Abstract
Purpose
To evaluate the association between refractive error and the prevalence of glaucoma by race or ethnicity.
Design
Cross-sectional study.
Participants
Kaiser Permanente Northern California Health Plan members with refractive error measured at 35 years of age or older between 2008 and 2014 and with no history of cataract surgery, refractive surgery, or a corneal disorder.
Methods
We identified 34 040 members with glaucoma or ocular hypertension (OHTN; cases) and 403 398 members without glaucoma (controls). Glaucoma cases were classified as primary angle-closure glaucoma (PACG); 1 of the 4 forms of open-angle glaucoma: primary open-angle glaucoma (POAG), normal-tension glaucoma (NTG), pigmentary glaucoma (PIGM), and pseudoexfoliation glaucoma (PEX); or OHTN. Refractive error, expressed as spherical equivalent (SE), was coded as a continuous trait and also as categories. Logistic regression analyses were used to estimate the association between refractive error and the prevalence of glaucoma overall and in specific racial or ethnic groups.
Main Outcome Measures
The association between refractive error and glaucoma subtypes evaluated as odds ratios (ORs) with 95% confidence intervals (CIs).
Results
In controls, the mean SE was −0.59 diopters (D) (standard deviation, 2.62 D). Each 1-D reduction in SE was associated with a 22% decrease in the odds of PACG (OR, 0.78; 95% CI, 0.77–0.80) and with increases in the odds of open-angle glaucoma ranging from 1.23 (95% CI, 1.20–1.26) for PIGM, to 1.07 (95% CI, 1.03–1.11) for PEX, and to 1.05 (95% CI, 1.04–1.06) for OHTN. In addition, we observed a stronger association between myopia and POAG among non-Hispanic whites (OR, 1.12; 95% CI, 1.11–1.13) and NTG among Asians (OR, 1.17; 95% CI, 1.15–1.20) and non-Hispanic whites (OR, 1.19; 95% CI, 1.15–1.22).
Conclusions
Myopia was associated with an increased prevalence of all forms of open-angle glaucoma and OHTN, whereas hyperopia was associated with a substantially increased prevalence of PACG. Although high myopia is a strong risk factor for glaucoma subtypes, low and moderate myopia also have a significant effect on glaucoma risk. Additionally, there were moderate racial differences in the association of myopia with the risk of POAG and NTG.
Glaucoma refers to a group of ocular disorders that are associated with progressive optic neuropathy. It is the second leading cause of blindness worldwide.1 Known risk factors include advanced age, black race, positive family history, and elevated intraocular pressure (IOP).2–6 Several large cross-sectional studies have reported a higher prevalence of primary open-angle glaucoma (POAG), the most common form of glaucoma, among myopic individuals compared with those without myopia,7–13 indicating that refractive error may play a role in the pathogenesis of glaucoma. Yet, the possible etiologic link between refractive error and glaucoma is poorly understood. Individuals with axial myopia may have weaker scleral support at the optic nerve, which may result in greater susceptibility of the optic nerve to glaucomatous damage.14 In some studies, myopic eyes have been reported to have slightly higher IOP and thinner central corneal thickness (CCT) than emmetropic or hyperopic eyes.7,15 If myopia partially mediates the risk of POAG through weaker scleral support, elevated IOP, or both, it also may predispose individuals to other forms of glaucoma.
Compared with individuals of European descent, African ancestry is associated with a higher risk of POAG developing,4 whereas Japanese have a higher incidence and prevalence of normal-tension glaucoma (NTG).16 In addition, some East Asian populations may be more susceptible anatomically to primary angle-closure glaucoma (PACG),17 although reasons for this racial difference are unclear, and the role of refractive error in this difference has not been well studied. Indeed, the relationships between refractive error and the risks of glaucoma subtypes in different racial and ethnic groups are only poorly understood.
The purpose of this study was to assess the associations between refractive error and the prevalence of glaucoma subtypes, specifically, PACG; several forms of open-angle glaucoma, including POAG, NTG, pigmentary glaucoma (PIGM), and pseudoexfoliation glaucoma (PEX); and ocular hypertension (OHTN), a condition of high IOP without signs of glaucomatous damage. The study was based on 437 438 Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) members 35 years of age or older who underwent refractive error measurement during the study period from 2008 through 2014. We further examined whether the observed association of refractive error with the prevalence of glaucoma varied by race or ethnicity. Recent studies have documented an increased prevalence of myopia in younger birth cohorts worldwide, including the United States18; thus, it is important to understand how refractive error influences the risk of glaucoma.
Methods
Setting
Study participants were identified from the KPNC, a large nonprofit integrated healthcare delivery system with 3.5 million active members comprising approximately 30% of the population of Northern California. The KPNC membership has been shown to be representative of the general population with respect to demographic characteristics, including racial or ethnic diversity, with some underrepresentation at the extremes of income.19 Since 1995, KPNC has recorded diagnoses, prescriptions, and procedures in a comprehensive electronic health record (EHR) system. The EHR was enhanced in 2007 to capture refractive errors, IOP, CCT, and cup-to-disc ratio (CDR) data.
Glaucoma was diagnosed by KPNC ophthalmologists through comprehensive eye examinations, which typically included measurements of visual acuity, IOP by tonometry, CCT by pachymetry, CDR by ophthalmoscopy and visual field testing, photography of the optic nerve head, and evaluation of the nerve fiber layer by optical coherence tomography. A diagnosis of glaucoma was established on the basis of optic nerve defects and corresponding visual field loss.
Study Population
Institutional Review Board approval was obtained. Eligible cases and controls were drawn from KPNC members who had refractive errors measured at 35 years of age or older between 2008 and 2014 and did not have conditions or procedures that can influence either the measurement or accuracy of refractive error. Specifically, we excluded individuals with histories of cataract surgery (in either eye), refractive surgery, keratitis, or corneal diseases (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes, 370.xx or 371.xx). We also excluded subjects who had diagnoses of borderline glaucoma (ICD-9-CM code, 365.0), preglaucoma (ICD-9-CM code, 365.00), or unspecified glaucoma (ICD-9-CM codes, 365.7 and 365.9) without a more specific diagnosis. In addition, we required controls to have 5 years or more of KPNC membership between 2008 and 2014 to ensure adequate length of observation for glaucoma. Glaucoma cases were not required to have 5 years of KPNC membership because their refractive error was measured at the time that glaucoma was recorded.
Glaucoma Cases. To investigate the association of refractive error with glaucoma subtypes, subjects with glaucoma were divided into 6 subgroups based on the most specific diagnoses recorded in the EHR. The POAG cases had 1 or more diagnoses of POAG (ICD-9-CM codes, 365.01, 365.05, 365.1, 365.10, 365.11, and 365.15) and no diagnosis of any other type of glaucoma, including secondary glaucoma. The NTG cases had at least 1 diagnosis of ICD-9-CM 365.12. The OHTN cases had an OHTN diagnosis (ICD-9-CM code, 365.04) with no diagnoses of POAG. The PEX, PIGM, and PACG cases were identified by ICD-9-CM codes 365.52, 365.13, and 365.2, respectively.
Controls. Eligible controls had no diagnosis of any type of glaucoma (ICD-9-CM code, 365.xx), no documented IOP of 22 mmHg or more in either eye, and no interocular CDR difference of 0.2 or more.
Data Collection
We categorized subjects as non-Hispanic white, black, Asian, Latino or Hispanic, or other based on self-reported race or ethnicity information recorded in the EHR.
Electronic Health Record System and Refractive Error Measures. Measurement of refractive error was a standard workflow component in most ophthalmology and optometry encounters. Intraocular pressure, CCT, and CDR usually were measured as part of the diagnostic workup for glaucoma, although IOP and CDR also were recorded commonly during routine eye examinations. Most subjects had multiple measures for both eyes, and these measures were highly correlated. For this study, we used measurements obtained from the right eye only. We selected for analysis the first documented spherical equivalent (SE) refractive error (calculated as sphere + cylinder/2), the maximum of all recorded CDR, and the median of all recorded IOP and CCT measurements.
Approximately half of the glaucoma cases were diagnosed before 2007; their SEs were recorded into the EHR after 2007, when the refractive error module in the EHR was implemented. Refractive errors generally are stable throughout adulthood. In the case of myopia, the typical onset occurs during childhood or adolescence, well before the time of onset of the glaucoma subtypes investigated in this study. Glaucoma patients usually receive IOP-lowering medications, but these treatments generally do not alter refractions, with the exception of parasympathetic mitotics.20,21 Hence, our analysis included prevalent glaucoma cases with SE measured after the glaucoma diagnosis.
Statistical Analysis
Analyses were conducted using R software version 3.1.1 (https://www.r-project.org/). After univariate analysis, we conducted multivariate logistic regression, stratifying by race or ethnicity, to evaluate the association between SE and the prevalence of each glaucoma subtype, specifically modeling SE as a continuous variable to estimate the effect of a per-diopter (D) decrease in SE, including as covariates age at the first refractive error measurement and gender. Heterogeneity across races was tested using the I2 statistic and Cochran's Q statistic. If no significant heterogeneity was detected across racial or ethnic groups, we then combined odds ratios (ORs) across racial or ethnic groups using a fixed-effects inverse variance weighted model; otherwise, a random effects model was applied. To evaluate further how low, moderate, and high myopia, as well as degrees of hyperopia, influenced the prevalence of glaucoma subtypes, we divided SE into 6 groups, (≤−6.00 D, −5.99 to −3.00 D, −2.99 to −1.00 D, −0.99 to 1.00 D, 1.01–3.00 D,≥3.01 D) and conducted logistic regression, adjusting for age at measurement of refractive error (as continuous), gender, and race or ethnicity (5 groups) to calculate ORs and 95% confidence intervals (CIs). All tests of statistical significance were 2 sided and considered statistically significant at P ≤ 0.05.
Results
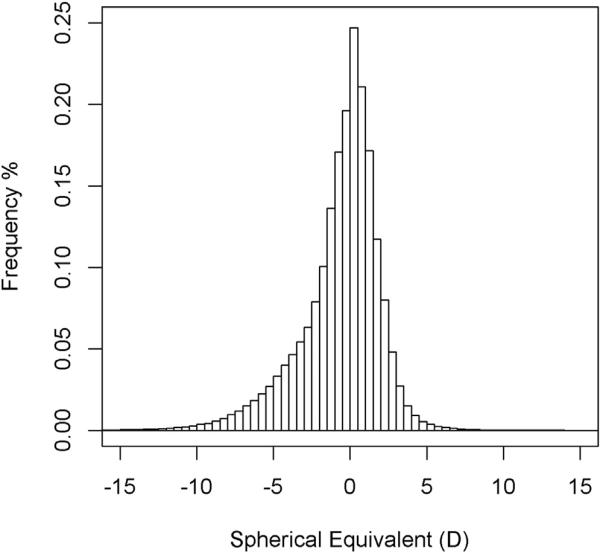
A total of 1 679 185 KPNC members were eligible for the study. Of those, 52% of the glaucoma cases (n = 69 939) and 36% of the controls (n = 536 761) had refractive errors measured at 35 years of age or older (Table 1). A history of cataract surgery was the most common reason for exclusion, and the proportion of cases that were excluded for this reason varied by glaucoma subtype, ranging from 32% for OHTN to 76% for PEX. After these exclusions, a total of 34 040 glaucoma cases (49%) and 403 398 controls (75%) remained in the study. The average length of enrollment during the study period from 2008 through 2014 was 6.2 years (standard deviation [SD], 1.6 years) in glaucoma cases and 6.7 years (SD, 0.7 years) in controls. The mean numbers of SE measurements per subject were 3.13 (SD, 2.41) for POAG, 3.51 (SD, 2.54) for NTG, 3.14 (SD, 2.30) for OHTN, and 2.84 (SD. 2.10) for controls. The mean SE was −0.6 D (SD, 2.6 D) among controls (Fig 1), 1.1 D (SD, 2.3 D) in PACG patients, and −0.6 D (SD, 2.8 D) in the combined open-angle glaucoma and OHTN patients. Table 2 summarizes the characteristics of the 6 glaucoma case groups and the glaucoma-free controls. Glaucoma patients were older than controls (58 ± 12 years), with PEX patients being substantially older (76 ± 9 years) and PIGM patients being the youngest (62 ± 11 years). Women constituted 57% of controls, 70% of PACG patients, and 44% of PIGM patients.
Table 1.
Eligible Glaucoma Cases and Controls, Kaiser Permanente Northern California, 2008–2014
| Characteristic | Controls* | Primary Angle-Closure Glaucoma |
Pseudoexfoliation Glaucoma |
Primary Open-Angle Glaucoma |
Normal- Tension Glaucoma |
Pigmentary Glaucoma |
Ocular Hypertension |
|---|---|---|---|---|---|---|---|
| Age at first spherical equivalent measure ≥35 years of age | 536 761 | 6320 | 2436 | 29 093 | 3396 | 1267 | 26 817 |
| Underwent cataract surgery | 85 153 (15.9%) | 3632 (57.5%) | 1856 (76.2%) | 14 774 (50.8%) | 1473 (43.4%) | 576 (45.5%) | 8543 (31.9%) |
| Underwent refractive surgery (and no cataract surgery) | 16 298 (3.0%) | 43 (0.7%) | 6 (0.2%) | 298 (1.0%) | 68 (2.0%) | 22 (1.7%) | 364 (1.4%) |
| Corneal disorder (and no cataract surgery or refractive surgery) | 31 912 (5.9%) | 306 (4.8%) | 71 (2.9%) | 1459 (5.0%) | 180 (5.3%) | 74 (5.8%) | 1544 (5.8%) |
| Eligible in the analysis† | 403 398 (75.2%) | 2339 (37.0%) | 503 (20.6%) | 12,562 (43.2%) | 1675 (49.3%) | 595 (47.0%) | 16 366 (61.0%) |
Controls were Kaiser Permanente members ≥35 years of age as of June 30, 2014, with at least 5 years of membership from 2008–2014.
Eligible glaucoma cases and controls were subjects with valid spherical equivalent measures at age 35 or old and without history of intraocular lens placement, refractive surgery, or corneal disorder.
Figure 1.
Histogram showing the spherical equivalent in right eyes in diopters (D) among controls.
Table 2.
Characteristics of Glaucoma Cases and Controls, Kaiser Permanente Northern California, 2008–2014
| Controls | Primary Angle-Closure Glaucoma |
Pseudoexfoliation Glaucoma |
Primary Open-Angle Glaucoma |
Normal-Tension Glaucoma |
Pigmentary Glaucoma |
Ocular Hypertension |
|
|---|---|---|---|---|---|---|---|
| Total (no.) | 403 398 | 2339 | 503 | 12 562 | 1675 | 595 | 16 366 |
| Race (%) | |||||||
| Asian | 16.70 | 20.50 | 7.20 | 12.60 | 30.90 | 2.40 | 11.20 |
| Black | 7.00 | 8.90 | 2.40 | 18.30 | 11.00 | 3.50 | 12.10 |
| Hispanic/Latino | 14.60 | 16.10 | 10.90 | 14.60 | 15.20 | 14.60 | 13.70 |
| Non-Hispanic white | 54.20 | 45.70 | 71.20 | 47.50 | 34.60 | 72.40 | 55.70 |
| Other | 4.80 | 7.60 | 6.80 | 5.30 | 6.40 | 4.70 | 5.30 |
| Gender (%) | |||||||
| Female | 57.10 | 69.90 | 61.20 | 50.60 | 53.30 | 44.20 | 58.60 |
| Male | 42.90 | 30.10 | 38.80 | 49.40 | 46.70 | 55.80 | 41.40 |
| Age in yrs (%) | |||||||
| 35–49 | 26.40 | 4.30 | 0.20 | 6.40 | 5.90 | 14.80 | 10.30 |
| 50–64 | 41.00 | 29.00 | 10.50 | 32.20 | 33.60 | 42.90 | 39.70 |
| 65+ | 32.60 | 66.70 | 89.30 | 61.40 | 60.50 | 42.40 | 50.00 |
| Mean (SD) | 58.38±12.3 | 68.72±10.84 | 76.21±9.32 | 67.41±11.27 | 66.85±10.86 | 62.03±11.11 | 64.10±11.29 |
| CDR*,† | |||||||
| Subjects with at least 1 measure (%) | 33.40 | 51.60 | 52.30 | 53.10 | 61.20 | 54.80 | 43.70 |
| Mean±SD | 0.31±0.12 | 0.44±0.21 | 0.47±0.20 | 0.55±0.2 | 0.63±0.18 | 0.50±0.21 | 0.41±0.17 |
| IOP (mmHG)†,‡ | |||||||
| Subjects with at least 1 measure (%) | 78.40 | 95.90 | 97.20 | 97.10 | 99.00 | 97.00 | 88.60 |
| Mean±SD | 14.98±2.73 | 16.55±3.33 | 17.24±3.76 | 17.10±3.47 | 14.29±2.45 | 17.11±3.45 | 18.40±3.55 |
| CCT (μm)†,‡ | |||||||
| Subjects with at least 1 measure (%) | 1.60 | 51.50 | 69.80 | 71.30 | 83.90 | 67.40 | 42.60 |
| Mean±SD | 560.96±37.87 | 550.70±39.05 | 549.30±38.06 | 550.28±38.42 | 539.89±35.73 | 557.06±38.27 | 570.13±39.52 |
| First spherical equivalent (D) in the right eye | |||||||
| Mean±SD | –0.59±2.62 | 1.14±2.33 | 0.10±2.55 | –0.67±2.91 | –1.37±3.22 | –1.96±2.92 | –0.51±2.73 |
CCT = central corneal thickness; CDR = cup-to-disc ratio; D = diopters; IOP = intraocular pressure; SD = standard deviation.
Maximum value for each subject.
All measures were of the right eye for each subject.
Median value for each subject.
The prevalence of glaucoma subtypes varied by race and ethnicity. The PACG and NTG cases were disproportionately common among Asian subjects, whereas POAG and OHTN were disproportionately common in black subjects. Both PEX and PIGM were less common and overrepresented among non-Hispanic white subjects.
Clinical features also varied with the subtype of glaucoma. As expected, glaucoma cases generally had higher CDRs and higher IOPs than controls. Also as expected, the OHTN patients had relatively high IOP, low CDR, and thick CCT, whereas NTG patients had relatively low IOP, high CDR, and thin CCT.
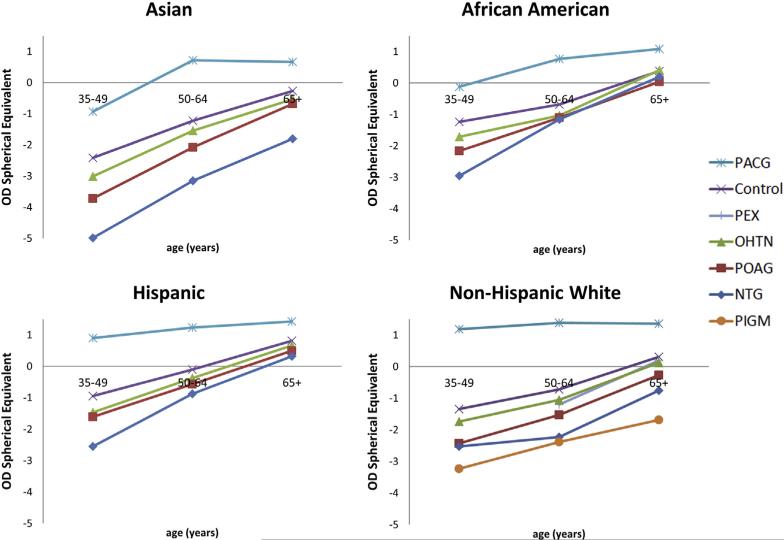
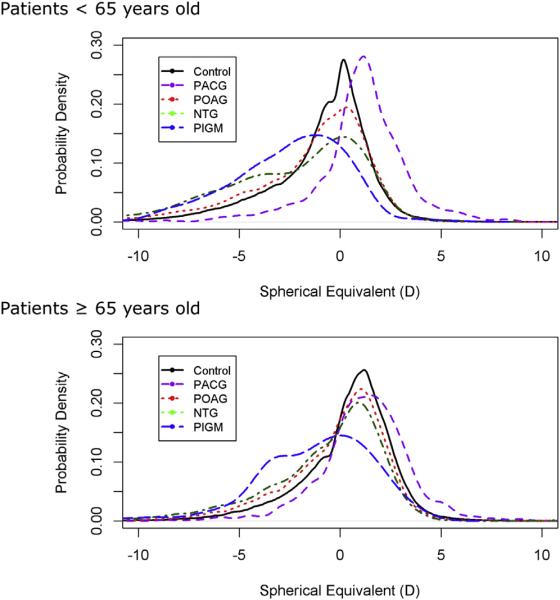
The mean SEs are shown graphically by race and ethnicity and age for glaucoma cases and controls in Figure 2. Because of the small numbers of PEX and PIGM cases in minority racial or ethnic groups, relevant SE data are shown only for non-Hispanic whites. Overall, the NTG and PIGM groups were the most myopic. Hyperopic shifts with age across all glaucoma subtypes and controls are evident. The mean SEs of the group 35 to 49 years of age and the group 65 years of or older differ in absolute terms by 2.15 D for Asians, 1.62 D for blacks, 1.76 D for Hispanics or Latinos, and 1.65 D for non-Hispanic whites. Strikingly, across all age groups, PACG patients were far more hyperopic, and patients with all forms of open-angle glaucoma were more myopic than controls, although observed differences for open-angle glaucoma were substantially greater for younger persons than for older persons. These trends were consistent across all racial and ethnic groups. Although mean SE provides a summary measure of refractive error, it does not describe the shape of the distribution. To understand better how the full range of refractive error differs across glaucoma subtypes, we examined the distributions of SE in glaucoma cases with that in controls for 2 age categories, younger than 65 years and 65 years of age or older (Fig 3). We observed larger differences in SE between patients and controls in the younger age group (younger than 65 years), especially PACG patients.
Figure 2.
The comparison of mean spherical equivalent in right eyes (OD) among glaucoma cases and controls by age and racial or ethnic groups. The pattern is consistent across age and racial or ethnic groups: primary angle-closure glaucoma (PACG) cases are more hyperopic, whereas among all forms of open-angle glaucoma, cases are more myopic than controls. NTG = normal-tension glaucoma; OHTN = ocular hypertension; PEX = pseudoexfoliation glaucoma; PIGM = pigmentary glaucoma; POAG = primary open-angle glaucoma.
Figure 3.
The comparison of spherical equivalent distribution in right eyes (OD) between glaucoma cases and controls. There were greater differences in spherical equivalent among glaucoma cases and controls younger than 65 years than those 65 years of age or older. Distributions are plotted as probability density. NTG = normal-tension glaucoma; PACG = primary angle-closure glaucoma; PIGM = pigmentary glaucoma; POAG = primary open-angle glaucoma.
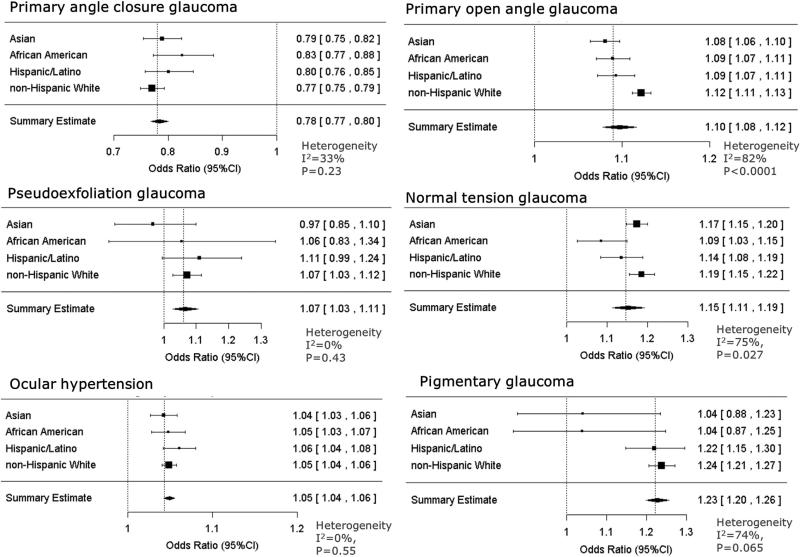
For each glaucoma subtype, we estimated the prevalence associated with each 1-D decrease in SE in each racial or ethnic group separately, adjusting for age (coded as continuous variable) and gender and examining the overall effect of refractive error on glaucoma prevalence (Fig 4). Each 1-D decrease in SE (i.e., less hyperopic) was associated with a 22% reduction in the prevalence of PACG (OR, 0.78; 95% CI, 0.77–0.80), indicating that PACG cases were more likely to be hyperopic than controls. The prevalence of each of the open-angle glaucoma subtypes was associated with a greater level of myopia (more negative SE), with ORs ranging from 1.05 (95% CI, 1.04–1.06) for OHTN to 1.23 (95% CI, 1.20–1.26) for PIGM. We observed significant heterogeneity in the OR with race or ethnicity among POAG patients (I2 = 82%; P < 0.0001) and NTG patients (I2 = 75%; P = 0.027). For POAG, the OR was larger in non-Hispanic white patients (OR, 1.12; 95% CI, 1.11–1.13) than other groups. For NTG, the effect of refractive error on prevalence was smaller in black patients (OR, 1.09; 95% CI, 1.03–1.15) than other groups.
Figure 4.
Analyses of the effect of a per-diopter decrease in spherical equivalent refractive error on the risk of glaucoma, stratified by race and ethnicity, for 5 different types of glaucoma as well as ocular hypertension. CI = confidence interval.
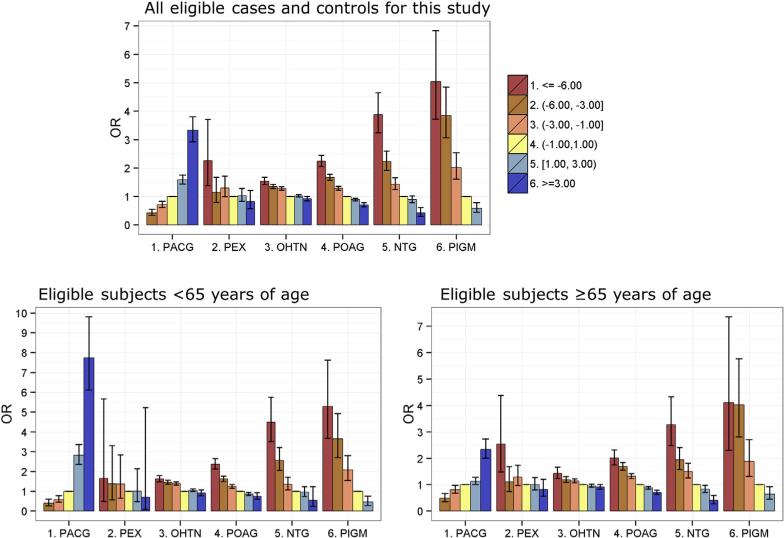
An important question about the association between refractive error and the prevalence of glaucoma is whether the observed effects are driven by a shift in the entire distribution of refractive error, or instead are the result of an increased prevalence of glaucoma in a subset of subjects with pathologic or high myopia (here defined as SE ≤ −6.00 D in the right eye). For this reason, we also examined the effect of low (−2.99 to −1.00 D), moderate (−5.99 to −3.00 D), and high (≤ −6.00 D) myopia, as well as 2 categories of hyperopia (1.00–2.99 D and ≥3.01 D), in comparison with a reference group with minimal refractive error (SE, −0.99 to 1.00 D; Fig 5; Table 3, available at www.aaojournal.org). We observed significant associations between low myopia and increased prevalence of OHTN, POAG, NTG, and PIGM, along with a significant decrease in the prevalence of PACG. We also observed stronger effects for moderate and high myopia. The greater associations of NTG and PIGM with high myopia were particularly striking (ORs, 3.88 and 5.04, respectively). Conversely, hyperopia was associated inversely with POAG, NTG, and PIGM, but directly associated with a substantially increased prevalence of PACG. In the stratified analysis of subjects younger than 65 years and 65 years of age or older (Fig 5; Tables 4 and 5, available at www.aaojournal.org), the associations generally were stronger in the younger age group. Notably, PACG and PIGM showed much greater magnitude of the associations with high hyperopia and high myopia, respectively, in subjects younger than 65 years compared with those 65 years of age or older.
Figure 5.
Odds ratios (ORs) and 95% confidence intervals for the association of glaucoma subtypes with myopia and hyperopia categories, adjusted for age at the first recorded spherical equivalent measure, gender, and race or ethnicity. Estimates are not plotted for −6.00 or less for primary angle-closure glaucoma (PACG) and 3.01 or more for pseudoexfoliation glaucoma (PEX) because of the small numbers of subjects. NTG = normal-tension glaucoma; OHTN = ocular hypertension; PIGM = pigmentary glaucoma; POAG = primary open-angle glaucoma.
Discussion
In this large, community-based cross-sectional study, we assessed the associations between refractive error and the prevalence of several subtypes of glaucoma. Although hyperopia was associated with PACG, myopia was associated with an increased prevalence of all forms of open-angle glaucoma. The magnitudes of the associations were strongest for those with the greatest refractive error. In general, associations were stronger in younger persons, whereas some associations were strongest in racial or ethnic subgroups. An increased prevalence of open-angle glaucoma was associated with low, moderate, and high myopia, and these associations were strongest in the PIGM and NTG subtypes.
The SE distribution we observed in the controls (−0.59±2.62 D; Fig 1) was comparable with the distribution reported in a large German population-based study (mean, −0.401 D for the right eye), demonstrating the external validity of the study.22 Furthermore, the hyperopic shift in average SEs between younger (35–49 years of age) and older (65 years of age or older) persons observed in our study, ranging from 1.62 to 2.15 D across racial and ethnic groups, was slightly larger, but consistent with results from other studies. In the Blue Mountain Eye Study in Australia, Fotedar et al23 found a hyperopic shift over 10 years of 0.40 D and 0.33 D in their 49- to 54-year and 55- to 64-year age groups, respectively. Vitale et al18 reported a 1-D shift toward myopia in a United States population from 1999 through 2004 compared with a population born 30 years earlier. The observed differences in SE across age groups seem to reflect both a myopic shift in younger birth cohorts18,23 and a hyperopic shift that occurs with increasing age.23,24
These findings support the hypothesis of a relationship of refractive error with the prevalence of glaucoma subtypes. However, several limitations and alternative explanations exist. First, like most prior reports of this hypothesis, this study may be affected by misclassification of glaucoma in individuals with myopia because of the difficulty of distinguishing glaucomatous optic nerve head changes, visual field defects, or both from those associated with myopia.25,26 The discs of myopic eyes often appear glaucomatous, with larger-diameter cups, crescents, and nerve fiber layer defects,27,28 giving rise to a more frequent diagnosis of glaucoma in those with myopia compared with those without myopia. Overdiagnosis of glaucoma in myopic eyes could lead to an overestimation of the strength of the association between glaucoma and myopia, and most likely in a manner that creates the impression of a dose-dependent relationship. For example, those with high myopia and normal IOP but with ocular structural or functional changes, or both, similar to those seen in glaucoma, may be more likely to receive a diagnosis of NTG. The potential misclassification of high myopes as NTG is less likely to occur in low to moderate myopes, and yet we observed strong associations of SE with NTG even among those with low to moderate myopia. Although we cannot rule out the possibility of this misclassification, we do not believe it explains the strong association observed for NTG (1-D reduction: OR, 1.15).
Second, because individuals with myopia seek ophthalmic care more frequently than those without refractive error, the controls selected for the study could be more myopic than the underlying population from which the glaucoma patients were ascertained. This selection bias would shift the effect estimates toward the null. However, concern about selection bias is tempered by the external validity we observed in relation to other community-based and population-based studies, as described above.
Third, because the study examined prevalent and not incident glaucoma, it is possible that, all else being equal, prevalent glaucoma cases with myopia may have higher clinical use and may be more likely than those without myopia to have their refractive error recorded, shifting the ORs away from the null. We designed the study to reduce this bias by requiring refractive error measures and at least 5 years of membership enrollment so that persons with and without glaucoma would have adequate opportunity to have eye examinations. This study design feature was effective: both the average length of observation and the mean number of SE measurements per subject were similar between the glaucoma cases and controls. Nonetheless, to assess this potential bias further, we conducted an analysis stratified according to age (<65 years and ≥65 years), reasoning that older patients have regular vision examinations and less potential for bias. The stratified analysis showed larger differences in SE between cases and controls in the younger group (Fig 3) and greater differences in the magnitude of association (Fig 5; Tables 4 and 5, available at www.aaojournal.org), but most of the associations remained robust, suggesting that differences in the frequency of ophthalmology or optometry visits between those with and without glaucoma did not drive the observed association with refractive error. Even if some degree of selection bias was present, it would not explain the associations we observed for most of the glaucoma subgroups in the study. The large effect of high myopia on PIGM and high hyperopia on PACG in part may reflect the younger age of onset of these subtypes. Future studies may be able to evaluate these findings in more detail using longitudinal data and incident cases.
Our finding that moderate to high myopia is associated with open-angle glaucoma is consistent with results from other studies. Although a few studies have found no association between myopia and POAG or OHTN,29,30 most population-based studies, including the Blue Mountain Eye Study, Beijing Eye Study, Singapore Malay Eye Study, and National Health and Nutrition Examination Survey (NHANES) have reported a 2- to 4-fold increased prevalence of glaucoma among myopic subjects compared with that of nonmyopic subjects, with a stronger association noted for moderate-to-high myopia.7–13,31,32 Although clinical use related selection bias may be lessened in population-based studies, the number of subjects with POAG in these studies has been limited, typically to 150 or fewer, and even fewer for other, less common forms of glaucoma. In addition, these studies included only a small number of participants with high myopia, and as a result, they were underpowered to compare the relative contribution of high myopia to POAG or other less common types of glaucoma compared with less extreme forms of refractive error. In contrast, our study included 437 438 subjects, providing sufficient power to examine the association of refractive error and subtypes of glaucoma and OHTN across racial or ethnic groups and to compare the effects of high myopia with those of low and moderate myopia. Compared with the OR of 2.46 (95% CI, 1.93–3.15) reported in a meta-analysis of 7 cross-sectional studies for the association between moderate myopia (SE, ≤3.00 D) and open-angle glaucoma,14 we found lower estimated ORs for both moderate myopia (OR, 1.67; 95% CI, 1.57–1.77) and high myopia (OR, 2.24; 95% CI, 2.06–2.44). These differences could result from a number of factors, including differences in the definition of open-angle glaucoma, in the use of clinical diagnoses of glaucoma, in the control of bias, in the proportion of NTG cases, in the exclusion of subjects with conditions or procedures that affect the measurement of refractive error, and in different cutoff points for high and low myopia used in the current and previous studies, among other reasons.
The associations between refractive error and glaucoma were similar across racial or ethnic groups, with 2 exceptions. The effect of refractive error on the risk of POAG was modestly but significantly stronger in non-Hispanic whites than in other groups, and its effect on the risk of NTG was significantly stronger in Asians and non-Hispanic whites. These findings may represent differences in the distribution of etiologic factors across racial or ethnic groups and glaucoma subtypes. In our study, most Asian subjects were East Asian (70%), with a smaller proportion being South Asian (2%) and Southeast Asian (<1%), whereas 27% did not have more specific racial or ethnic data available. As such, our findings largely reflect the East Asian group. Further investigation in Asian subpopulations could clarify the effects we observed. Additionally, to our knowledge, studies of the relationship between refractive error and glaucoma in Hispanic or Latino and black populations have been limited. The examination of refractive error and glaucoma in these populations, such as the Latino Eye Study or the Baltimore Eye Study, may clarify the findings we report here.
Several plausible mechanisms may underlie the effect of refractive error on the risk of glaucoma. The optic nerve heads of myopic eyes have been postulated to be structurally more susceptible to glaucomatous damage, partly because of their increased axial lengths and thinner scleras.33–35 The reduced retinal nerve fiber layer thickness, in combination with reduced scleral support at the optic nerve head in myopic eyes, may contribute to the increased prevalence of open-angle glaucoma.36 Accordingly, the thinner corneas (reduced CCT) observed in NTG cases could be linked to thinner scleras and weaker support for the optic nerve head in myopic eyes.37 Future genetic studies on refractive error, open-angle glaucoma subtypes, axial length, and CCT may illuminate further the possible hereditary basis for the racial or ethnic differences in the strength of the association between refractive error and the risk of POAG and NTG.
Pigmentary glaucoma is a rare disorder that mostly occurs in young men with high myopia.38 To our knowledge, this is the largest observational study to quantify the association between myopia and PIGM. In PIGM, the posterior iris rubs against the anterior surface of the crystalline lens, causing iris pigment to be shed into the aqueous humor, ultimately clogging the trabecular meshwork and increasing IOP.38–40 The current finding suggests that myopia may alter the shape of the anterior segment of the eye, at least in a subgroup of eyes, and may lead to greater susceptibility to PIGM.
We observed an association of hyperopia with PACG that contrasted with the association we observed with open-angle glaucoma. Previous epidemiologic evidence for this relationship has been limited and inconclusive. Some studies reported significant associations between hyperopia and PACG,41,42 whereas others did not.43–45 The strong association observed in our study confirmed the clinical impression that hyperopia predisposes to PACG. Anterior chamber depth and axial length have been found to be associated inversely with refractive error, that is, hyperopic eyes have shallower chambers,46,47 suggesting an increased prevalence in such eyes for angle-closure glaucoma.48–50 That anterior chamber depth is affected by race and ethnicity as well as age and gender suggests a potential role for genetic influences, which is consistent with a recent report of a genetic variant within the ABCC5 gene that influences anterior chamber depth and the risk of PACG among Asians.51 The possibility that the risk of certain subtypes of glaucoma could be mediated by the genetic variants linked to refractive errors or the susceptibility to the same warrants further investigation.
Our study has a number of significant strengths. First, it is the largest study to evaluate comprehensively the association between refractive error and the risk of glaucoma subtypes and, to our knowledge, the first to examine differences across racial and ethnic groups. Second, we were able to ensure the subjects were categorized correctly in terms of their refractive errors by screening out those with prior histories of cataract surgery, refractive surgery, or corneal disorders. Third, the distribution in our study population of characteristics known to be associated with each of the glaucoma subtypes, including IOP, CCT, and CDR, is consistent with previous reports in the literature,52,53 highlighting the validity of the case and control definitions based on the EHR. Fourth, we designed the study to reduce any potential bias and analyzed the potential for bias using subgroup analysis. Finally, the study is generalizable, because the population is socioeconomically and ethnically representative of the general population in Northern California.
In summary, we observed that myopia was associated with an increased prevalence of all forms of open-angle glaucoma and OHTN, whereas hyperopia was associated strongly with an increased prevalence of angle-closure glaucoma across all racial or ethnic groups. The strongest association of myopia was with PIGM, NTG among Asians and non-Hispanic whites, and POAG. These findings argue for further investigation into the relationship of refractive errors and glaucoma using longitudinal data. In addition, future studies should aim to elucidate how genetic factors, potentially through methods such as Mendelian randomization, and biological mechanisms underlying eye growth and the development of refractive errors potentially influence the risk of glaucoma.
Supplementary Material
Acknowledgments
Supported by a research grant from the Kaiser Permanente Medical Care Plan, Northern California Region, Community Benefit program, Oakland, California.
Abbreviations and Acronyms
- CCT
central corneal thickness
- CDR
cup-to-disc ratio
- CI
confidence interval
- D
diopter
- EHR
electronic health record
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IOP
intraocular pressure
- KPNC
Kaiser Permanente Medical Care Plan, Northern California Region
- NTG
normal-tension glaucoma
- OHTN
ocular hypertension
- OR
odds ratio
- PACG
primary angle-closure glaucoma
- PEX
pseudoexfoliation glaucoma
- PIGM
pigmentary glaucoma
- POAG
primary open-angle glaucoma
- SD
standard deviation
- SE
equivalent. standard
Footnotes
Supplemental material is available at www.aaojournal.org.
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Author Contributions:
Conception and design: Shen, Jorgenson
Analysis and interpretation: Shen, Melles, Metlapally, Barcellos, Schaefer, Risch, Herrinton, Wildsoet, Jorgenson
Data collection: Shen, Melles, Jorgenson
Obtained funding: Schaefer, Jorgenson
Overall responsibility: Shen, Melles, Metlapally, Barcellos, Schaefer, Risch, Herrinton, Wildsoet, Jorgenson
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tielsch JM, Katz J, Sommer A, et al. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 3.Kong X, Chen Y, Chen X, Sun X. Influence of family history as a risk factor on primary angle closure and primary open angle glaucoma in a Chinese population. Ophthalmic Epidemiol. 2011;18:226–32. doi: 10.3109/09286586.2011.595040. [DOI] [PubMed] [Google Scholar]
- 4.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–74. [PubMed] [Google Scholar]
- 5.Wolfs RC, Klaver CC, Ramrattan RS, et al. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998;116:1640–5. doi: 10.1001/archopht.116.12.1640. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MR, Hertzmark E, Walker AM, et al. A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol. 1987;105:1066–71. doi: 10.1001/archopht.1987.01060080068030. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Klein BE, Klein R, et al. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003;110:211–7. doi: 10.1016/s0161-6420(02)01260-5. [DOI] [PubMed] [Google Scholar]
- 9.Perera SA, Wong TY, Tay WT, et al. Refractive error, axial dimensions, and primary open-angle glaucoma: the Singapore Malay Eye Study. Arch Ophthalmol. 2010;128:900–5. doi: 10.1001/archophthalmol.2010.125. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Wang Y, Wang S, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007;114:216–20. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Grodum K, Heijl A, Bengtsson B. Refractive error and glaucoma. Acta Ophthalmol Scand. 2001;79:560–6. doi: 10.1034/j.1600-0420.2001.790603.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuzin AA, Varma R, Reddy HS, et al. Ocular biometry and open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2010;117:1713–9. doi: 10.1016/j.ophtha.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SY, Nemesure B, Leske MC. Refractive errors in a black adult population: the Barbados Eye Study. Invest Ophthalmol Vis Sci. 1999;40:2179–84. [PubMed] [Google Scholar]
- 14.Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994e2. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Chang SW, Tsai IL, Hu FR, et al. The cornea in young myopic adults. Br J Ophthalmol. 2001;85:916–20. doi: 10.1136/bjo.85.8.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolan WP. Prevention of primary angle-closure glaucoma in Asia. Br J Ophthalmol. 2007;91:847–8. doi: 10.1136/bjo.2006.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971e1972 and 1999e2004. Arch Ophthalmol. 2009;127:1632–9. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 19.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai JC, Chang HW. Refractive change after dorzolamide use in patients with primary open-angle glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2001;17:499–504. doi: 10.1089/10807680152729185. [DOI] [PubMed] [Google Scholar]
- 21.Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Wolfram C, Hohn R, Kottler U, et al. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS) Br J Ophthalmol. 2014;98:857–61. doi: 10.1136/bjophthalmol-2013-304228. [DOI] [PubMed] [Google Scholar]
- 23.Fotedar R, Mitchell P, Burlutsky G, Wang JJ. Relationship of 10-year change in refraction to nuclear cataract and axial length findings from an older population. Ophthalmology. 2008;115:1273–8. 8e1. doi: 10.1016/j.ophtha.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Gudmundsdottir E, Arnarsson A, Jonasson F. Five-year refractive changes in an adult population: Reykjavik Eye Study. Ophthalmology. 2005;112:672–7. doi: 10.1016/j.ophtha.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Loyo-Berrios NI, Blustein JN. Primary-open glaucoma and myopia: a narrative review. WMJ. 2007;106:85–9. 95. [PubMed] [Google Scholar]
- 26.Chang RT, Singh K. Myopia and glaucoma: diagnostic and therapeutic challenges. Curr Opin Ophthalmol. 2013;24:96–101. doi: 10.1097/ICU.0b013e32835cef31. [DOI] [PubMed] [Google Scholar]
- 27.Dichtl A, Jonas JB, Naumann GO. Histomorphometry of the optic disc in highly myopic eyes with absolute secondary angle closure glaucoma. Br J Ophthalmol. 1998;82:286–9. doi: 10.1136/bjo.82.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay E, Seah SK, Chan SP, et al. Optic disk ovality as an index of tilt and its relationship to myopia and perimetry. Am J Ophthalmol. 2005;139:247–52. doi: 10.1016/j.ajo.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 29.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829e30. [DOI] [PubMed] [Google Scholar]
- 30.Chao DL, Shrivastava A, Kim DH, et al. Axial length does not correlate with degree of visual field loss in myopic Chinese individuals with glaucomatous appearing optic nerves. J Glaucoma. 2010;19:509–13. doi: 10.1097/IJG.0b013e3181d12dae. [DOI] [PubMed] [Google Scholar]
- 31.Sommer A, Tielsch JM. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1996;114:235. doi: 10.1001/archopht.1996.01100130229029. [DOI] [PubMed] [Google Scholar]
- 32.Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population. Invest Ophthalmol Vis Sci. 2013;54:830–5. doi: 10.1167/iovs.12-11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott R, Grosvenor T. Structural model for emmetropic and myopic eyes. Ophthalmic Physiol Opt. 1993;13:41–7. doi: 10.1111/j.1475-1313.1993.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 34.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 35.Fong DS, Epstein DL, Allingham RR. Glaucoma and myopia: are they related? Int Ophthalmol Clin. 1990;30:215–8. doi: 10.1097/00004397-199030030-00009. [DOI] [PubMed] [Google Scholar]
- 36.Chang RT. Myopia and glaucoma. Int Ophthalmol Clin. 2011;51:53–63. doi: 10.1097/IIO.0b013e31821e5342. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed-Noor J, Bochmann F, Siddiqui MA, et al. Correlation between corneal and scleral thickness in glaucoma. J Glaucoma. 2009;18:32–6. doi: 10.1097/IJG.0b013e31816b2fd1. [DOI] [PubMed] [Google Scholar]
- 38.Niyadurupola N, Broadway DC. Pigment dispersion syndrome and pigmentary glaucoma—a major review. Clin Experiment Ophthalmol. 2008;36:868–82. doi: 10.1111/j.1442-9071.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 39.Farrar SM, Shields MB. Current concepts in pigmentary glaucoma. Surv Ophthalmol. 1993;37:233–52. doi: 10.1016/0039-6257(93)90008-u. [DOI] [PubMed] [Google Scholar]
- 40.Campbell DG, Schertzer RM. Pathophysiology of pigment dispersion syndrome and pigmentary glaucoma. Curr Opin Ophthalmol. 1995;6:96–101. doi: 10.1097/00055735-199504000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970;54:161–9. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L, Cao WF, Wang YX, et al. Anterior chamber depth and chamber angle and their associations with ocular and general parameters: the Beijing Eye Study. Am J Ophthalmol. 2008;145:929–36. doi: 10.1016/j.ajo.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Senthil S, Garudadri C, Khanna RC, Sannapaneni K. Angle closure in the Andhra Pradesh Eye Disease Study. Ophthalmology. 2010;117:1729–35. doi: 10.1016/j.ophtha.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Kim YY, Lee JH, Ahn MD, Kim CY. Angle closure in the Namil study in central South Korea. Arch Ophthalmol. 2012;130:1177–83. doi: 10.1001/archophthalmol.2012.1470. [DOI] [PubMed] [Google Scholar]
- 45.van Romunde SH, Thepass G, Lemij HG. Is hyperopia an important risk factor for PACG in the Dutch Population? A case control study. J Ophthalmol. 2013;2013:630481. doi: 10.1155/2013/630481. Available at: http://dx.doi.org/10.1155/2013/630481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosny M, Alio JL, Claramonte P, et al. Relationship between anterior chamber depth, refractive state, corneal diameter, and axial length. J Refract Surg. 2000;16:336–40. doi: 10.3928/1081-597X-20000501-07. [DOI] [PubMed] [Google Scholar]
- 47.Caprioli J, Spaeth GL, Wilson RP. Anterior chamber depth in open angle glaucoma. Br J Ophthalmol. 1986;70:831–6. doi: 10.1136/bjo.70.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alsbirk PH. Anterior chamber depth and primary angle-closure glaucoma. I. An epidemiologic study in Greenland Eskimos. Acta Ophthalmol (Copenh) 1975;53:89–104. doi: 10.1111/j.1755-3768.1975.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhao JL. [Depth of the anterior chamber in primary angle closure glaucoma] Zhonghua Yan Ke Za Zhi. 1985;21:1–5. [PubMed] [Google Scholar]
- 50.Zhao JL. [Anterior chamber depth measurement in the early diagnosis of primary angle closure glaucoma] Zhonghua Yan Ke Za Zhi. 1986;22:89–92. [PubMed] [Google Scholar]
- 51.Nongpiur ME, Khor CC, Jia H, et al. ABCC5, a gene that influences the anterior chamber depth, is associated with primary angle closure glaucoma. PLoS Genet. 2014;10:e1004089. doi: 10.1371/journal.pgen.1004089. Available at: http://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53(Suppl 1):S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma. 2014;23:606–12. doi: 10.1097/IJG.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.