Abstract
Introduction
The relative roles of surgery and stereotactic body radiation therapy in stage I non-small cell lung cancer (NSCLC) are evolving particularly for marginally operable patients. Since there is limited prospective comparative data for these treatment modalities, we evaluated their relative use and outcomes at the population level using a national database.
Methods
Patient variables and treatment-related outcomes were abstracted for patients with clinical stage I NSCLC from the National Cancer Database. Patients receiving surgery were compared to those undergoing SBRT in exploratory unmatched and subsequent propensity matched analyses.
Results
Between 1998 and 2010, 117618 patients underwent surgery or SBRT for clinical stage I NSCLC. Of these, 111731 (95%) received surgery while 5887 (5%) underwent SBRT. Patients in the surgery group were younger, more likely to be males, and had higher Charlson comorbidity scores. SBRT patients were more likely to have T1 (vs.T2) tumors and receive treatment at academic centers. Thirty-day surgical mortality was 2596/109485 (2.4%). Median overall survival favored the surgery group in both unmatched (68.4 months vs. 33.3 months, p<.001) and matched analysis based on patient characteristics (62.3 months vs. 33.1months, p<.001). Disease specific survival was unavailable from the dataset.
Conclusion
In a propensity matched comparison, patients selected for surgery have improved survival compared with SBRT. In the absence of information on cause of death and with limited variables to characterize comorbidity, it is not possible to assess the relative contribution of patient selection or better cancer control towards the improved survival. Rigorous prospective studies are needed to optimize patient selection for SBRT in the high-risk surgical population.
Keywords: non-small cell lung cancer, surgery, stereotactic body radiation therapy, outcomes
Introduction
Surgical resection has been traditionally considered the gold standard in patients with clinical stage I non-small cell lung cancer (NSCLC). Stereotactic body radiation therapy (SBRT) was introduced over a decade ago as an alternative to conventionally fractionated radiation therapy in patients considered medically inoperable. Since then, the application of SBRT has expanded and it is often considered in patients who may be surgical candidates but face a potentially higher risk of perioperative morbidity or mortality. Several retrospective institutional studies have compared early and intermediate-term outcomes after these two treatment modalities (1–9) yet high-quality prospective trial data remain elusive.
Most comparative studies have generally found patients undergoing surgery to have longer overall survival (OS) compared to SBRT patients (3, 7, 10) particularly when the operation performed is a lobectomy. However, studies comparing local/regional recurrence and disease-free survival after surgery or SBRT have shown mixed results. (1, 3, 8, 9) A part of the problem comparing locoregional control is the lack of uniformity between treating specialties (surgeons and radiation oncologists) and individual studies in definitions of local and regional recurrence as well as the varying schedules of follow-up imaging studies employed after surgery or SBRT. Additionally, both treatment modalities have evolved with the widespread use of thoracoscopic techniques in surgery and the improvement of radiation doses and fractions in SBRT.
The literature contrasting SBRT and surgery has largely come from major academic centers with leading radiation oncology and thoracic surgery programs. Another criticism of the institutional studies has been the relatively short follow-up in the SBRT cohorts. Recent claims database analyses have studied surgery and SBRT for lung cancer in the elderly population.(11, 12)
The National Cancer Data Base (NCDB), a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, is a nationwide oncology outcomes database for more than 1,500 Commission-accredited cancer programs. About 70 percent of all newly diagnosed cases of cancer in the US are captured at the institutional level and reported to the NCDB.(13, 14) We aimed to study the actual practice patterns of treatment for stage I NSCLC in the United States and to understand the relative efficacy of surgical resection and stereotactic body radiation therapy in this population using the NCDB.
Methods
Using de-identified patient information from the NCDB participant user file, we abstracted information on patients with clinical stage I NSCLC who received treatment between 1998 and 2010 with either surgical resection (Surgery) or stereotactic body radiation therapy (SBRT). Patients who did not receive either one of these two treatment plans (Surgery or SBRT) were excluded. Specifically, the surgical cohort began in 1998 while the first SBRT case was from 2003.Patients who received only palliative treatment (as coded in the database) were also excluded from the analysis. For both the surgery and SBRT arms, patients with tumors greater than 5 cm in size, clinical T2b disease or clinical N1/2/unknown or clinical M1/unknown status were excluded. Additionally, surgical patients who received any neoadjuvant therapy (chemotherapy or radiation) were excluded. Patients who were eventually pathologically upstaged or received adjuvant therapy after surgery or SBRT were included. The study was exempted by the institutional review board.
For each patient, we obtained information on patient-related variables, tumor-related variables, treatment, and short- (30-day mortality, readmission), and long-term (overall survival) outcomes. Using information on race and income, we formed dichotomized groups in which a patient was either Caucasian or not Caucasian, and had an annual income less than or greater than $35000. Additionally, based on the population size of the area from which a patient presented rural (regional population less than 250000) and urban locations were defined. Comorbidity was annotated using the Charlson/Deyo score, categorized as 0, 1 or greater than or equal to 2. The NCDB combined those with scores of 2 or greater into one group as very few patients had scores greater than 2. Treatment facilities are classified as in the NCDB as community cancer programs, comprehensive community cancer programs, and academic/research centers in the NCDB, and the former two were categorized as non-academic centers for the purpose of this analysis.
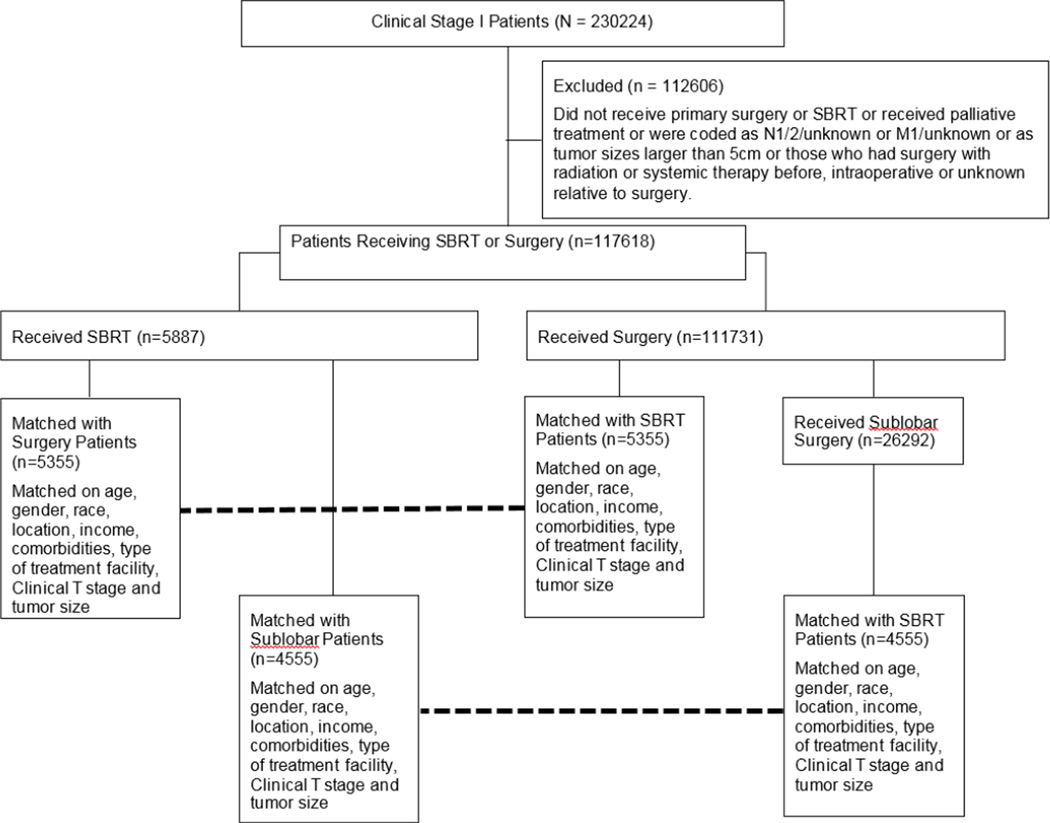
Last known vital status and the time between diagnosis and the last known follow-up date were used to determine survival. We initially contrasted patients receiving surgery to those who received SBRT in an unmatched comparison. Patients in the surgery group were then matched to those in the SBRT group using a propensity score based technique. The propensity score was the probability of receiving SBRT during the study period, estimated using a logistic regression model including age at diagnosis, gender, race, income, rural versus urban status, Charlson/Deyo score, tumor size, T1 vs. T2 status, and type of facility where treatment was administered. These variables were selected from an initial univariate analysis comparing the surgery and SBRT groups and variables significantly different between the groups were chosen for propensity matching. Patients for whom the propensity scores matched to the fourth decimal place were matched in 1:1 fashion. Automated matching was performed using the Fuzzy extension command in SPSS (SPSS 21.0 for Windows, SPSS Inc, Chicago, IL).(15) Recognizing that surgery in the form of sublobar resection (wedge resection or segmentectomy) is a closer anatomic approximation to the volume of lung parenchyma treated with SBRT and often offered to patients at higher risk from lobectomy, we performed a secondary analysis (unmatched and propensity score matched) restricting surgery patients only to those who underwent sublobar resection. (Figure 1)
Figure 1.
Consort Diagram showing schema of study subject selection and analysis.
All statistical analyses were performed using SPSS 21.0. Descriptive statistics were expressed as mean ± standard deviation unless otherwise specified. Independent samples t tests and one-way ANOVA were used to compare continuous variables. Chi-square tests were used to compare categorical data. Overall survival was estimated by the Kaplan-Meier method. The log-rank test was used to determine differences in overall survival. All statistical tests were two-sided and a 0.05 level of significance was used.
Results
Between 1998 and 2010, 230224 patients were diagnosed with clinical stage I NSCLC at 1600 institutions. A total of 117618/230224 met study criteria (figure 1) and were treated with primary surgery (n=111731, 95.0%) or SBRT (n=5887, 5.0%). The mean follow-up for the entire study group was 36.5 months. The median follow-up was longer for surgical patients (27.5 months vs. 16.6 months, p<0.001).
Patients in the surgery group were younger, and were more likely to be males and non-Caucasians. (Table 1) Surgical patients were also more likely to be from rural areas, had higher Charlson comorbidity scores, and slightly larger tumors. SBRT patients were more likely to have T1 (vs.T2) tumors and receive treatment at academic centers. In the surgery cohort, lobectomy (82749/111731, 74.1%) was the most common operation, while the remaining patients underwent a sublobar resection (26292/111731, 23.5%) or pneumonectomy (2690/111731, 2.4%). Median postoperative hospital stay was 6 days and the 30-day surgical mortality was 2596/109485 (2.4%). In surgical patients 1-year overall survival was 90.0%. One-year survival after SBRT was 85.5%.
Table 1.
Baseline characteristics, treatment-related variables, and long-term outcomes in all patients with clinical stage I NSCLC who received surgery vs. SBRT. This table shows an unmatched comparison.
| Surgery (n=111731) | SBRT (n=5887) | P value |
|||
|---|---|---|---|---|---|
| Age (years) | 67.9 ± 9.9 | 74.7 ± 8.7 | <.001 | ||
| Female Gender | 59338 (53.1%) | 3208 (54.5%) | 0.039 | ||
| Caucasian | 110560 (90.0%) | 5354 (90.9%) | 0.019 | ||
| Urban Location | 73644 (65.9%) | 3998 (67.9%) | 0.002 | ||
| Income >$35000/year | 71481 (67.5%) | 3806 (68.0%) | 0.473 | ||
| Charlson/Deyo Score (available total n=90391, surgery 84504, SBRT 5887) | 0 | 42761 (50.6%) | 0 | 3489 (59.3%) | <.001 |
| 1 | 30401 (36.0%) | 1 | 1603 (27.2%) | ||
| 2 | 11342 (13.4%) | 2 | 795 (13.5%) | ||
| Tumor Size (mm) | 24.1 ± 10.7 | 23.4 ± 9.5 | <.001 | ||
| Clinical T stage | T1 | 80184 (71.8%) | T1 | 4477 (76.0%) | |
| T2 | 31547 (28.2%) | T2 | 1410 (24.0%) | ||
| Facility Reporting Case | Nonacademic 72174 (64.6%) | Nonacademic 3470 (58.9%) | <.001 | ||
| Academic 39557 (35.4%) | Academic 2417 (41.1%) | ||||
| Type of operation | Lobectomy 82749 (74.1%) Wedge 22010 (19.7%) Segment 4282 (3.8%) Pneumonectomy 2690 (2.4%) |
||||
| Chemotherapy administered | 12514 (11.3%) | 241 (4.1%) | <0.001 | ||
| Radiation administered | 5694 (5.1%) | 5887 (100%) | <0.001 | ||
| Median radiation dose (cGy) | 5400 (n=2798) | 5400 (n=5887) | |||
| Median Survival (months) | 68.4 ± 0.4 | 33.3 ± 0.8 | <.001 | ||
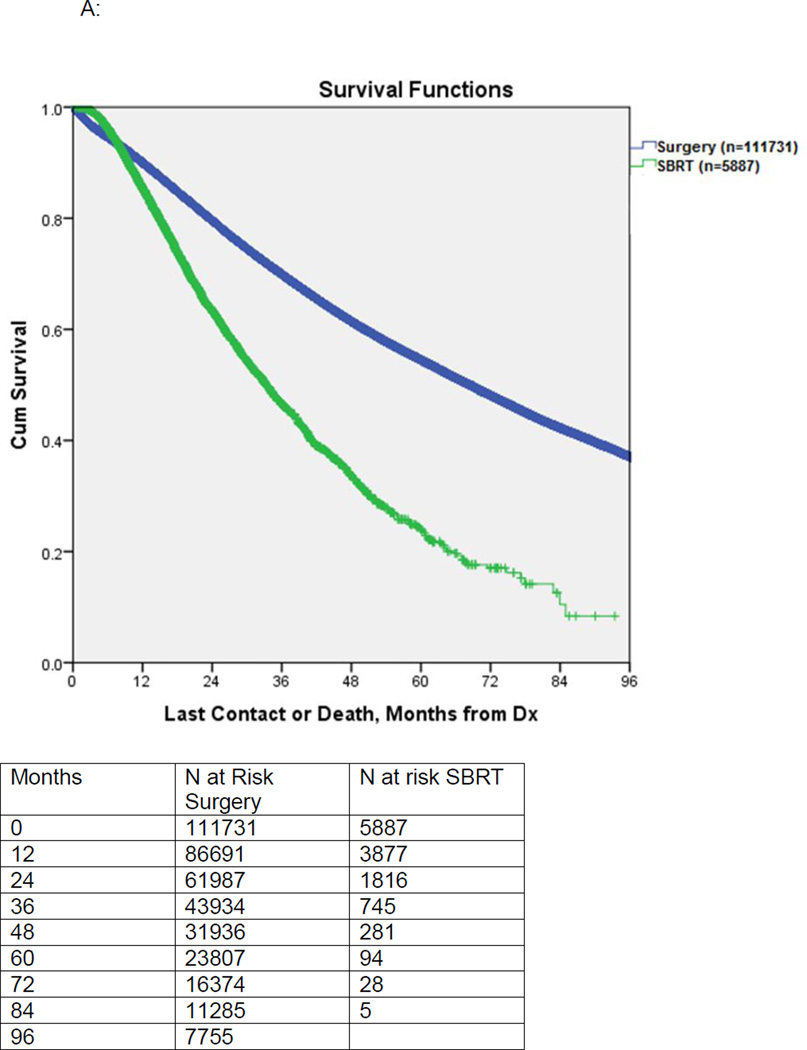
Postoperatively, 13610 of 94086 (14.5%) surgical patients with pathologic staging data available were found to have pathologic upstaging (final pathologic stage II or higher). Overall 9.1% of surgical patients received adjuvant chemotherapy only, 2.8% received adjuvant radiation alone, while 2.3% received both adjuvant chemotherapy and radiation. In the SBRT cohort, the mean radiation dose was 5383 ± 678 Gy and 4.1% of patients received chemotherapy. Median survival for unmatched patients receiving surgery vs. SBRT was 68.4 months vs. 33.3 months, respectively, (p<0.001). (Figure 2A)
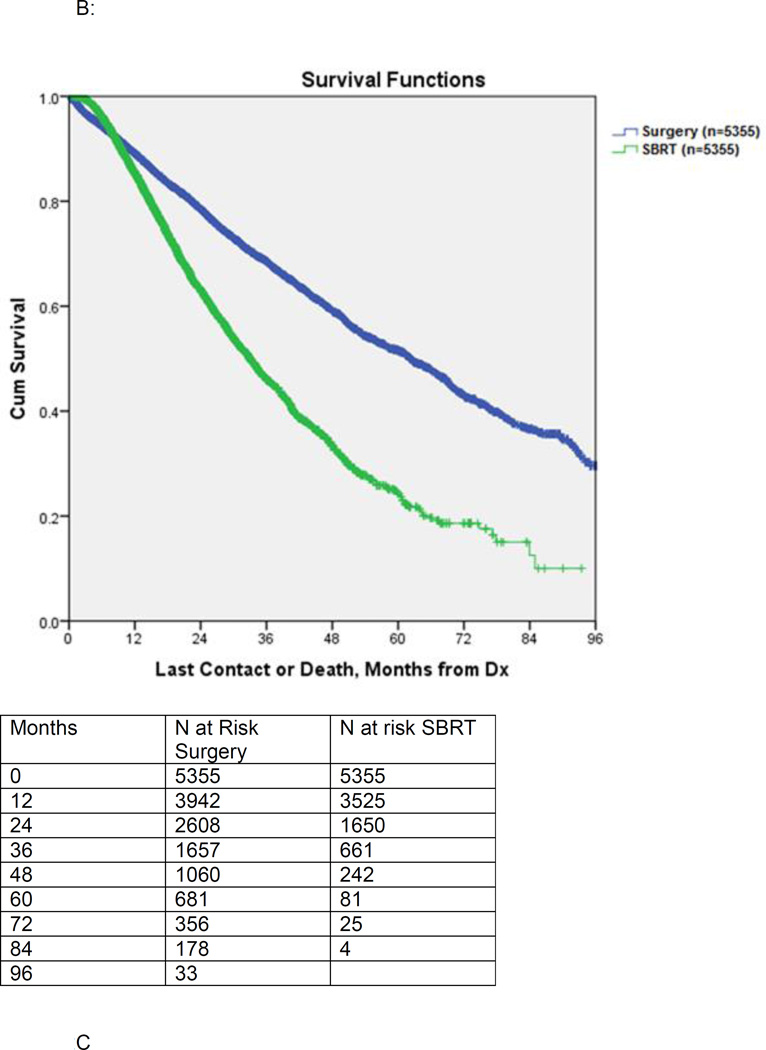
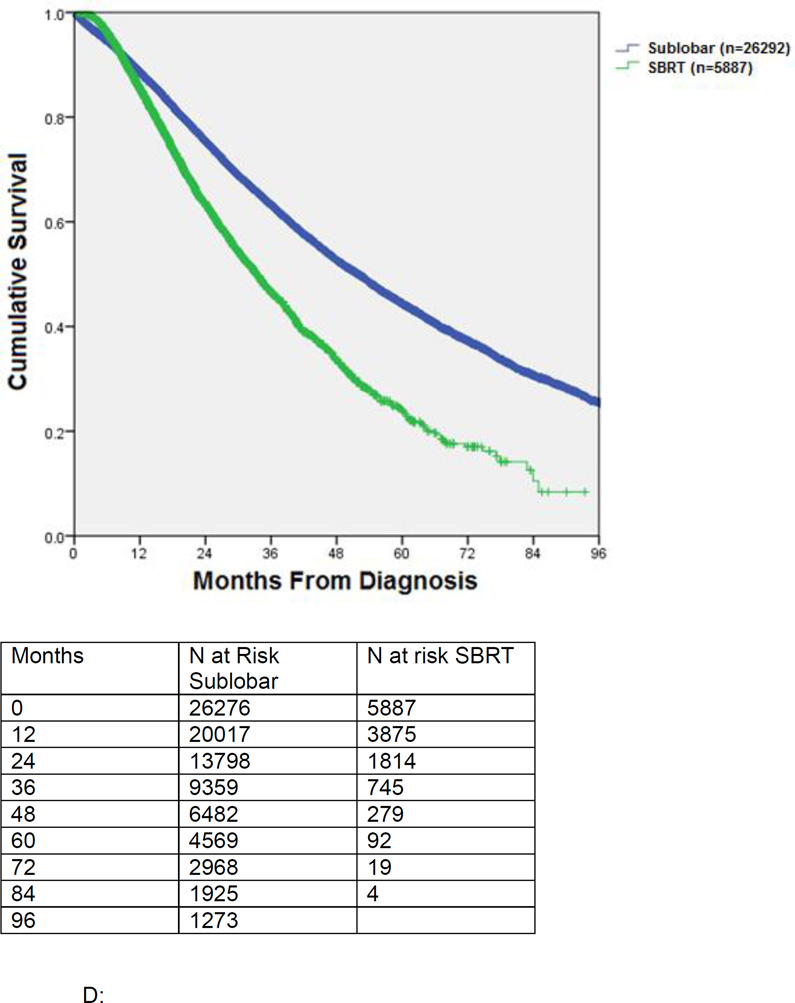
Figure 2.
A: Kaplan-Meier survival of patients undergoing surgery versus SBRT. This is an unmatched comparison.
B: Kaplan-Meier survival of patients undergoing surgery versus SBRT: propensity score matched comparison.
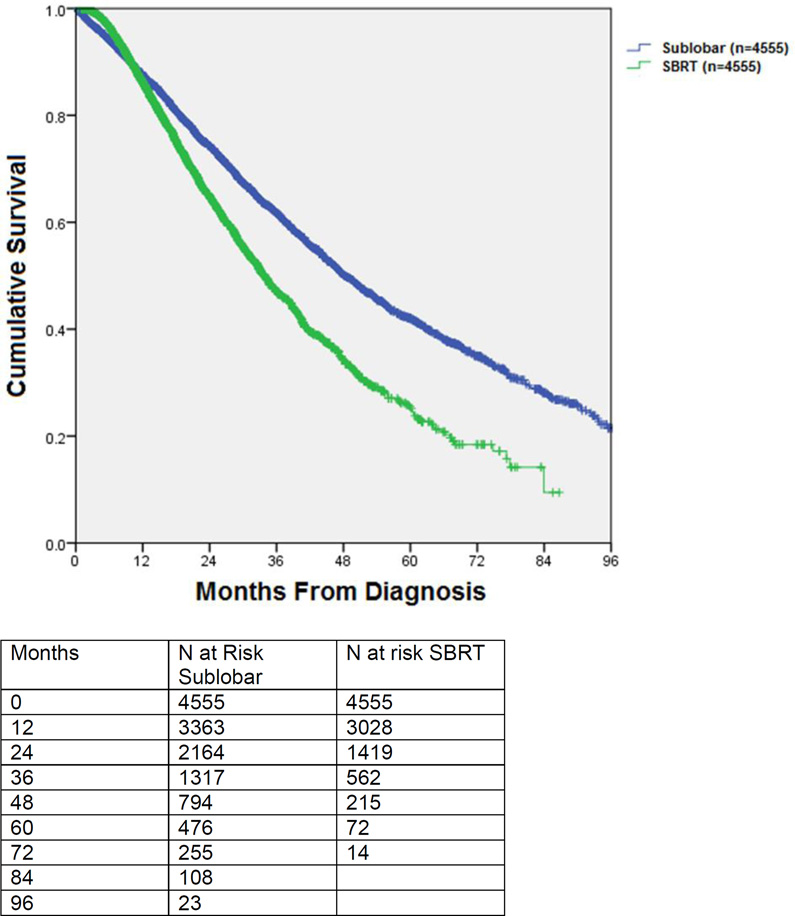
C Kaplan-Meier survival of patients undergoing sublobar resection (wedge or segmentectomy) versus SBRT. This is an unmatched comparison.
D: Kaplan-Meier survival of patients undergoing sublobar resection (wedge or segmentectomy) versus SBRT: propensity score matched comparison.
Propensity score matching between the surgery and SBRT groups yielded 5355 matched pairs. These groups were comparable in age, gender, race, location (rural versus urban), income, Charlson/Deyo comorbidity, treatment facility type, tumor size, and T1 vs. T2 status. (Table 2) The median postoperative hospital stay was 6 days and 227/5208 (4.4%) patients experienced unplanned readmissions after surgery. The 30-day surgical mortality was 136/5355 (2.5%). Median survival for matched patients receiving surgery versus SBRT was 62.3 months vs. 33.1 months, respectively (p<0.001). (Figure 2B) The 3-year survival for matched patients receiving surgery was 68.5%, compared to 46.0% for SBRT. Partitioning the matched patients into quintiles based on propensity score, the median OS for surgical patients from the lowest to the highest propensity score was 79.3 months, 69.5 months, 62.0 months, 57.5 months, and 49.9 months respectively. In the matched SBRT group the corresponding median survival was 36.2 months, 34.8 months, 30.6 months, 32.6 months, and 32.2 months respectively.
Table 2.
Baseline characteristics, treatment-related variables, and long-term outcomes in propensity score matched patients with clinical stage I NSCLC who received surgery vs. SBRT.
| Surgery (n=5355) | SBRT (n=5355) | P value |
|||
|---|---|---|---|---|---|
| Age (years) | 74.2 ± 8.4 | 74.3 ± 8.5 | 0.522 | ||
| Female Gender | 2973 (55.5%) | 2948 (55.1%) | 0.627 | ||
| Caucasian | 4838 (90.3%) | 4864 (90.8%) | 0.390 | ||
| Urban Location | 3806 (71.1%) | 3785 (70.7%) | 0.671 | ||
| Income >$35000/year | 3661 (68.4%) | 3661 (68.4%) | 1 | ||
| Charlson/Deyo Score | 0 | 3160 (59.0%) | 0 | 3102 (57.9%) | 0.406 |
| 1 | 1446 (27.0%) | 1 | 1507 (28.1%) | ||
| 2 | 749 (14.0%) | 2 | 746 (13.9%) | ||
| Tumor Size (mm) | 23.3 ± 10.3 | 23.4 ± 9.5 | 0.739 | ||
| Clinical T stage | T1 | 4099 (71.8%) | T1 | 4063 (75.9%) | 0.414 |
| T2 | 1256 (28.2%) | T2 | 1292 (24.1%) | ||
| Facility Reporting Case | Nonacademic 3146 (58.7%) | Nonacademic 3183 (59.4%) | 0.479 | ||
| Academic 2209 (41.3%) | Academic 2172 (40.6%) | ||||
| Type of operation | Lobectomy 3824 (71.4%) Wedge 1230 (23.0%) Segment 236 (4.4%) Pneumonectomy 65 (1.2%) |
||||
| Chemotherapy administered | 486 (9.2%) | 226 (4.3%) | <0.001 | ||
| Radiation administered | 198 (3.7%) | 5355 (100%) | <0.001 | ||
| Median radiation dose (cGy) | 5600 (n=141) | 5400 (n=5355) | |||
| Median Survival (months) | 62.3 ± 1.7 | 33.1 ± 0.8 | <.001 | ||
In an unmatched comparison of surgical patients undergoing sublobar resection (wedge resection or segmentectomy, n=26292) to those undergoing SBRT (n=5887), surgical patients were younger, more likely to be treated at non-academic centers, and had higher Charlson comorbidity scores (Table 3). Surgical patients also had smaller tumors and were more likely to have clinical T1 (vs. T2) tumors. Median postoperative hospital stay was 5 days and the 30-day surgical mortality was 716/19339 (3.7%). Overall 6.1% of surgical patients received adjuvant chemotherapy only, 5.4% received adjuvant radiation alone, while 2.3% received both adjuvant chemotherapy and radiation. In the SBRT cohort, the mean radiation dose was 5383 ± 678 Gy and 4.1% of patients received chemotherapy. Median survival for unmatched patients receiving surgery versus SBRT was 51.9 months vs. 33.3 months, respectively (p<0.001). (Figure 2C)
Table 3.
Baseline characteristics, treatment-related variables, and long-term outcomes in all patients with clinical stage I NSCLC who received sublobar resection (wedge resection or segmentectomy) vs. SBRT. This table shows an unmatched comparison.
| Surgery (n=26292) | SBRT (n=5887) | P value |
|||
|---|---|---|---|---|---|
| Age (years) | 70.1 ± 9.3 | 74.7 ± 8.7 | <.001 | ||
| Female Gender | 14670 (55.8%) | 3208 (54.5%) | 0.070 | ||
| Caucasian | 24016 (91.3%) | 5354 (90.9%) | 0.332 | ||
| Urban Location | 17710 (67.4%) | 3998 (67.9%) | 0.415 | ||
| Income >$35000/year | 17172 (68.8%) | 3806 (68.0%) | 0.202 | ||
| Charlson/Deyo Score (available total n=25846, surgery 19959, SBRT 5887) | 0 | 9087 (45.5%) | 0 | 3489 (59.3%) | <.001 |
| 1 | 7662 (38.4%) | 1 | 1603 (27.2%) | ||
| 2 | 3211 (16.1%) | 2 | 795 (13.5%) | ||
| Tumor Size (mm) | 19.3 ± 9.0 | 23.4 ± 9.5 | <.001 | ||
| Clinical T stage | T1 | 80184 (71.8%) | T1 | 4477 (76.0%) | <.001 |
| T2 | 31547 (28.2%) | T2 | 1410 (24.0%) | ||
| Facility Reporting Case | Nonacademic 16353 (62.2%) | Nonacademic 3470 (58.9%) | <.001 | ||
| Academic 9939 (37.8%) | Academic 2417 (41.1%) | ||||
| Type of operation | Wedge 22010 (83.7%) Segment 4282 (16.3%) |
||||
| Chemotherapy administered | 2193 (8.4%) | 241 (4.1%) | <0.001 | ||
| Radiation administered | 2015 (7.7%) | 5887 (100%) | <0.001 | ||
| Median radiation dose (cGy) | 5750 (n=1033) | 5400 (n=5887) | |||
| Median Survival (months) | 51.9 ± 0.5 | 33.3 ± 0.8 | <.001 | ||
Propensity score matching between sublobar resection only and SBRT groups yielded 4555 matched pairs. These groups were comparable in age, gender, race, location (rural versus urban), income, comorbidities, treatment facility type, tumor size, and T1 vs. T2 status. (Table 4) The median postoperative hospital stay was 5 days and 176/4421 (4.0%) patients experienced unplanned readmissions after surgery. The 30-day surgical mortality was 89/4555 (2.0%). Median survival for matched patients receiving surgery versus SBRT was 48.3 months vs. 33.9 months, respectively (p<0.001). (Figure 2D) The 3-year survival for matched patients receiving sublobar resection was 61.7%, compared to 47.0% for SBRT. Partitioning the matched patients into quintiles based on propensity score, the median OS for sublobar resection surgery patients from the lowest to the highest propensity score was 69.5 months, 51.2 months, 47.3 months, 43.9 months, and 39.3 months respectively. In the matched SBRT group the corresponding median survival was 40.9 months, 39.7 months, 32.9 months, 32.2 months, and 28.9 months respectively. A separate subgroup analysis of patients with Charlson score 2 was also performed (supplementary table).
Table 4.
Baseline characteristics, treatment-related variables, and long-term outcomes in propensity score matched patients with clinical stage I NSCLC who received sublobar resection (wedge resection or segmentectomy) vs. SBRT.
| Surgery (n=4555) | SBRT (n=4555) | P value |
|||
|---|---|---|---|---|---|
| Age (years) | 73.7 ± 8.2 | 73.8 ± 8.5 | 0.751 | ||
| Female Gender | 2504 (55.0%) | 2556 (56.1%) | 0.282 | ||
| Caucasian | 4166 (91.5%) | 4164 (91.4%) | 0.940 | ||
| Urban Location | 3214 (70.6%) | 3193 (70.1%) | 0.646 | ||
| Income >$35000/year | 3119 (68.5%) | 3139 (68.9%) | 0.651 | ||
| Charlson/Deyo Score | 0 | 2525 (55.4%) | 0 | 2459 (54.0%) | 0.366 |
| 1 | 1378 (30.3%) | 1 | 1414 (31.0%) | ||
| 2 | 652 (14.3%) | 2 | 682 (15.0%) | ||
| Tumor Size (mm) | 21.8 ± 9.4 | 21.9 ± 8.6 | 0.481 | ||
| Clinical T stage | T1 | 3641 (79.9%) | T1 | 3647 (80.1%) | 0.896 |
| T2 | 914 (20.1%) | T2 | 908 (19.9%) | ||
| Facility Reporting Case | Nonacademic 2674 (58.7%) | Nonacademic 2726 (59.8%) | 0.268 | ||
| Academic 1881 (41.3%) | Academic 1829 (40.2%) | ||||
| Type of operation | Wedge 3745 (82.2%) | ||||
| Segment 810 (17.8%) | |||||
| Chemotherapy administered | 394 (8.7%) | 187 (4.2%) | <0.001 | ||
| Radiation administered | 360 (7.9%) | 4555 (100%) | <0.001 | ||
| Median radiation dose (cGy) | 5940 (n=256) | 5400 (n=4555) | |||
| Median Survival (months) | 48.3 ± 1.2 | 33.9 ± 0.9 | <.001 | ||
With the understanding, that patients recorded in the most recent time period included in the NCDB may have less complete follow-up that those from the prior period, we undertook several subset analyses to confirm the validity of our findings in the overall cohort. We propensity matched patients undergoing surgery to those undergoing SBRT in the time period prior to 2007. Additionally, we performed a separate propensity matched analysis where the year of diagnosis was also included as a matching variable to compare patients undergoing surgery or SBRT. Finally, we performed these 2 analyses (pre 2007 era, and matching by year of diagnosis) comparing patients undergoing SBRT or sublobar resection. In all of these subset analyses, our findings from the overall cohort of patients were similar in direction and magnitude.
Discussion
These data suggest that patients with clinical stage I NSCLC selected to undergo surgery have longer overall survival versus those who undergo stereotactic body radiation therapy in both unmatched and propensity score matched comparisons. In subgroup analyses of sublobar lung resection versus SBRT, the difference persists albeit at a smaller magnitude. Lobectomy with mediastinal lymph node sampling/dissection is considered the standard of care for low-risk patients with early-stage NSCLC. More limited lung resection (wedge/segmentectomy) has been reserved for patients with suboptimal lung function or other significant comorbidities or very small/ ground-glass nodules. SBRT was initially introduced as an alternative to conventionally fractionated radiation therapy for medically inoperable patients with early-stage NSCLC.SBRT has become the preferred treatment in the medically inoperable population based upon high rates of local control, convenience, and suggestion of better survival compared to historic outcomes after conventional radiation. (16, 17) The application of SBRT has expanded to patients who are medically operable (2, 5) but may be at an elevated risk for perioperative adverse outcomes and these decisions for treatment allocation in high-risk patients are often made subjectively on an individual basis due to lack of evidence based guidelines.
A number of retrospective institutional studies have compared surgery and SBRT for clinical stage I NSCLC using propensity score matched analyses (1–4, 9) or multivariate regression models.(7) While most authors, including those of a recent meta-analysis, have noted a longer OS in the surgical patients (3, 7–10), others have found equivalent intermediate-term OS. (2, 4) Evaluating cancer-specific outcomes, some studies have reported a longer disease-free survival (DFS) with surgery (9), while others have found no differences between the treatment arms. (3) Additionally, previous publications have noted similar cancer-specific survival after surgery or SBRT. (8, 10) Several retrospective studies comparing locoregional tumor control between surgery and SBRT have been criticized for having inconsistent definitions for locoregional failure between the two arms. Two recent analyses using uniform definitions of locoregional failure have reported better local control rates with surgery, however regional and distant control rates appear to be similar. (8, 9) Most of the above-mentioned studies have included lobectomy and sublobar resection patients in the surgical cohorts. Only two direct comparisons of wedge resection and SBRT have been reported with one study showing no differences in recurrence patterns for the two arms and longer OS with surgery (7) and the other demonstrating superior disease control with surgery but equivalent OS. (6) A recent propensity matched analysis based on institutional data revealed similar cause specific survival in patients treated with sublobar resection or SBRT. (18) The same group published a propensity matched analysis of patients treated with VATS lobectomy and SBRT and noted better overall and cause specific survival in the VATS lobectomy group.(19) A potential problem in any retrospective comparison of treatment modalities is the difference in radiographic follow-up that typically defines locoregional recurrences. CT or PET scans are often employed every 3 months for patients treated with SBRT and typically at 6 month intervals for surgical patients.
Two recent comparative studies have utilized the Surveillance, Epidemiology, and End Results database linked to Medicare to compare surgery and SBRT. Shirvani and colleagues studied elderly patients with NSCLC treated between 2003 and 2009 in multivariate and propensity matched analyses and concluded that lobectomy was associated with better outcomes than sublobar resection and propensity score matching suggested that SBRT may be a good option among patients with very advanced age and multiple comorbidities. (11) Yu and colleagues studied patients treated between 2007 and 2009 and noted that for patients with short life expectancies (< 5 years), there was no difference in lung cancer mortality between surgery and SBRT. However, for patients with long life expectancies, there was greater overall mortality as well as a trend toward greater lung cancer mortality with SBRT versus surgery. (12) Our study adds to the information from these analyses by involving a larger national dataset, including all age groups of patients and not only the elderly lung cancer population, as well as by conducting distinct analyses for various subgroups including those undergoing sublobar resection as well as patients with higher comorbidity scores.
We noted longer survival in surgical patients in this national sample however the database does not include information about DFS or cancer-specific survival (CSS). Additionally, data concerning locoregional or distant recurrences are unavailable. Based on the aforementioned studies, any anticipated advantage of surgery regarding recurrence patterns would likely be moderate, and therefore unlikely to completely account for the large differences in OS observed in our study (median survival 62 months vs. 33 months). Similarly, the more frequent administration of chemotherapy in the surgical cohort (9.2% vs. 4.3%) and the relative survival benefit of this adjuvant therapy cannot explain the difference in OS.(20) This observation suggests an inherent selection bias whereby healthier patients are more likely to have been allocated to the surgical arm despite our efforts to match these patients. We suspect there still must be unmeasured covariates that influence treatment allocation for which this propensity score matching process cannot completely account.
In studying the sublobar resection propensity score matched patients by quintiles of propensity score (with higher propensity scores likely reflecting increased perceived surgical risk), we noted that median OS for the highest risk quintile sublobar resection group patients was 39 months and approached the survival of that of the overall matched SBRT cohort including all matched patients (34 months). These findings indicate that the highest propensity score quintile includes the greatest risk surgical patients and comprises those where a multidisciplinary team may have equipoise about the treatment modality especially if sublobar resection is being considered. The characterization of this high risk group of patients however is not possible with the information available in the current database.
Previous studies, including national cooperative group trials, have utilized arbitrary parameters based on expert opinion to classify possible surgical patients as high risk. (21, 22) These high-risk parameters for auditing a clinical trial has been shown to be misleading if used to guide clinical care. (23) A number of risk stratification models have been developed for patients undergoing lung resection using large national and international databases for model development and validation.(24–29) Amongst these models, the European Society Objective Score (ESOS) (24), the Thoracoscore (27), and a model derived from the Society of Thoracic Surgeons database (25) are the most prominent. The Thoracoscore was developed from a large French database and is composed of 9 variables predicting hospital mortality.(27) Kozower and colleagues al have described a regression model that associated more than 15 variables with a composite outcome of major morbidity or mortality.(25) Unfortunately, these models have found very limited application in daily clinical surgical practice while no such models have been proposed for the SBRT population. It is likely that the previously utilized risk models based upon surgical patients alone are inadequate at guiding treatment allocation (i.e. operative vs. non-operative therapy) because they fail to capture the spectrum of patients that are turned down for surgery. This subjectivity in treatment allocation is reflected even in the current analysis where there is a greater proportion of patients with Charlson score 1 in the surgical cohort, though the distribution of those with Charlson score 2 is similar between the surgical and SBRT patients. It is therefore imperative to have clinically applicable, validated risk models built with data from medically inoperable and high-risk surgical patients so that those designated in each category can be offered the appropriate treatment or counselled on the relative risk and benefits of each therapy when outcomes are deemed to be equivalent.
It is apparent, based on the limitations of the existing literature, that rigorous, prospective studies can better show the relative efficacy of surgery and SBRT in high-risk patients while simultaneously providing us with objective definitions of inoperable and high-risk patients. Unfortunately three trials attempting to answer these questions have failed to accrue and have been closed. These included the ROSEL (Radiosurgery Or Surgery for operable Early stage non-small cell Lung cancer) trial in Holland, STARS (Randomized Study to Compare CyberKnife Stereotactic Radiotherapy With Surgical Resection in Stage I Non-small Cell Lung Cancer) trial, and the American College of Surgeons Oncology Group (ACOSOG) Z4099/Radiation Therapy Oncology Group (RTOG) 1021. In light of these failures, a reasonable, stepwise alternative approach may be to create a multi-institutional registry of all patients with clinical Stage I NSCLC being evaluated for SBRT or surgery.
Our study has some strengths and limitations when compared to prior publications. It includes information from a national database that reflects actual practice patterns for all environments where patients with early-stage NSCLC receive care. Thus the findings are more likely to generalize to the population when compared to reports from highly specialized treatment centers. The large sample size available for primary and secondary analyses is another advantage compared with institutional studies where subset analysis may be underpowered. However, despite propensity score matching, our retrospective analysis likely misses a significant selection bias in treatment allocation, such that lower risk patients may have been preferentially allocated to surgery. The accuracy of individual observations in large databases is arguably lower than that in closely monitored clinical trials however the general trend of short- and long-term outcomes we observed is similar to prior cohort studies. Additionally, DFS and CSS, important parameters in long-term follow-up of a cohort of cancer patients, are unavailable from the NCDB.
We conclude that patients with clinical stage I NSCLC selected for surgery appear to show better long-term overall survival compared to SBRT. Outcomes in both treatment cohorts are strongly influenced by comorbidity and risk. Unmeasured co-variates likely play an important part in treatment allocation and may be associated with survival. Rigorous prospective studies are urgently needed to accurately define the high-risk surgical patient and optimize patient selection for SBRT in this population.
Supplementary Material
Acknowledgments
Grant support - Varun Puri -NIH K07CA178120, K12CA167540-02 (Paul Calabresi award)
References
- 1.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 2.Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I–II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013;24:1543–1548. doi: 10.1093/annonc/mdt026. [DOI] [PubMed] [Google Scholar]
- 3.Mokhles S, Verstegen N, Maat AP, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: Results from propensity score analysis. Lung Cancer. 2015 doi: 10.1016/j.lungcan.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer. 2013;119:2683–2691. doi: 10.1002/cncr.28100. [DOI] [PubMed] [Google Scholar]
- 5.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 6.Port JL, Parashar B, Osakwe N, et al. A propensity-matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. Ann Thorac Surg. 2014;98:1152–1159. doi: 10.1016/j.athoracsur.2014.04.128. [DOI] [PubMed] [Google Scholar]
- 7.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28:928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 8.Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol. 2013;8:192–201. doi: 10.1097/JTO.0b013e31827ce361. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg. 2014;147:1183–1191. doi: 10.1016/j.jtcvs.2013.11.057. discussion 1191-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Zhu F, Ma X, et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;112:250–255. doi: 10.1016/j.radonc.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014;149:1244–1253. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JB, Soulos PR, Cramer LD, et al. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer. 2015;121:2341–2349. doi: 10.1002/cncr.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller D, Guilfoyle C, Sariego J. Geographical influence on racial disparity in breast cancer presentation in the United States. Am Surg. 2011;77:933–936. [PubMed] [Google Scholar]
- 14.Raigani S, Ammori J, Kim J, Hardacre JM. Trends in the Treatment of Resectable Pancreatic Adenocarcinoma. J Gastrointest Surg. 2013 doi: 10.1007/s11605-013-2335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan J, Rosenfeldt F, Chaudhuri K, Marasco S. Cardiac surgery in patients with a history of malignancy: increased complication rate but similar mortality. Heart Lung Circ. 2012;21:255–259. doi: 10.1016/j.hlc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanni TB, Jr, Grills IS, Kestin LL, Robertson JM. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol. 2011;34:494–498. doi: 10.1097/COC.0b013e3181ec63ae. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer. 2014;50:2932–2938. doi: 10.1016/j.ejca.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg. 2015;99:1122–1129. doi: 10.1016/j.athoracsur.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 21.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg. 2011;142:1143–1151. doi: 10.1016/j.jtcvs.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012;144:S35–S38. doi: 10.1016/j.jtcvs.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puri V, Crabtree TD, Bell JM, et al. National cooperative group trials of "high-risk" patients with lung cancer: are they truly "high-risk"? Ann Thorac Surg. 2014;97:1678–1683. doi: 10.1016/j.athoracsur.2013.12.028. discussion 1683-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrisford R, Brunelli A, Rocco G, Treasure T, Utley M. The European Thoracic Surgery Database project: modelling the risk of in-hospital death following lung resection. Eur J Cardiothorac Surg. 2005;28:306–311. doi: 10.1016/j.ejcts.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 25.Kozower BD, Sheng S, O'Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875–881. doi: 10.1016/j.athoracsur.2010.03.115. discussion 881–873. [DOI] [PubMed] [Google Scholar]
- 26.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg. 2012;256:487–493. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg. 2007;133:325–332. doi: 10.1016/j.jtcvs.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg. 2008;85:1857–1865. doi: 10.1016/j.athoracsur.2008.03.024. discussion 1865. [DOI] [PubMed] [Google Scholar]
- 29.Harpole DH, Jr, DeCamp MM, Jr, Daley J, et al. Prognostic models of thirty-day mortality and morbidity after major pulmonary resection. J Thorac Cardiovasc Surg. 1999;117:969–979. doi: 10.1016/S0022-5223(99)70378-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.