Abstract
Structural covariance, the examination of anatomic correlations between brain regions, has emerged recently as a valid and useful measure of developmental brain changes. Yet the exact biological processes leading to changes in covariance, and the relation between such covariance and behavior, remain largely unexplored. The steroid hormone testosterone represents a compelling mechanism through which this structural covariance may be developmentally regulated in humans. Although steroid hormone receptors can be found throughout the central nervous system, the amygdala represents a key target for testosterone-specific effects, given its high density of androgen receptors. In addition, testosterone has been found to impact cortical thickness (CTh) across the whole brain, suggesting that it may also regulate the structural relationship, or covariance, between the amygdala and CTh. Here we examined testosterone-related covariance between amygdala volumes and whole-brain CTh, as well as its relationship to aggression levels, in a longitudinal sample of children, adolescents, and young adults 6 to 22 years old. We found: (1) testosterone-specific modulation of the covariance between the amygdala and medial prefrontal cortex (mPFC); (2) a significant relationship between amygdala-mPFC covariance and levels of aggression; and (3) mediation effects of amygdala-mPFC covariance on the relationship between testosterone and aggression. These effects were independent of sex, age, pubertal stage, estradiol levels and anxious-depressed symptoms. These findings are consistent with prior evidence that testosterone targets the neural circuits regulating affect and impulse regulation, and show, for the first time in humans, how androgen-dependent organizational effects may regulate a very specific, aggression-related structural brain phenotype from childhood to young adulthood.
Keywords: Androgen, Amygdala, Prefrontal Cortex, Structural Covariance, Human Brain, Puberty
1. INTRODUCTION
Structural covariance, the examination of anatomic correlations between brain regions, has emerged recently as a useful addition to existing modalities investigating brain function and structure (Alexander-Bloch et al., 2013). Maturational coupling has been demonstrated in both structural covariance and functional connectivity networks (Raznahan et al., 2011), reflecting progressive network integration between 5–18 years old (Zielinski et al., 2010). Such findings have significantly contributed toward support for the use of structural covariance as a valid measure of developmental brain changes. Yet the exact biological processes leading to changes in covariance, and the relation between such covariance and behavior, remain largely unexplored.
The steroid hormone testosterone represents a compelling mechanism through which this anatomical covariance may be developmentally regulated in humans (Nguyen et al., 2013a). Indeed, brain masculinization of limbic brain regions primarily relies on a perinatal testosterone surge in rodents (Zuloaga et al., 2008). In addition, testosterone can act on both androgen and estrogen receptors through its conversion to other steroid hormones (Zuloaga et al., 2008), potentially carrying out widespread effects in the CNS.
Many limbic structures have been shown to be exquisitely sensitive to the effects of testosterone, but among them, the amygdala shows the most significant reorganization through androgen receptor-dependent processes during the pubertal transition (De Lorme et al., 2012; Johnson et al., 2013). More recent evidence shows that the cortex also displays a high density of androgen receptors (Goldstein et al., 2002), and there have been several reports of testosterone-related differences in cortical maturation in children, adolescents and young adults (Herting et al., 2014; Nguyen et al., 2013a). Taken together, these findings suggest that testosterone may regulate the organization and function of both the amygdala and the cortex.
In particular, both exogenously administered and endogenous testosterone levels have been associated with a decrease in functional connectivity between the amygdala and medial prefrontal cortex (mPFC), effectively uncoupling the amygdala from the regulatory influences of the mPFC (Bos et al., 2012; Goetz et al., 2014; Stanton et al., 2009; van Wingen et al., 2009; Volman et al., 2011). Testosterone-related changes in amygdala-mPFC functional connectivity were, in turn, shown to correlate with levels of aggression, risk-taking and reactivity to threat, establishing a direct link to behavioral changes (Peters et al., 2015; Spielberg et al., 2015). Interestingly, our group also found a negative structural covariance between the amygdala and mPFC (Albaugh et al., 2013). This anatomical relationship may well represent the structural foundation underlying the functional connectivity between the amygdala and mPFC.
Herein we aim to examine whether testosterone levels during childhood, adolescence and young adulthood is associated with structural covariance between amygdala volume and cortical thickness (CTh) in a longitudinal sample of 216 developmentally healthy subjects 6–22 years old‥ In addition, we aim to examine whether structural covariance correlates with aggression levels in the same sample. A conservative, whole-brain approach was used, hypothesizing that the volume of the amygdala will be most significantly associated with CTh of the mPFC, and that, in turn, this covariance will be associated with differences in aggression levels.
2. MATERIAL AND METHODS
2.1 Sampling and Recruitment
The National Institutes of Health (NIH) MRI Study of Normal Brain Development is a multi-site project that aimed to provide a normative database to characterize healthy brain maturation. Subjects were recruited across the United States with a population-based sampling method seeking to achieve a representative sample in terms of income level, race and ethnicity (Evans, 2006). All experiments on human subjects were conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the adequate understanding and written parental consent, as well as assent of the subjects (or consent, if >=18 years old). Subjects underwent repeated magnetic resonance brain imaging (MRI) every 2 years, with a maximum of 3 scans over 4 years. The sample was limited to developmentally healthy children with rigorous exclusion criteria, described in detail elsewhere (Evans, 2006). After strict quality control of MRI data (see 2.3, ‘Image Processing’) and the exclusion of scans without paired hormonal measurements or behavioral parameters, 216 subjects were used for hormonal-related analyses and 207 subjects for behavioral analyses (see Table 1 and 2).
Table 1.
Sample Characteristics for Testosterone-Related Analyses
| Measures | Total sample n=216 324 scans |
Males n=93 134 scans |
Females n=123 190 scans |
Male-female comparisons* |
|---|---|---|---|---|
| 1 MRI scan | n=129 129 scans |
n=59 59 scans |
n=70 70 scans |
χ2=1.31 p=0.51 |
| 2 MRI scans | n=66 132 scans |
n=27 54 scans |
n=39 78 scans |
|
| 3 MRI scans | n=21 63 scans |
n=7 21 scans |
n=14 42 scans |
|
| Age (years) | 13.5 Range 6.1–22.3 SD 3.6 |
13.6 Range 6.1–22.1 SD 3.6 |
13.4 Range 6.2–22.3 SD 3.6 |
df=322 p=0.64 |
|
Total brain volume (cm3) |
1274.2 SD 129.1 |
1364.5 SD 104.0 |
1210.5 SD 105.0 |
df=322 p<0.0001* |
| Pubertal stage | 2.7 SD 1.5 |
2.4 SD 1.3 |
2.9 SD 1.6 |
df=322 p=0.004* |
| Testosterone (pg/mL) | 92.4 SD 76.5 |
112.22 SD 89.6 |
78.4 SD 62.3 |
df=322 p<0.0001* |
|
Collection time (min after midnight) |
697.8 SD 127.3 |
695.3 SD 119.4 |
699.5 SD 132.9 |
df=322 p=0.77 |
| Handedness | R= 299 L= 25 |
R= 119 L= 15 |
R= 180 L= 10 |
χ2=3.88 p=0.05 |
|
Left amygdala (mm3) |
1074.8 SD 131.8 |
1160.2 SD 119.7 |
1014.6 SD 104.0 |
df=322 p<0.0001* |
|
Right amygdala (mm3) |
1095.0 SD 123.9 |
1175.8 SD 112.3 |
1038.0 SD 97.1 |
df=322 p<0.0001* |
|
Cortical thickness mPFCa (mm) |
3.9053 SD 0.2464 |
3.9257 SD 0.2429 |
3.8909 SD: 0.2485 |
df=288 p=0.253 |
Between males and females in the complete sample: using chi-squared or T-tests, significance threshold p<0.05
Thickness of the medial prefrontal cortex, in the region found to be significant in testosterone-related analyses
Table 2.
Sample Characteristics for Aggression-Related Analyses
| Measures | Total sample n=207 290 scans |
Males n=86 118 scans |
Females n=121 172 scans |
Male-female comparisons* |
|---|---|---|---|---|
| 1 MRI scan | n=139 139 scans |
n=59 59 scans |
n=80 80 scans |
χ2=0.46 p=0.79 |
| 2 MRI scans | n=53 106 scans |
n=22 44 scans |
n=31 62 scans |
|
| 3 MRI scans | n=15 45 scans |
n=5 15 scans |
n=10 30 scans |
|
| Age (years) | 12.8 Range 6.1–19.7 SD 3.1 |
12.8 Range 6.1–19.2 SD 3.0 |
12.8 Range 6.2–19.7 SD 3.1 |
df=288 p=0.94 |
|
Total brain volume (cm3) |
1276.4 SD 130.7 |
1371.4 SD 101.3 |
1211.2 SD 106.4 |
df=288 p<0.0001* |
| Pubertal stage | 2.6 Range 1–5 SD 1.5 |
2.4 Range 1–5 SD 1.3 |
2.8 Range 1–5 SD 1.6 |
df=288 p=0.008* |
| Testosterone (pg/mL) | 88.0 SD 73.7 |
103.3 SD 86.2 |
77.5 SD 61.9 |
df=288 p=0.003* |
|
Collection time (min after midnight) |
698.3 SD 125.1 |
701.9 SD 114.0 |
695.8 SD 132.5 |
df=288 p=0.69 |
| Handedness | R= 266 L= 24 |
R= 104 L= 14 |
R= 162 L= 10 |
= 2=3.38 p=0.07 |
|
Left amygdala (mm3) |
1073.7 SD 133.0 |
1161.5 SD 121.1 |
1013.5 SD 104.4 |
df=288 p<0.0001* |
|
Right amygdala (mm3) |
1092.6 SD 124.0 |
1175.0 SD 111.0 |
1036.0 SD 98.3 |
df=288 p<0.0001* |
|
CBCLa Aggressive Behavior |
2.4 SD 2.7 |
2.6 SD: 2.8 |
2.3 SD: 2.5 |
df=288 p=0.246 |
|
CBCLa Anxious-Depressed |
0.1 SD 2.0 |
−0.1 SD 1.9 |
0.2 SD 2.0 |
df=288 p=0.102 |
|
Cortical thickness mPFCb (mm) |
3.0334 SD 0.2866 |
3.0566 SD 0.3013 |
3.0174 SD: 0.2759 |
df=288 p=0.253 |
Between males and females in the complete sample: using chi-squared or T-tests, significance threshold p<0.05
Child Behavior Checklist
Thickness of the medial prefrontal cortex, in the region found to be significant in both aggression-related and testosterone-related analyses
2.2 MRI Protocol
A three-dimensional T1-weighted (T1W) Spoiled Gradient Recalled (SPGR) echo sequence from 1.5 Tesla scanners was obtained on each participant, with 1mm isotropic data acquired sagittally from the entire head for most scanners. In addition, T2-weighted (T2W) and proton density-weighted (PDW) images were acquired using a two-dimensional (2D) multi-slice (2mm) dual echo fast spin echo (FSE) sequence.
2.3 Cortical Thickness: Image Processing
All quality-controlled MR images were processed through the CIVET pipeline (version 1.1.9) developed at the MNI for fully automated structural image analysis. The pipeline processing steps have been described at length in other publications (Nguyen et al., 2013a; Nguyen et al., 2013b). In addition to the CIVET pipeline, a multistage quality control (QC) process was implemented, as described previously (Nguyen et al., 2013a; Nguyen et al., 2013b), excluding subjects with white or gray matter artifacts.
2.4 Amygdala Volume: Image Processing
Volumetric measures were obtained using a validated, fully automatic segmentation method for the amygdala in human subjects from MRI data (Collins and Pruessner, 2010). The method utilizes a large, manually labeled MRI dataset (n = 80) of young healthy adults that serves as a template library (Pruessner et al., 2001). The manual segmentation was done by four different raters, and intra-class intra-rater and inter-rater reliability varied between r=0.83 for the right and r=0.95 for the left amygdala (Pruessner et al., 2000). The segmentation method is characterized by label fusion techniques that combine segmentations from a subset of ‘n’ most similar templates. Specifically, each template is used to produce an independent segmentation of the subject using the ANIMAL procedure (Collins and Evans, 1997) followed by a thresholding step to eliminate CSF, which results in ‘n’ different segmentations. To fuse the segmentations at each voxel, a voting strategy is used; the label with the most votes from the ‘n’ templates is assigned to the voxel. The rationale for combining multiple segmentations is to minimize errors and maximize consistency between segmentations. When using n = 11 templates, the label fusion technique has been shown to yield an optimal median Dice Kappa of 0.826 and Jaccard similarity of 0.703 for the amygdala (Collins and Pruessner, 2010).
2.5 Hormonal Measurement
During each MRI visit, children provided two separate 1–3 cm3 samples of saliva, collected on the day of the scan, which were assayed by enzyme-linked immunosorbent assay (ELISA) methods, and the average results used as a measure of testosterone levels. The intra-assay coefficient was 6.1%, and the inter-assay coefficient was 13.5% for testosterone. Intra-assay coefficients of less than 10% and inter-assay coefficients of less than 15% are considered acceptable by the manufacturer (Salimetrics Salivary DHEA, ELISA, State College, PA; Salimetrics Salivary Testosterone ELISA Kit, State College, PA). At the next MRI, a similar procedure was followed and the child again provided two separate saliva samples for hormonal measurement. Each child was scheduled for three repeat MRI scans separated by two year-intervals. Each scan was paired with the hormonal sample collected at the time of the scan, thereby covering all relevant developmental ages. All steroid hormones easily cross the blood brain barrier when not bound to proteins such as the sex hormone-binding globulin or alpha-fetoprotein, and salivary sampling measures the free, biologically active portions of circulating hormonal levels relevant to studies of brain-hormone associations (Khan-Dawood et al., 1984; Worthman et al., 1990). In addition, because testosterone levels have been shown to follow diurnal and seasonal patterns in response to the pulsatile release of adrenocorticotropic hormone and gonadotropin-releasing hormone, particularly in boys (Brambilla et al., 2009; Stanczyk, 2006), we have controlled for time collection, sex and season in all hormonal-related analyses (see 2.9 ‘Statistical Analyses’). Finally, to ensure that sex differences in the range for testosterone levels did not unduly influence our results, we have also standardized testosterone levels within each sex and rerun all analyses with sex-standardized hormonal values.
2.6 Behavioral Measures
The Child Behavior Checklist (CBCL) and Young Adult Self-Report (YASR) are age-appropriate instruments extensively used for assessing psychopathology and competence worldwide, and ask parents or young adults themselves to report on specific behaviors exhibited within the previous 6 months (Achenbach, 1991; Achenbach and Rescorla, 2001b). The YASR was derived from items on the CBCL and serves as a self-report extension of the CBCL for young adults (Achenbach, 1997). Parent-rated CBCL scores have been showed to be predictive of YASR scores at 8-year follow-up (Ferdinand and Verhulst, 1995). Both the CBCL and YASR are reliable measures with high stability over time, validated in multiple cultures (Achenbach and Rescorla, 2001a). These scales demonstrate excellent psychometric properties, with high internal consistency (Aggressive Behavior subscale [Cronbach’s alpha = .91]; Anxious-Depressed subscale [Cronbach’s alpha = .83]) (Achenbach and Rescorla, 2001b). All behavioral measures were obtained within 6 months of the scanning session with which it was paired. The Aggressive Behavior subscale was selected as the primary behavioral outcome measure in this study, given prior evidence of testosterone-related modulation of amygdala-frontal connectivity (Bos et al., 2012; Manuck et al., 2010) and associated anger and threat responses (Goetz et al., 2014; Spielberg et al., 2015; Spielberg et al., 2014; Stanton et al., 2009), as well as risk-taking behavior (Peters et al., 2015). The Anxious-Depressed subscale was selected as a secondary behavioral outcome measure in light of reports observing associations between amygdala reactivity and fearful responses to threat (Campbell, 2013; Carre et al., 2012; Derntl et al., 2009).
2.7 Pubertal Staging
The Pubertal Development Scale (PDS) was administered by a physician to all subjects included in this study (Petersen et al., 1988). This scale has been shown to have good reliability (coefficient alpha: 0.77) and validity (r2=0.61–0.67) compared to physical examination (Petersen et al., 1988). During an interview with the child/adolescent, questions were asked about physical development. We computed a puberty variable consisting of 5 stages, representing increasing levels of physical maturity similar to Tanner staging, previously described (Nguyen et al., 2013a; Nguyen et al., 2013b).
2.8 Handedness
A measure of hand preference was adapted from the Edinburgh Handedness Inventory, including handwriting and seven gestural commands. The criterion for hand preference was defined as at least seven of eight responses with the same hand.
2.9 Statistical Analyses
Statistical analyses were done using SurfStat (Mathlab toolbox designed by Keith J. Worsley) and SPSS 21.0 (SPSS, Inc., Chicago, Illinois). Mixed effects designs were used to model the relationship between variables of interest (i.e. testosterone and aggression levels) and covariance of the amygdala and native-space CTh, taking into account the within- and between- individual variances in this longitudinal sample, and controlling for the effects of age, sex, total brain volume, scanner, handedness with the addition of collection time of salivary samples in hormone-related analyses. All continuous variables were centered using their respective means.
In a first step, a whole-brain correction, using random field theory (RFT, p<0.05), was used in all analyses to control for multiple comparisons. Results using this correction were considered to be significant.
Both linear and quadratic mixed effects models were examined for variables for interest. This approach was preferred and considered more conservative given the variability in developmental trajectories observed across different research groups. Here we prioritized the examination of linear and quadratic models because: (1) mean and local CTh trajectories, as well as testosterone-related cortical maturation, have been previously found to be best described by first-order linear functions in our sample (Brain Development Cooperative Group, 2012; Nguyen et al., 2012; Nguyen et al., 2013a); and (2) the relationship between aggression levels and CTh of the anterior cingulate cortex in our sample has been previously found to be best described by second-order linear functions (i.e. quadratic models) (Ducharme et al., 2011). Therefore we hypothesized that, similar to previous observations relating to this sample, linear models would best describe testosterone-related associations, and quadratic models would best describe aggression-related associations.
2.9.1 Testosterone-Related Analyses
To examine associations between testosterone and structural covariance of the amygdala, we examined the significance of the terms “Testosterone*Amygdala” and “Testosterone-Squared*Amygdala” in separate analyses, while controlling for all the aforementioned control variables (see examples below, with the terms of interest underlined). We examined testosterone-related structural covariance of the right and left amygdala volumes, as well as the average volume of both amygdalae. To examine any distinct effects of testosterone above and beyond those related to pubertal stage, estradiol or season of collection, these variables were also included as control variables in additional testosterone-related models.
Linear model: Whole-brain CTh= 1+Testosterone*Amygdala+Testosterone+Amygdala+Collection Time+Age+Sex+Scanner+Handedness+Total Brain Volume+random (Subj)+I*
Quadratic model: Whole-brain CTh= 1+Testosterone*Testosterone*Amygdala+Testosterone*Testosterone+Testosterone*Amygdala+Testoster one+Amygdala+Collection Time+Age+Sex+Scanner+Handedness+Total Brain Volume+random (Subj)+I*
*Where ‘Subj’ refers to a specific subject and ‘I’ to the identity matrix of the mixed effects model.
To examine sex and age differences in testosterone-related structural covariance, we examined the significance of the terms “Testosterone*Sex*Amygdala”, “Testosterone*Age*Amygdala”, “Testosterone*Testosterone*Sex*Amygdala”, and “Testosterone*Testosterone*Age*Amygdala” in separate analyses, while controlling for all the aforementioned control variables, e.g.:
Linear model: Whole-brain CTh=1+Testosterone*Sex*Amygdala+Testosterone*Sex+Testosterone*Amygdala+Sex*Amygdala+Testostero ne+Sex+Amygdala+Collection Time+Age+Sex+Scanner+Handedness+Total Brain Volume+random (Id)+I*
Quadratic model: Whole-brain CTh= 1+Testosterone*Testosterone*Sex*Amygdala+Testosterone*Testosterone*Sex+Testosterone*Testostero ne*Amygdala+Testosterone*Sex*Amygdala+Testosterone*Testosterone+Testosterone*Sex+Testosteron e*Amygdala+Sex*Amygdala+Testosterone+Sex+Amygdala+Collection
Time+Age+Sex+Scanner+Handedness+Total Brain Volume+random (Id)+I*
*Where ‘Subj’ refers to a specific subject and ‘I’ to the identity matrix of the mixed effects model.
2.9.2 Aggression-Related Analyses
To examine associations between aggression and structural covariance of the amygdala, we examined the significance of the terms “Aggression*Amygdala” and “Aggression*Aggression*Amygdala” in separate analyses, while controlling for all the aforementioned control variables (similar to models for testosterone shown above). We examined aggression-related structural covariance of the right and left amygdala, as well as the average volume of both amygdalae. To examine any distinct effects of aggressive behavior above and beyond those related to threat-related fearful responses, ‘anxious-depressed’ scores were also included as control variables in additional aggression-related analyses.
To examine sex and age differences in aggression-related structural covariance, we examined the significance of the terms “Aggression*Sex*Amygdala”, “Aggression*Age*Amygdala”, “Aggression*Aggression*Sex*Amygdala”, and “Aggression*Aggression*Age*Amygdala” in separate analyses, while controlling for all the aforementioned control variables (similar to models for testosterone shown above). Of note, the prefrontal area of overlap between testosterone-related and aggression-related analyses was used as the dependent variable.
2.9.3 Mediation Effects
According to the criteria established by Baron and Kenny (Baron and Kenny, 1986), for a mediation effect to be established: (1) testosterone has to be associated with amygdala-prefrontal covariance; (2) amygdala-prefrontal covariance has to be associated with aggression; (3) testosterone has to be associated with aggression; and (4) the significance of the relation between testosterone and aggression has to decrease when controlling for amygdala-prefrontal covariance. For criteria (1) and (2), we extracted the coefficients and p-values of peak voxels (i.e. voxels with the highest coefficient) from the region of overlap between testosterone-brain and brain-aggression analyses (see 2.9.1 and 2.9.2; full range of coefficients in the region of overlap also available in the ‘Results’ section). This region of overlap only includes voxels that survived whole-brain RFT correction in both analyses. Therefore, examining coefficients from this region allows us to address the question of whether testosterone levels correlate with a specific structural brain phenotype that would be in turn be related to aggression. Of note, no additional analyses were run for criteria (1) and (2) in addition to the whole-brain analyses already described in 2.9.1 and 2.9.2; the coefficients and p-values were simply extracted from existing analyses.
For criteria (3) and (4), we ran two additional analyses consisting of (1) a mixed effects model of the relationship between testosterone and aggression, controlling for age, sex, and collection time; (2) a mixed effects model of the relationship between testosterone and aggression, controlling for amygdala-prefrontal covariance and related neuroimaging control variables (amygdala, prefrontal CTh, amygdala*prefrontal CTh, total brain volume, scanner, handedness) in addition to age, sex, and collection time. Finally, we carried a formal test of the significance of the mediation effect (Sobel test [http://quantpsy.org/sobel/sobel.htm]).
2.9.4 Separate Examination of Sex and Age effects
To assess the direct effects of sex and age on structural covariance of the amygdala, we examined the significance of the terms “Sex*Amygdala”, “Age*Amygdala”, “Sex*Sex*Amygdala”, and “Age*Age*Amygdala” in separate analyses, while controlling for all the aforementioned control variables (similar to models for testosterone shown above).
3. RESULTS
3.1 Sample Characteristics
Table 1 details sample characteristics (n=216), including number of longitudinal scans and covariates of interest. There were no significant sex differences in the number of completed scans as well as no sex differences in age. As shown in Table 2, sample characteristics for the restricted sample (n=207) used for behavioral analyses were not significantly different from those of the complete sample. There were no sex differences in CBCL Aggressive Behavior or Anxious-Depressed scores, consistent with recent analyses of temperament confirming the absence of sex difference in anger (Archer, 2009; Else-Quest et al., 2006) and depression rates when accounting for the higher rates of externalizing symptoms in males (Martin et al., 2013). Detailed social and demographic comparisons of males and females in this sample have been published elsewhere (Nguyen et al., 2012).
3.2 Testosterone Correlates with Structural Covariance between the Amygdala and Prefrontal Cortex
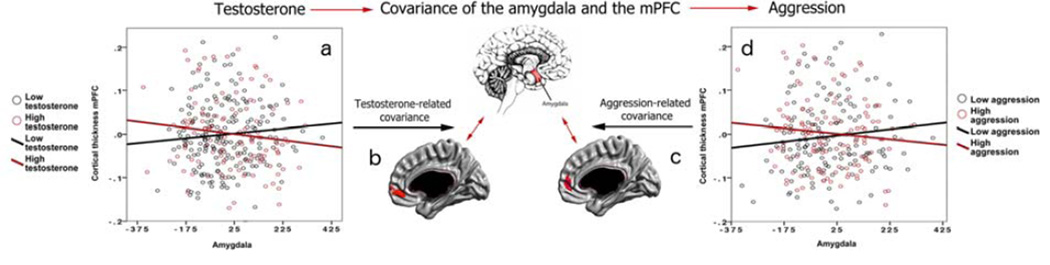
As shown in Figure 1, whole-brain analyses, controlling for the effects of age, sex, total brain volume, scanner, handedness and collection time of salivary samples, revealed that testosterone correlates with structural covariance between the left amygdala and CTh of the right rostral anterior cingulate cortex and orbitofrontal cortex (linear models, Brodmann 10 and 11, cluster-level p=0.037, 166 voxels, peak vertex id 57584 [x=7.2, y=45.0, z=−9.8], p=4*10−5). Lower testosterone levels (<87.5 pg/mL) were associated with a positive covariance between the amygdala and CTh of this prefrontal region, and higher testosterone levels (>=87.5 pg/mL) were associated with a negative correlation between these two regions. No other brain region met the threshold for significance (RFT, p<0.05).
FIGURE 1. Testosterone-Related and Aggression-Related Amygdala-Prefrontal Covariance.
LEGEND: This figure displays testosterone-related covariance and aggression-related covariance of the amygdala and prefrontal cortex.
Testosterone-related covariance Graph 1a displays the relationship between the volume of the left amygdala (X axis) and cortical thickness of the right medial prefrontal cortex (mPFC, Y axis) for subjects with low testosterone levels (<87.5 pg/mL; black lines) and those with high testosterone levels (>=87.5 pg/mL; red lines).
Lower testosterone levels were associated with a positive covariance between the amygdala and CTh of this prefrontal region, and higher testosterone levels were associated with a negative correlation between these two regions.
Figure 1b shows the specific region of the mPFC (right rostral anterior cingulate cortex and orbitofrontal cortex) where the relationship between cortical thickness and amygdala varies according to testosterone levels, as shown in Graph 2a. Of note, no other brain region met the threshold for significance (RFT, <0.05).
Aggression-related covariance Graph 1d displays the relationship in the complete sample between the average of the left and right amygdala volumes (X axis) and cortical thickness of the right mPFC (Y axis), for subjects with low aggression scores (< 2.42; black lines) and those with high aggression scores (>=2.42; red lines). Of note, aggression-squared values were used for Graph 2d to facilitate comparison with testosterone-related analyses.
Lower aggression scores were associated with a positive covariance between the amygdala and CTh of the right anterior cingulate cortex, and higher aggression scores were associated with a negative correlation between these regions.
Figure 1c shows the specific region of the mPFC (right rostral anterior cingulate cortex), where the relationship between cortical thickness and amygdala volume varies according to aggression levels, as shown in Graph 1d. This prefrontal area shows extensive overlap with the area found to be significant in testosterone-related analyses. Of note, no other brain region met the threshold for significance (RFT, <0.05)
Note that standardized residuals were used for cortical thickness values on the Y axes of Graphs 1a and 1d. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
Of note, there were no significant sex-related or age-related effects on testosterone-related structural covariance. In addition, after controlling for estradiol levels, pubertal stage, season of collection, or using testosterone values standardized within each sex, findings were also not quantifiably different and significance level remained the same. Finally, there were no significant quadratic relationships between testosterone and amygdala-CTh covariance across the whole brain.
3.3 Aggression Levels Correlate With Structural Covariance between the Amygdala and Prefrontal Cortex
As shown in Figure 1, whole-brain analyses, controlling for the effects of age, sex, total brain volume, scanner and handedness, revealed that aggression levels are related to structural covariance between the amygdala, bilaterally, and the right rostral anterior cingulate cortex (quadratic models, Brodmann 10, cluster-level p=0.029, 172 voxels, peak vertex id 58332 [x=12.0, y=51.8, z=0.9], p=8*10−5). Lower aggression scores (<2.42) were associated with a positive covariance between the amygdala and CTh of the right anterior cingulate cortex, and higher aggression scores (>=2.42) were associated with a negative correlation between these regions. No other brain region met the threshold for significance (RFT, p<0.05).
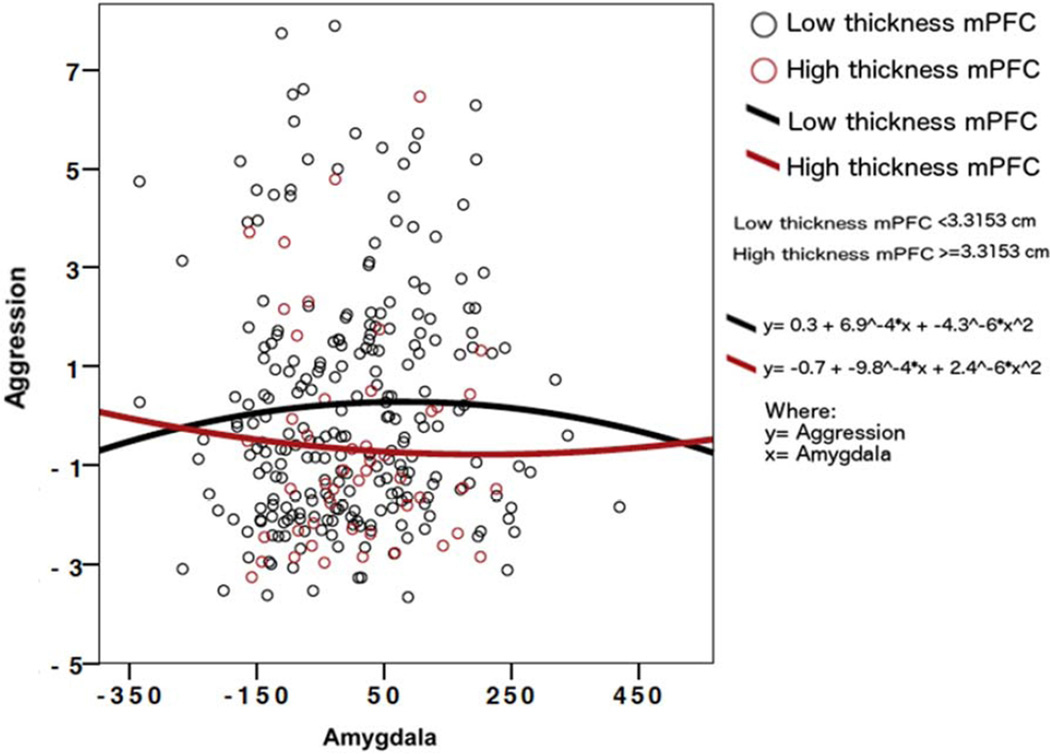
Subjects with low aggression levels displayed positive amygdala-prefrontal covariance relationships analogous to those with low testosterone levels, and those with high aggression levels displayed negative covariance relationships analogous to those with high testosterone levels. More specifically, as shown in Figure 2, higher prefrontal CTh was associated with a U-shape quadratic function between amygdala volume and aggression levels, while lower prefrontal CTh was associated with an inverted U-shape curve. In other words, for the great majority of individuals in this sample, higher cortical thickness of the mPFC was associated with lower aggression levels at a given amygdala volume, but this effect diminished greatly and disappeared at more extreme amygdala values, i.e. very low or very high amygdala volumes more than 2 standard deviations from the average amygdala volume.
FIGURE 2. Aggression-Related Covariance: the Quadratic Relationship between Amygdala and Aggression Varies depending on Thickness of the Prefrontal Cortex.
LEGEND: This graph displays the quadratic interaction between amygdala volume (X axis), aggression levels (Y axis) and thickness of the medial prefrontal cortex (mPFC).
Subjects with lower cortical thickness values (<3.3153 mm) are represented in black dots and those with higher cortical thickness (>=3.3153 mm) in red dots. Quadratic curves for subjects with lower cortical thickness (black curve) and higher cortical thickness (red curve), as well as equations for each curve, where x=aggression scores, and y=amygdala volume, are also shown.
For the great majority of individuals in this sample, higher thickness of the mPFC was associated with lower aggression levels at a given amygdala volume, but this effect diminished greatly and disappeared at more extreme amygdala values, i.e. very low or very high amygdala volumes more than 2 standard deviations from the average amygdala volume. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
Of note, there were no significant sex-related or age-related effects on aggression-related amygdala-prefrontal covariance. In addition, after controlling for anxious-depressed symptoms, findings were not quantifiably different and significance level remained the same. Finally, there were no significant linear relationships between aggression and amygdala-CTh covariance across the whole brain.
3.4 Mediation Effects: Testosterone, Amygdala-Prefrontal Covariance and Aggression
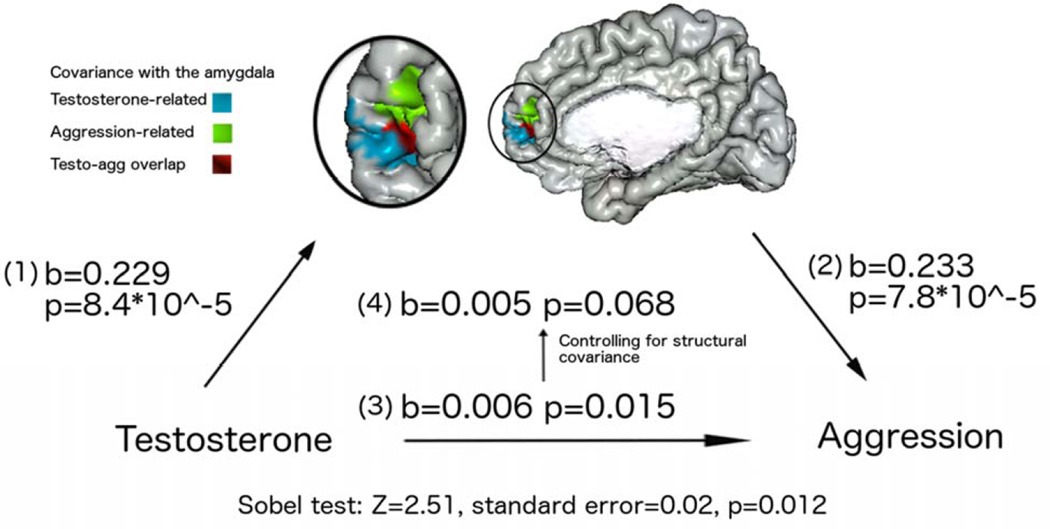
As shown in Figure 3, the relationship between testosterone and amygdala-prefrontal covariance (linear model: peak voxel id 45145 [x=7.3, y=43.8, z=−10.3], beta coefficient=0.229, standard error=0.06, p=8.4*10−5, range of correlations within area of overlap=0.1740–0.229), as well as the relationship between aggression levels and covariance of these same regions (quadratic model: peak voxel 58332 [x=12.0, y=51.8, z=0.9], beta coefficient=0.233, standard error=0.07, p=7.8*10−5, range of correlations within area of overlap=0.165–0.233) were more significant and of greater magnitude than the relationship between testosterone and aggression (linear model: beta coefficient=5.6*10−3, p=0.015). In addition, controlling for brain parameters resulted in a decreased significance of the relationship between testosterone and aggression levels (linear model: beta coefficient=4.9*10−3, p=0.068). Of note, there was no significant effect of sex on the testosterone-aggression relationship, as well as no difference in the results of analyses run with unstandardized testosterone values (including sex as a covariate) and analyses using values of testosterone standardized within each sex. Finally, the formal mediation test (Sobel test) was also significant (Z=2.51, standard error=0.02, p=0.012), confirming significant mediation effects of the brain phenotype on the relationship between testosterone and aggression.
Figure 3. Mediation Effects: Testosterone, Amygdala-Prefrontal Covariance and Aggression.
LEGEND: This figure displays mediation effects of amygdala-prefrontal covariance on the relationship between testosterone and aggression levels.
To examine associations between: (1) testosterone and amygdala-prefrontal covariance; and (2) amygdala-prefrontal covariance and aggression, we extracted the beta coefficients and p-values of peak voxels (i.e. voxels with the highest coefficient) from the region of overlap (displayed in red) between the areas significant for testosterone-brain (displayed in blue) and brain-aggression (displayed in green) analyses. Of note, this step only included an extraction of coefficients and p-values, without additional analyses being run for parts (1) and (2) of the mediation effects. The region of overlap includes voxels that survived whole-brain RFT correction, from analyses outlined in Figures 1 and 2. The beta coefficients and related p-values are displayed, for testosterone-related linear covariance on the left-hand side, and aggression-related quadratic covariance on the right-hand side.
To examine associations between: (3) testosterone and aggression; and (4) testosterone and aggression, controlled for amygdala-prefrontal covariance, we ran two additional analyses consisting of: a mixed effects model of the relationship between testosterone and aggression; and a mixed effects model of the relationship between testosterone and aggression, controlling for amygdala-prefrontal covariance. The central set of coefficients and p-values represent the relationship between testosterone and aggression, before (lower set of values) and after (higher set of values) controlling for amygdala-prefrontal covariance.
Finally, we carried a formal test of the significance of the mediation effect (Sobel test [http://quantpsy.org/sobel/sobel.htm]), for which results are listed at the bottom of the figure (Z=2.51, standard error=0.02, p=0.012). Of note, positive coefficients indicate a positive correlation, with −1 representing a perfect negative fit and + 1 representing a perfect positive fit between the variables of interest. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
3.5 Age and Sex Do Not Correlate with Structural Covariance between Amygdala and Cortical Thickness
To compare the effects of age and sex to those of testosterone and aggression, we examined their main effects on structural covariance of the amygdala with whole-brain CTh, and found no significant linear or quadratic effects of these variables on structural covariance.
4. DISCUSSION
This report provides evidence for a significant relationship between endocrine mechanisms, cortico-limbic covariance, and behavioral effects. Effects of testosterone on structural covariance were found to be more significant than sex- and age-related models that do not include this hormone. These results are consistent with evidence that amygdala volumes and gray matter of the medial prefrontal cortex show no alterations in relation to sex chromosome status (Savic, 2014). With respect to developmental age, our data, combined with recent reports that adults show changes in amygdala structure and reactivity in response to testosterone, suggest that the influence of testosterone is maintained across the lifespan (De Lorme et al., 2012; Goetz et al., 2014).
Next, we show that effects of testosterone on structural covariance are above and beyond those related to estradiol alone. It is difficult to disambiguate the effects of androgens and estrogens on the brain, as testosterone can be converted through aromatization to estradiol (Zuloaga et al., 2008). Nevertheless, a growing body of literature is supporting the notion that testosterone and estradiol have distinct effects on brain function and structure (Juraska et al., 2013). Human males with mutations rendering the aromatase enzyme dysfunctional present with typical male-like patterns of behavior, suggesting that the importance of aromatization on CNS development is much less significant in humans than it is in lower animals (Grumbach and Auchus, 1999). In addition, androgen-dependent processes appear to have the greatest impact during the pubertal transition, through an increase in complexity of the astrocyte arbor in the medial amygdala (Johnson et al., 2013), whereas estrogen-dependent processes appear to play a more prominent role during the prenatal and perinatal period (Zuloaga et al., 2008).
In fact, several hormones increase during the pubertal transition, and therefore it is critical to differentiate the effects of a single steroid hormone, in this case testosterone, from the overall effects of pubertal maturation. The present study demonstrates that testosterone-related associations remain significant above and beyond any effects related to pubertal stage alone, supporting the notion that structural covariance between the amygdala and prefrontal cortex stems from a distinct, androgen-dependent process.
More specifically, we found testosterone-specific associations between amygdala volume and key prefrontal areas involved in emotional regulation and impulse control. Findings highlight the anatomical relationships that may underlie testosterone-related functional connectivity. For example, lower testosterone levels were associated with a positive covariance between the amygdala and the mPFC in both males and females. It is possible that this enhanced structural relationship underlies the increased functional coupling between the amygdala and mPFC, previously demonstrated in low-testosterone states (Volman et al., 2011). In turn, the emergence of a negative covariance between the amygdala-mPFC may underlie the shift toward a functional uncoupling of these regions in high-testosterone states (Volman et al., 2011). Taken together, these findings suggest that testosterone-related anatomical networks formed during childhood and adolescence may underlie testosterone-sensitive functional networks shown to persist until adulthood (Goetz et al., 2014).
Additionally, findings link a very similar intermediate brain phenotype, i.e. covariance between the amygdala and mPFC, to aggression levels. This is not surprising, given the plethora of evidence that exist linking amygdala-prefrontal connectivity to the regulation of responses to threat, anger, and risk-taking (Goetz et al., 2014; Peters et al., 2015; Spielberg et al., 2015; Stanton et al., 2009). There were no sex and age effects on the relationship between aggression and covariance, further highlighting the stability of anger traits and the lack of sex differences in anger reported in systematic analyses of the literature (Else-Quest et al., 2006; Ferdinand and Verhulst, 1995). These findings were also independent of anxious-Depressed symptoms, further establishing that the association between these brain regions and aggressive behavior is independent of anxiety levels or fear-related reactive anger. Finally, the quadratic relationship seen between aggression levels and anatomical covariance shows that: (1) for the great majority of individuals in this sample, higher thickness of the mPFC was associated with lower aggression levels at a given amygdala volume; but that (2) this effect diminished greatly and disappeared at more extreme amygdala values, i.e. very low or very high amygdala volumes more than 2 standard deviations from the average amygdala volume. These results are consistent with our group’s prior findings of a quadratic relationship between aggression and mPFC CTh, such that the highest aggression levels were seen with thinner prefrontal cortices (Ducharme et al., 2011). In addition, the current findings support the notion that extremes in amygdala volumes are associated with early life stress, more severe psychopathology and psychopathic traits (Pardini et al., 2014; Pechtel et al., 2014), and thus, potentially, a reduced effect of the mPFC in protecting against increased aggressive drive.
Further, results from this study show that relationships between testosterone and amygdala-prefrontal covariance, as well between amygdala-prefrontal covariance and aggression levels, are stronger than the relationship between testosterone and aggression. From these findings, one may speculate that testosterone contributes, at least in part, in shaping a specific intermediate brain phenotype involved in the regulation of aggressive behavior. This predominance, across the pubertal transition, of a testosterone-specific brain phenotype in the regulation of aggression is reminiscent of a specific type of juvenile aggressive behavior in rodents shown to be androgen receptor-dependent (Meaney, 1988; Zuloaga et al., 2008).
Still, what are the potential mechanisms through which testosterone may affect structural covariance of the amygdala and the prefrontal cortex, and how can it act differentially on these brains regions? The beginning of an answer can be found in studies showing that at very high levels of testosterone, rapid non-genomic changes following androgen receptor activation can lead to accelerated, calcium-induced, cell death, while relatively lower levels tend to be associated with slower, genomic signaling associated with preserved numbers of astrocytes and neurons (Foradori et al., 2008). These findings thus support the notion that brain regions with differences in androgen receptor expression may exhibit a variable response to testosterone, with the same hormone triggering apoptotic cascades in one structure and neuroprotective changes in another.
4.1 Limitations
The scans were done on 1.5T scanners, which have lower resolution compared to newer 3T models. However, all quality-controlled MR images were processed using the highest standards (see 2.3 and 2.4, Methods). No functional MRI data was available to directly test the relationship between structural covariance and functional connectivity in this sample. Nonetheless, the structural brain phenotype involves regions whose functional connectivity was previously observed to relate directly to aggression levels. Another noted limitation includes the use of an adult template library (as opposed to a pediatric template) to augment the ANIMAL-based segmentation of the amygdala. Still, the segmentation method used in this study has been shown to be fairly resistant to volumetric deviations, particularly for an ovoid structure such as the amygdala with a lower surface-to-volume ratio (Collins and Pruessner, 2010). In addition, previous comparisons between pediatric and adult templates only demonstrated volumetric bias in the youngest age group (4.5–6.9 years) when using an adult template, with no other systematic bias demonstrated for older age groups (Fonov et al., 2011). The age group susceptible to bias therefore falls mostly below the age range of the children, adolescents and young adults included in this study, supporting the notion that no systematic error was introduced in the volumetric measurement of the amygdala.
Another common concern in hormone-brain association studies is the presence of intra-individual hormonal variation due to known, or unknown, causes. Reassuringly, testosterone levels have been shown to remain highly correlated for several days, weeks and possibly even an entire year, and to reliably correlate with stable measures of personality (Dabbs Jr, 1990; Granger et al., 2004; Sellers et al., 2007). Still, to limit any systematic bias related to intra-individual hormonal variation, we have controlled for diurnal and seasonal variation as well as for sex, with no significant changes in the results. We have also attempted to account for as-yet-unknown causes of intra-individual variation through the use of repeated hormonal sampling within the same MRI visit, longitudinal hormonal sampling every 2 years and the use of mixed effects statistical modeling. In addition, to fully account for the effect of sex, we standardized testosterone values within each sex and reran all analyses with no significant changes in the results. Finally, no umbilical cord or amniotic measurements were available in this study and we therefore cannot control for testosterone levels in utero, a period during which significant testosterone-related changes in brain structure are thought to occur. However, more recent observations have identified continuing and distinct organizational effects of testosterone even throughout adolescence and young adulthood, supporting the notion that postnatal brain changes related to testosterone may be of the same, or even greater, order of magnitude than prenatal changes (Bramen et al., 2011; Neufang et al., 2009; Paus et al., 2010; Peper et al., 2009).
4.2 Conclusions
We found testosterone to be associated with cortico-limbic anatomical covariance in key affective anatomical networks. Further, these anatomical networks appear to be involved, at least in part, in the regulation of testosterone-related aggression, supporting the notion that androgen-related effects on brain structure and behavior may be distinct from those of sex, age, pubertal stage and estradiol from childhood to early adulthood.
HIGHLIGHTS.
Testosterone regulates amygdala-prefrontal covariance from childhood to adulthood
Amygdala-prefrontal covariance mediates the testosterone-aggression relationship
These effects are distinct from those of sex, age, estradiol and pubertal stage
A testosterone-specific structural brain phenotype predicts aggressive behavior
ACKNOWLEDGEMENTS
This work was supported by Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02–3343, N01-MH9–0002, and N01-NS-9–2314, −2315, −2316, −2317, −2319 and −2320). The authors would like to thank Aurélie Labbé, Assistant Professor of Epidemiology, Biostatistics and Occupational Health, McGill University, who was consulted with regards to the statistical methods of this paper.
ROLE OF FUNDING
The funding served to support the Brain Development Cooperative Group, primarily involved in protocol development and data collection.
***Appendix A
Brain Development Cooperative Group Key is a multi-site research group formed from personnel from several pediatric study centers.
Data was collected from 6 sites across the United States:
(1) Children’s Hospital Medical Center of Cincinnati, Cincinnati, OH, USA, 45229:
Principal Investigator William S. Ball, M.D., Investigators Anna Weber Byars, Ph.D., Mark Schapiro, M.D., Wendy Bommer, R.N., April Carr, B.S., April German, B. A., and Scott Dunn, R.T.;
(2) Children’s Hospital Boston, Boston, MA, USA, 02115:
Principal Investigator Michael J. Rivkin, M.D., Investigators Deborah Waber, Ph.D., Robert Mulkern, Ph.D., Sridhar Vajapeyam, Ph.D., Abigail Chiverton, B.A., Peter Davis, B.S., Julie Koo, B.S., Jacki Marmor, M.A., Christine Mrakotsky, Ph.D., M.A., Richard Robertson, M.D., and Gloria McAnulty, Ph.D.;
(3) University of Texas Health Science Center at Houston, Houston, TX, USA, 77030: :
Principal Investigators Michael E. Brandt, Ph.D., Jack M. Fletcher, Ph.D., and Larry A. Kramer, M.D., Investigators Grace Yang, M.Ed., Cara McCormack, B.S., Kathleen M. Hebert, M.A., and Hilda Volero, M.D.;
(4) Washington University in St. Louis, St. Louis, MO, USA, 63110:
Principal Investigators Kelly Botteron, M.D. and Robert C. McKinstry, M.D., Ph.D., Investigators William Warren, Tomoyuki Nishino, M.S., C. Robert Almli, Ph.D., Richard Todd, Ph.D., M.D., and John Constantino, M.D.;
(5) University of California Los Angeles, Los Angeles, CA, USA, 90024:
Principal Investigator James T. McCracken, M.D., Investigators Jennifer Levitt, M.D., Jeffrey Alger, Ph.D., Joseph O’Neil, Ph.D., Arthur Toga, Ph.D., Robert Asarnow, Ph.D., David Fadale, B.A., Laura Heinichen, B.A., and Cedric Ireland B.A.;
(6) Children’s Hospital of Philadelphia, Philadelphia, PA, USA, 19104:
Principal Investigators Dah-Jyuu Wang, Ph.D. and Edward Moss, Ph.D., Investigator Robert A. Zimmerman, M.D., and Research Staff Brooke Bintliff, B.S., Ruth Bradford, and Janice Newman, M.B.A.
In addition, the Brain Development Cooperative Group also included: a data coordinating center, a neurostatistics center, a clinical coordinating center, a diffusion tensor processing center, a scientific review center and a spectroscopy processing center:
(1) Data Coordinating Center, McGill University, Montreal, QC, Canada, H3A 1A1:
The Principal Investigator is Alan C. Evans, Ph.D., Investigators are Rozalia Arnaoutelis, B.S., G. Bruce Pike, Ph.D., D. Louis Collins, Ph.D., Gabriel Leonard, Ph.D., Tomas Paus, M.D., and Alex Zijdenbos, Ph.D., and Research Staff are Samir Das, B.S., Vladimir Fonov, Ph.D., Luke Fu, B.S., Jonathan Harlap, Ilana Leppert, B.E., Denise Milovan, M.A., and Dario Vins, B.C., and at Georgetown University, Thomas Zeffiro, M.D., Ph.D. and John Van Meter, Ph.D.
(2) Neurostatistics Laboratory, Harvard University/McLean Hospital, Belmont, MA, USA, 02478:
Investigators include Nicholas Lange, Sc.D. and Michael P. Froimowitz, M.S., who work with data coordinating center staff and all other team members on biostatistical study design and data analyses.
(3) Clinical Coordinating Center, Washington University in St. Louis, St. Louis, MO, USA, 63110:
The Principal Investigator is Kelly Botteron, M.D., Investigators C. Robert Almli Ph.D., Cheryl Rainey, B.S., Stan Henderson M.S., Tomoyuki Nishino, M.S., William Warren, Jennifer L. Edwards M.SW., Diane Dubois R.N., Karla Smith, Tish Singer and Aaron A. Wilber, M.S.
(4) Diffusion Tensor Processing Center, National Institutes of Health, Bethesda, MD, USA, 20892:
The Principal Investigator is Carlo Pierpaoli, M.D., Ph.D., Investigators Peter J. Basser, Ph.D., Lin-Ching Chang, Sc.D., Chen Guan Koay, Ph.D. and Lindsay Walker, M.S.
(5) Scientific Review, National Institutes of Health, Bethesda, MD, USA, 20892:
The Principal Collaborators are Lisa Freund, Ph.D. (NICHD), Judith Rumsey, Ph.D. (NIMH), Lauren Baskir, Ph.D. (NIMH), Laurence Stanford, Ph.D. (NIDA), and Karen Sirocco, Ph.D. (NIDA) and from NINDS, Katrina Gwinn-Hardy, M.D. and Giovanna Spinella, M.D.
(12) Spectroscopy Processing Center, University of California Los Angeles, Los Angeles, CA, USA, 90024:
The Principal Investigator is James T. McCracken, M.D.; Investigators are Jeffry R. Alger, Ph.D., Jennifer Levitt, M.D., and Joseph O’Neill, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist 4–18 and 1991 profile. Burlington VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM. Manual for the young adult self-report and young adult behavior checklist. Burlington (VT): University of Vermont, Department of Psychiatry; 1997. [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA: school-age forms and profiles. Burlington VT: University of Vermont, Department of Psychiatry; 2001a. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington VT: University of Vermont, Research Center for Children, Youth, and Families; 2001b. [Google Scholar]
- Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ. Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: implications for the neural substrates of emotion regulation. NeuroImage. 2013;71:42–49. doi: 10.1016/j.neuroimage.2012.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. Does sexual selection explain human sex differences in aggression? Behav Brain Sci. 2009;32:249-+. doi: 10.1017/S0140525X09990951. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Ramsey NF, van Honk J. The neural mechanisms by which testosterone acts on interpersonal trust. NeuroImage. 2012;61:730–737. doi: 10.1016/j.neuroimage.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group BDCG. Total and Regional Brain Volumes in a Population-Based Normative Sample from 4 to 18 Years: The NIH MRI Study of Normal Brain Development. Cereb Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The Effect of Diurnal Variation on Clinical Measurement of Serum Testosterone and Other Sex Hormone Levels in Men. J Clin Endocr Metab. 2009;94:907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty Influences Medial Temporal Lobe and Cortical Gray Matter Maturation Differently in Boys Than Girls Matched for Sexual Maturity. Cereb Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. The evolutionary psychology of women’s aggression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130078. doi: 10.1098/rstb.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc Cogn Affect Neurosci. 2012;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Evans AC. Animal: Validation and Applications of Nonlinear Registration-Based Segmentation. Int J Pattern Recogn. 1997;11:1271–1294. [Google Scholar]
- Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. NeuroImage. 2010;52:1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiol Behav. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- De Lorme KC, Schulz KM, Salas-Ramirez KY, Sisk CL. Pubertal testosterone organizes regional volume and neuronal number within the medial amygdala of adult male Syrian hamsters. Brain Res. 2012;1460:33–40. doi: 10.1016/j.brainres.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, Collins DL, Albaugh MD, Evans AC, Karama S. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol Psychiat. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. Gender differences in temperament: a meta-analysis. Psychol Bull. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Evans AC. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Ferdinand RF, Verhulst FC. Psychopathology from adolescence into young adulthood: an 8-year follow-up study. Am J Psychiatry. 1995;152:1586–1594. doi: 10.1176/ajp.152.11.1586. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carre JM. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol Psychiat. 2014;76:324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS, Jr, Faraone SV, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp. 2014;35:5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL. Androgen receptors mediate masculinization of astrocytes in the rat posterodorsal medial amygdala during puberty. J Comp Neurol. 2013;521:2298–2309. doi: 10.1002/cne.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64:203–210. doi: 10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Khan-Dawood FS, Choe JK, Dawood MY. Salivary and plasma bound and “free” testosterone in men and women. Am J Obstet Gynecol. 1984;148:441–445. [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology. 2010;35:94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Neighbors HW, Griffith DM. The Experience of Symptoms of Depression in Men vs Women Analysis of the National Comorbidity Survey Replication. Jama Psychiatry. 2013;70:1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. The sexual differentiation of social play. Trends Neurosci. 1988;11:54–58. doi: 10.1016/0166-2236(88)90164-6. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex Differences and the Impact of Steroid Hormones on the Developing Human Brain. Cereb Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013a;23:1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci. 2013b;33:10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Raine A, Erickson K, Loeber R. Lower Amygdala Volume in Men is Associated with Childhood Aggression, Early Psychopathic Traits, and Future Violence. Biol Psychiat. 2014;75:73–80. doi: 10.1016/j.biopsych.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Nawaz-Khan I, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Susman E, Veillette S, Pausova Z. Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav. 2010;57:63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. NeuroImage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen A, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Pol HEH. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Peters S, Dietsje JJ, Van Duijvenvoorde ACK, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortext connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A Self-Report Measure of Pubertal Status: Reliability, Validity, and Initial Norms. J Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lerch Jason P, Lee N, Greenstein D, Wallace Gregory L, Stockman M, Clasen L, Shaw Phillip W, Giedd Jay N. Patterns of Coordinated Anatomical Change in Human Cortical Development: A Longitudinal Neuroimaging Study of Maturational Coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I. Asymmetry of cerebral gray and white matter and structural volumes in relation to sex hormones and chromosomes. Front Neuroendocrinol. 2014:8. doi: 10.3389/fnins.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers JG, Mehl MR, Josephs RA. Hormones and personality: Testosterone as a marker of individual differences. J Res Pers. 2007;41:126–138. [Google Scholar]
- Spielberg JM, Forbes EE, Ladouceur CD, Worthman CM, Olino TM, Ryan ND, Dahl RE. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc Cogn Affect Neurosci. 2015;10:408–415. doi: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Olino TM, Forbes EE, Dahl RE. Exciting fear in adolescence: does pubertal development alter threat processing? Dev Cogn Neurosci. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ. Measurement of Androgens in Women. Semin Reprod Med. 2006;24:078–085. doi: 10.1055/s-2006-939566. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Wirth MM, Waugh CE, Schultheiss OC. Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biol Psychol. 2009;81:118–122. doi: 10.1016/j.biopsycho.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, Fernandez G. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;34:539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- Volman I, Toni I, Verhagen L, Roelofs K. Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cereb Cortex. 2011;21:2282–2290. doi: 10.1093/cercor/bhr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clin Chem. 1990;36:1769–1773. [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]