Abstract
Purpose
To identify the proteins that are relevant to eye research and develop assays for the study of a set of these proteins.
Experimental Design
We conducted a bibliometric analysis by merging gene lists for human and mouse from the National Center for Biotechnology Information FTP site and combining them with PubMed references that were retrieved with the search terms “eye”[MeSH Terms] OR “eye”[All Fields] OR “eyes”[All Fields].
Results
For human and mouse eye studies, respectively, the total number of publications was 13,525 and 23,895, and the total number of proteins was 4,050 and 4,717. For proteins in human and mouse eye studies, respectively, 88.7% and 81.7% had five or fewer citations. The top fifty most intensively studied proteins for human and mouse eye studies were generally in the areas of photoreceptors and phototransduction, inflammation and angiogenesis, neurodevelopment, lens transparency, and cell cycle and cellular processes. We proposed selected reaction monitoring assays that were developed in silico for the top fifty most intensively studied proteins in human and mouse eye research.
Conclusions and clinical relevance
We conclude that scientists engaged in eye research tend to focus on the same proteins. Newer resources and tools in proteomics can expand the investigations to lesser-known proteins of the eye.
Keywords: biological processes, eye, human proteome project, proteomics, mass spectrometry, mouse
1 Introduction
Proteomics is beginning to gain greater attention in the field of eye research, owing to recent advances that have been made in protein chemistry, mass spectrometry, and bioinformatics [1]. Although proteins are an essential link between genotype and phenotype, the mechanisms by which genomic variation is translated to disease phenotypes through proteins is not well understood in general [2]. The level of complexity between the genome and specific phenotypes increases tremendously at the protein level due to protein isoforms, single nucleotide polymorphisms, post-translational modifications (PTMs), and protein degradation.
The biology and disease oriented branch of the Human Proteome Project (B/D-HPP) was organized in 2010. The goal of the B/D-HPP is to support “the broad application of state-of-the-art measurements of proteins and proteomes by life scientists studying the molecular mechanisms of biological processes and human disease. This will be accomplished through the generation of research and informational resources that will support the routine and definitive measurement of the process or disease relevant proteins.” [2]. Specifically, the B/D-HPP seeks to identify proteins that are relevant to a particular field and generate assays and reagents for these proteins [2]. The dissemination of selected reaction monitoring (SRM) assays may help accelerate research in many different fields.
Our specific aims were to identify the proteins that have been most intensively studied in eye research and provide new tools for the investigation of the top fifty proteins in human and mouse eye research, respectively. The number of scientific publications was used as the indicator of how intensively a protein was studied in eye research.
2 Materials and Methods
In order to identify the proteins that have received the greatest attention in eye research, human and mouse gene information was retrieved from the National Center for Biotechnology Information FTP site. PubMed references with the search terms “eye”[MeSH Terms] OR “eye”[All Fields] OR “eyes”[All Fields] were downloaded from PubMed. The lists of human and mouse gene were then combined to creative respective lists of proteins for human and mouse eye research, respectively. The earliest publication on PubMed was from 1813, and the earliest reference to a gene was from 1924. There were few publications prior to 1970 (only 3 for human and 24 for mouse eye research).
PANTHER was used to classify protein function. For the top fifty proteins in human and mouse eye research, respectively, heat maps were used to show the number of publications per year, and STRING was used to examine functional protein networks. NeXtProt was used as the main reference for human proteins and their associated diseases, number of isoforms, variants, and PTMs using gold level criteria. UniProt was used as the main reference for mouse proteins and their associated diseases and number of isoforms. REACTOME and Gene Ontology were used to identify groups of proteins involved in specific biological pathways studied in human eye research: complement cascade, Wnt signaling, VEGF signaling, apoptosis, visual phototransduction, etc. We did not find published SRM assays for forty-eight of the top fifty proteins in human eye research and forty-nine of the top fifty proteins in mouse eye research in the peer-reviewed scientific literature. SRM assays were constructed in silico using Skyline (MacCoss Lab, University of Washington, Seattle, WA), a commonly used theoretical prediction and selection algorithm [3] and following the guidelines for SRM assay development of Kuzyk and colleagues [4]. None of the SRM assays have been applied in vivo.
3 Results
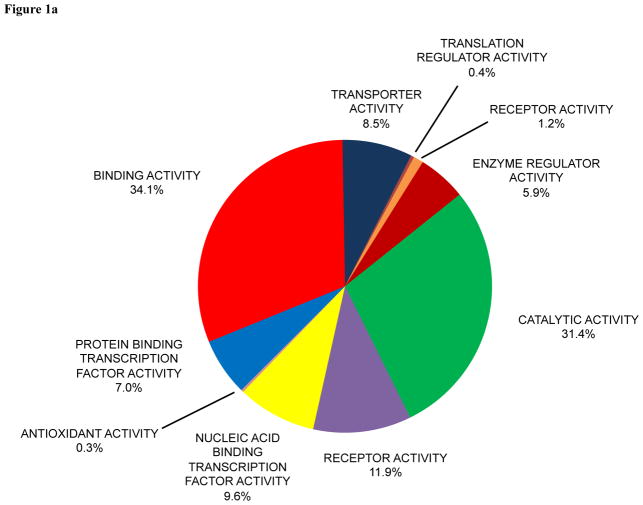
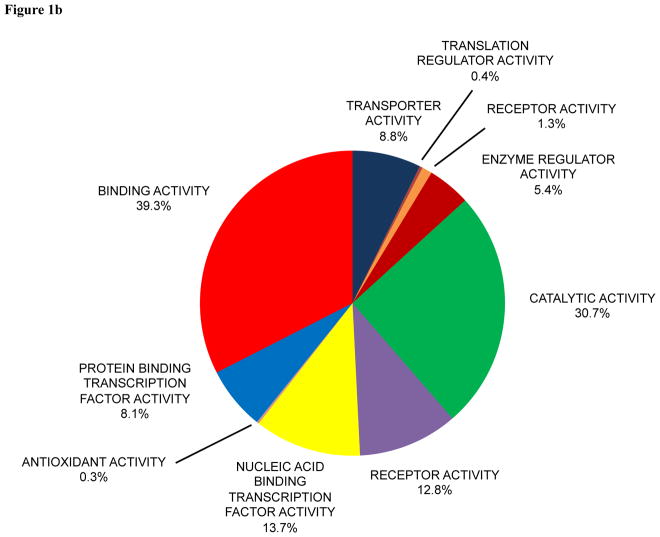
A total of 4,050 proteins were found in human eye studies (Supporting Information Table 1). A total of 4,717 proteins were found in mouse eye studies (Supporting Information Table 2). The total number of publications for human and mouse eye studies, respectively, was 13,525 and 23,895. PANTHER was used to classify protein function for the 4,050 proteins in human eye studies (Figure 1a) and 4,717 proteins in mouse eye studies (Figure 1b). The molecular functions and detection of the top fifty human eye proteins in the different tissues and biofluids of the human eye are presented in Supporting Information Table 3. The molecular functions of the top fifty mouse eye proteins are present in Supporting Information Table 4.
Figure 1.
Pie diagram of protein functions in (a) human and (b) mouse eye research classified by PANTHER.
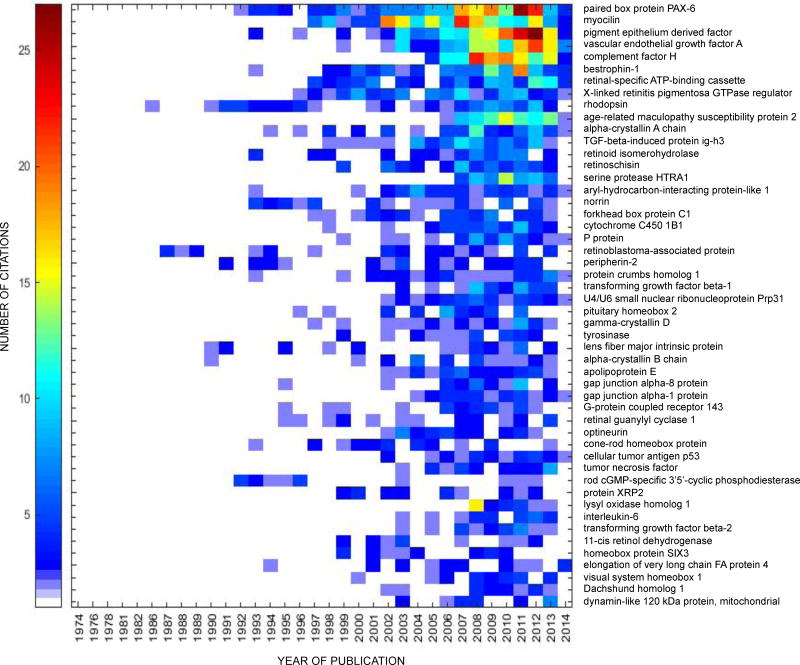
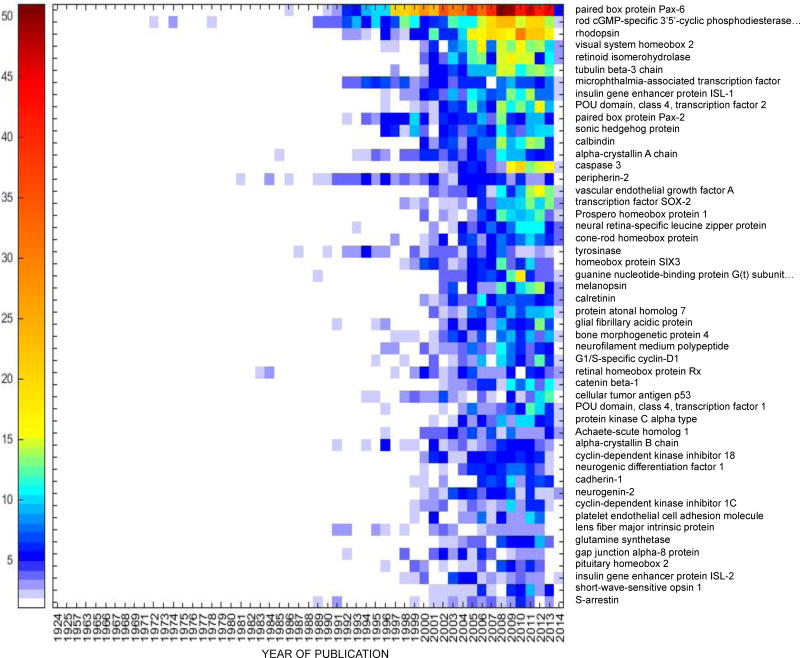
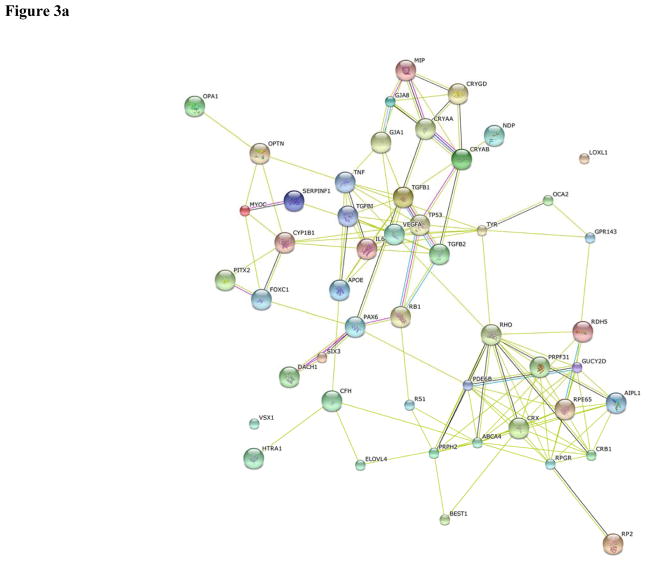
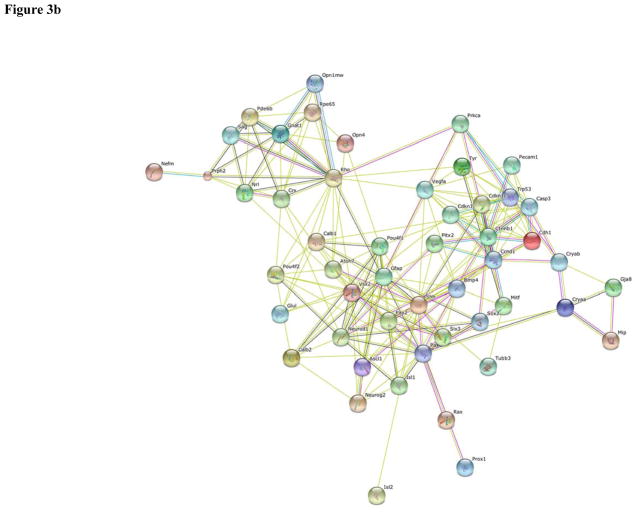
PAX-6 was the top among the fifty most intensively studied proteins in both human (Table 1) and mouse (Table 2) eye research. Heat maps showing the frequency of publication per year for the top fifty proteins in human and mouse eye research are shown in Figures 2a and 2b, respectively. The functional protein networks of the top fifty most intensively studied proteins in human eye research revealed three clusters representing photoreceptors and phototransduction, inflammation and angiogenesis, and proteins involved in lens transparency (Figure 3a). The functional protein networks of the top fifty most intensively studied proteins in mouse eye research revealed three clusters that represented photoreceptors and phototransduction, neurodevelopment, and cell cycle and cellular processes (Figure 3b).
Table 1.
| UniProt ID | Gene | Protein name | Citations | Functions | Associated diseases | Isoforms, PTMs, variants |
|---|---|---|---|---|---|---|
| P26367 | PAX6 | paired box protein PAX-6 | 204 | transcription factor | aniridia; Peters anomaly; foveal hypoplasia 1; keratitis hereditary; coloboma of iris, choroid, and retina; coloboma of optic nerve; bilateral optic nerve hypoplasia; aniridia, cerebellar ataxia, and mental deficiency | 2 isoforms 1 PTM 85 variants |
| Q99972 | MYOC | myocilin | 198 | regulates the activation of different signaling pathways; secreted glycoprotein | glaucoma 1, open angle, A; glaucoma 3, primary congenital, A | 1 isoform 2 PTMs 148 variants |
| P36955 | SERPINF1 | pigment epithelium-derived factor | 180 | neurotrophic protein; inhibits angiogenesis | osteogenesis imperfecta 6 | 1 isoform 5 PTMs 38 variants |
| P15692 | VEGFA | vascular endothelial growth factor A | 138 | growth factor that plays a role in angiogenesis, vasculogenesis, and endothelial cell growth | microvascular complications of diabetes | 17 isoforms 6 PTMs 21 variants |
| P08603 | CFH | complement factor H | 132 | cofactor in alternative complement pathway | basal laminar drusen; complement factor H deficiency; hemolytic uremic syndrome atypical 1; macular degeneration, age-related, 4 | 2 isoforms 51 PTMs 255 variants |
| O76090 | BEST1 | bestrophin-1 | 108 | forms calcium-sensitive chloride channels | vitelliform macular dystrophy 2; retinitis pigmentosa 50; adult onset vitelliform macular dystrophy; Bestrophinopathy, autosomal recessive; vitreoretinochoroidopathy, autosomal dominant | 3 isoforms 0 PTMs 172 variants |
| P78363 | ABCA4 | retinal-specific ATP-binding cassette transporter | 96 | inward-directed retinoid flipase | Stargardt disease 1; fundus flavimaculatus; macular degeneration, age-related, 2; cone-rod dystrophy 3; retinitis pigmentosa 19 | 1 isoform 10 PTMs 479 variants |
| Q92834 | RPGR | X-linked retinitis pigmentosa GTPase regulator | 94 | plays a role in ciliogenesis | retinitis pigmentosa 3; retinitis pigmentosa and sinorespiratory infections with or without deafness; cone-rod dystrophy, X-linked 1; macular degeneration, X-linked, atrophic | 6 isoforms 4 PTMs 155 variants |
| P0C7Q2 | ARMS2 | age-related maculopathy susceptibility protein 2 | 87 | retina homeostasis | macular degeneration, age-related, 8 | 1 isoform 0 PTMs 7 variants |
| P08100 | RHO | rhodopsin | 87 | key role in visual process | retinitis pigmentosa 4; night blindness, congenital stationary, autosomal dominant 1 | 1 isoform 13 PTMs 111 variants |
| P02489 | CRYAA | alpha-crystallin A chain | 77 | plays a role in transparency and refractive index of the lens | cataract 9, multiple types | 1 isoform 13 PTMs 28 variants |
| Q15582 | TGFBI | transforming growth factor-beta-induced protein ig-h3 | 77 | binds to type I, II, and IV collagens | corneal dystrophy, epithelial basement membrane; corneal dystrophy, Groenouw type 1; corneal dystrophy, lattice type 1; corneal dystrophy, Thiel-Behnke type; corneal dystrophy, Reis-Bucklers type; corneal dystrophy, lattice type 3A; corneal dystrophy, Avellino type | 1 isoform 33 PTMs 110 variants |
| Q16518 | RPE65 | retinoid isomerohydrolase | 63 | roles in production of 11-cis retinal and visual pigment regeneration | Leber congenital amaurosis 2; retinitis pigmentosa 20; autosomal dominant retinitis pigmentosa with choroidal involvement | 1 isoform 9 PTMs 106 variants |
| O15537 | RS1 | retinoschisin | 62 | involved in cell adhesion processes during retinal development | retinoschisis juvenile X-linked 1 | 1 isoform 5 PTMS 99 variants |
| Q92743 | HTRA1 | serine protease HTRA1 | 60 | serine protease | macular degeneration, age-related 7; cerebral arteriopathy with subcortical infarcts and leukoencephalopathy, autosomal recessive | 1 isoform 0 PTMs 34 variants |
| Q9NZN9 | AIPL1 | aryl-hydrocarbon-interacting protein-like 1 | 58 | may be involved in protein trafficking and/or protein folding and stabilization | Leber congenital amaurosis 4 | 5 isoforms |
| Q12948 | FOXC1 | forkhead box protein C1 | 54 | regulator of cell viability and resistance to oxidative stress | Axenfeld-Rieger syndrome 3; iridogoniodysgenesis anomaly; Peters anomaly | 1 isoform 5 PTMs 37 variants |
| Q00604 | NDP | norrin | 54 | involved in canonical Wnt signaling pathway; role in retinal neovascularization | Norrie disease; vitreoretinopathy, exudative 2 | 1 isoform 5 PTMs 75 variants |
| Q16678 | CYP1B1 | cytochrome C450 1B1 | 53 | heme-thiolate monooxygenase | Peters anomaly; glaucoma 3, primary congenital, A; glaucoma, primary open angle; glaucoma 1, open angle, A | 1 isoform 2 PTMs 101 variants |
| Q04671 | OCA2 | P protein | 52 | role in melanin synthesis, melanosome maturation | albinism, oculocutaneous, 2 | 3 isoforms 5 PTMs 209 variants |
| P06400 | RB1 | retinoblastoma-associated protein | 51 | tumor suppressor | childhood cancer retinoblastoma; bladder cancer; osteogenic sarcoma | 1 isoform 30 PTMs 201 variants |
| P23942 | PRPH2 | peripherin-2 | 50 | involved in outer segment disk morphogenesis | retinitis pigmentosa 7; retinitis punctata albescens; adult-onset vitelliform macular dystrophy; patterned dystrophy of retinal pigment epithelium; choroidal dystrophy, central areolar 2; cone-rod dystrophy; retinitis pigmentosa; macular degeneration | 1 isoform 2 PTMs 46 variants |
| P82279 | CRB1 | protein crumbs homolog 1 | 49 | role in photoreceptor morphogenesis | retinitis pigmentosa 12; Leber congenital amaurosis 8; pigmented paravenous chorioretinal atrophy | 5 isoforms 82 PTMs 301 variants |
| P01137 | TGFB1 | transforming growth factor beta-1 | 46 | multifunctional; involvement in proliferation, differentiation, etc. | Camurati-Engelmann disease | 1 isoform 12 PTMs 40 variants |
| Q8WWY3 | PRPF31 | U4/U6 small nuclear ribonucleoprotein Prp31 | 46 | involved in pre-mRNA splicing | retinitis pigmentosa 11 | 3 isoforms 9 PTM 40 variants |
| Q99697 | PITX2 | pituitary homeobox 2 | 46 | involved in cell proliferation, morphogenesis | Axenfeld-Rieger syndrome 1; iridogoniodysgenesis 2; Peters anomaly; ring dermoid of cornea | 3 isoforms 1 PTM 47 variants |
| P07320 | CRYGD | gamma-crystallin D | 44 | component of lens | cataract 4, multiple types | 1 isoform 0 PTMs 21 variants |
| P14679 | TYR | tyrosinase | 43 | involved in formation of pigments | albinism, oculocutaneous, 1A; albinism, oculocutaneous, 1B | 2 isoforms 6 PTMs 225 variants |
| P30301 | MIP | lens fiber major intrinsic protein | 43 | water channel | cataract 15, multiple types | 1 isoform 4 PTMs 41 variants |
| P02511 | CRYAB | alpha-crystallin B chain | 42 | component of lens | myopathy, myofibrillar, 2; cataract 16, multiple types; myopathy, myofibrillar, fata infantile hypertonic, alpha-B crystallin-related; cardiomyopathy, dilated 1II | 1 isoform 7 PTMs 23 variants |
| P02649 | APOE | apolipoprotein E | 42 | mediates the binding, internalization, and catabolism of lipoprotein particles | hyperlipoproteinemia 3; Alzheimer disease 2; sea-blue histiocyte disease; lipoprotein glomerulopathy; familial hypercholesterolemia | 1 isoform 9 PTMs 46 variants |
| P48165 | GJA8 | gap junction alpha-8 protein | 42 | channel activity | cataract 1, multiple types | 1 isoform 0 PTMs 82 variants |
| P17302 | GJA1 | gap junction alpha-1 protein | 39 | gap junction protein that acts as regulator of bladder capacity | oculodentodigital dysplasia; oculodentaldigital dysplasia, autosomal recessive; syndactyly 3; hypoplastic left heart syndrome 1; Hallermann-Streiff syndrome; atrioventricular septal defect 3; craniometaphyseal dysplasia, autosomal recessive | 1 isoform 26 PTMs 113 variants |
| P51810 | GPR143 | G-protein coupled receptor 143 | 39 | receptor for tyrosine, L-DOPA, and dopamine | albinism ocular 1; nystagmus congenital X-linked 6 | 1 isoform 1 PTM 70 variants |
| Q02846 | GUCY2D | retinal guanylyl cyclase 1 | 39 | rossible functional role in rod/cone photoreceptors | Leber congenital amaurosis 1; cone-rod dystrophy 6 | 1 isoform 3 PTMs 128 variants |
| Q96CV9 | OPTN | optineurin | 39 | roles in maintaining Golgi complex, membrane trafficking, exocytosis | glaucoma 1, open angle, E; glaucoma, normal pressure; amyotrophic lateral sclerosis 12 | 3 isoforms 8 PTMs 60 variants |
| O43186 | CRX | cone-rod homeobox protein | 37 | transcription factor, upstream of several photoreceptor-specific genes | Leber congenital amaurosis 7; cone-rod dystrophy 2; retinitis pigmentosa | 1 isoform 0 PTMs 47 variants |
| P04637 | TP53 | cellular tumor antigen p53 | 37 | tumor suppressor | esophageal cancer; Li-Fraumeni syndrome; squamous cell carcinoma of the head and neck; lung cancer; papilloma of the choroid plexus; adrenocortical carcinoma; basal cell carcinoma 7 | 9 isoforms 34 PTMs 1706 variants |
| P01375 | TNF | tumor necrosis factor | 35 | proinflammatory cytokine | psoriatic arthritis | 1 isoform 5 PTMs 18 variants |
| P35913 | PDE6B | rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit beta | 34 | role in transmission and amplification of the visual signal | retinitis pigmentosa 40; night blindness, congenital stationary, autosomal dominant 2 | 3 isoforms 2 PTMs 104 variants |
| O75695 | RP2 | protein XRP2 | 33 | GTPase-activating protein | retinitis pigmentosa 2 | 1 isoform 4 PTMs 51 variants |
| Q08397 | LOXL1 | lysyl oxidase homolog 1 | 33 | active on elastin and collagen substrates | exfoliation syndrome | 1 isoform 7 PTMs 34 variants |
| P05231 | IL6 | interleukin-6 | 32 | multifunctional cytokine; induces acute phase response | rheumatoid arthritis systemic juvenile | 1 isoform 3 PTMs 32 variants |
| P61812 | TGFB2 | transforming growth factor beta-2 | 32 | suppresses interleukin-2 dependent T-cell growth | Loeys-Dietz syndrome 4 | 2 isoforms 8 PTMs 47 variants |
| Q92781 | RDH5 | 11-cis retinol dehydrogenase | 32 | catalyzes final step in biosynthesis of 11-cis retinaldehyde, the universal chromophore of visual pigments | retinitis punctata albescens | 1 isoform 1 PTM 50 variants |
| O95343 | SIX3 | homeobox protein SIX3 | 31 | transcriptional regulator | holoprosencephaly 2 | 1 isoform 0 PTMs 30 variants |
| Q9GZR5 | ELOVL4 | elongation of very long chain fatty acids protein 4 | 31 | elongates saturated and monosaturated very long chain fatty acids | Stargardt disease 3 | 1 isoform 1 PTM 38 variants |
| Q9NZR4 | VSX1 | visual system homeobox 1 | 31 | binds to the locus core region of the red/green visual pigment gene cluster | corneal dystrophy, posterior polymorphous, 1; keratoconus 1; craniofacial anomalies and anterior segment dysgenesis syndrome | 8 isoforms 0 PTMs 39 variants |
| Q9UI36 | DACH1 | Dachshund homolog 1 | 31 | transcription factor involved in regulation of organogenesis | -- | 4 isoforms 6 PTMs 84 variants |
| O60313 | OPA1 | dynamin-like 120 kDa protein, mitochondrial | 30 | role in mitochondrial fusion and regulation of apoptosis | optic atrophy 1; dominant optic atrophy plus syndrome | 2 isoforms 4 PTMs 142 variants |
Protein function(s), disease states, isoforms, PTMs, and variants based upon NeXtProt entries using gold level criteria. Disease states, isoforms, PTMs, and variants reported for NeXtProt curated entries.
It should be noted that although this list comprises fifty proteins, there are actually many more proteins due to isoforms and variants noted in the last column
Table 2.
| UniProt ID | Gene | Protein name | Citations | Functions | Associated diseases in mice | Isoforms |
|---|---|---|---|---|---|---|
| P63015 | Pax6 | paired box protein Pax-6 | 653 | transcription factor involved in development of eye and other organs | defects in Pax6 cause condition of small eye (Sey) with lack of eyes and nasal primordial | 3 isoforms |
| P23440 | Pde6b | rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit beta | 298 | role in transmission and amplification of visual signal | defects in Pd36b are cause of a retinal degeneration | 2 isoforms |
| P15409 | Rho | rhodopsin | 244 | photoreceptor required for vision at low light intensity | -- | 1 isoform |
| Q61412 | Vsx2 | visual system homeobox 2 | 162 | role in specification and morphogenesis of sensory retina | defects in Vsx2 are cause of ocular retardation (OR(J)), a disease with microphthalmia, retinal destruction, and absence of optic nerve | 1 isoform |
| Q91ZQ5 | Rpe65 | retinoid isomerohydrolase | 144 | role in visual pigment regeneration | defects in Rpe65 cause light damage susceptibility (LDS) of the retina | 1 isoform |
| Q9ERD7 | Tubb3 | tubulin beta-3 chain | 137 | major constituent of microtubules | -- | 1 isoform |
| Q08874 | Mitf | microphthalmia-associated transcription factor | 132 | transcription factor for genes that play essential roles in cell differentiation, proliferation, and survival | defects in Mitf cause microphthalmia (mi) | 9 isoforms |
| P61372 | Isl1 | insulin gene enhancer protein ISL-1 | 129 | regulates promoters of insulin, glucagon, and somatostatin genes | -- | 2 isoforms |
| Q63934 | Pou4f2 | POU domain, class 4, transcription factor 2 | 127 | transcription factor | -- | 1 isoform |
| P32114 | Pax2 | paired box protein Pax-2 | 126 | transcription factor | renal-coloboma syndrome | 2 isoforms |
| Q62226 | Shh | sonic hedgehog protein | 117 | intercellular signal essential for various patterning events during development | -- | 1 isoform |
| P12658 | Calb1 | calbindin | 115 | buffers cytosolic calcium | -- | 1 isoform |
| P24622 | Cryaa | alpha-crystallin A chain | 113 | contributes to transparency and refractive index of the lens | -- | 2 isoforms |
| P70677 | Casp3 | caspase-3 | 112 | role in activation cascade of caspases involved in apoptosis | -- | 1 isoform |
| P15499 | Prph2 | peripherin-2 | 111 | may function as adhesion molecule in outer segment disks | responsible for retinal degeneration slow (Rds) | 1 isoform |
| Q00731 | Vegfa | vascular endothelial growth factor A | 104 | growth factor involved in angiogenesis, vasculogenesis, and endothelial cell growth | -- | 6 isoforms |
| P48432 | Sox2 | transcription factor SOX-2 | 95 | transcription factor for some genes involved in embryonic development | -- | 1 isoform |
| P48437 | Prox1 | Prospero homeobox protein 1 | 89 | transcription factor involved in developmental processes | -- | 1 isoform |
| P54846 | Nrl | neural retina-specific leucine zipper protein | 88 | transcription factor involved in expression of several rod-specific genes | -- | 1 isoform |
| P11344 | Tyr | tyrosinase | 87 | involved in formation of pigments such as melanins | defects in Tyr results in various forms of albinism | 1 isoform |
| Q62233 | Six3 | homeobox protein SIX3 | 87 | transcriptional regulator | -- | 2 isoforms |
| O54751 | Crx | cone-rod homeobox protein | 87 | transcription factor that transactivates a sequence upstream of several photoreceptor-specific genes | -- | 1 isoform |
| P20612 | Gnat1 | guanine nucleotide-binding protein G(t) subunit alpha-1 | 86 | modulator or transducer of various transmembrane signaling systems | -- | 1 isoform |
| Q9QXZ9 | Opn4 | melanopsin | 85 | photoreceptor required for regulation of circadian rhythm | -- | 2 isoforms |
| Q08331 | Calb2 | calretinin | 84 | calcium-binding protein | -- | 1 isoform |
| Q9Z2E5 | Atoh7 | protein atonal homolog 7 | 83 | transcription factor involved in the differentiation of most ganglion cells | -- | 1 isoform |
| P03995 | Gfap | glial fibrillary acidic protein | 81 | class-III intermediate filament | -- | 2 isoforms |
| P21275 | Bmp4 | bone morphogenetic protein 4 | 81 | induces cartilage and bone formation | -- | 1 isoform |
| P25322 | Ccnd1 | G1/S-specific cyclin-D1 | 78 | phosphorylates and inhibits members of the retinoblastoma (RB) protein family | 1 isoform | |
| P08553 | Nefm | neurofilament medium polypeptide | 78 | component of neurofilaments | -- | 1 isoform |
| O35602 | Rax | retinal homeobox protein Rx | 77 | regulates initial specification of retinal cells and/or their subsequent proliferation | -- | 1 isoform |
| Q02248 | Ctnnb1 | catenin beta-1 | 73 | downstream component of canonical Wnt signaling pathway | -- | 1 isoform |
| P02340 | Tp53 | cellular tumor antigen p53 | 72 | tumor suppressor | -- | 1 isoform |
| P17208 | Pou4f1 | POU domain, class 4, transcription factor 1 | 71 | probable transcription factor | -- | 1 isoform |
| Q02067 | Ascl1 | Achaete-scute homolog 1 | 70 | transcription factor | -- | 1 isoform |
| P20444 | Prkca | protein kinase C alpha type | 70 | calcium-activated, phospholipid- and diacylglycerol (DAG)-dependent serine/threonine-protein kinase | expression of mutant form UV25 causes malignant transformation of cells | 1 isoform |
| P23927 | Cryab | alpha-crystallin B chain | 69 | may contribute to transparency and refractive index of lens | -- | 1 isoform |
| P46414 | Cdkn1b | cyclin-dependent kinase inhibitor 18 | 67 | regulator of cell cycle progression | -- | 1 isoform |
| Q60867 | Neurod1 | neurogenic differentiation factor 1 | 64 | transcriptional activator | Neurod1 null mice are deaf and die shortly after birth | 1 isoform |
| P09803 | Cdh1 | cadherin-1 | 63 | calcium-dependent cell adhesion protein | -- | 1 isoform |
| P70447 | Neurog2 | neurogenin-2 | 62 | transcriptional regulator | -- | 1 isoform |
| P49919 | Cdkn1c | cyclin-dependent kinase inhibitor 1C | 57 | inhibitor of several G1 cyclin/CDKcomplexes and mitotic cyclin B-CDC2 | 2 isoforms | |
| Q08481 | Pecam1 | platelet endothelial cell adhesion molecule | 56 | cell adhesion molecule needed for leukocyte transedothelial migration | -- | 4 isoforms |
| P51180 | Mip | lens fiber major intrinsic protein | 55 | water channel | defects in Mip cause autosomal dominant cataract | 1 isoform |
| P15105 | Glul | glutamine synthetase | 53 | essential for proliferation of fetal skin fibroblasts | -- | 1 isoform |
| P97474 | Pitx2 | pituitary homeobox 2 | 52 | involved in cell proliferation and morphogenesis | mice embryos lacking isoform Ptx2c show left-right patterning defects and severe development abnormalities | 5 isoforms |
| P28236 | Gja8 | gap junction alpha-8 protein | 52 | component of gap junction | -- | 1 isoform |
| Q9CXV0 | Isl2 | insulin gene enhancer protein ISL-2 | 52 | transcriptional factor | -- | 1 isoform |
| P51491 | Opn1sw | short-wave-sensitive opsin 1 | 50 | visual pigment | -- | 1 isoform |
| P20443 | Sag | S-arrestin | 50 | binds to photoactivated-phosphorylated rhodopsin | -- | 1 isoform |
Protein function(s), disease states, and isoforms based upon UniProt entries.
It should be noted that although this list comprises fifty proteins, there are actually many more proteins due to isoforms and variants noted in the last column
Figure 2.
(a) Heat map of the fifty most studied proteins in human eye research, 1974–2014. The first publication associated with both a gene and eye for human research in PubMed appeared in 1974. (b) Heat map of the fifty most studied proteins in the mouse eye research, 1924–2014. The first publication associated with both a gene and eye for mouse research in PubMed appeared in 1924. The heat map does not represent a full year for 2014, but only what was published on PubMed by 10/20/14.
Figure 3.
Functional protein networks among the top 50 most studied proteins for (a) human and (b) mouse eye.
We further examined the overlap between the fifty most intensively studied proteins in human and mouse eye research. There were fifteen proteins that were common to both human and mouse eye studies: paired box protein PAX-6, vascular endothelial growth factor A, rhodopsin, alpha-crystallin A chain, retinoid isomerohydrolase, peripherin-2, pituitary homeobox 2, tyrosinase, lens fiber major intrinsic protein, alpha-crystallin B chain, gap junction alpha-8 protein, cone-rod homeobox protein, cellular tumor antigen p53, rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit beta, and homeobox protein SIX3.
The least-studied proteins comprised a large proportion of the proteins in both human and mouse eye studies, as mentioned above. Of the 4,050 proteins in human eye studies, the percentages of proteins with 5, 4, 3, 2, or 1 citation(s) were 2.5%, 3.8%, 7.6%, 17.6%, and 57.2%, respectively. Of the 4,717 proteins in mouse eye studies, the percentages of proteins with 5, 4, 3, 2, or 1 citation(s) were 3.2%, 4.4%, 8.3%, 16.0%, and 49.8%, respectively. In other words, 88.7% of proteins in human eye studies and 81.7% of proteins in mouse eye studies had five or fewer citations.
To facilitate the use of mass spectrometry for the quantification of these top proteins, we have proposed SRM assays for the top fifty proteins in human and mouse eye research as presented in Supporting Information, Tables 5 and 6. The list of the top proteins as characterized by a bibliometric approach, corresponds to what Van Eyk has called “popular proteins.” A complementary approach is to identify “priority proteins” based upon biological pathways. Biological pathways that are currently under intensive investigation in eye research include the complement cascade, Wnt signaling, VEGF signaling, apoptosis, visual phototransduction, degradation of extracellular matrix, cell response to hypoxia, oxidative stress-induced senescence, ERK activation, signaling by the TGF-beta receptor complex, and the inflammasome. A provisional list of 1416 “priority proteins” is shown in Supporting Table 7. Only 16 of the top 50 most intensively studied human eye proteins overlapped with the provisional list of priority proteins.
4 Discussion
In the present study, we identified over 4000 proteins that have been studied in human eye research and over 4700 proteins that have been studied in mouse eye research. There were nearly 80% more scientific publications for proteins in eye research for mouse than for humans. The underlying reason for the difference is not clear, but one could speculate that mouse eye proteins have been more frequently studied due to the greater available of eye tissues from mice than from humans. The ten most intensively studied proteins in human and mouse eye research are discussed below.
Paired box protein PAX-6 (PAX6) has been the most intensively studied protein in both human and mouse eye studies. PAX6 plays a multi-level role in the morphogenesis of the eye, especially in the development of the lens, cornea, and retina [5]. PAX6 is a transcriptional factor that binds with DNA through interactions with two N- and C-terminal domains, termed PAI and RED, respectively. Three isoforms of PAX6 are produced via alternative splicing. The ratio between the canonical form, isoform 1, and isoform 5a varies among tissue types [5]. PTMs of PAX6 include phosphorylation and ubiquitination. Multiple variants have been reported in PAX6. Mutations in Pax-6 are associated with small eye (Sey) in mouse [6] and aniridia (partial or complete absence of the iris) in humans [7].
Myocilin is a 504 amino acid glycoprotein that was initially identified because it is induced in the eye by glucocorticoid treatment [8,9]. Myocilin is found in the trabecular meshwork, cornea, lamina cribosa, ciliary body, iris, vitreous, retina, optic nerve, and aqueous humor [10]. The structure of myocilin includes a signal peptide sequence for cleavage as a secreted protein and a C-terminal olfactomedin domain. Over 70 glaucoma-associated variants have been identified in myocilin, of which >90% are located in exon 3 that codes for the olfactomedin domain [9]. Most myocilin variants that contain an amino acid substitution are not secreted but accumulate within the endoplasmic reticulum as homo- or heterodimers [9]. The function of myocilin is not well understood [9,10].
Pigment epithelium-derived factor (PEDF) is a secreted glycoprotein that belongs to the serpin (serine protease inhibitor) family [11]. PEDF has heparin- and collagen-binding sites, and an unusual asymmetric distribution of charged amino acid residues, with basic and acidic regions on the opposite poles of the protein [12,13]. PEDF has neurotrophic and anti-angiogenic effects [11] and provides protection against oxidative stress in diabetic retinopathy [14]. PEDF inhibits retinal neovascularization induced by vascular endothelial growth factor [15].
Vascular endothelial growth factor-A (VEGF-A), a member of the vascular permeability factor/VEGF family, is a disulfide-bonded dimeric glycoprotein that plays a central role in angiogenesis [16]. VEGF-A has 17 isoforms that arise from alternative promoter usage, alternative splicing, and alternative initiation. The VEGF-A164/165 isoform, named after the total number of amino acid residues in mouse and human proteins, respectively, has been most intensively studied because of its role in angiogenesis [16]. VEGF-A binds with VEGF receptors 1 and 2, two high affinity tyrosine kinase receptors [17], and with neuropilin-1 [18]. Neutralization of VEGF-A with ranibizumab, a recombinant monoclonal antibody, was shown to prevent visual loss in neovascular age-related macular degeneration (AMD) [19]. Other antibodies against VEGF-A have shown similar effects in treatment of AMD.
Complement factor H (CFH) is a 1213 amino acid glycoprotein that plays a central role in the complement system. The complement system of innate immunity is involved in cellular integrity, microbial killing, immune surveillance, tissue homeostasis, and mediation of inflammatory responses [20]. Complement is involved in the recognition of diseased or damaged host cells, regulation of cellular immune responses, and interaction with the coagulation cascade. CFH plays a role in limiting complement-mediated damage to healthy host cells [21]. CFH has multiple binding sites, including those for C3b, heparin, C-reactive protein, and sialic acid. Two variants of CFH, Y402H and I62V, are strongly associated with the risk of AMD [22]. Immunohistochemical studies have demonstrated that CFH is present within vascular lumens and perivascular spaces around large blood vessels, in the choriocapillaris, intercapillary septa, Bruch’s membrane, and in large choroidal vessels and stroma in eyes with AMD [23].
Bestrophin-1 (BEST1) is a 585 amino acid transmembrane protein that is involved in intracellular calcium signaling [24]. There are three isoforms that arise through alternative splicing. BEST1 is most strongly localized in the cytosol close to the basolateral membrane of the retinal pigment epithelium (RPE) [24]. Mutations in BEST1 cause a variety of retinal degenerations, the best known being Best’s vitelliform macular dystrophy, or Best’s disease. Mutations in BEST1 are associated with increased accumulation of lipofuscin, a yellow aging-associated pigment, in the RPE, but the underlying pathophysiology is not well understood [25].
Retinal-specific ATP binding cassette transformer (ABCA4) is in the family of ABC transporters, a ubiquitous set of integral membrane proteins present in all living organisms [26]. ABCA4 has two transmembrane domains, two nucleotide-binding domains (ATP-binding cassettes), and two extracellular domains [26]. ABCA4 is located in the disk margins of photoreceptor outer segments. The reason for the restricted localization of ABCA4 within the rod outer segments is not clear [26]. ABCA4 seems to play a role in the clearance of all-trans-retinal from disk membranes after photoexcitation of rhodopsin [26]. Mutations in ABCA4 are associated with Stargardt disease [27], fundus flavimaculatus [28], cone-rod dystrophy, and a form of retinitis pigmentosa [26].
X-linked retinitis pigmentosa GTPase regulator (RPGR) is a 1020 amino acid protein that has six isoforms arising from alternative splicing. Isoform 6, or RPGRORF15 is highly expressed in photoreceptors and is implicated in retinal disease. RPGR contains a glycine/glutamic-acid rich domain near the C-terminal end that accounts for up to 80% of RPGR mutations [29]. RPGR is found in centrioles, ciliary axonemes, and microtubular transport complexes [29]. RPGR plays a role in microtubular transport through the ciliary structures that connect the inner and outer segments of photoreceptors [29,30]. X-linked forms of cone-rod dystrophy, cone dystrophy, and macular atrophy have been associated with RPGRORF15 mutations.
Age-related maculopathy susceptibility protein 2 (ARMS2) is a 107 amino acid protein that has been implicated in AMD. ARMS2, a recent gene in evolution, is present only in humans and higher primates [31]. No homologous gene has been annotated in lower vertebrates or other organisms [32]. ARMS2 has nine predicted phosphorylation sites but no remarkable structural motifs. Recent studies show there are two isoforms of ARMS2: isoform A, the canonical form and isoform B that arises as a splice variant [33,34]. The function of ARMS2 is not well understood. ARMS2 has been localized to retina and RPE [35]. The A69S risk variant of ARMS2 is strongly associated with AMD [22]. Since ARMS2 is in strong linkage disequilibrium with serine protease HTRA1, it is unclear whether ARMS2, HTRA1, or both proteins are involved in the pathogenesis of AMD.
Rhodopsin (RHO), a visual pigment found in rod photoreceptors in the retina, is essential for the process of vision. RHO is a member of the G-protein-coupled-receptor family. The structure of RHO includes a transmembrane protein moiety, opsin, which contains a ligand-binding site for retinal on the extracellular side of the transmembrane bundle [36]. The absorption of photons causes the isomerization of 11-cis retinal to all-trans retinal, conformation changes in rhodopsin, and downstream signal transduction [36]. Mutations in rhodopsin are associated with congenital stationary night blindness and retinitis pigmentosa (RP) [37].
Rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit beta (Pde6b) has been extensively studied in mouse models for autosomal recessive retinitis pigmentosa [38]. Rod phosphodiesterase (PDE) is a membrane-associated protein that consists of two catalytic subunits, rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit alpha (Pde6a) and Pde6b, and two gamma inhibitory subunits [38]. PDE plays a role in phototransduction by hydrolyzing the cGMP second messenger. Natural mouse models with the Pde6b mutations have been used to evaluate pharmacological treatments and gene therapy for protecting photoreceptors from apoptosis [38].
Visual system homeobox 2 (Vsx2) is a transcription factor that controls the morphogenesis of the eye [39,40]. Vsx2 is a 361 amino acid protein that contains a 60 amino acid homeodomain, or DNA binding module composed of three alpha helices [39]. Mutations in Vsx2 are associated with microphthalmia in humans [39,40] and mice [41].
Retinoid isomerohydrolase (Rpe65) is an RPE-specific protein that plays an important role in the visual cycle by converting all-trans retinyl esters to 11-cis-retinol [42]. Rpe65 is bound to smooth endoplasmic reticulum in RPE cells, but the exact mechanism of this binding is unclear [42]. In humans, mutations of Rpe65 are associated with Leber’s congenital amaurosis, recessive RP, fundus albipunctatus, and autosomal dominant RP with choroidal involvement [43].
Tubulin beta-3 chain (Tubb3) is a component of microtubules. Microtubules form the cytoskeleton and consist of heterodimers of alpha- and beta-tubulin. Tubulin has a wide range of PTMs, including acetylation, phosphorylation, detyrosination, polyglycylation, and polyglutamylation [44]. Tubb3, one of six tubulins found in mammals, has expression mainly limited to neurons [45]. In humans, TUBB3 mutations are associated with congenital oculomotor nerve hypoplasia and later-onset peripheral axon degeneration [45].
Microphthalmia-associated transcription factor (Mitf), a member of the family of basic helix-loop-helix leucine-zipper microphthalmia-related transcription factors, is a regulator of melanocytes and has pleiotrophic roles in RPE cells, mast cells, and osteocytes [46]. There are nine isoforms of Mitf. PTMs of Mitf include phosphorylation, sumoylation, and ubiquitination. The target genes for Mitf include those involved in pigmentation, cell cycle, survival, motility and invasion, metabolism, and oxidative stress [47]. Defects in Mitf cause microphthalmia in mice.
Insulin gene enhancer protein ISL-1 (Isl1), a transcription factor of the LIM-homeodomain protein family, is a 349 amino acid that is essential in development of many cell types, including retina [48]. Isl1 has two tandemly arrayed LIM domains near the N terminus that mediate protein-protein interactions and an adjacent homeodomain that binds DNA [49]. ISl1 regulates promoters of insulin, glucagon, and somatostatin genes.
POU domain, class 4, transcription factor 2 (Pou4f2) is a 411 amino acid transcription factor that is expressed in developing and adult retinal ganglion cells [50]. Pou4f2 is one of three members of the POU4F family, all of which are expressed only in ganglion cells of the retina [51]. Selective ablation of Pou4f2 had no impact on long-term survival of retinal ganglion cells in adult mice [51]. Pouf4f2 has been of interest in glaucoma research, since glaucomatous optic atrophy is characterized by a progressive loss of retinal ganglion cells.
Paired box protein Pax-2 (Pax2) is a transcription factor that is required for optic fissure closure in the developing eye [52]. Astrocytes, the earliest glial cell population in optic nerve development, play a role in retinal angiogenesis and formation of the brain-retinal barrier [53]. Pax2 mutations are associated with a renal-coloboma syndrome that involves the eye, ear, central nervous system, and urogenital tract in humans and mice [54,55].
A bibliometric analysis conducted in 2011 showed that about three-quarters of protein research focuses on the 10% of proteins that were known before the human genome was mapped [56]. Most of the diseases or processes associated with the ten most intensively studied proteins of the human eye are related to development or single gene disorders such as retinitis pigmentosa. Some of the proteins that are being investigated, such as ARMS2, have gained recent attention mainly because of strong disease associations at the genetic level. As noted by the investigators of the 2011 bibliometric analysis, scientists have an apparent reluctance to work on unknown or lesser-known proteins. The reasons for this are unclear but may possibly have to do with greater risk in grant applications, as it is harder to explain rationale and significance for proteins that have unknown functions [56]. In addition, the intensity with which certain proteins were studied was related to the availability of chemical probes for the particular protein [56]. The present analysis corroborates the observation that most proteins of the eye have not been well studied: over 57% of proteins in human eye research and nearly 50% of proteins in mouse eye research had one citation only. A limitation of this study is that many older references may have had less stringent quality control than are currently used in claiming identification of proteins. Another limitation is that the proteins identified as “priority proteins” for human eye research will likely grow and change in the future. What we have proposed here is a starting point based upon some of the most intensely studied biological pathways in human eye research.
Recent advances in proteomics, bioinformatics, and mass spectrometry instrumentation should help expand scientific investigations to lesser-known proteins in eye research. Discovery work on proteomes of specific tissues and cell types has been greatly facilitated by data-dependent acquisition approach using Orbitrap mass spectrometers [57] or data-independent approaches (e.g. SWATH) [58]. Targeted methods for selective protein quantitation such as SRM most often use triple quadrupole mass spectrometers. SRM assays were recently used for quantification of a large number of human tear proteins [59]. The SRM assays proposed in the present paper for human and mouse eye proteins can be applied for protein quantification. All instruments can quantitate proteins without the need for antibodies or specific chemical probes, although these can employed when increased sensitivity is required to deal with dynamic range constraints [60,61]. Protein interaction studies can be used to determine binding partners and infer their functional networks [62,63]. Many strategies are available to identify and quantify different PTMs, such as phosphorylation, O-GlcNAcylation, and glycosylation [64–66]. Specific isoforms and variants arising from single nucleotide polymorphisms can be targeted by SRM assays. Application of these new advances should help both discovery and hypothesis-based research about the rich diversity of proteins involved in biological processes of the eye and vision and in health and disease. These newer tools will help scientists investigate proteins that remain “hidden in plain sight” [56].
Supplementary Material
Statement of clinical relevance.
Research on the biology of the eye and underlying molecular mechanisms of eye disease can be advanced through the larger application of state-of-the-art quantification and characterization of protein and proteomes. This study utilized a bibliometric analysis to identify the most intensively studied proteins in human and mouse eye research. Selected reaction monitoring assays have been developed in silico for the top fifty most intensively studied proteins in human and mouse eye research.
Acknowledgments
This work was supported by the National Institutes of Health grants R01 EY024596, R01 AG027012, the Joint King Khaled Eye Specialist Hospital and Wilmer Eye Institute Research Grant Program, the Edward N. & Della L. Thome Memorial Foundation, and Research to Prevent Blindness.
List of abbreviations
- ABCA4
retinal-specific ATP binding cassette transformer
- AMD
age-related macular degeneration
- ARMS2
age-related maculopathy susceptibility protein 2
- B/D-HPP
Biology/Disease - Human Proteome Project
- BEST1
bestrophin-1
- CFH
complement factor H
- Isl1
insulin gene enhancer protein ISL-1
- Mitf
microphthalmia-associated transcription factor
- Pax2
paired box protein Pax-2
- PAX6
paired box protein PAX-6
- Pde6a
rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit alpha
- Pde6b
rod cGMP-specific 3′,5′-cyclic phosphodiesterase subunit beta
- PEDF
pigment epithelium-derived factor
- PDE
phosphodiesterase
- Pou4f2
POU domain, class 4, transcription factor 2
- RHO
rhodopsin
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- RPE65
retinoid isomerohydrolase
- RPGR
X-linked retinitis pigmentosa GTPase regulator
- SRM
selected reaction monitoring
- Tubb3
tubulin beta-3 chain
- VEGF-A
vascular endothelial growth factor-A
- Vsx2
visual system homeobox 2
Footnotes
The authors have declared no conflict of interest.
References
- 1.Semba RD, Enghild JJ, Venkatraman V, Dyrlund TF, Van Eyk JE. The Human Eye Proteome Project: perspectives on an emerging proteome. Proteomics. 2013;13:2500–2511. doi: 10.1002/pmic.201300075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebersold R, Bader GD, Edwards AM, Van Eyk JE, et al. The biology/disease-driven human proteome project (B/D-HPP): enabling protein research for the life sciences community. J Proteome Res. 2013;12:23–27. doi: 10.1021/pr301151m. [DOI] [PubMed] [Google Scholar]
- 3.Bereman MS, MacLean B, Tomazela DM, Liebler DC, MacCoss MJ. The development of selected reaction monitoring methods for targeted proteomics via empirical refinement. Proteomics. 2012;12:1134–1141. doi: 10.1002/pmic.201200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuzyk MA, Parker CE, Domanski D, Borchers CH. Development of MRM-based assays for the absolute quantitation of plasma proteins. Methods Mol Biol. 2013;1023:53–81. doi: 10.1007/978-1-4614-7209-4_4. [DOI] [PubMed] [Google Scholar]
- 5.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Ret Eye Res. 2012;31:351–376. doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Hill RE, Favor J, Hogan BL, Ton CC, et al. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 7.Ton CC, Hiryonen H, Miwa H, Weil MM, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 8.Polansky JR, Fauss DJ, Chen P, Chen H, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- 9.Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009;88:704–712. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menaa F, Braghini CA, Vasconcellos JP, Menaa B, et al. Keeping an eye on myocilin: a complex molecule associated with primary open-angle glaucoma susceptibility. Molecules. 2011;16:5402–5421. doi: 10.3390/molecules16075402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 12.Simonovic M, Gettins PGW, Volz K. Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor. Proc Natl Acad Sci USA. 2001;98:11131–11135. doi: 10.1073/pnas.211268598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasui N, Mori T, Morito D, Matsushita O, et al. Dual-site recognition of different extracellular matrix components by anti-angiogenic/neurotrophic serpin, PEDF. Biochemistry. 2003;42:3160–3167. doi: 10.1021/bi0206558. [DOI] [PubMed] [Google Scholar]
- 14.Elahy M, Baindur-Hudson S, Cruzat VF, Newsholme P, Dass CR. Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy. J Endocrinol. 2014;222:R129–R139. doi: 10.1530/JOE-14-0065. [DOI] [PubMed] [Google Scholar]
- 15.Duh EJ, Yang HS, Suzuma I, Miyagi M, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–829. [PubMed] [Google Scholar]
- 16.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol Mech Dis. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker MW, Xu P, Li X, Vander Kooi CW. Structural basis for selective vascular endothelial growth factor-1 (VEGF-A) binding to neuropilin-1. J Biol Chem. 2012;287:11082–11089. doi: 10.1074/jbc.M111.331140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 20.Makou E, Herbert AP, Barlow PN. Functional anatomy of complement factor H. Biochemistry. 2013;52:3949–3962. doi: 10.1021/bi4003452. [DOI] [PubMed] [Google Scholar]
- 21.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeAngelis MM, Silveira AC, Carr EA, Kim IK. Genetics of age-related macular degeneration: current concepts, future directions. Sem Ophthalmol. 2011;26:77–93. doi: 10.3109/08820538.2011.577129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutto IA, Baba T, Merges C, Juriasinghani V, et al. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss O, Müller C, Reichhart N, Tamm ER, Gomez NM. The role of bestrophin-1 in intracellular Ca++ signaling. Adv Exp Med Biol. 2014;801:113–119. doi: 10.1007/978-1-4614-3209-8_15. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Q, Hartzell HC, Yu K. Bestrophins and retinopathies. Pflugers Arch Eur J Physiol. 2010;460:559–569. doi: 10.1007/s00424-010-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Biol Med. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allikmets R, Singh N, Sun H, Shroyer NF, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 28.Rozet JM, Gerber S, Souied E, Perrault I, et al. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet. 1998;6:291–295. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- 29.Wright AF, Shu X. Focus on molecules: RPGR. Exp Eye Res. 2006;85:1–2. doi: 10.1016/j.exer.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Hosch J, Lorenz B, Stieger K. RPGR: role in the photoreceptor cilium, human retinal disease, and gene therapy. Ophthalmic Genet. 2011;32:1–11. doi: 10.3109/13816810.2010.535889. [DOI] [PubMed] [Google Scholar]
- 31.Francis PJ, Appukuttan B, Simmons E, Landauer N, et al. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Hum Mol Genet. 2008;17:2673–2680. doi: 10.1093/hmg/ddn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minor EA, Court BL, Dubovy S, Wang G. AMD-associated variants at the chromosome 10q26 locus and the stability of ARMS2 transcripts. Invest Ophthalmol Vis Sci. 2013;54:5913–5919. doi: 10.1167/iovs.13-12273. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Scott WK, Whitehead P, Court BL, et al. A novel ARMS2 splice variant is identified in human retina. Exp Eye Res. 2012;94:187–191. doi: 10.1016/j.exer.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kortvely E, Hauck SM, Duetsch G, Gloeckner CJ, et al. ARMS2 is a constituent of the extracellular matrix providing a link between familial and sporadic age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:79–88. doi: 10.1167/iovs.09-3850. [DOI] [PubMed] [Google Scholar]
- 36.Zhou XE, Melcher K, Xu HE. Structure and activation of rhodopsin. Acta Pharmacol Sin. 2012;33:291–299. doi: 10.1038/aps.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawamura S, Colozo AT, Ge L, Müller DJ, Park PS. Stuctural, energetic, and mechanical perturbations in rhodopsin mutant that causes congenital stationary night blindness. J Biol Chem. 2012;287:21826–21835. doi: 10.1074/jbc.M112.340182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han J, Dinculescu A, Dai X, Du W, et al. The history and role of naturally occurring mouse models with Pde6b mutations. Mol Vis. 2013;19:2579–2589. [PMC free article] [PubMed] [Google Scholar]
- 39.Zou C, Levine EM. Vsx2 controls eye organogenesis and retinal progenitor identify via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLOS Genet. 2012;8:e1002924. doi: 10.1371/journal.pgen.1002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang L, Sandell JH. Focus on molecules: Homeobox protein Chx10. Exp Eye Res. 2008;86:541–542. doi: 10.1016/j.exer.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Burmeister M, Novak J, Liang MY, Basu S, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- 42.Kiser PD, Palczewski K. Membrane-binding and enzymatic properties of RPE65. Prog Ret Eye Res. 2010;29:428–442. doi: 10.1016/j.preteyeres.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ripamonti C, Henning GB, Ali RR, Bainbridge JW, et al. Nature of the visual loss in observers with Leber’s congenital amaurosis caused by specific mutations in RPE65. Invest Ophthalmol Vis Sci. 2014;55:6817–6828. doi: 10.1167/iovs.14-14923. [DOI] [PubMed] [Google Scholar]
- 44.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tischfield MA, Baris HN, Wu C, Rudolph G, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnheiter H. The discovery of the microphthalmia locus and its gene, Mitf. Pigment Cell Melanoma Res. 2010;23:729–735. doi: 10.1111/j.1755-148X.2010.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014;563:28–34. doi: 10.1016/j.abb.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gadd MS, Jacques DA, Nisevic I, Craig VJ, et al. A structural basis for the regulation of the LIM-homeodomain protein islet 1 (Isl1) by intra- and intermolecular interactions. J Biol Chem. 2013;288:21924–21935. doi: 10.1074/jbc.M113.478586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gan L, Xiang M, Zhou L, Wagner DS, et al. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci USA. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang L, Hu F, Xie X, Harder J, et al. Pou4f1 and Pou4f2 are dispensible for the long-term survival of adult retinal ganglion cells in mice. PLOS One. 2014;9:e94173. doi: 10.1371/journal.pone.0094173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viringipurampeer IA, Ferreira T, DeMaria S, Yoon JJ, et al. Pax2 regulates a fadd-dependent molecular switch that drives tissue fusion during eye development. Hum Mol Genet. 2012;15:2357–2369. doi: 10.1093/hmg/dds056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao C, Zhang X. Development of astrocytes in the vertebrate eye. Dev Dyn. 2014;243:1501–1510. doi: 10.1002/dvdy.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Favor J, Sandulache R, Neuhäuser-Klaus A, Pretsch W, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci USA. 1996;93:1387–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schimmenti LA, Cunliffe HE, McNoe LA, Ward TA, et al. Further delineation of renal-coloboma syndrome in patients with extreme variability of phenotype and identical PAX2 mutations. Am J Hum Genet. 1997;60:869–878. [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P, Dufresne C, Turner R, Ferri S, et al. The proteome of human retina. Proteomics. 2014 Nov 19; doi: 10.1002/pmic.201400397. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillett LC, Navarro P, Tate S, Röst H, et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics. 2012;11:1–17. doi: 10.1074/mcp.0111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong L, Zhou WY, Jylha A, Aapola U, et al. Quantitation of 47 human tear proteins using high resolution multiple reaction monitoring (HR-MRM) based-mass spectrometry. J Proteomics. 2015;115:36–48. doi: 10.1016/j.jprot.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Liebler DC, Zimmerman LJ. Targeted quantitation of proteins by mass spectrometry. Biochemistry. 2013;52:3797–3806. doi: 10.1021/bi400110b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surinova S, Hüttenhain R, Chang CY, Espona L, Vitek O, Aebersold R. Automated selected reaction monitoring data analysis workflow for large-scale targeted proteomic studies. Nat Prot. 2013;8:1602–1619. doi: 10.1038/nprot.2013.091. [DOI] [PubMed] [Google Scholar]
- 62.Miteva YV, Budayeva HG, Cristea IM. Proteomics-based methods for discovery, quantification, and validation of protein-protein interactions. Anal Chem. 2013;85:749–768. doi: 10.1021/ac3033257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Budayeva HG, Cristea IM. A mass spectrometry view of stable and transient protein interactions. Adv Exp Med Biol. 2014;806:263–282. doi: 10.1007/978-3-319-06068-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hart GW. Three decades of research on O-GlcNAcylation – a major nutrient sensor that regulates signaling, transcription and cellular metabolism. Front Endocrinol (Lausanne) 2014;5:183. doi: 10.3389/fendo.2014.00183. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leitner A, Sturm M, Lindner W. Tools for analyzing the phosphoproteome and other phosphorylated biomolecules: a revew. Anal Chim Acta. 2011;703:19–30. doi: 10.1016/j.aca.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Pan S, Chen R, Aebersold R, Brentnall TA. Mass spectrometry based glycoproteomics – from a proteomics perspective. Mol Cell Proteomics. 2011;10:R110.003251. doi: 10.1074/mcp.R110.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.