatin C and BTP-B2M | 10.2 (9.5, 11.0) | 9.6 (8.0, 11.4) | 25.0% (22.6%, 27.6%) | 0.186 (0.177, 0.195) |
Note: Shown are performance of equations developed in the development dataset (2/3) and tested in the internal validation dataset (1/3). Bias is not shown as it is expected to be near zero, since both the development and the internal validation dataset are random samples of the total dataset.
Abbreviations and definitions: B2M, β2-microgobulin; BTP, β-trace protein; CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate; IQR, interquartile range of difference between measured GFR and estimated GFR; 1−P30 (1−P20), percentage of estimates greater than 30% (20%) different from measured GFR; RMSE, root mean squared error calculated on log scale
1−P30 significantly different (p<0.001) from CKD-EPI creatinine-cystatin C equations
Discussion
It is crucial that assessment of GFR is performed accurately for interpretation of symptoms, signs and laboratory abnormalities associated with CKD as well as for drug dosing, and for detection, staging, management and prognostication of these diseases. Currently used filtration markers, creatinine and cystatin C, are recommended for general use, however, greater accuracy of GFR estimates could facilitate better clinical decision making29. Blood levels of BTP and B2M were first mentioned as possible endogenous filtration marker at least two decades ago7–9,30–32. They have been used in GFR estimating equations in selected populations, including children or transplant recipients30–32 and investigated as prognostic markers in CKD studies and in the general population10–18. As such, availability of rigorously developed GFR estimating equations using BTP and B2M would enable expression of these markers on the GFR scale for comparison with established markers in evaluation of kidney function and risk associations. In this report, we present new CKD-EPI equations using BTP and B2M alone and in combination with each other and compare their performance to existing CKD-EPI equations based on creatinine and cystatin C in a large population of adults with CKD. Our main findings are that BTP and B2M, like cystatin C, are less influenced by age, sex and race than creatinine and less influenced by race than cystatin C, do not vary across subgroups of body mass index and diabetes, but BTP and B2M do not improve GFR estimation beyond currently available equations including creatinine and cystatin C. These findings provide valuable lessons for development of GFR estimation equations and may have important applications in research as we will describe.
Steady-state serum levels of endogenous filtration markers are used to estimate GFR. However, all endogenous filtration markers are affected by physiological processes other than GFR (ie non-GFR determinants), including generation, tubular reabsorption and secretion, and extra-renal elimination. The GFR estimating equations use easily measured demographic or clinical variables, such as age, sex and race, as surrogates for non-GFR determinants. Imprecision in the relationship of observed surrogates to the unmeasured physiological processes contribute significantly to imprecision of GFR estimates compared to mGFR. We hypothesize that combination of multiple markers could lead to more precise GFR estimating equations that require fewer surrogates if the markers are not highly correlated with each other and the non-GFR determinants of the markers are independent of each other. For creatinine, muscle mass is a major determinant of variation in generation; age, sex, and race are included as surrogates, but do not account sufficiently for individual variation, leading to a search for alternative markers. Like cystatin C, BTP and B2M appear to be less dependent upon muscle mass than creatinine32,33. Like cystatin C, BTP equation performance improves with inclusion of age and sex, but not race, but coefficients are weaker than that of creatinine-based equations. The B2M equation and the combined BTP-B2M equation do not require age, sex or race. The lack of improvement in precision of GFR estimation by BTP and B2M beyond that of creatinine and cystatin C suggests that factors other than age, sex and race affect BTP and B2M more than they affect creatinine and cystatin C; these factors are not well understood. It has been shown that BTP is increased with steroids and potentially in certain inflammatory states and B2M is increased in malignancy and inflammation7–9,31,32,34,35. If these factors could be captured by easily measured surrogates, precision of GFR estimation using BTP and B2M alone and in combination with creatinine or cystatin C could be improved. Failure to account for them could limit the use of these filtration markers for GFR estimation in patients with cancer or inflammatory states. Alternatively, lack of improvement in precision of GFR estimation by BTP and B2M compared to creatinine and cystatin C may reflect larger measurement error in the filtration markers. Prior studies have shown that BTP has greater within-person variability (CV, 11.6%) compared to creatinine, cystatin C or B2M (CVs of 7.6%, 6.8% and 8.4%, respectively)36. Possibly BTP and B2M may improve GFR estimation in populations with a higher range of GFR compared to what is seen here, since the CKD-EPI creatinine and cystatin C equations are known to be more accurate in populations with CKD and a lower range of GFR3.
Although the performance of these new CKD-EPI BTP and B2M equations was not shown to be better than that of the existing CKD-EPI equations based on creatinine and cystatin C, they may have potential utility. First, in conditions in which creatinine generation is altered, there would be a use for estimating GFR without creatinine. For example, in amputees, patients with disorders of muscle or extremes of diet, GFR estimates from cystatin C, BTP and B2M may be more accurate than estimates from creatinine37. Cystatin C is thought to be affected by fat mass, and therefore BTP and B2M may be more accurate than creatinine or cystatin C in obese patients. These applications would require explicit evaluation in other populations. Second, the combination of BTP and B2M without demographics provides equivalent results to use of creatinine-based eGFR. In some circumstances, there may be advantages of being able to compute eGFR without the use of demographics, especially race. Third, multiple studies have compared the associations of cystatin C, BTP or B2M with adverse outcome to associations with creatinine. Since the markers have different scales, it has been challenging to directly compare risk associations across markers alone or in combination. Expressing BTP and B2M on the GFR scale would facilitate these comparisons between markers. In addition, our finding of less accurate estimation of mGFR using BTP and B2M than creatinine suggests that prior findings of stronger risk associations of BTP and B2M than creatinine-based eGFR may be due to factors affecting BTP and B2M other than GFR10–14,18. Nevertheless, these and other uses of eGFR based on BTP and B2M must be weighed against the complexity and additional cost for their measurement. We would anticipate that use of these or other novel markers would be as a confirmatory test of creatinine-based eGFR. Of note, B2M assays are widely available and relatively inexpensive. At present, there is no commercially available assay in the United States for BTP.
A key strength of this study is our use of three large CKD cohorts for development and internal validation of the new equations. In addition, we used a pre-specified rigorous analytical plan for testing of all variables. All three studies used similar methods for measurement of GFR. Our study also has limitations. First, we did not evaluate the BTP and B2M equations in an external validation population. Other studies will be required to evaluate the validity of our findings. Second, the study population was restricted to CKD, did not include many participants of non-black, non-white race, and did not include the very old. The CKD-EPI creatinine and cystatin C equations perform very well in this population, which may have limited our ability to observe improvement of equations using all four markers. Ideally estimating equations are developed in diverse populations so that the estimates can be broadly applied to people with and without known CKD, across a wider range of age and race. Third, the observed imprecision may be due to heterogeneity among studies. However, pooling across studies allows for a more generalizable result than would be obtained from a single study. Fourth, the use of the average of the CKD-EPI creatinine-cystatin C equation with the BTP-B2M equation does not optimize the coefficients for all four markers. Development of new equations with BTP and B2M in combination with creatinine and cystatin C in a diverse pooled dataset is ongoing. Fifth, these populations were not selected for marked alterations in non-GFR determinants of the filtration markers, in which accuracy in equation performance would be lower. Sixth, error in the filtration markers or mGFR may account for some of the noted imprecision. Error in mGFR is especially important in evaluating improvements in precision of GFR estimates using multiple markers.
In conclusion, the CKD-EPI BTP and B2M equations are less accurate than the CKD-EPI creatinine and cystatin C equations in populations with CKD, but do not require use of demographic variables. Further evaluation is necessary to determine if these equations have utility in more diverse populations with and without CKD, nevertheless they provide a methodological advance for the study of these markers in ongoing and future research studies.
Supplementary Material
Acknowledgements
Support: This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK097020; NIDDK U01DK085689 (Chronic Kidney Disease Biomarkers Consortium; Drs. Coresh, Levey and Inker); NIDDK U01DK045388 and the NCMHHD M01RR00071 (AASK Study); NIDDK U01DK35073 (MDRD Study); and cooperative agreements from NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902); and is supported in part by the following institutional Clinical Translational Science Awards (CTSA) and other NIH grants: University of Pennsylvania NIH/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, K01DK092353, and K24DK002651, Johns Hopkins University UL1TR000424, University of Maryland General Clinical Research Center M01 RR16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco-Clinical and Translational Science Institute UL1 RR-024131 (CRIC Study). Dr. Shafi is supported by NIDDK grant K23 DK083514.
Dr. Inker reports funding to Tufts Medical Center for research and contracts with the NIH, National Kidney Foundation (NKF), Pharmalink AB and Gilead Sciences, a consulting agreement with Otsuka, and has a provisional patent (Drs Coresh, Inker and Levey) filed 8/15/2014 (Precise estimation of glomerular filtration rate from multiple biomarkers; licensing under negotiation). Dr. Levey reports funding to Tufts Medical Center for research and contracts with the NIH, NKF, Amgen, Pharmalink AB, Gilead Sciences, and has the aforementioned provisional patent. Dr. Coresh has the aforementioned provisional patent. Dr. Greene is a consultant for Jansen Pharmaceuticals and Pfizer, and reports grants from Nephrogenix, Keryx biopharmaceuticals, and Genkyotex S.A. Dr. Kusek works at the NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: LAI, JC, ASL; LAI, HT, JC, MCF, AHA, GJB, GC, TG, AK, JWK, JLash, JLewis, JRS, SDN, JS, TS, ASL; data analysis/interpretation: LAI, HT, JC, MCF, AHA, GJB, GC, TG, AK, JWK, JLash, JLewis, JRS, SDN, JS, TS, ASL; statistical analysis: LAI, HT, JC, MCF, TG; supervision or mentorship: LAI, JC, TG, ASL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. LAI takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Performance in internal validation dataset of Cr and CysC equations developed in development dataset, compared to existing CKD-EPI equations.
Table S2: Clinical characteristics of study population.
Table S3: Pearson correlation coefficients among log-transformed filtration markers and mGFR.
Table S4: Regression coefficients for GFR estimating equations developed in development dataset.
Table S5: Performance of GFR estimating equations including vs not including age, sex and race in development dataset.
Figure S1: ROC curves for prediction of mGFR < 30, <45 and < 60 for BTP, B2M, and BTP-B2M equations.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Performance in internal validation dataset of Cr and CysC equations developed in development dataset, compared to existing CKD-EPI equations.
Supplementary Table S2 (PDF). Clinical characteristics of study population.
Supplementary Table S3 (PDF). Pearson correlation coefficients among log-transformed filtration markers and mGFR.
Supplementary Table S4 (PDF). Regression coefficients for GFR estimating equations developed in development dataset.
Supplementary Table S5 (PDF). Performance of GFR estimating equations including vs not including age, sex and race in development dataset.
Supplementary Figure S1 (PDF).. ROC curves for prediction of mGFR < 30, <45 and < 60 for BTP, B2M, and BTP-B2M equations.
References
- 1.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 2.College of American Pathologists. Current Status Of Reporting Estimated Glomerular Filtration Rate (eGFR) [Accessed October 8, 2013];2012 http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt%7BactionForm.contentReference%7D=committees%2Fchemistry%2Fchemistry_resources.html&_state=maximized&_pageLabel=cntvwr. [Google Scholar]
- 3.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. 1997;7(4):499–506. doi: 10.1093/glycob/7.4.499. [DOI] [PubMed] [Google Scholar]
- 8.White CA, Ghazan-Shahi S, Adams MA. beta-Trace Protein: A Marker of GFR and Other Biological Pathways. Am J Kidney Dis. 2015;65(1):131–146. doi: 10.1053/j.ajkd.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Viberti GC, Keen H, Mackintosh D. Beta 2-microglobulinaemia: a sensitive index of diminishing renal function in diabetics. Br Med J (Clin Res Ed) 1981;282(6258):95–98. doi: 10.1136/bmj.282.6258.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astor BC, Shafi T, Hoogeveen RC, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59(5):653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012;23(2):351–359. doi: 10.1681/ASN.2011070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhavsar NA, Appel LJ, Kusek JW, et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. 2011;58(6):886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster MC, Coresh J, Hsu CY, et al. Serum β-trace protein and β-2 microglobulin as Predictors End-stage Renal Disease in Adults with Chronic Kidney Disease [ASN Abstract SA-OR012] J Am Soc Nephrol. 2014;25:83A. [Google Scholar]
- 14.Foster MC, Inker LA, Hsu CY, et al. Filtration Markers as Predictors of ESRD and Mortality in Southwestern American Indians With Type 2 Diabetes. Am J Kidney Dis. 2015;66(1):75–83. doi: 10.1053/j.ajkd.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17(2):546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 16.Spanaus KS, Kollerits B, Ritz E, Hersberger M, Kronenberg F, von Eckardstein A. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem. 2010;56(5):740–749. doi: 10.1373/clinchem.2009.138826. [DOI] [PubMed] [Google Scholar]
- 17.Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 18.Foster MC, Inker LA, Levey AS, et al. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis. 2013;62(1):42–51. doi: 10.1053/j.ajkd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) Cystatin C Equations for Estimating GFR With Standardized Serum Cystatin C Values. Am J Kidney Dis. 2011;58(4):682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: 2014. http://www.R-project.org. [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter., Suppl. 2013;3(1):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 30.White CA, Akbari A, Doucette S, et al. Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int. 2009;76(7):784–791. doi: 10.1038/ki.2009.262. [DOI] [PubMed] [Google Scholar]
- 31.White CA, Akbari A, Doucette S, et al. Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis. 2009;54(5):922–930. doi: 10.1053/j.ajkd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Filler G, Kusserow C, Lopes L, Kobrzynski M. Beta-trace protein as a marker of GFR--history, indications, and future research. Clin Biochem. 2014;47(13–14):1188–1194. doi: 10.1016/j.clinbiochem.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Juraschek SP, Coresh J, Inker LA, et al. Comparison of serum concentrations of beta-trace protein, beta2-microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013;8(4):584–592. doi: 10.2215/CJN.08700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melegos DN, Grass L, Pierratos A, Diamandis EP. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53(1):32–37. doi: 10.1016/s0090-4295(98)00453-1. [DOI] [PubMed] [Google Scholar]
- 35.Abbink FC, Laarman CA, Braam KI, et al. Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem. 2008;41(4–5):299–305. doi: 10.1016/j.clinbiochem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. Am J Kidney Dis. 2013;61(5):716–722. doi: 10.1053/j.ajkd.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurlow JS, Abbott KC, Linberg A, Little D, Fenderson J, Olson SW. SCr and SCysC concentrations before and after traumatic amputation in male soldiers: a case-control study. Am J Kidney Dis. 2014;63(1):167–170. doi: 10.1053/j.ajkd.2013.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.