Abstract
Our previous study indicated that attenuation of endoplasmic reticulum (ER) stress by administration of 4-phenylbutyric acid (4-PBA) could prevent cardiac rupture and remodeling in a mouse model of myocardial infarction (MI). However, whether 4-PBA is protective in hypertrophic heart disease is unclear. Thus, we tested the therapeutic effect of 4-PBA on pressure-overload induced myocardial hypertrophy. Transverse aortic constriction (TAC) was used to create myocardial hypertrophy in C57BL/6 male mice for 4 weeks. Immediately after surgery, the mice were administrated either 4-PBA (20 mg/kg/day) or 0.9% NaCl by intraperitoneal injection. At the end of 4 weeks, the mice underwent high-resolution echocardiographic imaging. Our results showed that both the left ventricular posterior wall thickness at end systole (LVPWs) and diastole (LVPWd) were increased in the TAC group, compared to control. 4-PBA administration attenuated hypertrophy and decreased the heart weight over body weight ratio. Masson’s trichrome staining showed that myocardial interstitial fibrosis and collagen deposition were also decreased by 4-PBA. We next detected the ER stress response in the heart tissues of TAC mice in different time points. Western blotting showed that the expression of ER stress marker, GRP78, CHOP and phosphor-PERK, were persistently increased 4 weeks after TAC. The treatment of 4-PBA inhibited the expression of ER stress markers. We also demonstrated that the 4-PBA at 20 mg/kg/day had no effect on histone 3 deacetylation inhibition, while attenuating ER stress and TAC-induced hypertrophy. These findings suggest that 4-PBA may be a therapeutic strategy to consider in preventing pressure-overload induced myocardial hypertrophy and interstitial fibrosis by selectively attenuating ER stress.
Keywords: Myocardial hypertrophy, fibrosis, ER stress, 4-Phenylbutyric acid
1. Introduction
Endoplasmic reticulum (ER) is an organelle that is mainly responsible for the synthesis, folding and modification of proteins in eukaryotic cells [1]. The properly folded and modified proteins are then transported to Golgi apparatus, fused with the plasma membrane and released into the extracellular space. Alterations in the intracellular environment can interrupt the protein processing and result in the accumulation of unfolded proteins in the ER as well as activate the unfolded protein response (UPR) [2]. Triggers for this stress response include ischemia, hypoxia, glucose deprivation and oxidative stress, among many. While the UPR initially aims to restore ER functions and promote cell survival, when ER stress persists, it can activate apoptosis and cell death [3].
The activation of ER stress in the heart is associated with myocardial apoptosis [4,5], hypertrophy [6,7], and fibrosis [8,9], the pathological processes common in development of ischemic and hypertrophic heart diseases. Therefore, inhibiting ER stress may be an important consideration in developing treatments for ischemic or hypertrophic cardiomyopathy. To that effect, our previous study has indicated that attenuation of ER stress by administration of 4-Phenylbutyric acid (4-PBA) at 20 mg/kg/day in a mouse model of myocardial infarction (MI) prevents cardiac rupture and remodeling by inhibiting cardiac apoptotic and fibrotic signal pathways [10]. However, little is known whether the administration of 4-PBA is effective on mitigating hypertrophic heart disease.
As an inhibitor of ER stress, 4-PBA directly targets mutant and/or misfolded proteins in cells and aids proper protein trafficking, thus eliminating intracellular UPR [11–16]. The underlying mechanisms by which 4-PBA attenuates ER stress are associated with modulation of several molecules, including inhibition of GRP78, protein kinase RNA-like ER kinase (PERK), c-Jun NH2-terminal kinase (c-JNK) phosphorylation, X-box-binding protein 1 (XBP1) splicing, and CCAAT/enhancer-binding protein homologous protein (CHOP) expression [17]. Some of the ER stress indicators, in particular, GRP78 and CHOP, are significantly upregulated during the pathogenesis of left ventricular hypertrophy (LVH) [6]. Therefore, inhibiting ER stress using 4-PBA may be a promising therapeutic approach to mitigate hypertrophic heart disease.
Current research shows that orally administering 4-PBA daily for a week at a high dosage (100 mg/kg/day) plays a protective role against cardiac hypertrophy and fibrosis in mice [18]. However, whether the protective effect by 4-PBA may be sustained chronically at a lower dosage is unclear. In the present study, we tested the long term therapeutic effect of 4-PBA on pressure-overload induced myocardial hypertrophy at a much lower dosage. Our results showed that 4-PBA when administrated for 4 weeks intraperitoneally at 20 mg/kg/day, was effective in preventing pressure overload hypertrophy and interstitial fibrosis of the heart by inhibiting ER stress.
2. Materials and methods
2.1 Animal
All procedures were performed in accordance with our institutional guidelines for animal research that conforms to the Guide for the care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Briefly, 8-week-old adult C57/BL6 male mice (18–22 g) were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg) by intraperitoneal injection. After successful endotracheal intubation (tidal volume: 0.3 ml; respiratory rate: 110 per minute), the chest cavity was opened in the second intercostal space at the left upper sternal border through a small incision. When the aortic arch was exposed, transverse aortic constriction (TAC) was then performed between brachiocephalic artery and left common carotid artery by tying a 7-0 nylon suture ligature against a 27-gauge needle to yield a narrowing 0.4 mm in diameter when the needle was removed and a reproducible TAC of 65–70% (see the diagram in Figure 1A). At the end of the procedure, the chest was closed by sutures.
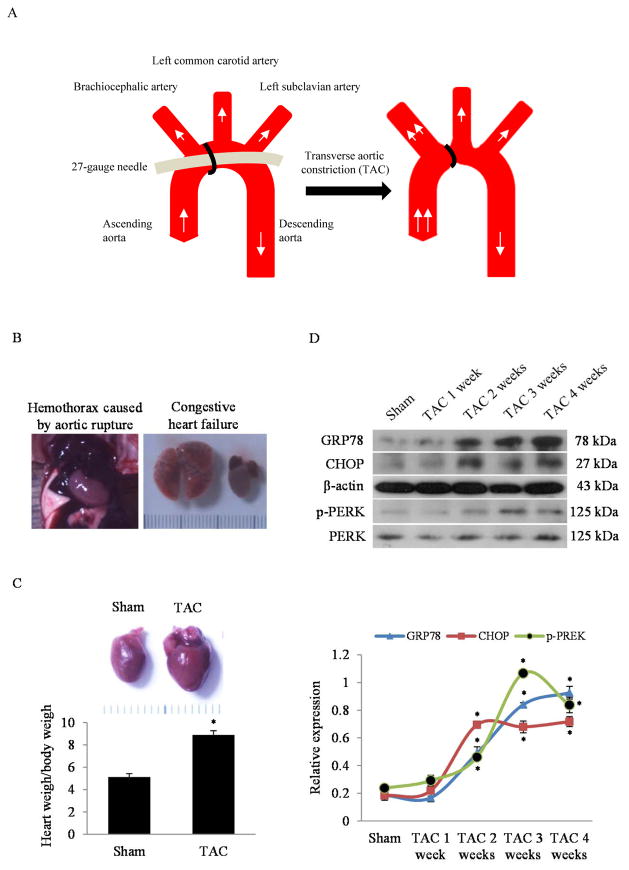
Fig. 1. Cardiac hypertrophy and ER stress response in mice following TAC.
(A) The diagram of transverse aortic constriction (TAC) in mouse. (B) The representative autopsy pictures of aortic rupture and congestive heart failure within the 4 weeks of TAC. (C) Representative autopsy pictures of the normal heart and hypertrophic heart induced by pressure overload for 4 weeks and the corresponding quantitative analysis of heart weight over body weight ratio. *P<0.05 vs. Sham; n=8 in each group. (D) Western blots and quantitative analysis of ER stress response markers (GRP78, CHOP and p-PERK) in the whole heart homogenates of mice in designated time points after TAC. *P<0.05 vs. Sham; n=3.
After recovery from the surgery, the mice were intraperitoneally injected with 4-PBA (Sigma–Aldrich, St. Louis, MO) daily for 4 weeks at the dosage of 20 mg/kg/day. 4-PBA was first dissolved by DMSO then diluted in 0.9% NaCl. The animals were housed in the 12-12 cycle environment, and checked three times each day. The dosage of 4-PBA was determined according to our previous study [10]. At the end of 4 weeks, the surviving animals were subjected to echocardiographic imaging, then euthanized for autopsy, and histological analysis, and western blotting.
2.2 Echocardiographic imaging
At the end of 4 weeks after TAC, the mice were anesthetized with a mixture of ketamine and xylazine as described above and placed on a warmed platform. Then transthoracic echocardiographic imaging was performed using Siemens ultrasonic instrument (Acuson Sequoia 512) with Siemens Acuson 15L8 probe. The hearts were scanned using M-mode at the levels of papillary muscle for cardiac function analysis.
2.3 Autopsy and Histology
After mice were sacrificed, the hearts were rapidly excised, rinsed with PBS, fixed in 4% paraformaldehyde and embedded in paraffin. Then, 5μm-sections at the levels of papillary muscle were cut for histology. To evaluate the extent of cardiac fibrosis, each heart section was stained with Masson’s trichrome kit (#HT15, Sigma-Aldrich). The extent of vascular fibrosis and interstitial fibrosis were determined as the ratio of the Masson’s trichrome-stained area to the total vascular area and total left ventricular area, respectively.
2.4 Western blot analysis
The heart samples in the sham and TAC group with or without 4-PBA were excised and homogenized. The following antibodies were used for western blotting: anti-GRP78 (sc-376768, Santa Cruz), anti-CHOP (#2895, Cell Signaling Technology), anti-β-actin (sc-47778, Santa Cruz), anti-phospho-PERK (sc-32577, Santa Cruz), anti-PERK (sc-13073, Santa Cruz), anti-Acetyl-Histone 3 (#9649, Cell Signaling Technology) and anti-Histone 3 (#4499, Cell Signaling Technology). Samples containing equal amounts of protein were separated by SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% skim milk at room temperature for 2 h and then incubated overnight at 4°C with the primary antibody. After being incubated with anti-mouse secondary antibody (#7076S, Cell Signaling Technology) or anti-rabbit secondary antibody (#7074S, Cell Signaling Technology) for 1 h at room temperature, the blots were detected in the dark room using autoradiography film (Denville Scientific, Inc) and quantified by densitometry using the Image J Analysis software (National Institutes of Health).
2.5 Statistical analysis
All data are expressed as mean ± SEM, and P< 0.05 was considered to be statistically significant. Statistical differences were evaluated by one-way ANOVA followed by Bonferroni’s multiple comparison exact probability test. All analyses were performed using SPSS 13.0 software (SPSS Inc, Chicago, IL, USA).
3. Results
3.1 ER stress response persists in the development of myocardial hypertrophy
As illustrated in Fig. 1A, we induced myocardial hypertrophy in C57BL/6 male mice by using the classic TAC method. After surgery, the aortic rupture and congestive heart failure were occasionally observed within the 4 weeks of pressure overload post TAC (Fig. 1B). The death rate in the TAC-treated mice was 20%. Of the 10 TAC-treated mice, 1 died from aortic rupture in the first 2 weeks and another died from congestive heart failure in the second half of the 4 week period. The dead mice were excluded from the experiment. The surviving animals developed pressure overload hypertrophy of the heart post TAC. As shown in Fig. 1C, the heart weight over body weight ratio (HW/BW) in the TAC group was significantly increased, compared with the sham group at 4 weeks. After the establishment of the TAC model, we investigated the temporal relationship of the ER stress response in the development of myocardial hypertrophy. The TAC mice were sacrificed at different time points, and the ER stress markers (GRP78, CHOP and phosphor-PERK) in the heart were examined by western blotting. As shown in Fig. 1D, the protein levels of GRP78, CHOP and phosphor-PERK were persistently increased in the entire period up to 4 weeks after TAC. These results suggest that ER stress remains activated in the pathogenesis of cardiac hypertrophy.
3.2 Low-dose 4-PBA selectively attenuates ER stress response while unaltering histone H3 acetylation
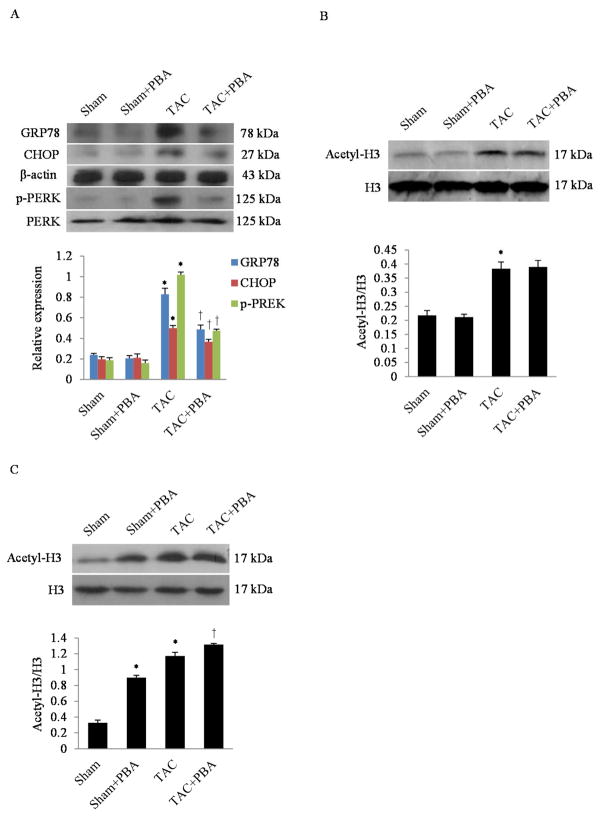
Because 4-PBA is an inhibitor of both ER stress [19] and histone deacetylase (HDAC) [20], we investigated whether 4-PBA had differential effects on ER stress and histone 3 deacetylation during development of pressure-load hypertrophy. In our TAC model, GRP78, CHOP, phosphor-PERK, and acetyl-histone 3 were all significantly increased post TAC. However, the treatment of 4-PBA at the dosage of 20 mg/kg/day for 4 weeks decreased ER stress response, while it did not affect histone 3 acetylation (Fig. 2A and B). Of note, when the 4-PBA dosage was increased to 100 mg/kg/day, the histone 3 acetylation was enhanced both in the sham and TAC group (Fig. 2C). These findings suggest that 4-PBA at the lower dose of 20 mg/kg/day is effective at selectively inhibiting ER stress response while leaving histone H3 acetylation unaffected.
Fig. 2. Effects of 4-PBA treatment on ER stress response and histone acetylation.
(A) Representative western blots and quantitative analysis of ER stress response markers (GRP78, CHOP and p-PERK) in the whole heart homogenates of mice with or without 4-PBA at 4 weeks after TAC. *P<0.05 vs. Sham; †P<0.05 vs. TAC; n=3. (B) Western blots and quantitative analysis of Histone 3 acetylation in the whole heart homogenates of mice with or without 4-PBA (20 mg/kg/day) after 4 weeks of TAC. *P<0.05 vs. TAC; n=3. (C) Western blots and quantitative analysis of Histone 3 acetylation in the whole heart homogenates of mice with or without 4-PBA (100 mg/kg/day) after 4 weeks of TAC. *P<0.05 vs. Sham; †P<0.05 vs. TAC; n=3.
3.3 4-PBA ameliorates pressure-overload hypertrophy of the left ventricle
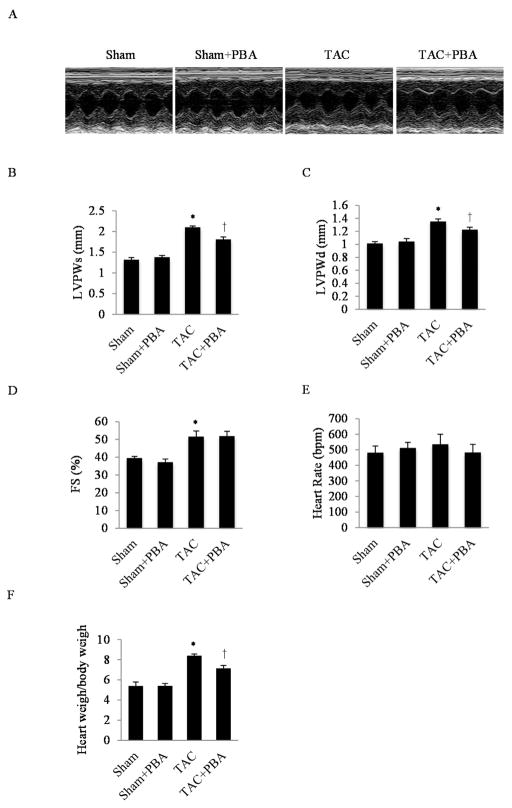
Based on our findings that ER stress was elevated in the development of cardiac hypertrophy and that 4-PBA efficiently inhibited the ER stress response, we tested if 4-PBA mitigated pressure overload hypertrophy in vivo. 4-PBA (20 mg/kg/day) was intraperitoneally injected into the TAC-treated mice for 4 weeks, followed by echocardiographic imaging. At the end of 4 weeks, the left ventricular posterior wall thickness at end systole (LVPWs), left ventricular posterior wall thickness at end diastole (LVPWd) and fractional shortening in percentage (FS%) in the TAC-treated mice were significantly increased, as expected, compared to the sham group. Administration of 4-PBA significantly inhibited this hypertrophic response while preserving the systolic function (Fig. 3A, B, C and D). Of note, 4-PBA did not affect the heart rate between the two treatment groups (Fig. 3E). Consistent with the echocardiographic data, findings from autopsy demonstrated that the HW/BW was lower in the TAC+4-PBA group, compared to the TAC-treated group without 4-PBA (Fig. 3F).
Fig. 3. Effects of 4-PBA treatment on cardiac hypertrophy.
(A) Representative pictures of M-mode echocardiographic imaging in mice after 4 weeks of TAC. (B) Quantitative analysis of left ventricular posterior wall thickness at systole (LVPWs). (C) Quantitative analysis of left ventricular posterior wall thickness at diastole (LVPWd). (D) Quantitative analysis of left ventricular fractional shortening (FS%). (E) Quantitative analysis of heart rate. (F) Quantitative analysis of the heart weight over body weight ratio (HW/BW). *P<0.05 vs. Sham; †P<0.05 vs. TAC; n=5 in each group.
3.4 4-PBA inhibits TAC-associated myocardial interstitial fibrosis
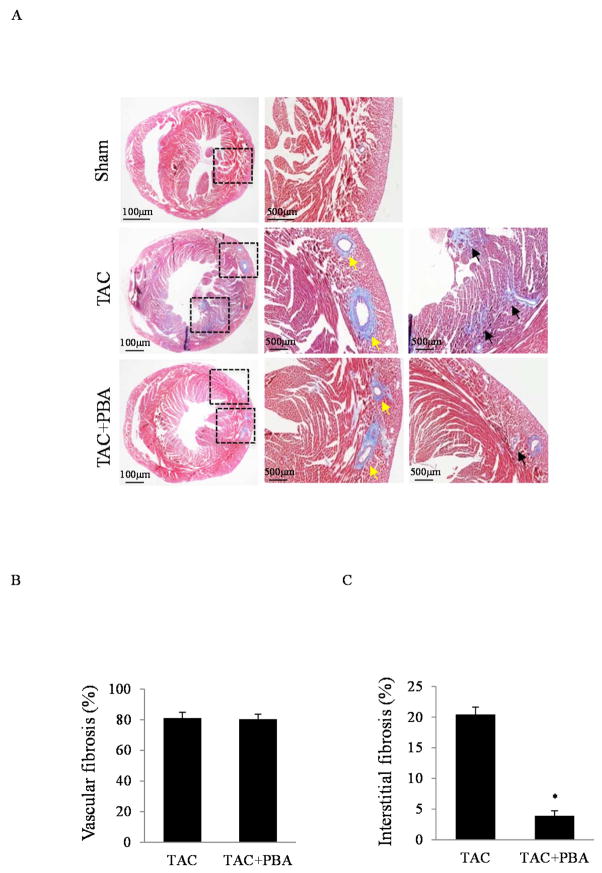
We further examined whether 4-PBA had impact on the extent of cardiac fibrosis. As shown in Fig. 4A, we found extensive peri-vascular and interstitial fibrosis in the hearts of the TAC-treated mice at the end of 4 weeks. The administration of 4-PBA did not change the extent of peri-vascular fibrosis (Fig. 4B) but significantly decreased the interstitial fibrosis (Fig. 4C).
Fig. 4. Effects of 4-PBA treatment on cardiac fibrosis.
(A) Masson staining of cardiac fibrosis from the hearts of mice with or without 4-PBA administration at the end of 4 weeks after TAC. Perivascular fibrosis (yellow arrow) and interstitial fibrosis (black arrow) were identified. (B) Quantitative analysis of vascular fibrosis. (C) Quantitative analysis of interstitial fibrosis. *P<0.05 vs. TAC.
4. Discussion
Our present study supports that ER stress is an important factor in the pathogenesis of pressure overload hypertrophy of the heart. First, we demonstrated in vivo that the ER stress response (manifested by increased protein levels of GRP78, CHOP and p-PERK) was persistent 4 weeks after TAC. We also demonstrated that TAC-induced ER stress was inhibited by long-term administration of 4-PBA at a low dose of 20 mg/kg/day. Furthermore, 4-PBA attenuated TAC-induced LVH and decreased the extent of interstitial fibrosis in the myocardium.
Cardiac hypertrophy and fibrosis accompany many forms of heart disease. While the etiology behind the hypertrophy and fibrosis is diverse, they may intersect in a shared cellular mechanism in the form of the ER stress response. For example, in cultured neonatal rat cardiomyocytes, application of ER stress inducer thapsigargin (TG) produced cellular hypertophy in a dose- and time-dependent manner [7]. TG also triggered the UPR and accumulation of intracellular procollagen in cultured rat cardiac fibroblasts [21]. Furthermore, accumulating in-vivo data from the pressure overload model implicates ER stress as a central player in the development of cardiac hypertrophy [22–24]. In this study, we shed light on the temporal relationship between ER stress and LVH, demonstrating that the ER stress response persists up to 4 weeks after TAC. Although mechanisms by which ER stress modulates myocardial hypertrophy and interstitial fibrosis remain unclear, some suggest involvement of CaN-MEF2c signaling pathways in cardiomyocytes [7] and TGFβ1-Smad2/3 in cardiac fibroblasts [10]. Further investigations are needed to detail the putative mechanisms.
Interestingly, although ER stress response was reported to be responsible for proliferation, migration and collagen synthesis in vascular smooth muscle cells (VSMCs) in atherosclerosis and vascular injury [25,26], we did not observe significant modulation of peri-vascular fibrosis by 4-PBA in the hearts of TAC-treated mice in the present study. It is possible that 4-PBA, at 20 mg/kg/day, may be insufficient to mitigate the fibrotic signaling transduction in VSMCs under mechanical stress created by pressure overload or hypertension.
4-PBA, also called sodium phenylbutyrate or Buphenyl, is a low-molecular-weight fatty acid and a non-toxic pharmacological compound that was originally used as a nitrogen-scavenging medication for chronic management of urea cycle disorders [27]. In the last decade, it was also shown to be a histone deacetylase inhibitor (HDACI) and chemical chaperone, leading to its use in clinical trials and preclinical research studying various conditions, including sickle cell anemia [28], beta thalassemia [29], cirrhosis and hepatic encephalopathy [30], acute myeloid leukemia [31], solid tumors [32] and various motor neuron disorders [33]. It should be noted that most of these studies used 4-PBA at much higher dosages (>100 mg/kg/day) [34]. On the contrast, our study showed that the in-vivo administration of low dose 4-PBA at 20 mg/kg/day selectively attenuated ER stress response and reduced LVH associated with pressure overload while having no effect on histone 3 acetylation. When the dosage was increased to 100 mg/kg/day, the acetylation of histone 3 was upregulated. Though HDACI actions of 4-PBA have been linked to attenuation of hypertension or pressure overload-induced cardiac hypertrophy and heart failure [35,36], our data show that 4-PBA selectively dosed as an ER stress inhibitor may be behind and/or overlap with the HDACI effect on its anti-hypertrophic actions.
4-PBA metabolizes to phenylacetate (PAA) by β-oxidation in the liver. Then PAA is conjugated with glutamine to form phenylacetylglutamine (PAG), which is eliminated in the urine. There are few studies reporting pharmacokinetics of 4-PBA in animal models. In clinical trials, when 4-PBA was intravenously administrated in patients with refractory solid tumors for 5 days at 410 mg/kg/day, the plasma concentration of 4-PBA and PAA was maintained at 500 μmol/liter and 1000 μmol/liter, respectively, throughout the duration of the infusion [34]. The high concentration of 4-PBA was required to influence histone deacetylation and antitumor effects. Our current study shows that the low dosed 4-PBA effectively inhibited TAC-triggered ER stress and LVH, while histone deacetylation remained unaffected.
In conclusion, our study is the first to demonstrate that (1) cardiac ER stress response is sustained during the development of myocardial hypertrophy and fibrosis up to 4 weeks after the onset of pressure overload, (2) the inhibitory effect of 4-PBA on ER stress can be differentiated from that on HDAC by selective dosing, and (3) attenuation of ER stress by 4-PBA significantly reduces pressure-induced LVH and associated myocardial fibrosis. These novel findings may provide a potential target for innovate treatments of hypertensive heart disease.
Acknowledgments
This study was supported by the grants from the Project of National Natural Science Foundation of China (No. 81400190, to Dr. Wang, http://www.nsfc.gov.cn/) and from the Key Research Project of Natural Science Foundation of Guangdong Province, China (No. 05z002, to Prof. Yan).
Abbreviations
- 4-PBA
4-Phenylbutyric acid
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- MI
myocardial infarction
- PERK
protein kinase RNA-like ER kinase
- c-JNK
c-Jun NH2-terminal kinase
- XBP1
X-box-binding protein 1
- CHOP
CCAAT/enhancer-binding protein homologous protein
- TAC
transverse aortic constriction
- LVH
left ventricular hypertrophy
- LVPWs
left ventricular posterior wall thickness at end systole
- LVPWd
left ventricular posterior wall thickness at end diastole
- HDAC
histone deacetylase
- HDACI
histone deacetylase inhibitor
- TG
thapsigargin
- VSMCs
vascular smooth muscle cells
- PAA
phenylacetate
- PAG
phenylacetylglutamine
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 2.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 3.Dufey E, Sepulveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol. 2014;307:C582–594. doi: 10.1152/ajpcell.00258.2014. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Zhang T, Li L, Wang J. Induction of Apoptosis by Hypertension Via Endoplasmic Reticulum Stress. Kidney Blood Press Res. 2015;40:41–51. doi: 10.1159/000368481. [DOI] [PubMed] [Google Scholar]
- 5.Isodono K, Takahashi T, Imoto H, Nakanishi N, Ogata T, Asada S, Adachi A, Ueyama T, Oh H, Matsubara H. PARM-1 is an endoplasmic reticulum molecule involved in endoplasmic reticulum stress-induced apoptosis in rat cardiac myocytes. PLoS One. 2010;5:e9746. doi: 10.1371/journal.pone.0009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan HS, Shangguan HJ, Shang Z, Yang L, Meng XM, Qiao SB. Endoplasmic reticulum stress caused by left ventricular hypertrophy in rats: effects of telmisartan. Am J Med Sci. 2011;342:318–323. doi: 10.1097/MAJ.0b013e3182112baf. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZY, Liu XH, Hu WC, Rong F, Wu XD. The calcineurin-myocyte enhancer factor 2c pathway mediates cardiac hypertrophy induced by endoplasmic reticulum stress in neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;298:H1499–1509. doi: 10.1152/ajpheart.00980.2009. [DOI] [PubMed] [Google Scholar]
- 8.Chaanine AH, Gordon RE, Kohlbrenner E, Benard L, Jeong D, Hajjar RJ. Potential role of BNIP3 in cardiac remodeling, myocardial stiffness, and endoplasmic reticulum: mitochondrial calcium homeostasis in diastolic and systolic heart failure. Circ Heart Fail. 2013;6:572–583. doi: 10.1161/CIRCHEARTFAILURE.112.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenna S, Trojanowska M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol. 2012;24:663–668. doi: 10.1097/BOR.0b013e3283588dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo T, Kim JK, Chen B, Abdel-Latif A, Kitakaze M, Yan L. Attenuation of ER stress prevents post-infarction-induced cardiac rupture and remodeling by modulating both cardiac apoptosis and fibrosis. Chem Biol Interact. 2015;225:90–98. doi: 10.1016/j.cbi.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem. 2007;282:27905–27912. doi: 10.1074/jbc.M702672200. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol. 2000;278:C259–267. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein RC, Lyons BM. Sodium 4-phenylbutyrate downregulates HSC70 expression by facilitating mRNA degradation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L43–51. doi: 10.1152/ajplung.2001.281.1.L43. [DOI] [PubMed] [Google Scholar]
- 14.Murray LS, Lu Y, Taggart A, Van Regemorter N, Vilain C, Abramowicz M, Kadler KE, Van Agtmael T. Chemical chaperone treatment reduces intracellular accumulation of mutant collagen IV and ameliorates the cellular phenotype of a COL4A2 mutation that causes haemorrhagic stroke. Hum Mol Genet. 2014;23:283–292. doi: 10.1093/hmg/ddt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Gonzales E, Grosse B, Schuller B, Davit-Spraul A, Conti F, Guettier C, Cassio D, Jacquemin E. Targeted pharmacotherapy in progressive familial intrahepatic cholestasis type 2: Evidence for improvement of cholestasis with 4-phenylbutyrate. Hepatology. 2015;62:558–566. doi: 10.1002/hep.27767. [DOI] [PubMed] [Google Scholar]
- 17.Malo A, Kruger B, Goke B, Kubisch CH. 4-Phenylbutyric acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Pancreas. 2013;42:92–101. doi: 10.1097/MPA.0b013e318259f6ca. [DOI] [PubMed] [Google Scholar]
- 18.Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim do H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun. 2012;421:578–584. doi: 10.1016/j.bbrc.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Carlisle RE, Brimble E, Werner KE, Cruz GL, Ask K, Ingram AJ, Dickhout JG. 4-Phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS One. 2014;9:e84663. doi: 10.1371/journal.pone.0084663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AC, Cohen S, Stewart M, Rivas R, Lison P. Radioprotection by the histone deacetylase inhibitor phenylbutyrate. Radiat Environ Biophys. 2011;50:585–596. doi: 10.1007/s00411-011-0384-7. [DOI] [PubMed] [Google Scholar]
- 21.Humeres C, Montenegro J, Varela M, Ayala P, Vivar R, Letelier A, Olmedo I, Catalan M, Rivas C, Baeza P, Munoz C, Garcia L, Lavandero S, Diaz-Araya G. 4-Phenylbutyric acid prevent cytotoxicity induced by thapsigargin in rat cardiac fibroblast. Toxicol In Vitro. 2014;28:1443–1448. doi: 10.1016/j.tiv.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, Wei Y, Cavener DR, Hu X, Hall J, Bache RJ, Chen Y. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension. 2014;64:738–744. doi: 10.1161/HYPERTENSIONAHA.114.03811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sari FR, Widyantoro B, Thandavarayan RA, Harima M, Lakshmanan AP, Zhang S, Muslin AJ, Suzuki K, Kodama M, Watanabe K. Attenuation of CHOP-mediated myocardial apoptosis in pressure-overloaded dominant negative p38alpha mitogen-activated protein kinase mice. Cell Physiol Biochem. 2011;27:487–496. doi: 10.1159/000329970. [DOI] [PubMed] [Google Scholar]
- 24.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 25.Noda T, Maeda K, Hayano S, Asai N, Enomoto A, Takahashi M, Murohara T. New endoplasmic reticulum stress regulator, gipie, regulates the survival of vascular smooth muscle cells and the neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2015;35:1246–1253. doi: 10.1161/ATVBAHA.114.304923. [DOI] [PubMed] [Google Scholar]
- 26.Ishimura S, Furuhashi M, Mita T, Fuseya T, Watanabe Y, Hoshina K, Kokubu N, Inoue K, Yoshida H, Miura T. Reduction of endoplasmic reticulum stress inhibits neointima formation after vascular injury. Sci Rep. 2014;4:6943. doi: 10.1038/srep06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.g Urea Cycle Disorders Conference, Consensus statement from a conference for the management of patients with urea cycle disorders. J Pediatr. 2001;138:S1–5. doi: 10.1067/mpd.2001.111830. [DOI] [PubMed] [Google Scholar]
- 28.Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;84:339–343. [PubMed] [Google Scholar]
- 29.Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–49. [PubMed] [Google Scholar]
- 30.McGuire BM, Zupanets IA, Lowe ME, Xiao X, Syplyviy VA, Monteleone J, Gargosky S, Dickinson K, Martinez A, Mokhtarani M, Scharschmidt BF. Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology. 2010;51:2077–2085. doi: 10.1002/hep.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maslak P, Chanel S, Camacho LH, Soignet S, Pandolfi PP, Guernah I, Warrell R, Nimer S. Pilot study of combination transcriptional modulation therapy with sodium phenylbutyrate and 5-azacytidine in patients with acute myeloid leukemia or myelodysplastic syndrome. Leukemia. 2006;20:212–217. doi: 10.1038/sj.leu.2404050. [DOI] [PubMed] [Google Scholar]
- 32.Camacho LH, Olson J, Tong WP, Young CW, Spriggs DR, Malkin MG. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Invest New Drugs. 2007;25:131–138. doi: 10.1007/s10637-006-9017-4. [DOI] [PubMed] [Google Scholar]
- 33.Iannitti T, Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D. 2011;11:227–249. doi: 10.2165/11591280-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carducci MA, Gilbert J, Bowling MK, Noe D, Eisenberger MA, Sinibaldi V, Zabelina Y, Chen TL, Grochow LB, Donehower RC. A Phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin Cancer Res. 2001;7:3047–3055. [PubMed] [Google Scholar]
- 35.Kang SH, Seok YM, Song MJ, Lee HA, Kurz T, Kim I. Histone Deacetylase Inhibition Attenuates Cardiac Hypertrophy and Fibrosis through Acetylation of Mineralocorticoid Receptor in Spontaneously Hypertensive Rats. Mol Pharmacol. 2015;87:782–791. doi: 10.1124/mol.114.096974. [DOI] [PubMed] [Google Scholar]
- 36.Gallo P, Latronico MV, Gallo P, Grimaldi S, Borgia F, Todaro M, Jones P, Gallinari P, De Francesco R, Ciliberto G, Steinkuhler C, Esposito G, Condorelli G. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res. 2008;80:416–424. doi: 10.1093/cvr/cvn215. [DOI] [PubMed] [Google Scholar]