Abstract
Purpose
Titmus stereoacuity testing has been used to estimate visual acuity in the evaluation of non-organic visual loss. Previous predictions were derived from optical degradation of visual acuity in normal subjects and may not account for the variability seen in patients with neuro-ophthalmic pathologies included in the differential diagnosis of non-organic visual loss. The purpose of this study was to evaluate the relationship between Titmus stereoacuity and minimal visual acuity based on a real-world testing environment.
Design
Cross-sectional, observational study.
Subjects
All patients presenting to our neuro-ophthalmology service between 4/25/2014 and 7/31/2014.
Methods
All subjects underwent routine neuro-ophthalmic examination, including Titmus stereoacuity measurements. A compound Bayesian logit-lognormal model accounting for heteroskedasticity was used to determine 95% and 99% prediction intervals of the worse eye’s near visual acuity based on stereoacuity. LogMAR acuity and log stereoacuity were analyzed.
Main Outcome Measures
Titmus stereoacuity and worse eye visual acuity.
Results
Of 561 patients, 364 subjects aged 11 to 91 years were included. Titmus stereoacuity was positively associated with VA: 9 circles correct (40 seconds of arc) indicated visual acuity of at least 20/40 with 95% confidence and 20/79 with 99% confidence; 6 circles correct (80 seconds of arc): 20/62 and 20/180, respectively; and 4 circles correct (140 seconds of arc): 20/110 and 20/570, respectively.
Conclusions
When fully accounting for individual variation and the full spectrum of neuro-ophthalmic diseases affecting visual acuity, stereoacuity remains associated with visual acuity, but previous commonly-used visual acuity estimates based on stereoacuity overestimated visual acuity. Our results more accurately predict minimum visual acuity from Titmus stereoacuity and should be preferentially used when evaluating patients with suspected non-organic visual loss. We demonstrate that Titmus stereoacuity cannot definitively prove normal visual acuity, and therefore can suggest, but not fully establish, the diagnosis of non-organic visual loss.
Stereopsis is a feature of human vision that is the result of higher order visual processing. It takes advantage of the lateral separation of the globes in space and the differential stimulation of retinal regions adjacent to fixation. It is strictly a binocular phenomenon, and it is one of many features of human vision that contributes to “depth perception.” Stereoacuity has been demonstrated to be affected by many features of viewed objects which can also affect monocular visual acuity, such as contour sharpness,1 object spacing,2 contrast, 3,4 exposure time,4 and luminance.5
There are many commercially available tests of stereoacuity that utilize different methods of disparity presentation and different object and background features.6 One of the most commonly used tests is the Titmus Stereotest (Stereo Optical Co., Inc. Chicago, IL) which is a vectographic test that utilizes polarized images and polarizing filters to display objects with differing degrees of disparity to each eye, in order to determine a threshold estimate of stereoacuity.
The measurement of stereoacuity has long been used in the field of neuro-ophthalmology in the diagnosis of non-organic visual loss.7 The logic of this is based on a supposed relationship between stereoacuity and visual acuity and the patient’s lack of knowledge of this supposed correlation. This use of stereoacuity began when Levy and Glick attempted to define the relationship between measured stereoacuity and visual acuity in 1974.8 Their study evaluated Titmus stereoacuity measures at many levels of visual acuity in 10 normal subjects with optically degraded visual acuity in one eye. The analyses utilized the average stereoacuity for each level of visual acuity in these normal subjects. They created a now commonly-used table in the field of neuro-ophthalmology for the diagnosis of nonorganic visual loss (Table 1). A subsequent study by Donzis, et al, in 1983,9 attempted to further define the relationship in normal subjects with different degrees of optically degraded binocular vision, and produced similar results.
Table 1.
Comparison of average Titmus stereoacuities as predicted by measured visual acuity of the worse eye in normal subjects with optically degraded visual acuity.
| Visual acuity of worse eye | Average stereopsis as measured in seconds of image disparity |
|---|---|
| 20/20 | 40 |
| 20/25 | 43 |
| 20/30 | 52 |
| 20/40 | 61 |
| 20/50 | 78 |
| 20/70 | 94 |
| 20/100 | 124 |
| 20/200 | 160 |
Adapted from Levy and Glick, 1974.8 For example, a patient who demonstrates a visual acuity in his worse eye of 20/60 and scores 7 circles (60 arcseconds) would be expected to have visual acuity in the worse eye no worse than 20/40. Inappropriate use of this table in this manner would suggest nonorganic visual loss.
These and other studies accurately define a relationship between mean stereoacuity and mean visual acuity in normal subjects, but they fail to predict visual acuity from stereoacuity at the level of the individual patient by failing to account for individual variation. The aim of this study is to better define this relationship and produce a more accurate tool to be used clinically for the individual patient in the diagnosis of non-organic visual loss.
Methods
This study was approved by our Institutional Review Board. This research was performed in accordance with Health Insurance Portability and Accountability Act regulations and adhered to the tenets of the Declaration of Helsinki.
The charts of all consecutive patients evaluated by our institution’s Neuro-ophthalmology service between April 25, 2014 and July 31, 2014 were reviewed. These patients had been referred for diagnosis and treatment of a variety of complaints thought to be neuro-ophthalmic in nature. While obtaining a complete history, the presence or absence of a history of childhood strabismus or amblyopia was systematically assessed by the examining physician. All patients underwent systematic detailed neuro-ophthalmic evaluation including measurement of visual acuity both at distance and near, stereoacuity, color vision, amsler grid, confrontation visual field testing, pupillary evaluation, ocular motility assessment, measurement of ocular alignment, cranial nerve testing, automated or manual perimetry when indicated, slit-lamp examination, and funduscopy. Distance visual acuity was measured using Snellen letters on a computer monitor. Near visual acuity was measured at 40 cm using numbers on a Rosenbaum near card. Diagnoses, when made, were done so based on the judgment of the examining neuro-ophthalmologist.
Stereoacuity was assessed using the Titmus polarized vectographic stereogram (Stereo Optical Co., Inc. Chicago, IL) at a distance of 40 cm, with the patients’ own near correction in place, when available. Testing distance was not measured but estimated by the testing physician. While wearing polarized glasses, patients were presented with 9 examples of four circles. Testing physicians were trained to ask the patient to “look at each of the circles and tell me which one seems to come out of the page closer to you: the top, the bottom, the right, or the left.” The test was scored based on the highest number circle set (1–9) answered correctly without missing two consecutive lower numbered circle sets, as per the manufacturer’s recommendation.
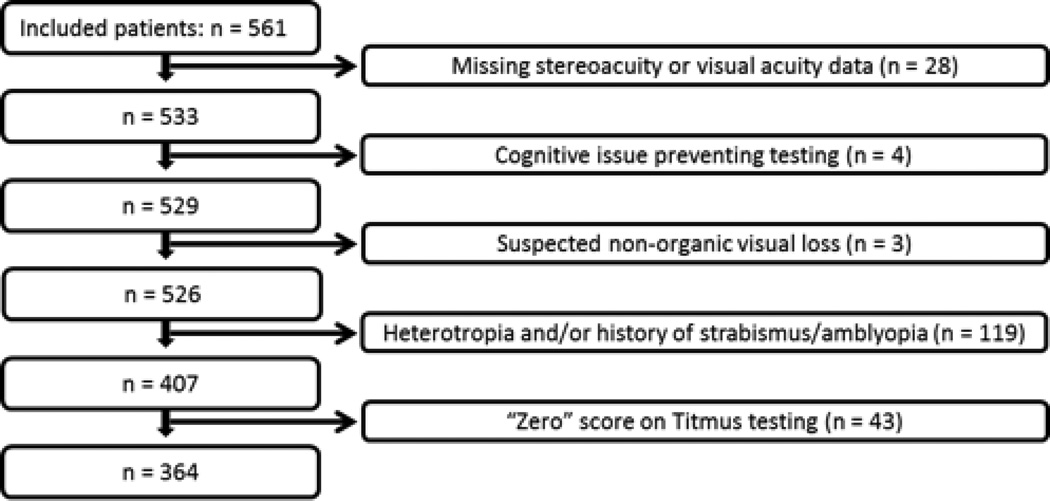
We excluded patients if there was no record of either near visual acuity of both eyes or Titmus stereoacuity, when results of such testing were unreliable because of an underlying diagnosis affecting cognition, when non-organic vision loss was suspected, when there was a history of childhood strabismus or amblyopia, when a heterotropia was present, or when eye movements were abnormal (figure 1). We also excluded from analyses patients with a Titmus stereoacuity score of zero circles because the test does not provide an estimated stereoacuity for these patients. Outliers were scrutinized for the possibility of non-organic disease, whether isolated or in combination with organic disease, and if non-organic disease was suspected these patients were excluded.
Figure 1.
Exclusion Criteria of the 561 included subjects. After exclusions for missing data, cognitive issues, suspected non-organic visual loss, the presence of a heterotropia or history of strabismus/amblyopia, and a “zero” score on the Titmus test, a total of 364 subjects remained for analysis.
The primary outcome of this study was the relationship between Titmus stereoacuity and worse eye near visual acuity. We sought to determine prediction intervals of a given confidence as opposed to averages of visual acuity to provide a tool for individual patient assessment.
Statistical analysis was performed by BBB with R: a language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org) version 3.1.2 and JAGS version 3.4.0 (Martyn Plummer, http://mcmc-jags.sourceforge.net/). Summary statistics of median, mean and frequency were calculated with complementary measures of variance (e.g., interquartile ranges, ranges). Median, 95% prediction intervals, and 99% prediction intervals of the worse eye’s visual acuity based on measured stereoacuity were determined using the posterior predictive distribution of a compound Bayesian logit-lognormal model accounting for heteroskedasticity fit to the data (commented model source code available in Supplemental Appendix). Models used three chains which had good mixing and low autocorrelation. For the purpose of statistical analysis Snellen ratios were converted to the logarithm of the minimum angle of resolution (logMAR) value and the number of circles identified was converted to log arcseconds of stereoacuity.
Results
Of the 561 patients seen in our neuro-ophthalmology clinic, 364 were included in the final analyses (figure 1). The median age of the cohort was 45 years (range, 11–91 years). There were 258 women, accounting for 71% of the total.
The mean logMAR worse eye visual acuity was 0.19 (mean Snellen equivalent 20/31). The interquartile range of logMAR worse eye visual acuity was 0.00 – 0.18 (Snellen equivalent range 20/20 – 20/30). The mean stereoacuity was 94 seconds of arc [median 60 seconds of arc (7 circles); interquartile range of 40 – 200 seconds of arc (9 circles – 3 circles)](Table 2). The frequency of diagnoses was typical for a tertiary-referral neuro-ophthalmology service (Table 3).
Table 2.
Subject characteristics (n = 364).
| Characteristics | Values |
|---|---|
| Median age | 45 years |
| Age range | 11 – 91 years |
| Gender | 71% women (n = 258) |
| Median visual acuity | 20/25 |
| Visual acuity interquartile range | 20/20 – 20/30 |
| Number of patients with acuity 20/70 or worse (%) | 35 (10%) |
| Number of patients with acuity 20/200 or worse (%) | 19 (5%) |
| Median stereoacuity | 7 circles (60 arcseconds) |
| Stereoacuity interquartile range | 40 – 200 arcseconds |
| Number of patients responding correctly to only 1–3 circles | 95 (26%) |
Table 3.
Most common diagnoses (n = 364).
| Diagnosis | Number (%) |
|---|---|
| Idiopathic Intracranial Hypertension | n =63 (17%) |
| Sellar mass | n =49 (13%) |
| Ischemic optic neuropathy | n =18 (5%) |
| Optic neuritis / multiple sclerosis | n =11 (3%) |
| Pseudo-disc edema | n =10 (2.7%) |
| Stroke | n =9 (2.5%) |
These values account for only the top 6 diagnoses, all others are not represented.
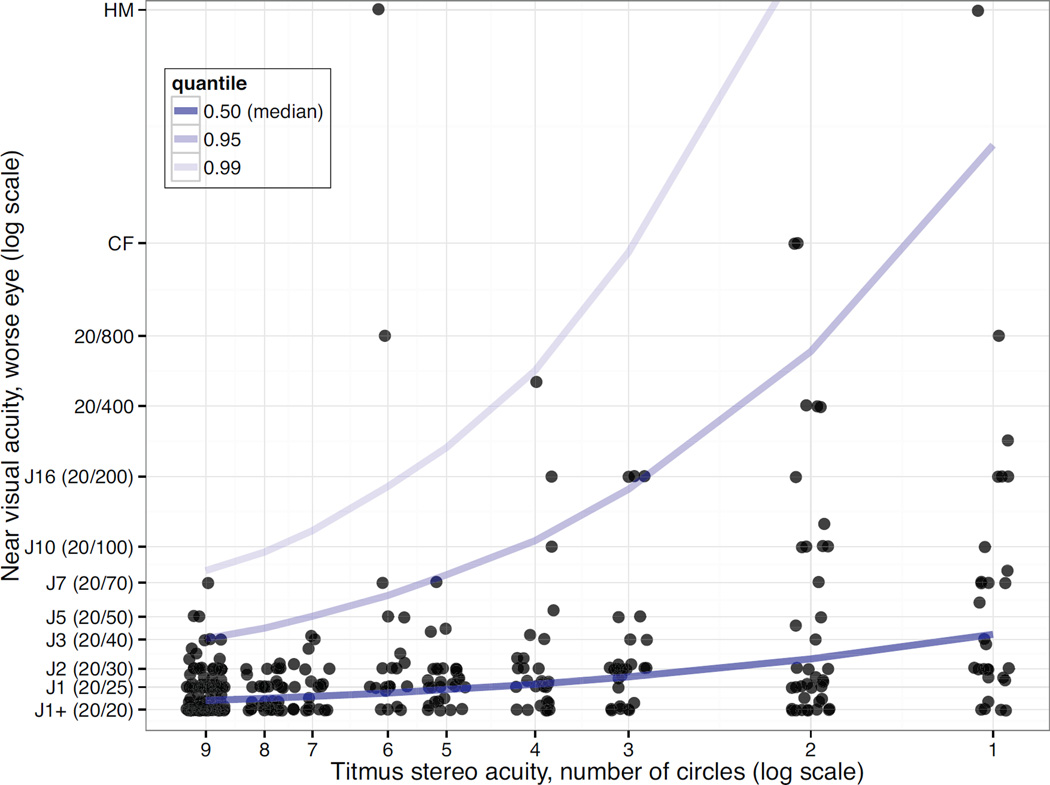
Prediction intervals for worse eye visual acuity of 95% and 99% percent were determined for all levels of stereoacuity (1 – 9 circles). Visual acuity was associated with stereoacuity (figure 2). A stereoacuity score of 9 circles (40 seconds of arc) predicted a visual acuity of 20/40 with 95% confidence and a visual acuity of 20/79 with 99% confidence (Table 4).
Figure 2.
Graphical depiction of worst eye near visual acuity (log scale) as a function of measured Titmus stereoacuity (log scale of circle score). Darkest blue line represents median visual acuity, medium blue line represents 95th prediction interval, and light blue line represents 99th prediction interval. Dots represent individual subjects and are jittered on the x-axis for visualization purposes.
Table 4.
Worse eye visual acuity as predicted from measured stereoacuity.
| Titmus Score (circles) | Worse eye visual acuity, median |
Worse eye visual acuity, 95% prediction interval (Snellen ratio) |
Worse eye visual acuity, 99% prediction interval (Snellen ratio) |
|---|---|---|---|
| 9 | 20/22 | 20/40 | 20/79 |
| 8 | 20/22 | 20/45 | 20/95 |
| 7 | 20/23 | 20/50 | 20/120 |
| 6 | 20/24 | 20/62 | 20/180 |
| 5 | 20/24 | 20/76 | 20/270 |
| 4 | 20/26 | 20/110 | 20/570 |
| 3 | 20/28 | 20/180 | 20/1800 |
| 2 | 20/33 | 20/690 | 20/39000 |
| 1 | 20/42 | 20/5300 | 20/7600000 |
Snellen equivalents converted from logMAR visual acuity and rounded to two significant digits. For example, a patient who demonstrates a visual acuity in his worse eye of 20/60 and scores 7 circles (60 arcseconds) would be expected to have visual acuity in the worse eye no worse than 20/50 with 95% certainty and no worse than 20/117 with 99% certainty. As such, non-organic visual loss would not be confirmed, but strongly suggested.
Discussion
Evaluating patients with suspected non-organic vision loss is a challenging diagnostic situation. When faced with such a patient, the examination techniques selected are often dependent on the reported visual acuity of the patient. When visual loss is reported as severe, at levels of “hand motions” to “no light perception,” effective tests rely on visual reflexes. These include optokinetic drum testing (a positive reflex suggests at least 20/400 vision), mirror reflection testing (the patient follows their own image in a mirror when vision is claimed to be hand motion or worse), and the visual threat test (a quiet but fast moving object approaching the patient's face elicits a protective movement).10 Other tests which offer no quantitative prediction of true visual acuity but rather qualitatively betray the non-organic nature of the complaint are non-visual task tests. These include having the patient attempt to touch their index fingers out in front of them, (relies solely on proprioception), or having them sign their name (relies solely on complex motor programming).10 The patient with non-organic visual loss misinterprets these as tests of vision and performs them poorly. For monocular visual loss at any level, many tests of “binocular” vision can be quantitative in addition to qualitative. These involve presenting visual optotypes to the patient while covertly masking the “normal” eye using fogging with high plus lenses, red-green lenses and a duochrome slide, or polarizing lenses and a Project-O-Chart slide.10 The patient perceives the testing to be binocular and, therefore, betrays the true visual acuity of the “bad” eye. In contrast, the monocular vertical prism dissociation test relies on the patient perceiving the test to be a monocular test of their “good” eye, and the patient betrays their true ability by perceiving two optotypes instead of one. 11
The stereoacuity test, which could be categorized as a “surrogate visual acuity test,” has the distinct advantage of being quantitative and having utility in both monocular and binocular visual loss cases. Levy and Glick’s data provided a long-accepted framework for predicting the lower limit of a patient’s worse eye visual acuity based on their measured Titmus stereoacuity.8 This framework allowed one to categorize a patient’s visual loss as being compatible or incompatible with visual loss of an organic etiology. Alternatively, measuring optokinetic nystagmus has also been proposed as a surrogate for direct measurement of visual acuity. Wester et al., in a 2007 study, described optokinetic nystagmus measurement by electrooculography to predict visual acuity.12 Their formula took into account a combination of central visual acuity and peripheral vision as measured by Goldmann manual perimetry. The need for an accurate measure of visual field limits its usefulness for predicting visual acuity in suspected non-organic visual loss.
We demonstrate that predictions of visual acuity based on the Levy and Glick data may give the examiner some false sense of security about the patient’s true ability. Levy and Glick’s table suggests that a patient who scored perfectly on the Titmus test would have a worse eye visual acuity of 20/20, or normal vision.8 Our analyses show that normal stereoacuity cannot be used to prove “normal visual acuity” as it has previously been used.7,8 However, appropriate use of stereoacuity data can still be a factor in the diagnostic process when non-organic visual loss is suspected. Like Levy and Glick8, we found that overall, worse eye visual acuity does correlate with stereoacuity, as estimated by Titmus score. However, because of substantial individual variation, one must be less strict in the assumptions about the level of a patient’s visual acuity based on their measured stereoacuity. For example, one patient with 20/70 acuity was able to score 9/9 circles, and the prediction intervals reflect that if a patient scores 9/9 circles we can be 95% certain that acuity is at least 20/40, and 99% certain that acuity is better than 20/90. This would obviously still be helpful if a patient suspected of non-organic vision loss claimed worse visual acuity, e.g. 20/200.
Of note, two subjects with visual acuity 20/200 or worse were able to score 6/9 circles. Perhaps counterintuitively, this number is about that expected by chance. When considering that each stimulus has only four responses, that correct responses to the first three stimuli require only monocular clues, and that a subject has to miss two consecutive stimuli before the test ends, subjects would be expected to reach 6 or more circles by chance about 15.6% of the time. Thus, we would have expected about 3 of our subjects with visual acuity 20/200 or worse to achieve 6 or more circles. About 2% of subjects should be able to achieve 9/9 circles by chance alone.
By analyzing the average stereopsis of normal subjects, previous studies created a way to predict the average stereoacuity of patients based on a given level of visual acuity. This, however, is not how the data have been used. Instead, people have generally used these studies to predict the best visual acuity of an individual patient based on stereoacuity. Our analyses take into account individual variation and more accurately predict visual acuity at the level of the individual patient. In addition, our data were acquired from a heterogeneous sample of patients presenting to a neuro-ophthalmology clinic, some normal, but most with some degree of ophthalmic or neuro-ophthalmic pathology. The pathology exhibited by these patients represents the relevant differential diagnoses of patients suspected of non organic visual loss, who often present to ophthalmology clinics. These data can be used to more accurately predict the lower limit of a patient’s visual acuity in the worse eye based on measured stereoacuity than can previous studies.
A weakness of our study is that the data were not acquired in a blinded fashion. Bias so introduced would tend to strengthen the association between visual acuity and stereoacuity and would therefore result in predictions of visual acuity better than the true acuity. However, since our predicted visual acuities are considerably lower than those of the available alternatives, we still feel our data have progressed in the direction of higher accuracy.
A clinician who suspects a non-organic component to a patient’s visual complaints can, and should, use a variety of testing methods to demonstrate inconsistencies in reported vision and proven visual abilities.
It is the burden of the examining physician to eliminate from the differential all true pathology in such cases. We believe that stereoacuity is still useful in evaluating patients with suspected non-organic vision loss; however, its use to demonstrate a certain range of vision is more accurate with our data. Normal stereoacuity cannot be used to predict normal visual acuity. Our analyses (Table 4) should be used preferentially when using stereoacuity in the evaluation of non-organic visual loss.
Supplementary Material
Acknowledgments
Financial Support: Supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York and by NIH/NEI core grant P30-EY006360 (Department of Ophthalmology) and NEI grant K23-EY019341 (Bruce). The sponsor or funding support had no role in the design and conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no proprietary or commercial interest in any materials discussed in this article
References
- 1.Stigmar G. Blurred visual stimuli II: The effect of blurred visual stimuli on Vernier and stereo acuity. Act Ophthalmol. 1971;49:364–379. doi: 10.1111/j.1755-3768.1971.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 2.Berry RN. Quantitative relations among Vernier, real depth, and stereoscopic depth acuities. J Exp Psychol. 1948;38:708–721. doi: 10.1037/h0057362. [DOI] [PubMed] [Google Scholar]
- 3.Geib T, Baumann C. Effect of luminance and contrast on stereoscopic acuity. Graefes Arch Clin Exp Ophthalmol. 1990;228:310–315. doi: 10.1007/BF00920053. [DOI] [PubMed] [Google Scholar]
- 4.Westheimer G, Pettet MW. Contrast and duration of exposure differentially affect Vernier and stereoscopic acuity. Proc R Soc Lond B. 1990;241:42–46. doi: 10.1098/rspb.1990.0063. [DOI] [PubMed] [Google Scholar]
- 5.Mueller CG, Lloyd VV. Stereoscopic acuity for various levels of illumination. Proc Natl Acad Sci U S A. 1948;34:223–227. doi: 10.1073/pnas.34.5.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fricke TR, Siderov J. Stereopsis, stereotests, and their relation to vision screening and clinical practice. Clin Exp Optom. 1997;80:165–172. [Google Scholar]
- 7.Miller NR. Neuro-ophthalmologic manifestations of nonorganic disease. In: Miller NR, Newman NJ, Biousse V, Kerrison JB, editors. Walsh & Hoyt’s Clinical Neuro-Ophthalmology. 6th ed. Philadelphia: Lippincott, Williams & Wilkins; 2005. pp. 1315–1336. [Google Scholar]
- 8.Levy NS, Glick EB. Stereoscopic perception and Snellen VA. Am J Ophthalmol. 1974;78:722–724. doi: 10.1016/s0002-9394(14)76312-3. [DOI] [PubMed] [Google Scholar]
- 9.Donzis PB, Rappazzo A, Burde RM, Gordon M. Effect of binocular variations of Snellen’s VA on Titmus stereoacuity. Arch Ophthalmol. 1983;101:930–932. doi: 10.1001/archopht.1983.01040010930016. [DOI] [PubMed] [Google Scholar]
- 10.Miller N. Functional neuro-ophthalmology. In: Aminoff MJ, Boller F, Swaab DF, editors. Handbook of Clinical Neurology. 3rd Series. New York, NY: Elsevier; 2011. pp. 493–502. [DOI] [PubMed] [Google Scholar]
- 11.Golnik KC, Lee AG, Eggenberger ER. The monocular vertical prism dissociation test. Am J Ophthalmol. 2004;137:135–137. doi: 10.1016/s0002-9394(03)00865-1. [DOI] [PubMed] [Google Scholar]
- 12.Wester ST, Rizzo JF, III, Balkwill MD, Wall C., III Optokinetic nystagmus as a measure of visual function in severely visually impaired patients. Invest Ophthalmol Vis Sci. 2007;48:4542–4548. doi: 10.1167/iovs.06-1206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.