Abstract
Introduction
Initial observation (IO) is a strategy to minimize prostate cancer overtreatment. We sought to evaluate contemporary trends in IO utilization for low-risk prostate cancer in the United States and to identify factors associated with its uptake.
Methods
Using the National Cancer Database, we identified men with low-risk prostate cancer diagnosed between 2004 and 2011. IO utilization was plotted over time. Multivariate logistic regression was performed to determine the influence of diagnosis year and other factors on IO selection.
Results
Of the 219 971 men with low-risk prostate cancer, 21 231 (9.7%) underwent IO. Beginning in 2008, IO use increased significantly with time (range: 7.5%–14.3%). Compared to 2004, patients diagnosed in 2011 had 2.5 times the odds of choosing IO (odds ratio [OR] 2.5, confidence interval [CI] 2.3–2.6, p < 0.01). Aside from diagnosis year, age, race, Charlson score, clinical T stage, and PSA level predicted IO use (p < 0.01). Other predictors of IO included hospital type, insurance provider, and household income. Specifically, comprehensive cancer centres, private insurance, and higher income predicted decreased IO usage (OR 0.5, CI 0.5–0.5, p < 0.01; OR 0.4, CI 0.4–0.4, p < 0.01; and OR 0.8, CI 0.8–0.9, p < 0.01, respectively). Less educated men were also less likely to undergo observation (OR 0.8, CI 0.8–0.9, p < 0.01). Treatment within the western United States was significantly, but weakly, associated with increased use of IO (p < 0.01).
Conclusions
In recent years, low-risk prostate cancer has been increasingly managed with IO, appropriately driven by patient and disease factors. Unexpectedly, observation usage also varies by race, hospital, insurance, income, and geography, suggesting that non-clinical factors may affect treatment selection.
Introduction
The overdiagnosis and overtreatment of prostate cancer has become a national public health concern.1 Early prostate cancer detection through widespread prostate-specific antigen (PSA) screening reduces cancer-specific mortality and the incidence of metastatic disease in a small proportion of men at the expense of exposing many more men to its associated risks.2,3 Early detection with PSA screening over-diagnoses 23% to 42% of prostate cancer, leading to over-treatment and its related harms.4–6 Citing this unfavourable risk-benefit ratio, the United States Preventative Services Task Force (USPSTF) recommended against screening in all men.1 While a ban on screening would eliminate overdiagnosis entirely, it also would permit 3000 to 4000 avoidable prostate cancer deaths annually.7 An alternative solution to overtreatment is to restrict screening to age-appropriate men and to limit treatment based on patient life expectancy and disease characteristics.
Watchful waiting (WW) and active surveillance (AS) minimize overtreatment by avoiding or delaying curative treatment in well-selected men, respectively. Both strategies involve a period of initial observation (IO) followed either by continued observation in elderly or sickly men who are unlikely to benefit from active treatment (WW) or by active monitoring with selective delayed intervention in men at higher risk for disease progression (AS). It is now clear that neither strategy sacrifices short-term cancer specific survival in men with low-risk disease.8–11 Seemingly, the primary barrier limiting the effectiveness of IO to combat overtreatment in this country has been its acceptance by urologists.12
Historically IO has been used at very low rates in the United States, largely relegated to use in older men.13,14 With the introduction of AS in 2002, IO became a feasible option for all men with low-risk prostate cancer.15 While recent IO usage appears to be robust in Scandinavia, little is known about its contemporary uptake in the United States or the factors that influence its utilization.11,16 We sought to examine IO utilization and its predictors in a national sample of men using the National Cancer Database (NCDB).
Methods
The NCDB, a joint project of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons, is a comprehensive clinical oncology dataset that captures 70% of all incident malignancies in the United States. It has been validated previously against the SEER (Surveillance, Epidemiology and End Results) database.17 The dataset contains only de-identified data, obviating the need for institutional review board approval.
We identified 1 666 913 patients with histologically confirmed prostate cancer based on ICD-O-3 primary site (C619) and histology (8140) codes. The study period was limited to diagnosis years 2004 to 2011 because PSA data were not available prior to 2004 (n = 973 558). Only patients with prostate cancer as their sole or first cancer diagnosis were included to avoid confounding from prior cancer treatments (n = 900 580). We limited our cohort to men with low-risk prostate cancer by the D’Amico criteria, defined as Gleason score ≤6 (no Gleason pattern 4 or 5), TNM clinical T stage T1–T2a, and PSA <10 (n = 220 187). After excluding nodal and metastatic disease, 219 971 patients were available for analysis.
The NCDB only includes data on “first course of treatment,” defined as all methods of treatment recorded in the treatment plan and administered to the patient before disease progression or recurrence. IO was defined as no first course treatment. Active treatment included prostatectomy, radiation therapy, androgen deprivation, and other unspecified treatments (which accounted for <3% of cases). To identify factors associated with IO utilization for low-risk prostate cancer, we compared men who underwent IO to those who received active treatment.
Univariate analysis was performed using the Pearson chi-square test. For multivariate logistic regression, adjusted odds ratios were calculated with IO as the response variable and diagnosis year, race, residence, education, income, insurance, age, comorbidity, hospital type, and hospital location as covariates. Statistical tests were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). P values <0.01 were considered statistically significant.
Results
Overall, from 2004–2011, 9.7% (21 231/219 971) of men with low-risk prostate cancer were managed with IO, while 198,740 were actively treated. The low-risk prostate cancer population was composed of predominantly more educated, wealthier, insured Caucasian men living in metropolitan areas who sought care at major academic and comprehensive cancer centers throughout the United States (Table 1). Patients were primarily healthy and younger than age 70. Most men had clinical T1c prostate cancer and PSA >4.
Table 1.
Characteristics of men with low-risk prostate cancer managed with initial observation vs. active treatment
| IO | % | T | % | Total | % | p value* | |
|---|---|---|---|---|---|---|---|
| Race | <0.01 | ||||||
| Non-Hispanic white | 16 262 | 76.6 | 160 041 | 80.5 | 176 303 | 80.1 | |
| Hispanic | 789 | 3.7 | 6429 | 3.2 | 7218 | 3.3 | |
| African-American | 2885 | 13.6 | 23 378 | 11.8 | 26 263 | 11.9 | |
| Other minority | 1295 | 6.1 | 8892 | 4.5 | 10 187 | 4.6 | |
| Patient residence | <0.01 | ||||||
| Metropolitan | 16 896 | 82.9 | 154 550 | 81.7 | 171 446 | 81.8 | |
| Rural | 417 | 2.0 | 4427 | 2.3 | 4844 | 2.3 | |
| Urban | 3073 | 15.1 | 30 224 | 16.0 | 33 297 | 15.9 | |
| Education level | <0.01 | ||||||
| Lowest | 2943 | 14.5 | 25 902 | 13.7 | 28 845 | 13.7 | |
| Lower middle | 4015 | 19.8 | 39 967 | 21.2 | 43 982 | 20.9 | |
| Upper middle | 4575 | 22.6 | 45 428 | 23.9 | 50 003 | 23.8 | |
| Highest | 8752 | 43.1 | 78 454 | 41.3 | 87 206 | 41.5 | |
| Income level | <0.01 | ||||||
| Lowest | 2500 | 12.3 | 21 440 | 11.3 | 23 940 | 11.4 | |
| Lower middle | 3392 | 16.7 | 31 048 | 16.4 | 34 440 | 16.4 | |
| Upper middle | 5341 | 26.3 | 51 491 | 27.1 | 56 832 | 27.1 | |
| Highest | 9054 | 44.6 | 85 786 | 45.2 | 94 840 | 45.2 | |
| Insurance | <0.01 | ||||||
| Private | 9244 | 81.4 | 110 638 | 56.5 | 119 882 | 55.4 | |
| Uninsured | 489 | 2.4 | 2201 | 1.1 | 2690 | 1.2 | |
| Federal/social | 10 867 | 52.8 | 82 844 | 42.3 | 93 711 | 43.3 | |
| Age, years | <0.01 | ||||||
| <50 | 656 | 3.1 | 9249 | 4.7 | 9905 | 4.5 | |
| 50–59 | 4487 | 21.1 | 57 948 | 29.2 | 62 435 | 28.4 | |
| 60–69 | 8680 | 40.9 | 86 371 | 43.5 | 95 051 | 43.2 | |
| 70–79 | 6168 | 29.1 | 41 894 | 21.1 | 48 062 | 21.8 | |
| >80 | 1240 | 5.8 | 3278 | 1.6 | 4518 | 2.1 | |
| Charlson score | <0.01 | ||||||
| 0 | 18 978 | 89.4 | 173 227 | 87.2 | 192 205 | 87.4 | |
| 1 | 1828 | 8.6 | 22 429 | 11.3 | 24 257 | 11.0 | |
| >1 | 425 | 2.0 | 3084 | 1.6 | 3509 | 1.6 | |
| Clinical T stage | <0.01 | ||||||
| T1a | 985 | 4.6 | 3698 | 1.9 | 4683 | 2.1 | |
| T1b | 251 | 1.2 | 1305 | 0.7 | 1556 | 0.7 | |
| T1c | 17 972 | 84.6 | 169 171 | 85.1 | 187 143 | 85.1 | |
| T2a | 2023 | 9.5 | 24 566 | 12.4 | 26 589 | 12.1 | |
| PSA | 0.11 | ||||||
| <4 | 5072 | 23.9 | 46818 | 23.6 | 51890 | 23.6 | |
| 4–10 | 16159 | 76.1 | 151922 | 76.4 | 168081 | 76.4 | |
| Hospital type | <0.01 | ||||||
| Academic | 10652 | 50.2 | 71332 | 35.9 | 81984 | 37.3 | |
| Community | 2100 | 9.9 | 16122 | 8.1 | 18222 | 8.3 | |
| Comprehensive | 8349 | 39.3 | 109727 | 55.2 | 118076 | 53.7 | |
| Other | 130 | 0.6 | 1559 | 0.8 | 1689 | 0.8 | |
| Hospital location | <0.01 | ||||||
| Midwest | 5017 | 23.6 | 50323 | 25.3 | 55340 | 25.2 | |
| Northwest | 5481 | 25.8 | 45897 | 23.1 | 51378 | 23.4 | |
| South | 6958 | 32.8 | 70341 | 35.4 | 77299 | 35.1 | |
| West | 3775 | 17.8 | 32179 | 16.2 | 35954 | 16.3 |
p values <0.01 considered significant. IO: initial observation; T: active treatment; PSA: prostate-specific antigen.
Diagnosis year was one of the strongest determinants of receiving IO. From 2004 to 2007, IO utilization remained relatively stable (range: 7.2–7.5%). Beginning in 2008, IO usage rose steadily, peaking at 14.3% by 2011 (Fig. 1). Compared to 2004, patients diagnosed in 2011 had 2.5 times the odds of receiving IO (odds ratio [OR] 2.5, confidence interval [CI] 2.3–2.6, p < 0.01).
Fig. 1.
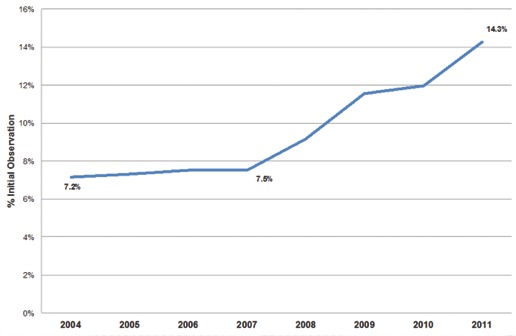
Initial observation utilization for low-risk prostate cancer.
Aside from diagnosis year, clinical factors were also significant predictors of IO usage (Table 2). In particular, age was the single greatest predictor of IO utilization. Compared to patients <50 years, patients >70 years had 2.5 times the odds of receiving IO (OR 2.5, CI 2.3–2.7, p < 0.01) and those >80 years had 7.2 times greater odds (OR 7.2, CI 6.4–8.0, p < 0.01). Charlson comorbidity index (CCI) had a small, but significant, impact on IO utilization as well. Patients with more than one comorbidity had 10% increased odds of receiving IO than those without comorbidities (p < 0.01). Men with PSA >4 or clinical T2 disease were significantly less likely (10% and 30% decreased odds, respectively) of undergoing IO than those with T1 disease or PSA <4 (p < 0.01).
Table 2.
Multivariate logistic regression analysis of predictors of initial observation utilization
| Variable | Adjusted OR | 95% CI | p value* |
|---|---|---|---|
| Diagnosis year | <0.01 | ||
| 2004 | 1.0 | (referent) | |
| 2005 | 1.0 | 1.0–1.1 | |
| 2006 | 1.1 | 1.0–1.1 | |
| 2007 | 1.1 | 1.0–1.2 | |
| 2008 | 1.4 | 1.3–1.5 | |
| 2009 | 1.9 | 1.8–2.0 | |
| 2010 | 2.0 | 1.9–2.1 | |
| 2011 | 2.5 | 2.3–2.6 | |
| Race | <0.01 | ||
| Non-Hispanic white | 1.0 | (referent) | |
| Hispanic | 1.1 | 1.0–1.2 | |
| African-American | 1.2 | 1.2–1.3 | |
| Other | 1.3 | 1.2–1.4 | |
| Patient residence | 0.10 | ||
| Metropolitan | 1.0 | (referent) | |
| Rural | 0.9 | 0.8–1.0 | |
| Urban | 1.0 | 0.9–1.0 | |
| Education level | <0.01 | ||
| Highest | 1.0 | (referent) | |
| Upper middle | 0.9 | 0.8–0.9 | |
| Lower middle | 0.8 | 0.8–0.8 | |
| Lowest | 0.8 | 0.8–0.9 | |
| Income level | <0.01 | ||
| Lowest | 1.0 | (referent) | |
| Lower middle | 1.0 | 0.9–1.1 | |
| Upper middle | 0.9 | 0.9–1.0 | |
| Highest | 0.8 | 0.8–0.9 | |
| Insurance | <0.01 | ||
| Private | 1.0 | (referent) | |
| Federal/social | 1.2 | 1.2–1.3 | |
| Uninsured | 2.5 | 2.3–2.8 | |
| Age, years | <0.01 | ||
| <50 | 1.0 | (referent) | |
| 50–59 | 1.2 | 1.1–1.3 | |
| 60–69 | 1.6 | 1.4–1.7 | |
| 70–79 | 2.5 | 2.3–2.7 | |
| >80 | 7.2 | 6.4–8.0 | |
| Charlson score | <0.01 | ||
| 0 | 1.0 | (referent) | |
| 1 | 0.7 | 0.7–0.7 | |
| >1 | 1.1 | 1.0–1.3 | |
| Clinical T stage | <0.01 | ||
| T1 | 1.0 | (referent) | |
| T2 | 0.7 | 0.7–0.8 | |
| PSA | <0.01 | ||
| <4 | 1.0 | (referent) | |
| >4 | 0.9 | 0.9–0.9 | |
| Hospital type | <0.01 | ||
| Academic | 1.0 | (referent) | |
| Community | 0.8 | 0.8–0.9 | |
| Comprehensive | 0.5 | 0.5–0.5 | |
| Other | 0.5 | 0.4–0.6 | |
| Hospital location | <0.01 | ||
| Midwest | 1.0 | (referent) | |
| Northwest | 1.1 | 1.1–1.2 | |
| South | 1.1 | 1.0–1.1 | |
| West | 1.3 | 1.3–1.4 |
p values <0.01 considered significant. PSA: prostate-specific antigen; OR: odds ratio; CI: confidence interval.
Albeit to a lesser extent, demographic factors were also important predictors of IO usage. African American and other, non-Hispanic minority men had 20% and 30% increased odds of receiving IO compared to Caucasian men (OR 1.2, CI 1.2–1.3; OR 1.3, CI 1.2–1.4; p < 0.01, respectively). Less educated men and wealthier men were both significantly less likely to receive IO (OR 0.8, CI 0.8–0.9; OR 0.8, CI 0.8–0.9; p < 0.01, respectively).
Non-clinical factors that significantly predicted IO use included: insurance provider, hospital type, and, to a lesser degree, hospital region. IO usage was highest in the uninsured and in patients with social insurance. Compared to patients with private insurance, patients with social insurance had 1.2 times the odds of receiving IO (OR 1.2, CI 1.2–1.3, p < 0.01), while uninsured patients had 2.5 times the odds (OR 2.5, CI 2.3–2.8, p < 0.01). IO was most frequently utilized at academic centres. Men treated at academic centres had 2.1, 1.2, and 1.9 times the odds of receiving IO compared to patients treated at comprehensive centres, community cancer programs, and other hospitals, respectively (p < 0.01). IO was most common in western United States, followed by the northeast, south, and midwest regions (p < 0.01) (See Appendix). Within a particular region, IO selection did not significantly depend on the patient’s place of residence (rural, urban, or metropolitan, using the typology published by the United States Department of Agriculture Economic Research Service).18
Discussion
From 2007 onward, IO usage increased gradually at a rate of 1.7% per year, peaking at 14.3%. This rise may be related to greater acceptance of AS, which was first included in clinical practice guidelines as an alternative to active treatment in 2007.19 In general, however, IO use was higher in men with limited life expectancies (age >70 and CCI ≥2), suggesting WW-predominant practice patterns.20 This is a change from pre-2008 trends, in which aggressive treatment was administered regardless of patient life expectancy.21 We found that academic centres in the United States led the way in IO adoption. Interestingly, men receiving IO were more likely to be poor or belong to a minority group and were less likely to have private health insurance than their counterparts.
While our study is one of the first to demonstrate the more recent rise in IO utilization in the United States, our baseline IO rate (7.4%) from 2004 to 2007 is consistent with prior research. Cooperberg and colleagues reported an 8.5% utilization rate of observation for low-risk prostate cancer from 2004 to 2007, using the CaPSURE database.22 On the contrary, Ritch and colleagues recently reported a much higher and rising rate of observation (from 18% in 2004 to 29% in 2009) in men with low-risk disease; however, since their data only included men age 65 and older, these results may have been influenced by selection bias.23 Loeb and colleagues also noted increasing AS utilization in Sweden over the same interval (2007–2011).16 However, AS rates for low- and very low-risk disease in Sweden were much higher (41%–59%) than our IO rates, possibly reflecting both cultural and financial disparities in practice patterns between the United States and Scandinavia.16
It is estimated that 38% to 60% of patients diagnosed with early prostate cancer are considered low risk by D’Amico criteria and thus are candidates for management with IO.11,24 Based on these estimates, our findings suggest that IO is still being underutilized in the United States despite its recent gains. The reasons behind underutilization are likely multifactorial.
The lack of clear recommendations favouring AS may have contributed to IO underutilization. For most of the diagnosis period (2004–2007), AS was not an accepted strategy for low-risk prostate cancer.18 Also, preliminary results from the largest prospective AS cohort (Prostate Cancer Research International: Active Surveillance, PRIAS), were not available until 2009.25 Moreover, the limited benefit of active treatment for low-risk, screen-detected prostate cancer was not yet known.8 Consequently, community urologists may have considered AS experimental, explaining why academic centres were primarily responsible for the rise in IO use.
Racial/ethnic and socioeconomic disparities in treatment selection for prostate cancer are well-recognized. It has been shown that African-American men are more likely to be managed expectantly than white men.26 Similarly, poor men and men with public health insurance are more likely to be treated conservatively.27,28 In keeping with these disparities, we found that minority men, especially African-Americans, men from lower socioeconomic groups, and uninsured or socially insured men preferentially received IO. While increased IO utilization as a whole should be considered an achievement, its preferential use in certain racial and socioeconomic groups is not evidence-based and may be detrimental. In particular, African-American men, who have a higher disease progression rate on AS, may not be the best candidates for this approach.29 Furthermore, poor men may experience inferior outcomes when managed conservatively.28
It is unclear why poor and uninsured or underinsured men were more inclined to receive IO. This disparity does not appear to be caused by local differences in resource availability, since area of residence did not affect IO selection. Given that IO is associated with lower upfront costs than active treatments, it is possible that financial considerations influenced treatment decisions.30 This also may explain why non-academic/non-research hospitals, which rely on fee-for-service reimbursement, preferentially utilized higher-cost treatments. In contrast, at government-sponsored Veterans Affairs hospitals, which are less influenced by reimbursement concerns, IO utilization is higher.31
There are significant barriers to widespread AS adoption in the United States. While patient anxieties may limit utilization, physician influence is the single most important factor influencing the decision to undergo AS.12,32 Interestingly, we found that education level was associated with treatment selection, with educated men more likely to choose IO. Men with poor prostate cancer knowledge have more decisional conflict and decision-making impairment than educated men, potentially explaining their reluctance to pursue AS.33 Urologists must promote AS, educating and reassuring low-risk patients on the benefits of IO relative to active treatment.
A clear strength of our study is the NCDB’s comprehensiveness, capturing over 70% of incident cancers in the United States regardless of age. Since hospitals included in the NCDB exhibit higher levels of cancer specialization than other hospitals, our findings may demonstrate the best-case scenario in terms of contemporary IO utilization.
The main limitation of this study is its retrospective nature. Since the NCDB does not code IO as a unique treatment, we used lack of treatment to define IO. This definition is somewhat flawed because treatment delay may be erroneously construed as IO, leading to misclassification and overestimation of the IO rate. Fortunately, the NCBD makes every effort to assign treatments appropriately to minimize this error. Similarly, the NCDB does not differentiate AS from WW, nor does it include data on number of positive cores, re-biopsies or second-course treatments, limiting our ability to selectively identify AS patients.
Conclusion
IO has been utilized increasingly for low-risk prostate cancer in the United States, especially in patients least likely to benefit from active treatment. Despite this progress, IO is still underutilized, possibly due to the influence of non-clinical factors. The future of AS and, for that matter, of prostate cancer diagnosis depends on the continued adoption of IO by urologists.
Acknowledgements
An abstract of the work was presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium in San Francisco, CA on January 30, 2014 and at the American Urological Association annual meeting in Orlando, FL on May 20, 2014. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Appendix 1.
Regions and their corresponding states
| Region | State/District |
|---|---|
| Northeast | Connecticut Maine Massachusetts New Hampshire New Jersey New York Pennsylvania Rhode Island Vermont |
| Midwest | Illinois Indiana Iowa Kansas Michigan Minnesota Missouri Nebraska North Dakota Ohio South Dakota Wisconsin |
| South | Alabama Arkansas Delaware Florida Georgia Kentucky Louisiana Maryland Mississippi North Carolina Oklahoma South Carolina Tennessee Texas Virginia Washington, D.C. West Virginia |
| West | Alaska Arizona California Colorado Hawaii Idaho Montana Nevada New Mexico Oregon Utah Washington Wyoming |
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. . Epub 2014 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: Importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandhu GS, Andriole GL. Overdiagnosis of prostate cancer. J Natl Cancer Inst Monogr. 2012;2012:146–51. doi: 10.1093/jncimonographs/lgs031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014;120:3519–26. doi: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 10.Bul M, van den Bergh RC, Zhu X, et al. Outcomes of initially expectantly managed patients with low or intermediate risk screen-detected localized prostate cancer. BJU Int. 2012;110:1672–7. doi: 10.1111/j.1464-410X.2012.11434.x. [DOI] [PubMed] [Google Scholar]
- 11.Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63:101–7. doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 12.Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87:369–74. doi: 10.1016/j.pec.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–9. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Sun L, Moul JW, et al. Watchful waiting and factors predictive of secondary treatment of localized prostate cancer. J Urol. 2004;171:1111–6. doi: 10.1097/01.ju.0000113300.74132.8b. [DOI] [PubMed] [Google Scholar]
- 15.Choo R, Klotz L, Danjoux C, et al. Feasibility study: Watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–9. doi: 10.1016/S0022-5347(05)65174-9. [DOI] [PubMed] [Google Scholar]
- 16.Loeb S, Berglund A, Stattin P. Population-based study of utilization and determinants of active surveillance and watchful waiting for low- and intermediate-risk prostate cancer. J Urol. 2013;190:1742–9. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 17.Mettlin CJ, Menck HR, Winchester DP, et al. A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer. 1997;79:2052–61. doi: 10.1002/(SICI)1097-0142(19970515)79:10<2052::AID-CNCR29>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.United States Department of Agriculture Economic Research Service 2003. [Accessed April 13, 2015]. http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.
- 19.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2012;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Vickers A, Bennette C, Steineck G, et al. Individualized estimation of the benefit of radical prostatectomy from the Scandinavian Prostate Cancer Group randomized trial. Eur Urol. 2012;62:204–9. doi: 10.1016/j.eururo.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daskivich TJ, Lai J, Dick AW, et al. Variation in treatment associated with life expectancy in a population-based cohort of men with early-stage prostate cancer. Cancer. 2014;120:3642–50. doi: 10.1002/cncr.28926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritch CR, Graves AJ, Keegan KA, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015;193:801–6. doi: 10.1016/j.juro.2014.08.102. . Epub 2014 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barocas DA, Cowan JE, Smith JA, Jr, et al. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180:1330–5. doi: 10.1016/j.juro.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 25.van den Bergh RC, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre ‘Prostate Cancer Research International: Active Surveillance’ study. BJU Int. 2010;105:956–62. doi: 10.1111/j.1464-410X.2009.08887.x. [DOI] [PubMed] [Google Scholar]
- 26.Underwood W, De Monner S, Ubel P, et al. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol. 2004;171:1504–7. doi: 10.1097/01.ju.0000118907.64125.e0. [DOI] [PubMed] [Google Scholar]
- 27.Schymura MJ, Kahn AR, German RR, et al. Factors associated with initial treatment and survival for clinically localized prostate cancer: Results from the CDC-NPCR Patterns of Care Study (PoC1) BMC Cancer. 2010;10:1–15. doi: 10.1186/1471-2407-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarts MJ, Koldewijn EL, Poortmans PM, et al. The impact of socioeconomic status on prostate cancer treatment and survival in the southern Netherlands. Urology. 2013;81:593–9. doi: 10.1016/j.urology.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Odom BD, Mir MC, Hughes S, et al. Active surveillance for low-risk prostate cancer in African American men: A multi-institutional experience. Urology. 2014;83:364–8. doi: 10.1016/j.urology.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran AT, Peele PB, Benoit RM. Cost comparison between watchful waiting with active surveillance and active treatment of clinically localized prostate cancer. Urology. 2010;76:703–7. doi: 10.1016/j.urology.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 31.Maurice MJ, Abouassaly R, Zhu H. American trends in expectant management utilization for prostate cancer from 2000–2009. Can Urol Assoc J. 2014;8:E775–82. doi: 10.5489/cuaj.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorin MA, Soloway CT, Eldefrawy A. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011;77:588–91. doi: 10.1016/j.urology.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan AL, Crespi CM, Saucedo JD, et al. Decisional conflict in economically disadvantaged men with newly diagnosed prostate cancer: Baseline results from a shared decision-making trial. Cancer. 2014;120:2721–7. doi: 10.1002/cncr.28755. [DOI] [PMC free article] [PubMed] [Google Scholar]


