Abstract
Background
Phospholipase A2s mediate the rate-limiting step in the formation of eicosanoids such as cysteinyl leukotrienes (CysLT). Group IVA cytosolic PLA2 (cPLA2α) is thought to be the dominant PLA2 in eosinophils; however, eosinophils also have secreted PLA2 (sPLA2) activity that has not been fully defined.
Objectives
To examine the expression of sPLA2 group X (sPLA2-X) in eosinophils, the participation of sPLA2-X in CysLT formation, and the mechanism by which sPLA2-X initiates CysLT synthesis in eosinophils.
Methods
Peripheral blood eosinophils were obtained from volunteers with asthma and/or allergy. A rabbit polyclonal anti-sPLA2-X antibody identified sPLA2-X by western blot. We used confocal microscopy to co-localize the sPLA2-X to intracellular structures. An inhibitor of sPLA2-X (ROC-0929) that does not inhibit other mammalian sPLA2s, as well as inhibitors of the mitogen activated kinase cascade (MAPK) and cPLA2α were used to examine the mechanism of N-formyl-methionyl-leucyl-phenylalanine (fMLP)-mediated CysLT formation.
Results
Eosinophils express the sPLA2-X gene (PLA2G10). The sPLA2-X protein is located in the endoplasmic reticulum (ER), golgi, and granules of eosinophils and moves to the granules and lipid bodies during fMLP-mediated activation. Selective sPLA2-X inhibition attenuated the fMLP-mediated release of arachidonic acid and CysLT formation by eosinophils. Inhibitors of p38, ERK1/2, JNK and cPLA2α also attenuated the fMLP-mediated CysLT formation. The sPLA2-X inhibitor reduced the phosphorylation of p38 and ERK1/2 as well as cPLA2α during cellular activation, indicating that sPLA2-X is involved in activating the MAPK cascade leading to CysLT formation via cPLA2α. We further demonstrate that sPLA2-X is activated prior to secretion from the cell during activation. Short-term priming with IL-13 and TNF/IL-1β increased the expression of PLA2G10 by eosinophils.
Conclusions
These results demonstrate that sPLA2-X plays a significant role in CysLTs formation by human eosinophils. The predominant role of the enzyme is the regulation of MAPK activation that leads to phosphorylation of cPLA2α. The sPLA2-X protein is regulated by proteolytic cleavage suggesting that an inflammatory environment may promote the formation of CysLTs through this mechanism. These results have important implications for the treatment of eosinophilic disorders such as asthma.
Keywords: eosinophil, leukotriene, phospholipase A2, mitogen-activated kinase, asthma, allergy
INTRODUCTION
Cysteinyl leukotrienes (CysLTs, C4, D4 and E4) are generated in increased quantities in the airways of patients with asthma (1–3). Eosinophils accumulate in the airways of many patients with asthma and serve as a key source of CysLTs (4). The rate-limiting step in CysLT formation is phospholipase A2 (PLA2)-mediated release of arachidonate from the sn-2 position of membrane phospholipids. It has been assumed that group IVA cytosolic PLA2 (i.e. cPLA2α) serves as the key regulator of endogenous CysLT synthesis in myeloid cells such as eosinophils (5,6); however, 10 mammalian secreted PLA2s (sPLA2s) have been identified, and in transfected cells, several sPLA2s have been found to coordinate eicosanoid synthesis with cPLA2α (7–9). Among the sPLA2s, groups V and X (i.e. sPLA2-V and sPLA2-X) have unique functional capacities to cleave phospholipids and initiate cellular eicosanoid synthesis (10,11). We have demonstrated that sPLA2-X is elevated in the airways of patients with asthma (12,13), and serves to activate CysLT synthesis in eosinophils when the recombinant enzyme is added exogenously to eosinophils (14).
Eosinophils also have endogenous sPLA2 activity that contributes to surfactant dysfunction through the cleavage of surfactant phospholipids (15,16). The gene expression of sPLA2 group IIA (sPLA2-IIA, PLA2G2A) and sPLA2-X (PLA2G10) were previously identified in human eosinophils (16). The sPLA2-IIA protein was identified in eosinophils (17), but does not contribute to leukotriene formation in these cells (18,19). The other high activity sPLA2, sPLA2-V, is not present in eosinophils (20). In the present study, we investigated the contribution of the sPLA2-X enzyme to sPLA2 activity in human primary eosinophils, and the role of this enzyme as a key control point in CysLT formation. The results support an important role of sPLA2-X in endogenous CysLT synthesis in eosinophils, and that sPLA2-X is involved in the activation of the MAPK cascade leading to the activation of cPLA2α.
METHODS
Materials
Ficoll-Paque PLUS (d 1.077) was from GE Healthcare (Piscataway, NJ). Antibodies for immunomagnetic selection were from Miltenyi Biotec (Auburn, CA). [3H]Arachidonate was from American Radiolabeled Chemicals (St. Louis, MO). N-formyl-methionyl-leucyl-phenylalanine (fMLP) was from Sigma-Aldrich (St. Louis, MO). Antibodies against phospho-cPLA2α [Ser505], cPLA2α, phospho-p44/42 MAPK (Erk 1/2)[Thr202/Tyr204], p44/42 MAPK (Erk 1/2), phospho-p38 MAPK [Thr180/Tyr182], p38 MAPK, phospho-stress-activated protein kinase/Jun-amino-terminal kinase (SAPK/JNK)[thr183/tyr185], and SAPK/JNK were from Cell Signaling Technologies (Beverly, MA). Inhibitors of MEK 1/2 (U0126), p38 (SB203580), JNK (SP600125), Furin I (344930, D-RVKR-CMK) and Furin II (344931, 6-D-R) were purchased from EMD Biosciences (San Diego, CA). Rabbit polyclonal anti-sPLA2-X antibody was developed and characterized in our laboratory (21). Dr. James J. Lee (Mayo Clinic, Scottsdale, AZ) provided the murine monoclonal anti-EPX antibody. Murine monoclonal anti-GM130 (35/GM130) was from BD Bioscience (San Jose, CA). Murine monoclonal anti-PDI (RL90) was from Abcam (Cambridge, MA). Murine monoclonal anti-ADRP (AP125) was from Fitzgerald (Acton, MA). Rabbit anti-5-LO antiserum was purchased from Cayman Chemical (Ann Arbor, MI) and rabbit anti-cPLA2α anti-serum was purchased from Santa Cruz Biotechnology (Dallas, TX). Cy3-conjugated goat anti-rabbit IgG was from Jackson ImmunoReasearch (West Grove, PA). Alexa Fluor 647 goat anti-mouse IgG was purchased from Invitrogen.
Isolation of human blood eosinophils and differentiation of HL15 cells
The University of Washington Institutional Review Board approved the study, and written informed consent was obtained from all participants. Peripheral blood eosinophils were obtained from volunteers with a physician diagnosis of asthma and/or allergy and ≥ 1.2 × 105 eosinophil/ml of peripheral blood. Granulocytes were isolated from peripheral blood by density gradient centrifugation followed by hypotonic lysis of red blood cells. Eosinophils were removed from the granulocyte fraction by negative immunomagnetic selection. The purity of eosinophils was determined by differential counts of Romanowski-stained (Diff-Quick) cytospin preparations. Eosinophil viability was assessed by trypan blue exclusion.
HL-60 clone-15 (HL-60-C15) and AML14.3D10-CCR3 cells were purchased from ATCC. Cells were cultured in RPMI with 10% FCS. HL-60-C15 cells were treated with and without 0.5 mM sodium butyrate for 7 days.
Selective PLA2 inhibitors
Because human eosinophils contain sPLA2 group IIA (sPLA2-IIA) (17) we used a sPLA2 inhibitor, known as ROC-0929, that is selective for sPLA2-X and does not inhibit other mammalian sPLA2s at nanomolar concentrations (22). The compound ROC-0929 is an analog of the well-known sPLA2 inhibitor LY315920 (22). Docking studies revealed that the isobutyl group of ROC-0929 sterically excludes this compound from the active site of sPLA2-IIA, but not the active site of sPLA2-X, resulting in > 80-fold difference in inhibitory potency of ROC-0929 between the sPLA2-X and sPLA2-IIA enzymes (22). To test for off target effects, we used a control compound, known as ROC-0428, that differs from ROC-0929 by one methyl group and is essentially devoid of sPLA2 inhibition (22). We also used a pan-sPLA2 inhibitor (0509A) and an inhibitor that is selective for the group IIA/IIE enzymes (0320). The inhibitory a ctivities of the sPLA2 inhibitors and the control compound are shown in Supplemental Table 1. Inhibition of cPLA2α was achieved with Pyr-2 (pyrrophenone) and Wyeth-2 (gifipladib) (23,24).
Secreted PLA2 activity assay
3H-labelled E. coli membranes were resuspended with reaction buffer at a final concentration of 2.0 × 106 dpm/mL (25). The eosinophil lysate was treated with inhibitors or DMSO control for 10 minutes at room temperature. The radio-labeled E. coli membrane substrate was added to each reaction tube and incubated at 37°C for 1 hour. The reaction was stopped by the addition of 0.1 M EDTA and the sample centrifuged at 14, 000 × g. The supernatant was submitted to scintillation counting.
Assessment of arachidonate release by eosinophils
The release of arachidonate and CysLT from eosinophils was determined in 24-well plates coated with 0.01% BSA. Eosinophils (1.8 × 105 per well) were resuspended in RPMI with 0.01% BSA, and the cells were incubated for 24 h with [3H]arachidonate (0.1 μCi/well). After the unincorporated arachidonate was washed three times, the cells were preincubated with inhibitors for 20 min at 37°C, and then stimulated with fMLP for 20 min. Supernatants were submitted to scintillation counting after being centrifuged to remove detached cells. The remaining eosinophils were detached with 0.25% trypsin with EDTA for 30 min at 37°C, pelleted, and submitted to scintillation counting. Arachidonate release was expressed as a percentage of counts/min in the supernatant to the total counts/min in the cells and supernatant.
Assessment of CysLT formation by eosinophils
Eosinophils were resuspended in HBSS with Ca2+ and Mg2+ at a concentration of 1.5 × 105 per well in a 5% CO2 incubator at 37°C. The cells were preincubated with either inhibitor or DMSO control for 20 min at 37°C and then stimulated with fMLP for 20 min. The synthesis of eicosanoids was stopped by the addition of 4 volumes of ice-cold methanol with 0.2% formic acid. An ELISA assay measured LTC4 or total CysLT levels after removal of the methanol by evaporation (Cayman Chemical, Ann Arbor, MI).
Selective Kinase Inhibitors
To examine the role of the MAPKs in LTC4 synthesis, we used a series of inhibitors that are specific with little cross inhibition of the other kinases (26–28). Eosinophils (1.5 × 105 per well) resuspended in HBSS with Ca2+ and Mg2+ were preincubated with either DMSO control or inhibitors of MEK1/2 (U0126, 10 μM), p38 (SB203580, 30 μM), or JNK (SP600125, 20 μM) for 20 min at 37°C in a 5% CO2 incubator and then stimulated with fMLP (100 nM). The synthesis of eicosanoids was stopped by the addition of 4 volumes of ice-cold methanol with 0.2% formic acid after 20 min.
Measurement of MAPK and cPLA2α phosphorylation
Phosphorylation of ERK1/2, p38, JNK and cPLA2α in eosinophils (2.0 × 106 cells) pretreated with DMSO control or ROC-0929 and then stimulated with 100nM fMLP for 20 min was determined by western blots of the phosphorylated protein relative to the total protein. The cells were pelleted and treated with lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, with protease and phosphatase inhibitors) on ice for 30 min. Equal amounts of cell lysate were heated for 10 min in LDS loading buffer with DTT and β-mercaptoethanol, and separated on 4–12% Bis-Tris gel under reducing conditions. The resolved proteins were transferred onto a polyvinylidene fluoride membrane using a semidry apparatus, blocked with 5% non-fat milk, and incubated with the primary antibody overnight at 4°C. ECL visualized the proteins of interest.
Pro-peptide convertase inhibitors
Eosinophils (1.5 × 105 per well) resuspended in HBSS with Ca2+ and Mg2+ were preincubated with either DMSO control or Furin I (D-RVKR-CMK, 10 μM) and Furin II (344931, 6-D-R, 10 μM) for 20 min at 37°C in a 5% CO2 incubator and then stimulated with fMLP (100 nM). The synthesis of eicosanoids was stopped by the addition of 4 volumes of iced methanol with 0.2% formic acid after 20 min.
Confocal Microscopy
Eosinophils (1.0 × 106 per well) were allowed to adhere to cover glasses coated with 0.01% BSA and treated with DMSO vehicle control or ROC-0929 for 20 min, and then treated with either vehicle control or fMLP (100 nM) for 20 min at 37°C in a 5% CO2 incubator. The cells were fixed in 3.7% formaldehyde in PBS at room temperature for 30 min. Eosinophils were permeabilized with 0.1% Triton X-100 and blocked with 10% goat serum. Immunostained cells were visualized with a Zeiss LSM510 Confocal Microscope.
Quantitative PCR
Real-time PCR analysis was conducted using TaqMan primer probe sets with quantification relative to an endogenous control gene using the delta Ct method. Primer-probe sets were obtained from the Applied Biosystems using a FAM probe for PLA2G10 (Hs00358567_m1) and a primer-limited VIC probe for HPRT1 (4326321E) as an endogenous control (Applied Biosystems, Foster City, CA). Real-time PCR was performed using a Mastercycler ep realplex system (Eppendorf, Hauppauge, NY). In some experiments, eosinophils were placed in RPMI at 37°C in a 5% CO2 incubator for 4 hours during treatment with buffer control, or TNFα, IL-β, IL-4, IL-5, IL-13, GMCSF, IL-33, and/or thymic stromal lymphopoietin (TSLP) (Peprotech, Rock Hill, NJ). Following cytokine treatment, Trizol isolated RNA was used to assay PLA2G10 gene expression.
Statistical Analysis
A one-way ANOVA assessed differences in eicosanoid formation with fMLP stimulation in the presence of specific inhibitors. Post-hoc comparisons were made between fMLP treated cells and other conditions with Dunnett’s multiple comparison test. A paired t-test assessed differences between PLA2G10 gene expression when two conditions were present.
Results
Subject characteristics
We isolated peripheral blood eosinophils 54 times from a total of 45 distinct donors for these studies. The mean viability was 99.5% (SD 1.58) and purity was 99.4% (SD 0.64) eosinophils. The mean age of the donors was 30.2 years old and 55.6% were female. The study population was 8.9% Hispanic, and 62.2% Caucasian, 8.9% African American, and 6.7% Asian. Both asthma and allergic diseases were present in 53.3% of the population, while 11.1% had asthma alone, and 35.6% had allergic diseases. The mean concentration of eosinophils in peripheral blood was 3.0 × 105 eosinophils/ml, and the mean percentage of eosinophils was 4.5%.
sPLA2-X is expressed by human eosinophils and localizes to structures involved in leukotriene formation
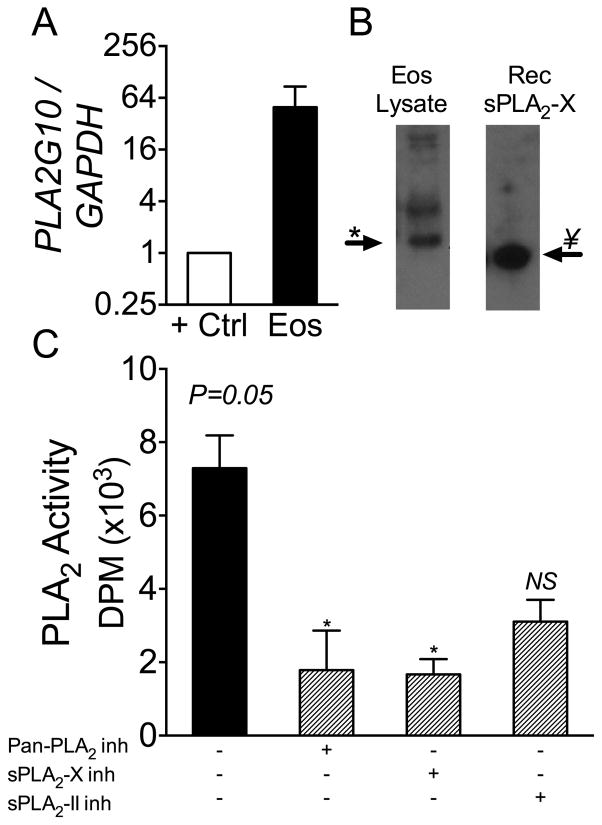
We assessed the expression of the sPLA2-X gene PLA2G10 in human eosinophils from 3 donors and found that human eosinophils strongly express PLA2G10 (Fig 1A). The positive control for PLA2G10 expression was RNA obtained from a human epithelial cell line as epithelial cells strongly express PLA2G10 (13). In cell lysates of eosinophils, the sPLA2-X protein can be identified on western blot using a rabbit polyclonal antibody that is specific for sPLA2-X (Fig 1B) (21,29). The native sPLA2-X protein runs on the gel at about 13.5 kDa, slightly larger than the recombinant mature sPLA2-X protein, indicating that some post-translational modification of the protein has occurred. Treatment with N-Glycosidase A to cleave N-glycans did not significantly change the gel shift of the protein (data not shown). A second larger band identified in the blot appears to be a dimer of the sPLA2-X enzyme that is also seen with the recombinant protein when run at a higher concentration (see Fig E3E). Either a sPLA2-X-specific inhibitor (ROC-0929) or a pan-sPLA2 inhibitor (0509A) significantly decreased the total sPLA2 activity in lysates from human eosinophils; however, the inhibitory activity of the sPLA2-IIA/IIE inhibitor (0320) did not reach statistical significance (Fig 1C; Table S1). It should be noted that the high-sensitivity PLA2 assay used in the present study has a specific activity for sPLA2-X that is relatively low due to the anionic phospholipid composition in the E. coli radiometric assay (30). In contrast, the release of free arachidonate from mammalian HEK293 cells is ~1,000 times higher for sPLA2-X over the sPLA2-IIA enzyme (11). These results indicate that sPLA2-X is expressed in human eosinophils and comprises the majority of sPLA2 activity in these cells.
Figure 1.
Identification of sPLA2-X in human eosinophils. A, Human eosinophils strongly express the sPLA2-X gene PLA2G10 relative to the positive control consisting of RNA isolated from an epithelial cell line. B, Western blot of eosinophil lysate demonstrates the sPLA2-X protein running as a single band at 13.5 kDa just above the mature recombinant protein. C, Total sPLA2 activity in eosinophils lysates was inhibited by a sPLA2-X-specific inhibitor (ROC-0929) or a pan-sPLA2 in hibitor (0509A), but the inhibition by a sPLA2 group IIA/IIE inhibitor did not reach statistical significance (P=0.05 0verall ANOVA, * P < 0.05 by post-hoc tests).
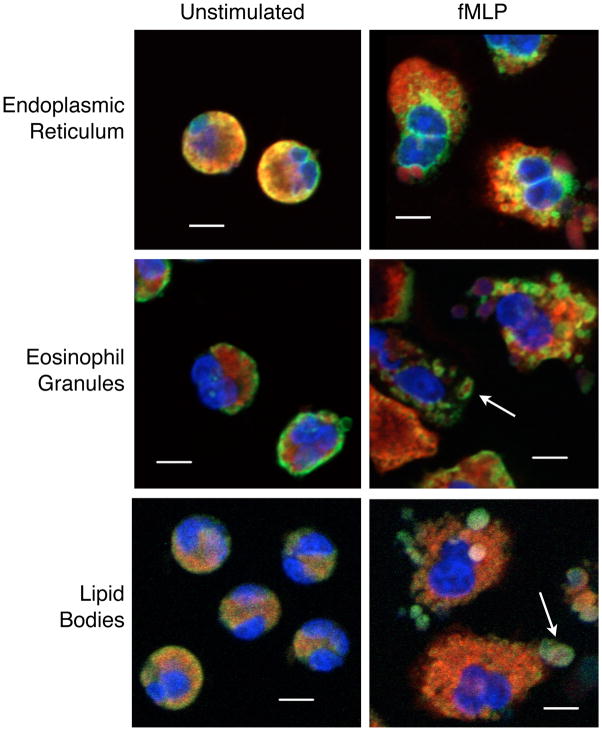
We used confocal microscopy to characterize the location of sPLA2-X in resting and fMLP-stimulated eosinophils. In resting cells the sPLA2-X protein co-localized predominantly to the endoplasmic reticulum (ER) and golgi (Fig 2 and Fig E1). There may also be some colocalization to eosinophil granules identified by immunostaining for EPX. The antibody used to identify lipid bodies (i.e. anti-ADRP) demonstrated only background immunostaining on the plasma membrane in resting cells.
Figure 2.
Localization of sPLA2-X in human eosinophils by confocal microscopy. Immunostaining demonstrates co-localization in yellow using antibodies directed against sPLA2-X (red) and endoplasmic reticulum (ER, anti-PDI), granules (anti-EPX) and lipid bodies (anti-ADRP), all in green. sPLA2-X co-localized predominantly to the endoplasmic reticulum (ER) in unstimulated eosinophils. Following fMLP stimulation, there is persistent colocalization to the ER and some colocalization to granules (arrow middle panel), and lipid bodies (arrow lower panel) that form after stimulation with fMLP. Some of the lipid bodies containing sPLA2-X appear extracellular. Further details of the immunostaining for sPLA2-X and eosinophil structures are presented in Figures E1 and E2. Scale bar is 5 μm.
Following stimulation of the cells with fMLP, there was persistent colocalization to the ER, and less co-localization to the golgi (Fig 2 and Fig E2). In stimulated cells, the granule staining by anti-EPX became more distinct, and there was some colocalization with sPLA2-X to the granules (arrow in middle panel of Fig 2). The immunostaining for sPLA2-X in granules is further delineated in Figure E2 demonstrating red immunostaining for sPLA2-X in structures labeled with the anti-EPX antibody for granules in green (arrows, third panel). With the anti-ADRP immunostaining of lipid bodies, fMLP stimulation caused the formation of lipid bodies that could be observed both within the cytoplasm of eosinophils as well as some lipid bodies that appear extracellular. Although there was limited colocalization of sPLA2-X to lipid bodies (arrow in lower panel of Fig 2), it was apparent that the sPLA2-X protein resides in these structures, but in a slightly different location from the target for the anti-ADRP (arrows in lower panel of Fig E2). These results indicate that sPLA2-X is located predominantly in the ER of resting eosinophils, but enters the secretory system and lipid bodies upon stimulation and may be externalized during activation either through release or eosinophil necrosis.
An active site-directed inhibitor of sPLA2-X inhibits fMLP-mediated eicosanoid synthesis
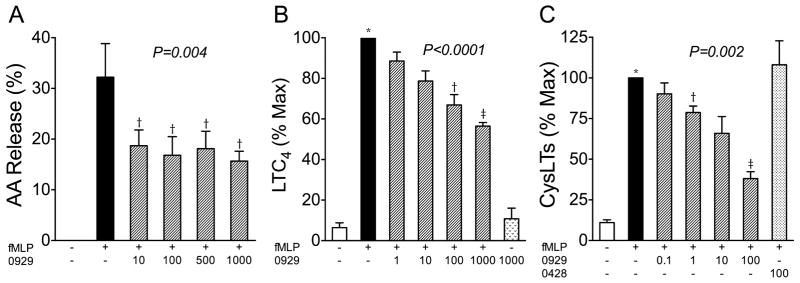
We activated human eosinophils with fMLP based in part on the observation that well-established allergens, house dust mite proteins, activate eosinophils via the formyl peptide receptors (31). After eosinophils were incubated with radiolabeled arachidonate, the percent of [3H]arachidonate released in response to fMLP was attenuated by pre-treatment of the cells with the sPLA2-X-specific inhibitor ROC-0929 (Fig 3A, P=0.004). In this assay, there was no significant difference between the effects of the inhibitor at concentrations ranging from 10 to 1000 nM. The release of the CysLT LTC4 from fMLP activated eosinophils was reduced in a dose dependent manner by increasing concentrations of ROC-0929 ranging from 1–1000 nM, although only the 100 and 1000 nM concentrations of the inhibitor caused a statistically significant reduction in LTC4 formation based on post hoc tests (Fig 3B, P<0.0001). The ROC-0929 inhibitor did not have any effect on the basal release of LTC4 from human eosinophils. We further quantified the total CysLT release from eosinophils following fMLP stimulation and found that there was dose dependent inhibition of CysLT formation starting at a concentration of just 1 nM (Fig 3C). As a further control for this experiment, we found that there was no alteration in CysLT formation mediated by the control inhibitor (ROC-0428) that is structurally similar to ROC-0929, but is unable to bind to the active site of sPLA2-X due to the addition of a methyl group (14,22).
Figure 3.
Eicosanoid formation mediated by endogenous sPLA2-X. A, An active-site directed sPLA2-X inhibitor (0929), reduced the amount of arachidonic acid released in response to fMLP across all concentrations of the inhibitor ranging from 10–1000 nM. B, The fMLP-mediated formation of LTC4 was reduced in a dose-related manner by the sPLA2-X inhibitor, but had no affect on basal LTC4 synthesis. C, The formation of total CysLTs was inhibited by the sPLA2-X inhibitor, but a structural analog that is not active as an inhibitor (0428), did not affect CysLT formation. *P<0.05, †P<0.01, ‡P<0.001.
We assessed the potential use of HL-60-C15 and AML14.3D10-CCR3 cells as potential cell lines that could be used to further understand the biology of sPLA2-X in eosinophils, but found that PLA2G10 expression in these cells was much lower than in primary human eosinophils (see supplemental results, Fig E3D). However, the HL-60-C15 cell line provides a further control for our results in primary human eosinophils (Fig 3) studies since this cell line has minimal expression of PLA2G10 and the ROC-0929 inhibitor had a negligible impact on CysLT formation in these cells (Fig E3B). We also assessed the prolonged maintenance of primary eosinophils in culture for potential RNA interference (RNAi) studies, and found that although the cells appeared viable, they failed to strongly activate in response to fMLP after 24 hours in culture, either with or without the addition of fetal calf serum (FCS) to the medium (Fig E4). We further found that treatment of eosinophils with IL-5 and GMCSF to prolong survival would not serve as an adequate model because of the impact on PLA2G10 expression (see below and Fig 5).
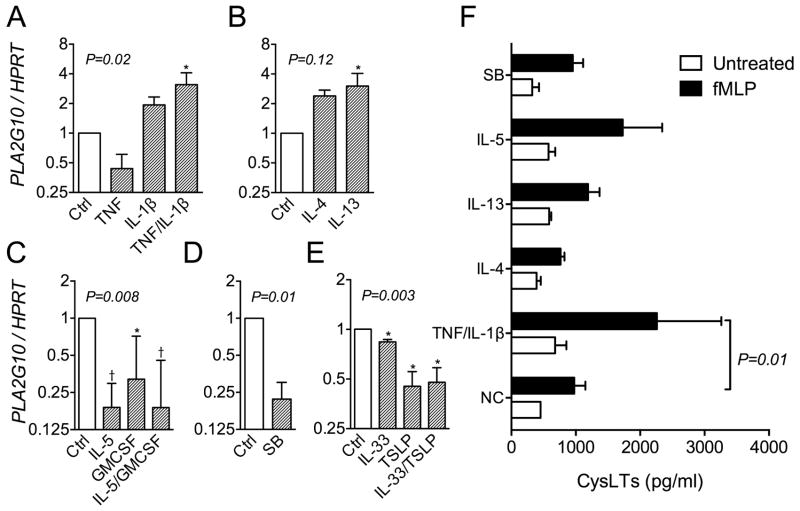
Figure 5.
Consequences of short-term cytokine treatment on PLA2G10 expression in eosinophils. A-E, Expression of PLA2G10 by eosinophils was increased by TNF and IL-1β and by IL-13 alone; while the expression was decreased by treatment with IL-5 with or without GMCSF, sodium butyrate, and TSLP, IL-33 or a combination of IL-33 and TSLP. F, The formation of CysLTs by fMLP-stimulated eosinophils was increased after priming with TNF/IL- β (P=0.01), but did not reach statistical significance for other selected cytokines. *P<0.05, †P<0.01
sPLA2-X is involved in the activation of the MAPK cascade and cPLA2α
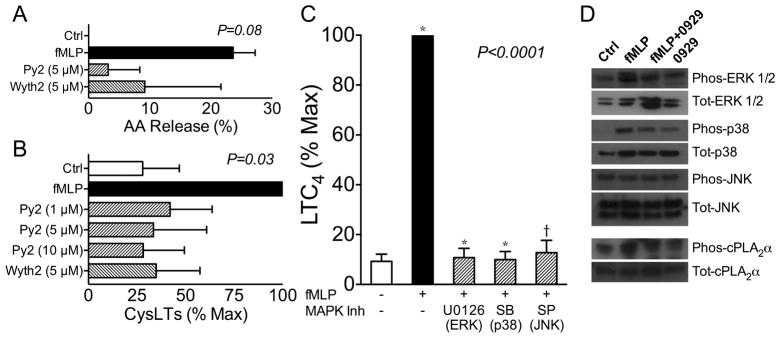
As we have demonstrated that exogenously added sPLA2-X acts in part via the activation of the MAPK cascade leading to phosphorylation at Ser505 of cPLA2α (14), we investigated the possibility that endogenous sPLA2-X serves as an initiator of the MAPK cascade and cPLA2α phosphorylation. The role of cPLA2α was examined using two different cPLA2α inhibitors Pyr-2 (pyrrophenone) and Wyeth-2 (girfiladib) during fMLP-mediated activation of eosinophils. Treatment of eosinophils with either cPLA2α inhibitor at a concentration of 5 μM decreased the release of radiolabeled arachidonate from eosinophils (Fig 4A, P=0.08). The generation of CysLTs in response to fMLP was also significantly inhibited in a dose-dependent manner by Pyr-2 in concentrations ranging from 1–10 μM, and by 5 μM Wyeth-2 (Fig. 4B, P=0.03). Selective inhibitors of MEK 1/2 (U0126 10 μM), p38 (SB203580 30 μM), and JNK (SP600125 20 μM) each suppressed LTC4 formation initiated by fMLP treated eosinophils (Fig 4C, P<0.0001).
Figure 4.
Effects of sPLA2-X mediated through the MAPK cascade. A, The fMLP-mediated release of [3H]arachidonate was attenuated by the cPLA2α inhibitors Pyr-2 (5 μM) and Wyeth-2 (5 μM). B, The formation of CysLTs in response to fMLP was also decreased by Pyr-2 (1–10 μM) and Wyeth-2 (5 μM). C, The fMLP-mediated LTC4 formation was inhibited by a MEK 1/2 inhibitor (U0126 10 μM), a p38 inhibitor (SB203580 30 μM), and by a JNK inhibitor (SP600125 20 μM). D, Western blots of cell lysates from eosinophils treated with buffer control (Ctrl), fMLP, or fMLP plus the sPLA2-X inhibitor (0929) demonstrates that the 0929 inhibitor attenuated the phosphorylation of ERK1/2 and p38 in response to fMLP. There were no changes in the phosphorylation of JNK, while the phosphorylation of cPLA2α at Ser505 relative to total cPLA2α was modestly reduced.
To connect the activation of CysLT formation by cPLA2α and the MAPK pathway to the endogenous role of sPLA2-X in this process, we examined western blots of eosinophil lysates following stimulation with fMLP, with or without the selective sPLA2-X inhibitor ROC-0929. We found that fMLP strongly initiated ERK1/2 and p-38 phosphorylation, and further that pre-treatment of the cells with ROC-0929 inhibited the phosphorylation of ERK1/2 and p-38 (Fig 4D). We were unable to identify any effects of fMLP or the ROC-0929 inhibitor on JNK phosphorylation, but did find that JNK was phosphorylated in these cells prior to fMLP treatment. We also found that the sPLA2-X inhibitor decreased phosphorylation of cPLA2α at Ser505 by approximately half as compared to the fMLP-stimulated cells without this inhibitor (Fig 4D). These results indicate that sPLA2-X initiates the phosphorylation of ERK1/2 and p-38, that is known to initiate phosphorylation of cPLA2α, leading to eicosanoid synthesis (32).
To further examine the sPLA2-X mediated activation of CysLT synthesis, we examined the effects of sPLA2-X inhibition on the localization of 5-LO and cPLA2α that form a complex in close proximity to nuclear membranes and lipid bodies during eosinophil leukotriene formation (33–35). Following fMLP-mediated activation, there was an increase in the signal for 5-LO and cPLA2α in the cytoplasmic space surrounding the nucleus and lipid bodies (Fig E5). Pre treatment of the cells with the sPLA2-X inhibitor ROC-0929 resulted in partial attenuation of the changes in 5-LO and cPLA2α immunostaining in these cells. These results further indicate that sPLA2-X is involved in the cascade of events leading to leukotriene formation. As we found that sPLA2-X resides in the secretory apparatus, and the ROC-0929 inhibitor is not readily cell permeable (8), we assessed western blots of the sPLA2-X protein in eosinophil supernatant and found that while the protein could be detected in extracellular fluid, there was no apparent difference in the amount of sPLA2-X in the eosinophil supernatant with activation (Fig E6A). One checkpoint in the activation of sPLA2-X is the cleavage of a pro-peptide that occurs at a furin consensus sequence (36,37). We found that a competitive inhibitor of furin-like propeptide convertases (6-D-R) (38) inhibited fMLP-mediated LTC4 formation while an irreversible pro-peptide convertase inactivator (D-RVKR-CMK) (39) did not alter LTC4 formation (Fig E6B).
PLA2G10 expression is increased by inflammatory signals and reduced by a HDAC inhibitor
Because sPLA2-X is upregulated by inflammatory stimuli such as TNF (40) and it is well known that eosinophils are ”primed” in the airways under the influence of IL-5 and GMCSF (41), we tested the effects of cytokines implicated in asthma on the expression of PLA2G10 in primary eosinophils. We found that the expression of PLA2G10 was increased by treatment of the cells with TNF and IL-1β together, but not by either cytokine alone (Fig 5A). Although the expression of PLA2G10 tended to be increased by both IL-4 and IL-13, the increase only reached statistical significance for IL-13 (Fig 5B). In contrast to our expectations, the expression of PLA2G10 was decreased by treatment with IL-5 with or without GMCSF (Fig 5C), and was also decreased by treatment with thymic stromal lymphopoietin (TSLP) and IL-33 (Fig 5D). In accord with our expectation, treatment with the HDAC inhibitor SB decreased the expression of PLA2G10 in primary eosinophils (Fig 5E). We also examined the effects of selected cytokines on the fMLP-mediated formation of CysLTs, and found that priming the eosinophils with TNF/IL- β was the only factor that led to a statistically significant increase in CysLTs (P=0.01, Fig 5F), while IL-5 tended to increase CysLT formation but this difference was not statistically significant (P=0.17). These data indicate that TNF/IL-1β as well as IL-13 increases the gene expression of PLA2G10 in eosinophils, and that TNF/IL-1β treatment increased fMLP-mediated CysLT formation.
DISCUSSION
Eosinophils are known to have endogenous sPLA2 activity (15,16), but the only previously characterized sPLA2 enzyme in eosinophils is sPLA2-IIA (17), an enzyme that does not contribute to leukotriene formation in these cells (18,19). A prior study found that the sPLA2-X gene PLA2G10 is expressed in eosinophils (16). In this study, we confirmed that PLA2G10 is expressed in human eosinophils, and demonstrate for the first time that sPLA2-X significantly contributes to sPLA2 activity, and is involved in the endogenous release of arachidonic acid and the formation of CysLTs. Based on confocal microscopy, we found that sPLA2-X resides within the secretory compartment, and appears to be externalized during activation. As it is known that fMLP-mediated CysLT formation is mediated by cPLA2α, we found that sPLA2-X was involved in the activation of the MAPK cascade, specifically ERK1/2 and p-38, which activate cPLA2α by phosphorylation at a Ser505 (32,42,43). These results show that sPLA2-X serves as a key regulator of CysLT formation by eosinophils, in part through the regulation of the MAPK cascade and cPLA2α. We further demonstrate that the gene expression of PLA2G10 is increased in response to TNFα/IL-β, and IL-13, and that TNFα/IL-β increased CysLT formation by eosinophils. These findings have important implications for asthma and allergic disease through the identification of a novel PLA2 enzyme that we show for the first time to regulate LT formation by eosinophils.
Human eosinophils undergo piecemeal degranulation in response to fMLP stimulation (44), suggesting that the sPLA2-X that we identified in the secretory compartment should be externalized during activation. Externalization may be very important since sPLA2-X has very high activity for phosphatidylcholine (PC) that is enriched on the extracellular facing plasma membrane (11) and a large portion of eicosanoid synthesis in transfected HEK293 cells occurred after secretion on the extracellular face of the cell (45). We did not observe immunostaining on the outer cell membrane by confocal studies probably because sPLA2-X does not bind to the plasma membrane or accumulate on the cell surface (8). The relatively large volume of cell culture medium that was used in vitro for the experiment also reduces cell surface binding. Because the ROC-0929 sPLA2-X inhibitor is cell impermeant and blocked CysLT formation, these studies support the finding that endogenous sPLA2-X is externalized where it can act on the outer cell membrane to generate free fatty acids including arachidonic acid, generate lysophospholipids and contribute to surfactant dysfunction. These results indicate the endogenous sPLA2-X acts in a manner that we have identified previously for exogenous sPLA2-X, causing CysLT formation via the MAPK cascade and cPLA2α, and also further amplifying CysLT formation by providing an additional source of arachidonic acid (14,46). The release of lysophospholipids is critical in the initiation of the MAPK cascade as we have previously demonstrated in eosinophils (14), and lysophospholipids were recently implicated as a major regulator of innate lymphoid cell responses in the periphery (47).
A key control point in sPLA2-X-mediated activation of eicosanoid synthesis is the proteolytic cleavage of a propeptide that must be removed to generate the active enzyme (48,49). Transgenic expression of full-length Pla2g10 in a mouse created no alteration in phenotype in the absence of an inflammatory environment (37). In contrast, transgenic expression of Pla2g10 without the propeptide (i.e. active sPLA2-X) in macrophages causes unrestrained inflammation (50). The propeptide contains a highly conserved RXn(K/R)R furin-recognition motif at its C-terminus (36). We found that a competitive furin propeptide convertase inhibitor (i.e. 6-D-R) that can act either within or following secretion from cells (38) inhibits LTC4 formation in these cells. In the cell lysate or the extracellular fluid, only a single band for the mature protein is seen (Figs 1B and E4E). These results suggest that sPLA2-X is activated within the cell prior to secretion. Intracellular inhibition of sPLA2-X could have even greater effects on eicosanoid formation in these cells; however, this hypothesis could not be further tested since a cell permeate sPLA2-X inhibitor is not available. As seen in supplemental Fig E3 the eosinophil-like cell lines HL-60-C15 and AML14.3D10-CCR3 have much lower expression of PLA2G10 compared to primary human eosinophils; thus, shRNA gene silencing studies were not attempted. We also found that the reduced activation of eosinophils after 24 hours in cell culture limited our ability to perform short-term siRNA studies (Fig E4). In a prior study, an irreversible furin propeptide convertase inhibitor (i.e. D-RVKR-CMK) prevented the secretion of the protein in HEK293 cells transfected with full-length human PLA2G10 or murine Pla2g10, suggesting that proteolytic conversion is regulating sPLA2-X release (36). This same irreversible furin propeptide convertase inhibitor did not alter LTC4 formation in eosinophils, suggesting that different pro-peptide convertases regulate LT formation in HEK293 cells and primary human eosinophils (38,51–53). As furins serve as checkpoints of inflammation, these results suggest that proteolytic control of sPLA2-X may be important in eosinophil-mediated inflammation (54).
It is well established that airway eosinophils relative to circulating eosinophils have increased ex vivo survival and markers of activation (41,55–57). Although IL-5 and GM-CSF have the most established effects on the production, differentiation and survival of eosinophils (58–60), eosinophil migration is also regulated by IL-4 and IL-13 (61,62). We found that IL-13 and IL-1β in combination with TNF were the major cytokines that increased PLA2G10 expression. We also found that TNF/IL-1β priming of eosinophils increased CysLT formation suggesting that such an increase in PLA2G10 may be involved in increasing CysLT formation by eosinophils. A number of cytokines including IL-5 and GMCSF actually decreased PLA2G10 expression. Further, the expression of PLA2G10 in eosinophils was decreased by a HDAC inhibitor and by IL-33 and TSLP. These findings were unexpected since IL-33 activates eosinophils via the transmembrane ST2 receptor (63–65), and TSLP activates both CD34+ progenitor cells and mature eosinophils (66,67).
A major question raised by these results is whether the increased levels of sPLA2-X identified in asthma (12,13) could be the result of secretion of this enzyme from eosinophils as suggested by the present study. Because we have shown that the epithelium (13) and airway macrophages (29) immunostain in human airway samples for sPLA2-X, and that primary epithelial cells in organotypic culture secrete the mature sPLA2-X protein (13), the precise cellular source of the enzyme in humans is unclear. The deletion of the murine sPLA2-X gene (pla2g10) (68) or an active site directed inhibitor of human sPLA2-X in a transgenic mouse model (69) inhibits the development of airway inflammation, hyperresponsiveness, and structural remodeling. Thus it will be important to identify the relative importance of the epithelium, macrophages and eosinophils in future murine studies examining the in vivo effects of sPLA2-X on allergic inflammation.
These studies have important potential therapeutic implications for asthma, as it is clear that lipid mediators including CysLTs play an important role in asthma (70,71), but therapies targeting CysLTs have only been modestly effective by virtue of blocking only one receptor (i.e. CysLT1 receptor that predominantly binds to LTD4), while these lipid mediators bind to multiple receptors (i.e. CysLT2 that preferentially binds LTC4, and the LTE4 receptors, CysLTE, P2Y12 and GPR99)(72,73). In this paper, we show for the first time in a primary human cell that an inhibitor of sPLA2-X can effectively block the formation of CysLTs. Further, the products of sPLA2s including lysophospholipids may also serve as important regulators of Th2-mediated inflammation (47). These findings further support sPLA2-X as a potential therapeutic target in asthma.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health grants R21HL111845 (TSH), and R37HL036235 (MHG).
Abbreviations used in this paper
- 5-LO
5-Lipoxygenase
- cPLA2α
Cytosolic phospholipase A2α
- CysLT
Cysteinyl leukotriene
- ER
endoplasmic reticulum
- ERK 1/2
extracellular-signal-regulated kinases 1/2 (p44/42 MAPK)
- HL-60-C15
HL-60 clone-15 cells
- LT
Leukotriene
- MAPK
mitogen activated protein kinase
- PC
Phosphatidylcholine
- PLA2
Phospholipase A2
- PLA2G10
mammalian sPLA2-X gene
- pla2g10
murine sPLA2-X gene
- RNAi
RNA interference
- SAPK/JNK
stress-activated protein kinase/Jun-amino-terminal kinase
- SB
sodium butyrate
- sPLA2
Secreted phospholipase A2
- sPLA2-IIA
sPLA2 group IIA
- sPLA2-V
sPLA2 group V
- sPLA2-X
sPLA2 group X
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hallstrand TS, Moody MW, Aitken ML, Henderson WR., Jr Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;116:586–593. doi: 10.1016/j.jaci.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavord ID, Ward R, Woltmann G, Wardlaw AJ, Sheller JR, Dworski R. Induced sputum eicosanoid concentrations in asthma. Am J Respir Crit Care Med. 1999;160:1905–1909. doi: 10.1164/ajrccm.160.6.9903114. [DOI] [PubMed] [Google Scholar]
- 3.Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, Godard P, Chanez P. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20:1370–1377. doi: 10.1183/09031936.02.00029202. [DOI] [PubMed] [Google Scholar]
- 4.Hallstrand TS, Henderson WR., Jr An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10:60–66. doi: 10.1097/ACI.0b013e32833489c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 6.Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 7.Balboa MA, Shirai Y, Gaietta G, Ellisman MH, Balsinde J, Dennis EA. Localization of group V phospholipase A2 in caveolin-enriched granules in activated P388D1 macrophage-like cells. J Biol Chem. 2003;278:48059–48065. doi: 10.1074/jbc.M305904200. [DOI] [PubMed] [Google Scholar]
- 8.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A2 occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A2-α. J Biol Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 9.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J Biol Chem. 2004;279:16488–16494. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese C, Triggiani M, Marone G, Mazzarella G. Arachidonic acid metabolism in inflammatory cells of patients with bronchial asthma. Allergy. 2000;55(Suppl 61):27–30. doi: 10.1034/j.1398-9995.2000.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 12.Hallstrand TS, Lai Y, Ni Z, Oslund RC, Henderson WR, Jr, Gelb MH, Wenzel SE. Relationship between levels of secreted phospholipase A2 groups IIA and X in the airways and asthma severity. Clin Exp Allergy. 2011 doi: 10.1111/j.1365-2222.2010.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallstrand TS, Lai Y, Altemeier WA, Appel CL, Johnson B, Frevert CW, Hudkins KL, Bollinger JG, Woodruff PG, Hyde DM, Henderson WR, Jr, Gelb MH. Regulation and function of epithelial secreted phospholipase A2 group X in asthma. Am J Respir Crit Care Med. 2013;188:42–50. doi: 10.1164/rccm.201301-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai Y, Oslund RC, Bollinger JG, Henderson WR, Jr, Santana LF, Altemeier WA, Gelb MH, Hallstrand TS. Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group x. J Biol Chem. 2011;285:41491–41500. doi: 10.1074/jbc.M110.153338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackerman SJ, Kwatia MA, Doyle CB, Enhorning G. Hydrolysis of surfactant phospholipids catalyzed by phospholipase A2 and eosinophil lysophospholipases causes surfactant dysfunction: a mechanism for small airway closure in asthma. Chest. 2003;123:355S. doi: 10.1378/chest.123.3_suppl.355s. [DOI] [PubMed] [Google Scholar]
- 16.Kwatia MA, Doyle CB, Cho W, Enhorning G, Ackerman SJ. Combined activities of secretory phospholipases and eosinophil lysophospholipases induce pulmonary surfactant dysfunction by phospholipid hydrolysis. J Allergy Clin Immunol. 2007;119:838–847. doi: 10.1016/j.jaci.2006.12.614. [DOI] [PubMed] [Google Scholar]
- 17.Blom M, Tool AT, Wever PC, Wolbink GJ, Brouwer MC, Calafat J, Egesten A, Knol EF, Hack CE, Roos D, Verhoeven AJ. Human eosinophils express, relative to other circulating leukocytes, large amounts of secretory 14-kD phospholipase A2. Blood. 1998;91:3037–3043. [PubMed] [Google Scholar]
- 18.Triggiani M, Granata F, Balestrieri B, Petraroli A, Scalia G, Del Vecchio L, Marone G. Secretory phospholipases A2 activate selective functions in human eosinophils. J Immunol. 2003;170:3279–3288. doi: 10.4049/jimmunol.170.6.3279. [DOI] [PubMed] [Google Scholar]
- 19.Munoz NM, Meliton AY, Lambertino A, Boetticher E, Learoyd J, Sultan F, Zhu X, Cho W, Leff AR. Transcellular secretion of group V phospholipase A2 from epithelium induces β2-integrin-mediated adhesion and synthesis of leukotriene C4 in eosinophils. J Immunol. 2006;177:574–582. doi: 10.4049/jimmunol.177.1.574. [DOI] [PubMed] [Google Scholar]
- 20.Munoz NM, Kim YJ, Meliton AY, Kim KP, Han SK, Boetticher E, O’Leary E, Myou S, Zhu X, Bonventre JV, Leff AR, Cho W. Human group V phospholipase A2 induces group IVA phospholipase A2-independent cysteinyl leukotriene synthesis in human eosinophils. J Biol Chem. 2003;278:38813–38820. doi: 10.1074/jbc.M302476200. [DOI] [PubMed] [Google Scholar]
- 21.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 22.Oslund RC, Cermak N, Gelb MH. Highly specific and broadly potent inhibitors of mammalian secreted phospholipases A2. J Med Chem. 2008;51:4708–4714. doi: 10.1021/jm800422v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seno K, Okuno T, Nishi K, Murakami Y, Yamada K, Nakamoto S, Ono T. Pyrrolidine inhibitors of human cytosolic phospholipase A2. Part 2: synthesis of potent and crystallized 4-triphenylmethylthio derivative ‘pyrrophenone’. Bioorg Med Chem Lett. 2001;11:587–590. doi: 10.1016/s0960-894x(01)00003-8. [DOI] [PubMed] [Google Scholar]
- 24.Lamothe J, Lee K, Schelling S, Stedman N, Leach M, McKew J, Clark J, Nickerson-Nutter C, Hegen M. Sa.29. Efficacy of Giripladib, a Novel Inhibitor of Cytosolic Phospholipase A2α, in Two Different Mouse Models of Rheumatoid Arthritis. Clinical Immunology. 2008;127:S89–S90. [Google Scholar]
- 25.Weiss J, Inada M, Elsbach P, Crowl RM. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]
- 26.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 28.DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- 29.Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR., Jr Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007;176:1072–1078. doi: 10.1164/rccm.200707-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touaibia M, Djimde A, Cao F, Boilard E, Bezzine S, Lambeau G, Redeuilh C, Lamouri A, Massicot F, Chau F, Dong CZ, Heymans F. Inhibition of secreted phospholipase A2. 4-glycerol derivatives of 4,5-dihydro-3-(4- tetradecyloxybenzyl)-1,2,4-4H-oxadiazol-5-one with broad activities. J Med Chem. 2007;50:1618–1626. doi: 10.1021/jm060082n. [DOI] [PubMed] [Google Scholar]
- 31.Svensson L, Redvall E, Bjorn C, Karlsson J, Bergin AM, Rabiet MJ, Dahlgren C, Wenneras C. House dust mite allergen activates human eosinophils via formyl peptide receptor and formyl peptide receptor-like 1. Eur J Immunol. 2007;37:1966–1977. doi: 10.1002/eji.200636936. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X, Sano H, Kim KP, Sano A, Boetticher E, Munoz NM, Cho W, Leff AR. Role of mitogen-activated protein kinase-mediated cytosolic phospholipase A2 activation in arachidonic acid metabolism in human eosinophils. J Immunol. 2001;167:461–468. doi: 10.4049/jimmunol.167.1.461. [DOI] [PubMed] [Google Scholar]
- 33.Ago H, Kanaoka Y, Irikura D, Lam BK, Shimamura T, Austen KF, Miyano M. Crystal structure of a human membrane protein involved in cysteinyl leukotriene biosynthesis. Nature. 2007;448:609–612. doi: 10.1038/nature05936. [DOI] [PubMed] [Google Scholar]
- 34.Martinez Molina D, Wetterholm A, Kohl A, McCarthy AA, Niegowski D, Ohlson E, Hammarberg T, Eshaghi S, Haeggstrom JZ, Nordlund P. Structural basis for synthesis of inflammatory mediators by human leukotriene C4 synthase. Nature. 2007;448:613–616. doi: 10.1038/nature06009. [DOI] [PubMed] [Google Scholar]
- 35.Mandal AK, Jones PB, Bair AM, Christmas P, Miller D, Yamin TT, Wisniewski D, Menke J, Evans JF, Hyman BT, Bacskai B, Chen M, Lee DM, Nikolic B, Soberman RJ. The nuclear membrane organization of leukotriene synthesis. Proc Natl Acad Sci U S A. 2008;105:20434–20439. doi: 10.1073/pnas.0808211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jemel I, Ii H, Oslund RC, Payre C, Dabert-Gay AS, Douguet D, Chargui K, Scarzello S, Gelb MH, Lambeau G. Group X secreted phospholipase A2 proenzyme is matured by a furin-like proprotein convertase and releases arachidonic acid inside of human HEK293 cells. The Journal of biological chemistry. 2011;286:36509–36521. doi: 10.1074/jbc.M111.268540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohtsuki M, Taketomi Y, Arata S, Masuda S, Ishikawa Y, Ishii T, Takanezawa Y, Aoki J, Arai H, Yamamoto K, Kudo I, Murakami M. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J Biol Chem. 2006;281:36420–36433. doi: 10.1074/jbc.M607975200. [DOI] [PubMed] [Google Scholar]
- 38.Peinado JR, Kacprzak MM, Leppla SH, Lindberg I. Cross-inhibition between furin and lethal factor inhibitors. Biochem Biophys Res Commun. 2004;321:601–605. doi: 10.1016/j.bbrc.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Garten W, Hallenberger S, Ortmann D, Schafer W, Vey M, Angliker H, Shaw E, Klenk HD. Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie. 1994;76:217–225. doi: 10.1016/0300-9084(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 40.Ni Z, Okeley NM, Smart BP, Gelb MH. Intracellular Actions of Group IIA Secreted Phospholipase A2 and Group IVA Cytosolic Phospholipase A2 Contribute to Arachidonic Acid Release and Prostaglandin Production in Rat Gastric Mucosal Cells and Transfected Human Embryonic Kidney Cells. J Biol Chem. 2006;281:16245–16255. doi: 10.1074/jbc.M513874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 42.Geijsen N, Dijkers PF, Lammers JJ, Koenderman L, Coffer PJ. Cytokine-mediated cPLA(2) phosphorylation is regulated by multiple MAPK family members. FEBS Lett. 2000;471:83–88. doi: 10.1016/s0014-5793(00)01373-9. [DOI] [PubMed] [Google Scholar]
- 43.Myou S, Leff AR, Myo S, Boetticher E, Meliton AY, Lambertino AT, Liu J, Xu C, Munoz NM, Zhu X. Activation of group IV cytosolic phospholipase A2 in human eosinophils by phosphoinositide 3-kinase through a mitogen-activated protein kinase-independent pathway. J Immunol. 2003;171:4399–4405. doi: 10.4049/jimmunol.171.8.4399. [DOI] [PubMed] [Google Scholar]
- 44.Malm-Erjefalt M, Persson CG, Erjefalt JS. Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation. Am J Respir Cell Mol Biol. 2001;24:352–359. doi: 10.1165/ajrcmb.24.3.4357. [DOI] [PubMed] [Google Scholar]
- 45.Murakami M, Kambe T, Shimbara S, Higashino K, Hanasaki K, Arita H, Horiguchi M, Arita M, Arai H, Inoue K, Kudo I. Different functional aspects of the group II subfamily (Types IIA and V) and type X secretory phospholipase A2s in regulating arachidonic acid release and prostaglandin generation. Implications of cyclooxygenase-2 induction and phospholipid scramblase-mediated cellular membrane perturbation. J Biol Chem. 1999;274:31435–31444. doi: 10.1074/jbc.274.44.31435. [DOI] [PubMed] [Google Scholar]
- 46.Feltenmark S, Gautam N, Brunnstrom A, Griffiths W, Backman L, Edenius C, Lindbom L, Bjorkholm M, Claesson HE. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci U S A. 2008;105:680–685. doi: 10.1073/pnas.0710127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanasaki K, Ono T, Saiga A, Morioka Y, Ikeda M, Kawamoto K, Higashino K, Nakano K, Yamada K, Ishizaki J, Arita H. Purified group X secretory phospholipase A2 induced prominent release of arachidonic acid from human myeloid leukemia cells. J Biol Chem. 1999;274:34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- 49.Cupillard L, Koumanov K, Mattei MG, Lazdunski M, Lambeau G. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J Biol Chem. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- 50.Curfs DM, Ghesquiere SA, Vergouwe MN, van der Made I, Gijbels MJ, Greaves DR, Verbeek JS, Hofker MH, de Winther MP. Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J Biol Chem. 2008;283:21640–21648. doi: 10.1074/jbc.M710584200. [DOI] [PubMed] [Google Scholar]
- 51.Becker GL, Sielaff F, Than ME, Lindberg I, Routhier S, Day R, Lu Y, Garten W, Steinmetzer T. Potent inhibitors of furin and furin-like proprotein convertases containing decarboxylated P1 arginine mimetics. J Med Chem. 2010;53:1067–1075. doi: 10.1021/jm9012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarac MS, Peinado JR, Leppla SH, Lindberg I. Protection against anthrax toxemia by hexa-D-arginine in vitro and in vivo. Infection and immunity. 2004;72:602–605. doi: 10.1128/IAI.72.1.602-605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komiyama T, Coppola JM, Larsen MJ, van Dort ME, Ross BD, Day R, Rehemtulla A, Fuller RS. Inhibition of furin/proprotein convertase-catalyzed surface and intracellular processing by small molecules. J Biol Chem. 2009;284:15729–15738. doi: 10.1074/jbc.M901540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seidah NG. What lies ahead for the proprotein convertases? Annals of the New York Academy of Sciences. 2011;1220:149–161. doi: 10.1111/j.1749-6632.2010.05883.x. [DOI] [PubMed] [Google Scholar]
- 55.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–7635. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 57.Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EA. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61:589–597. doi: 10.1111/j.1398-9995.2006.01060.x. [DOI] [PubMed] [Google Scholar]
- 58.Rothenberg ME. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- 59.Sedgwick JB, Quan SF, Calhoun WJ, Busse WW. Effect of interleukin-5 and granulocyte-macrophage colony stimulating factor on in vitro eosinophil function: comparison with airway eosinophils. J Allergy Clin Immunol. 1995;96:375–385. doi: 10.1016/s0091-6749(95)70057-9. [DOI] [PubMed] [Google Scholar]
- 60.Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol. 2001;107:679–685. doi: 10.1067/mai.2001.114245. [DOI] [PubMed] [Google Scholar]
- 61.Horie S, Okubo Y, Hossain M, Sato E, Nomura H, Koyama S, Suzuki J, Isobe M, Sekiguchi M. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern Med. 1997;36:179–185. doi: 10.2169/internalmedicine.36.179. [DOI] [PubMed] [Google Scholar]
- 62.Luttmann W, Matthiesen T, Matthys H, Virchow JC., Jr Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-alpha on eosinophil activation in vitro. Am J Respir Cell Mol Biol. 1999;20:474–480. doi: 10.1165/ajrcmb.20.3.3326. [DOI] [PubMed] [Google Scholar]
- 63.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 66.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM, Delespesse G. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–478. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 67.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 68.Henderson WR, Jr, Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, Rudensky AY, Gelb MH. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henderson WR, Jr, Oslund RC, Bollinger JG, Ye X, Tien YT, Xue J, Gelb MH. Blockade of human group X secreted phospholipase A2 (GX-sPLA2)-induced airway inflammation and hyperresponsiveness in a mouse asthma model by a selective GX-sPLA2 inhibitor. J Biol Chem. 2011;286:28049–28055. doi: 10.1074/jbc.M111.235812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffin M, Weiss JW, Leitch AG, McFadden ER, Jr, Corey EJ, Austen KF, Drazen JM. Effects of leukotriene D on the airways in asthma. N Engl J Med. 1983;308:436–439. doi: 10.1056/NEJM198302243080807. [DOI] [PubMed] [Google Scholar]
- 71.Gauvreau GM, Parameswaran KN, Watson RM, O’Byrne PM. Inhaled leukotriene E4, but not leukotriene D4, increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;164:1495–1500. doi: 10.1164/ajrccm.164.8.2102033. [DOI] [PubMed] [Google Scholar]
- 72.Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288:10967–10972. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J Allergy Clin Immunol. 2009;124:406–414. doi: 10.1016/j.jaci.2009.05.046. quiz 415–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.