Abstract
Obesity is associated with metabolic derangements in multiple tissues, which contribute to the progression of insulin resistance and the metabolic syndrome. The underlying stimulus for these metabolic derangements in obesity are not fully elucidated, however recent evidence in rodents and humans suggests that systemic, low level elevations of gut derived endotoxin (lipopolysaccharide, LPS) may play an important role in obesity related, whole-body and tissue specific metabolic perturbations. LPS initiates a well-characterized signaling cascade that elicits many pro-and anti-inflammatory pathways when bound to its receptor, Toll-Like Receptor 4 (TLR4). Low-grade elevation in plasma LPS has been termed “metabolic endotoxemia” and this state is associated with a heightened pro-inflammatory and oxidant environment often observed in obesity. Given the role of inflammatory and oxidative stress in the etiology of obesity related cardio-metabolic disease risk, it has been suggested that metabolic endotoxemia may serve a key mediator of metabolic derangements observed in obesity. This review provides supporting evidence of mechanistic associations with cell and animal models, and provides complimentary evidence of the clinical relevance of metabolic endotoxemia in obesity as it relates to inflammation and metabolic derangements in humans. Discrepancies with endotoxin detection are considered, and an alternate method of reporting metabolic endotoxemia is recommended until a standardized measurement protocol is set forth.
Keywords: Obesity, metabolic endotoxemia, substrate metabolism, inflammation, endotoxin detection
1. Introduction
Endotoxins are large, heat stable lipopolysaccharides (LPS), which are the major glycolipid component of the outer membrane of gram-negative bacteria1 that comprise approximately 70% of the total bacteria in the gut 2. Endotoxin can enter the blood by either local or systemic infection by exogenous gram-negative bacteria, through paracellular absorption following bacterial cell lysis of endogenous gram-negative bacteria in the gut, and through transcellular (via chlyomicrons) transport of endogenous endotoxin following diurnal feeding patterns3, 4. LPS contains a pathogen-associated molecular pattern, Lipid A, which initiates a signaling cascade resulting in activation of various pro-inflammatory pathways and increases oxidative stress upon binding to its pattern recognition receptor, Toll-like receptor 4 (TLR4)4–8. TLR4 resides on the cell surface of monocytes, other immune cells, and various other cell types (e.g., skeletal muscle, adipose tissue, and liver)9–11.
Bacterial infections are the leading cause of sepsis, of which gram-negative bacterial infections account for of the 45–60% of cases12, 13. In patients with sepsis, the concentration of circulating endotoxin is often elevated a hundred fold or higher compared to age-matched, healthy controls (e.g., 581±49 vs. 5.1±7.3 pg/mL, respectively)14. The elevation in endotoxin in bacterial infection (endotoxemia) results in mass overproduction of pro-inflammatory cytokines, which can lead to shock, cell damage, and potentially multiple organ failure15. Conversely, Cani et al.4 described “metabolic endotoxemia” as a condition of chronically elevated plasma LPS at levels 10–50 times lower than during septic conditions. Metabolic endotoxemia was observed in genetically obese (ob/ob) mice consuming normal chow, and could be induced in lean mice (C57bl6/J) consuming an obesogenic diet4, 16. Diet induced elevations in endotoxin were related to increased fat deposition, heighten pro-inflammatory and oxidative pathways and insulin resistance4, 16, and these perturbations could be partially or completely abrogated with antibiotic treatment4, 16. Thus, these findings were the first to indicate gut microbial endotoxin as a mediating source of a heightened pro-inflammatory milieu in obese rodent models16.
Approximately 65% of US adults and > 100 billion people worldwide are overweight or obese17, 18. Accompanying obesity is a state of chronic low-grade inflammation, characterized by elevated systemic and local pro-inflammatory cytokines and acute phase proteins19. Heightened activation of the immune system in obesity plays a role in the development of the Type II diabetes (T2D), the metabolic syndrome, and cardiovascular disease20, 21. Importantly, cardiovascular disease and T2D are the 1st and 7th leading cause of death in the United States, respectively22, 23.
Local adipose tissue inflammation and the secretion of a plethora of pro-inflammatory adipokines from visceral adipose tissue is indicated in the etiology of cardio-metabolic disease development during obesity24. However, the finding of Cani and colleagues, in association with recent evidence in humans, has lead to the hypothesis that metabolic endotoxemia may also participate in low-grade inflammation and the development of cardio-metabolic disease in obesity25–27. The present review will provide an overview of the evidence of the existence, consequences, and clinical relevance of metabolic endotoxemia in human obesity, and supports mechanistic associations with cell and rodent experiments. In addition, issues of endotoxin detection as it relates to measuring and reporting metabolic endotoxemia concentrations in the literature are discussed.
2. Metabolic endotoxemia and obesity: Evidence in rodents
2.1. Metabolic endotoxemia and its involvement in obesity, inflammation, and insulin resistance
It is important to note that the influence of bacterial endotoxin on metabolism has been studied for nearly a century28, 29. Initial studies explored the effects of lethal doses of endotoxin on metabolism, while later studies evolved to explore similar metabolic outcomes with sub-lethal concentrations of endotoxin or during bacterial sepsis30–33. More recently, the influence of chronically elevated plasma LPS, at levels 10–50 times lower than during septic conditions, on metabolism has been characterized and termed, “metabolic endotoxemia”4.
In a series of studies, Cani et al. showed that chronic, modest elevations (~ 1.5 fold) in endotoxin could be induced in lean mice (C57bl6/J) consuming a high-fat (72% of total calories)/high energy diet for four weeks or in genetically obese (ob/ob) mice consuming normal chow4, 16. In these studies, the diet induced elevations in endotoxin were related to increased fat deposition, systemic and tissue specific inflammation (e.g., liver, skeletal muscle, and adipose tissue) and insulin resistance4, 16.
The role of endotoxin as a mediator of adipose tissue development, systemic and local inflammatory processes and metabolic derangements was confirmed through low dose LPS (300 μg/kg/day) injection in lean mice on a normal chow diet4. Injection of 300 μg/kg/day of LPS in lean mice elicited similar derangements of diet induced obesity, however these mice developed slightly less glucose intolerance compared to mice consuming an obesogenic diet. Furthermore, lean mutants lacking the crucial LPS co-receptor, cluster of differentiation (CD) 14, were resistant to high fat diet induced weight gain, tissue specific inflammation, hepatic lipid deposition, and insulin resistance, therefore indicating TLR4 activation via LPS as a mediating event in high fat diet induced inflammation and metabolic derangements4.
Follow-up studies in lean mice (C57bl6/J) showed that consuming either high-carbohydrate (37% of total calories)/ high-energy or high-fat (72% of total calories)/high energy diets for four weeks lead to significant increases in circulating plasma endotoxin compared to mice consuming an isocaloric control diet for the same duration34. However, the increase in plasma endotoxin was significantly greater in the high-fat/ high-energy diet group compared to the high-carbohydrate/ high-energy group (~2.5 fold increase vs. ~1.5 fold increase, respectively) indicating that both dietary composition and energy intake influence the magnitude of elevation in circulating endotoxin.
2.1.2. The gut microbiota serves as the link between high fat feeding, endotoxin, and inflammation
Under normal physiological conditions, the gut microbiota promotes gut barrier function through a glucagon like peptide (GLP) 2 dependent mechanism35. However, a high fat diet can unfavorably alter the gut microbial composition, leading to increased intestinal permeability, as evidenced by less abundant and disorganized tight junction proteins, zonulin and occludin in the colon35. The gut microbiota dependent mechanisms mediating increased intestinal permeability are not fully elucidated, however a reduction in Bifidobacterium spp. and overactivation of the endocannabinoid (eCB) system seem to play an important role16, 35, 36. Furthermore, LPS, which is elevated due to increased intestinal permeability, can increase intestinal permeability and the peripheral eCB tone, thus completing a damaging positive feedback pathway36. Administration of antibiotics or prebiotics to genetically (ob/ob) or diet induced obese mouse models leads to a reduction in intestinal permeability and circulating plasma endotoxin16, 35, 36. Notably, selective modulation of the gut microbiota with prebiotics has been shown to decrease the mRNA expression of the eCB receptor, CB1 in the colon; decrease the eCB agonists, anandamine and 2-aracidonoylglycerol; decrease the expression of eCB agonist inhibitor, fatty acid amide hydrolase; and increase the endogenous production of the intestinotrophic, GLP2 in mice administered a high fat diet35, 36. Together the improvements in gut barrier function reduce circulating LPS, inflammation, and metabolic derangements; thus indicating that changes in the gut microbiota mediate metabolic endotoxemia and systemic and local inflammation.
2.2. Metabolic endotoxemia and altered adipose, liver and skeletal muscle metabolism
Endotoxin from LPS can initiate systemic and local inflammation and also result in reactive oxygen species (ROS) production upon binding with TLR4 and subsequent activation of NFκB6–8. TLR4 is abundant on immune cells, liver, adipose tissue, and skeletal muscle9–11. Collectively, these tissues play an important role in the regulation of glucose and lipid homeostasis, and it has been demonstrated that pro-inflammatory cytokines and ROS production interfere with normal metabolism in these tissues37–40. For instance, Cani et al.35 have reported increased expression of pro-inflammatory (e.g., PAI-1 TNFa, IL6, IL-1), oxidative stress (NADPHox, iNOS) and macrophage infiltration markers (CD86) in liver tissue of genetically obese mice with metabolic endotoxemia. In addition, increased expression of pro-inflammatory markers have been observed in visceral and subcutaneous adipose tissue of lean mice after chronic low dose LPS injection, with concomitant increases in weight gain, visceral and subcutaneous adipose tissue size, and increased liver weight4. Interestingly, CD14 knockout mice do not experience these inflammatory changes with LPS injection; nor do they develop changes in body weight, adipose tissue size, and increased liver weight. The mechanisms of altered lipid metabolism with LPS stimulation are unclear. However, increased adipose tissue size following LPS exposure appears to occur, at least in part, through a decrease in adipogenesis36.
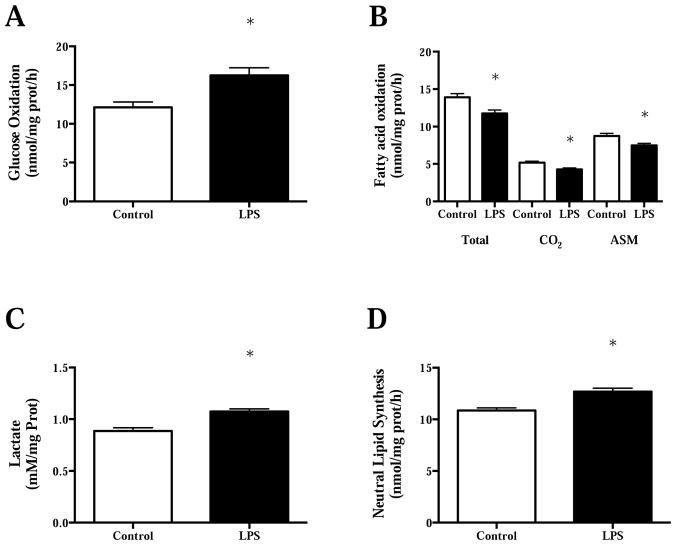
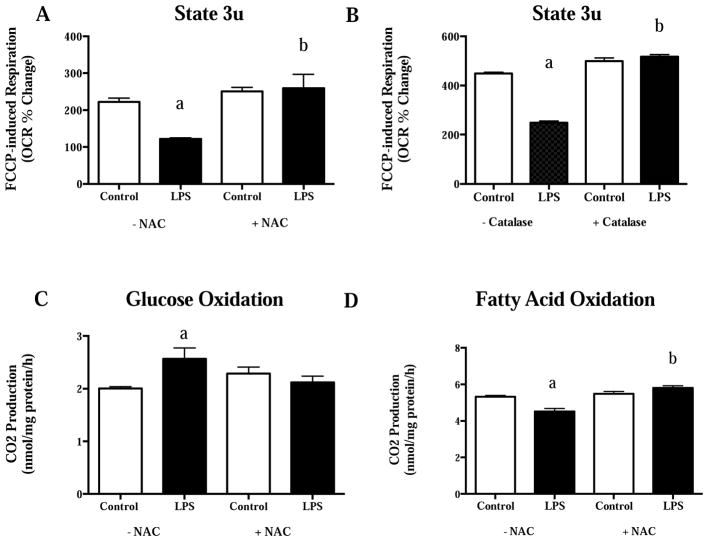
Although, some have reported increased pro-inflammatory expression in skeletal muscle following chronic low dose LPS injection (300 μg/kg/day)4, others report altered fasting skeletal substrate metabolism in the absence of pro-inflammatory expression in lean mice following a single low dose LPS injection (0.025 μg)41. Specifically, Frisard et al.41 demonstrated a switch in basal (fasting) substrate preference in rodent skeletal muscle cultures to increased glucose oxidation and decreased fatty acid oxidation after a single 2-hour LPS treatment (500 ng/mL of Escherichia coli 0111:B4) (Figure 1A–B). This switch in substrate preference was accompanied by an increase in lactate production and an increase in neutral lipid synthesis (Figure 1C–D). Interestingly, low dose LPS (50 pg/mL) elicited the same effects as 500 ng/mL after a single 2-hour exposure. The use of TLR4 mutant mice confirmed a TLR4 dependent pathway for changes in basal substrate metabolism in skeletal muscle since TLR4 mutant mice were resistant to reductions in basal fatty acid oxidation and increases in glucose oxidation after a single low dose LPS injection (0.025 μg/mouse). Treatment with parthenolide, an inhibitor of IκB Kinase (IKK), in C2C12 (mouse myotubes) cells resulted in complete abrogation of substrate metabolism changes with low dose LPS treatment. This data suggests that the metabolic perturbations in skeletal muscle are due, at least in part, to NFκB translocation via LPS-TLR4 activation. Follow-up studies in C2C12 cells demonstrated decrements in mitochondrial respiration following short-term (2 hour), low dose (50 pg/mL) LPS treatment42. In addition, co-treatment with LPS and the antioxidant, n-acetyl-cysteine (NAC), prevented changes in cellular mitochondrial respiration and changes in basal substrate metabolism previously reported (Figure 2A–D). Taken together, short term LPS exposure induces changes in basal skeletal muscle metabolism and mitochondrial oxygen consumption that seem to depend on TLR4 activation, NFκB translocation and ROS production. Indeed, LPS has been shown to increase ROS production via direct TLR4 activation and NFκB translocation in other cell types43. Furthermore, ROS has been demonstrated to activate NFκB translocation, therefore completing a noxious positive feedback pathway that is likely to participate in aberrant skeletal muscle metabolism following LPS exposure44.
Figure 1.
A) Glucose oxidation, B) fatty acid oxidation (Total= total palmitate oxidation (CO2 + ASM); CO2=complete palmitate oxidation; ASM= acid soluble metabolites and represents incomplete palmiate oxidation), C) lactate production, and D) neutral lipid synthesis in C2C12 cells following 2 hour treatment with lipopolysaccharide (500 ng/mL). *P<0.05 compared to control. Data are presented as mean± SE. Figure redrawn from Frisard et al.41.
Figure 2.
A) FCCP-stimulated maximal respiration with and without 20 mmol/L NAC, B) FCCP-stimulated maximal respiration with and without 25 U/mL of catalase, C) glucose oxidation with and without 20 mmol/L NAC, and D) fatty acid oxidation with and without 20 mmol/L NAC in C2C12 cells following 2-hour LPS treatment (50 pg/mL). a, significantly different from control without NAC/Catalase co-treatment, P<0.05. b, significantly different from LPS treatment without NAC/Catalase co-treatment, P<0.05. Data are presented as mean± SE. FCCP= Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; NAC=N-acetyl cysteine. Figure redrawn from Frisard et al.42.
The skeletal muscle metabolic changes in the absence of pro-inflammatory activation in our experiments may be due to the fact that our “low dose” LPS treatment is ~300x lower than what has been previously reported as “low dose” LPS by Cani et al.4. Standardizing the terminology of LPS dosage is needed to clarify mechanisms responsible for metabolic changes following LPS exposure. It appears that ‘very’ low concentrations of LPS are below a threshold needed to trigger inflammatory pathways, but not below a threshold to alter skeletal muscle metabolism. In accordance with this hypothesis, perturbations in metabolism have been observed in human leukocytes following “low dose” LPS exposure in the absence of a pro-inflammatory response, while higher doses elicit metabolic alterations in tandem with an increased pro-inflammatory signature45. Future studies are needed to determine if a similar phenomenon is apparent in other cell types.
3. Metabolic endotoxemia and obesity: Evidence in humans
3.1. Increased TLR4 tissue expression, elevated circulating endotoxin, and altered gut microbiota in obese vs. lean individuals
Obesity is characterized as a chronic state of low-grade inflammation46, 47. Complimentary cross-sectional evidence indicates that the LPS-TLR4 pathway is responsible, at least in part, for the heightened pro-inflammatory milieu in human obesity. For example, Creely et al.48 reported that TLR4 mRNA expression and protein content is elevated in adipose tissue from obese and T2D compared to lean donors. In addition, similar findings have been reported, in tandem with increased TLR4 signaling, in skeletal muscle from obese and T2D compared to normal weight donors41, 49. Furthermore, cross-sectional analyses have shown an elevation in circulating endotoxin in obese individuals compared to their lean counterparts 25, 50. To tie things together, Ley et al.51 were the first to describe compositional differences in the gut microbiota of obese compared to lean individuals. At the phylum level, it was found that obese individuals had a greater firmicutes/bacteroidetes ratio than aged matched controls and this difference was reversed with either carbohydrate-restricted or fat-restricted diets. Notably, and possibility due to differences in measurement techniques, not all have found these same differences in the gut microbiota of obese individuals, with some finding the opposite52, 53. More recently, there has been evidence of reduced bacterial diversity and specific genus and species differences in the gut microbiota of obese individuals54,55. Specifically, representatives of the genus bifidobacterium are reduced in the feces of obese subjects52, 56, 57. Interestingly, the abundance of bifidobacterium is associated with gut barrier function and reduced intestinal endotoxin levels in rodents58, 59. As mentioned previously, the gut microbiota serves as the link between high fat feeding, endotoxin translocation, and systemic inflammation in rodent models. Thus, it is possible that this relationship also exist in humans; however the cross-sectional nature of these studies limits this interpretation. Therefore, prospective and experimental studies have been designed and executed to further elucidate this relationship in humans, as summarized below (See Sections 3.2–3.5).
3.2. High fat meals, short-term high fat diets, and metabolic endotoxemia
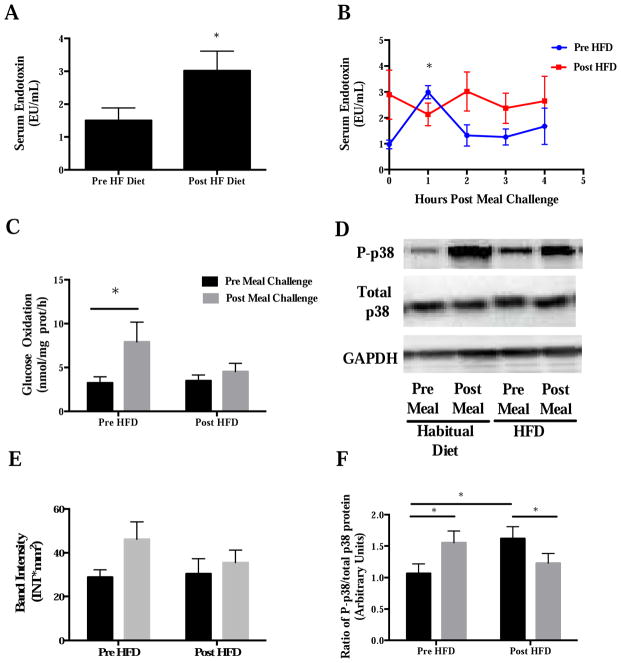
A high fat meal induces a modest elevation in circulating endotoxin in healthy, non-obese, obese and T2D individuals compared to fasting concentrations60–64. Postprandial elevations in circulating endotoxin peak between 1 and 5 hours, are transiently elevated (~ 1–2 hours), and appear to be more robust in metabolically impaired individuals60–64. In line with rodent studies, the elevation in postprandial endotoxin occurs in tandem with heightened pro-inflammatory pathway activation and ROS generation. For example, increased circulating sCD14 and IL-6; increased NFκB DNA binding; increased TLR4 protein expression; increased SOCS3, TNFα, and IL-1B mRNA expression in mononuclear cells; and increased protein expression of NADPH oxidase subunit p47phos in mononuclear cells has been observed in the postprandial period alongside elevations in circulating endotoxin61, 63, 65. In lean rodents, 4 weeks of an obesogenic diet leads to elevated fasting plasma endotoxin and a disruption in the natural diurnal rhythm of circulating plasma endotoxin4. Our group very recently corroborated these findings in lean, healthy humans fed a short-term (5 days) isocaloric high fat diet [55% fat, (25% of total energy as saturated fat), 30% carbohydrate, and 15% protein]64. Specifically, this high fat diet significantly increased fasting serum endotoxin (Figure 3A) and disrupted the normal fasted-to-fed serum endotoxin production following a high fat meal (63% fat) (Figure 3B). Interestingly, the high fat diet completely abrogated postprandial elevations in glucose oxidation, and phosphorylation status of p38 protein following a high fat meal (Figure 3C–F), which occurred in the absence of changes in whole body insulin sensitivity. Ongoing, larger studies are exploring the mechanisms behind these changes (NCT02328235- Hulver, P.I.) On the other hand, Hulson et al.66 report that an acute (7 days) hypercaloric (~ 50% above energy needs) high fat diet (65% fat) impairs insulin sensitivity in healthy, non-obese individuals and leads to significant weight gain; whereas normal insulin sensitivity and body weight is maintained in those consuming a probiotic mixture (Lactobacillus casei Shirota) for 4-weeks. Although these researchers did not measure circulating endotoxin, these findings suggest that maintenance of a favorable gut microbiota composition via probiotic supplementation likely prevent changes in insulin sensitivity. However, whether the maintenance of a healthy microbiota during high fat feeding prevented elevations in circulating endotoxin, and thus preserved whole body glucose metabolism merits future study.
Figure 3.
A) Fasting serum endotoxin, B) postprandial serum endotoxin, C) fasting and postprandial (4 hour post high fat [63%] meal) glucose oxidation, DE) fasting and postprandial p38 MAPK total and phosphorylated protein, and F) fasting and postprandial phospho- to total-p-38 ratio before and after a 5 day, eucaloric high fat (55%) diet in healthy, non-obese males (n=6). *P<0.05. Data are presented as mean± SE. Figure redrawn from Anderson et al.64.
3.3. Long-term high fat diets and metabolic endotoxemia
The reports of a relationship between longer term high fat feeding (4–8 weeks) and elevated circulating endotoxin are inconsistent. For instance, Pendyala and colleagues67 show that plasma endotoxin is significantly elevated (71% vs. baseline), independent of increases in circulating pro-inflammatory cytokines, after 1 month of isocaloric, high fat (40% fat of total energy) feeding in middle aged (55–66 years old), healthy adults. Conversely, 8 weeks of high fat (45% fat of total energy) overfeeding (+ 760 kcals/day) did not influence fasting plasma endotoxin in healthy, non-obese males 68. Furthermore, we recently corroborated the lack of change in circulating serum endotoxin, whole body insulin sensitivity, and skeletal muscle substrate metabolism after 4 weeks of high fat (55% fat of total energy) overfeeding (+1000 kcal/day) in healthy, young non-obese males (unpublished findings, in review). Obviously, the relationship between high fat feeding and metabolic endotoxemia is more complex in humans compared to rodents and appears to be influenced by the time course of feeding, the macronutrient (and possibly energy) composition, and the age of participants. Notably, each of the above mentioned studies measured endotoxin activity with different assays, thus likely contributing to these variable findings. Future studies are needed to determine whether short and long-term high fat diets exacerbate existing elevations in circulating endotoxin in obese and T2D individuals.
3.4. Observational and experimental clinical implications of metabolic endotoxemia in obesity and cardio-metabolic disease
Large prospective population based studies highlight the clinical relevance of elevated circulating endotoxin and proteins of the LPS-TLR4 pathway as they relate to cardio-metabolic disease development. For example, elevated baseline fasting serum endotoxin concentrations predicted coronary heart disease events at follow up (10 years) in middle aged, otherwise healthy individuals25. In addition, the risk of incident diabetes is increased at follow up (10 years) in healthy adults aged 25–75 with elevated serum endotoxin69. Furthermore, the risk for developing metabolic syndrome and most of its components (including central adiposity) is increased in middle aged and older healthy Chinese adults with elevated fasting plasma LPS binding protein (LBP) at baseline 70. Indeed, the role of endotoxin as a key mediator in the development cardio-metabolic disturbances cannot be gleaned from longitudinal observation studies. Fortunately, many studies have induced experimental endotoxemia via intravenous injection of endotoxin to address this issue.
Administration of lower to moderate doses (0.2–2 ng/kg body weight) of E coli LPS produce pro-inflammatory and metabolic disturbances that more closely resemble chronic diseases than do higher (3–5 ng/kg body weight) doses71. For instance, low dose (0.6 ng/kg body weight) intravenous LPS administration induces rapid, transient increases in plasma IL-6 (25-fold) and TNFα (100-fold), followed by modest induction of pro-inflammatory mRNA cytokine expression in adipose tissue (i.e., IL-6, TNFα, MCP-1, SOCS1 and SOCS3)72. In addition, whole body insulin sensitivity (Si) and HOMA- IR are significantly impaired post 24 hours LPS injection, independently of altered β-cell function to levels commensurate of metabolic syndrome and diabetes73, 74. Others have observed decreased basal and insulin stimulated skeletal muscle glucose uptake, in the absence of increased circulating pro-inflammatory cytokines, while continuously infusing low dose LPS (0.025 ng/kg/hour) over 360 minutes into the femoral artery75. Skeletal muscle biopsies did not reveal that LPS impaired insulin signaling compared to placebo in this study. A single intravenous dose (2 ng/kg of body weight) of LPS increases NEFA76, 77, indicative of increased adipose tissue lipolysis76, 77. According to the reverse Randle cycle, it is possible that the LPS induced rise in circulating FFA could reduce glucose uptake and oxidation without impairing insulin signaling78, 79. These observations are in contrast to a switch to preferential glucose oxidation over fatty acid oxidation in the fasting period in rodent skeletal muscle following acute LPS exposure41, 42. However it is important to note that these studies use different LPS dosing paradigms, therefore the continuous infusion of LPS over 360 minutes in the former study75 may alter skeletal muscle metabolism differently than a single LPS injection or short LPS exposure41, 42. Indeed, future studies are needed to elucidate these differences.
Single low-dose experimental endotoxemia models provide causal evidence of LPS induced metabolic disturbances that closely resemble those observed in obesity and the metabolic syndrome. However, this model has its limitations, as it does not accurately reflect low- grade chronically elevated endotoxin observed after high fat feeding and metabolic disease. In rodents, subcutaneously implanted mini-osmotic infusion pumps have been used to continuously deliver LPS at low dose (300 μg/kg/day) for 4-weeks, which lead to similar circulating endotoxin levels and metabolic derangements as observed following high fat feeding for the same duration4. Continuous infusion regimens have been performed in humans with other drugs, such as chemotherapy80. To our knowledge, continuous delivery of LPS at low doses has not been performed in human subjects, but warrants future study to more accurately model metabolic endotoxemia. The importance of developing these studies is reflected in the diminished the pro- and anti-inflammatory responses to LPS on day 5 compared to day 1following once daily intravenous injections of 2ng/kg/day of E. coli. LPS for 5 consecutive days81. Whether a resistance to inflammatory and metabolic changes develops to continuous low dose delivery of LPS is unknown.
3.5. Weight loss, gut microbiota changes, and reductions in metabolic endotoxemia
As reviewed above, the prevailing hypothesis is that high energy/fat intake leads to gut microbiota dysbiosis and leads to elevations in circulating endotoxin in humans through increased paracellular (increased intestinal permeability) and transcellular transport (chylomicron-LPS co-transport) 3, 4, 35. Therefore, reducing caloric intake and/or modulating the gut microbiota (e.g., pre-, pro-, or synbiotics) may reduce circulating endotoxin and metabolic perturbations. Some studies show that bariatric surgery leads to altered gut microbiota82, reductions in circulating endotoxin27, 83, reductions in pro-inflammatory pathways27, 82, 83, and improvements in glucose homeostasis27, 82, 83. Monte et al.27 showed that, in addition to reductions in circulating endotoxin, TLR4, TLR2, and CD14 mRNA expression, and NFκB binding to DNA in mononuclear cells were all significantly reduced following Roux-en-Y gastric bypass surgery (RYGB) surgery. Furthermore, by measuring bacterial DNA (broad range PCR and of prokaryote 16sRNA) in plasma, Ortiz et al.83 only showed reductions in pro-inflammatory pathways and insulin resistance following surgical (RYGB and sleeve gastrectomy) weight loss in subjects who had concomitant reductions in circulating bacterial DNA. However, these results should be interpreted with caution since only a small number of subjects had increases or no change in circulating bacterial DNA content following weight loss. Furthermore, in these studies it is unclear whether reduced caloric intake, changes in the gut microbiota, or a combination of both, are responsible for the reductions in circulation endotoxin following weight loss.
Diet-induced weight loss, with or without gut modulation therapy, has been shown to reduce circulating endotoxin, reduce systemic inflammation, and improve glucose homeostasis in obese individuals and T2D84–87. In one study, the changes in the proportions of firmicutes, bacilli, actinobacteria, Bifidobacterium and Faecalibacterium praausnitzii were negatively corrected to the change in circulating endotoxin in obese women following 8 weeks of prebiotic therapy (inulin-type fructans; 16g/day). In addition, Zhao et al.88 recently reported reductions in intestinal permeability, several genera of endotoxin producing gut bacteria, systemic pro-inflammatory tone, and improved insulin sensitivity following a 9-week multiple faceted weight loss intervention that included prebiotic consumption. Following the intervention, the proportion of Citrobacter (genera of bacteria containing opportunistic pathogens and LPS containing microbiota) was positively correlated to body mass, body mass index, and IL1-β after adjusting for gender, weight loss, and age. This evidence suggests that the gut microbiota may serve as the link between high fat intake, endotoxin translocation, and systemic inflammation in humans. To support this evidence further, prebiotic (xylo-oligosaccharide+ inulin; 1g and 3g/ day, respectively) treatment in young, healthy individuals consuming a moderately high fat diet was able to reduce circulating endotoxin concentrations, in the absence of weight loss89. In addition, the prebiotic treatment attenuated increases in IL1-β (pro-inflammatory) gene expression and lessened the decrease in IL-13 (anti-inflammatory) gene expression in whole blood stimulated with LPS during following the intervention. Taken together, since weight loss is known to alter the gut microbiota, and gut modulation therapy has been shown to reduce circulating endotoxin and its consequences without weight loss, it appears the gut microbiota alterations mediate endotoxin translocation and systemic inflammation in humans.
4. Endotoxin Detection: Limitations with measurement in blood
Multiple methods of endotoxin detection are available and the strengths and limitations of these methods have been reviewed elsewhere 1, 15, 90, 91. The FDA accepts the use of a Limulus Amoebocyte Lysate (LAL) test for detection of endotoxins. The LAL test is based on a conserved coagulation cascade that is initiated in amoebocytes of the Horseshoe Crab when LPS from gram-negative bacteria is introduced to the host15. The cascade involves three serine protease zymogens and one clottable protein zymogen. The first step of the clotting cascade, Factor C, is activated in the presence of LPS, leading to a series of zymogen activations leading to a coagulin gel clot. The degree of activation of this cascade is related to endotoxin activity1, 15. Endotoxin concentrations can be reported in a weight by volume measure (e.g., pg/mL) or by endotoxin units (EU) per unit volume (e.g., EU/mL). EU is a measure of endotoxin activity and is preferred to the weight per volume measurement since LPS from different gram-negative bacteria have different potencies that depend on multiple factors92.
Despite its wide spread use, the LAL has inherent drawbacks in seasonal preparation and contamination with 1–3 β-D glucan leading to decreased and sensitivity and specificity, respectively15. In addition, the detection of endotoxin in the blood is widely regarded as difficult and susceptible to inaccuracies, with many factors in plasma (bile salts, proteins, co-factors, and lipoproteins) inhibiting LPS or rendering it non-detectable with the LAL test1, 15. In addition, anticoagulants used in plasma collection, such as EDTA and heparin have been shown to interfere with the LAL reaction93, 94. Furthermore, both false positive reactions and enhancement of endotoxin activity have been noted with the LAL in plasma samples1. Some, but not all, have also reported endotoxin detection issues in serum95–97. The generation of a recombinant Factor C allowed for the development of a single step activation endotoxin detection assay, thus, reducing inference of blood factors on steps beyond Factor C98. However, the factors in blood that interferes with LPS detection are not avoided with this assay. Given the difficulties with blood samples, heat inactivation and/or dilution of the sample are necessary to reduce interference99. In addition, chemical treatments are used when inactivation with heat is not effective100. Non-standardized treatment of blood samples has lead to wildly variable reports of endotoxin concentration in the literature (see below). In addition, the physiological relevance of measuring endotoxin in the blood after inactivation procedures has been questioned, since the pre-inactivated blood sample may more closely represents in vivo conditions of endotoxin exposure101. Therefore, it is possible that inactivation procedures to lead spurious endotoxin values not seen by LPS-sensing cells and tissues.
4.1. A range of metabolic endotoxemia: Does it exist and does it matter?
The range of endotoxin concentrations in healthy and obese/T2D individuals is wildly variable in the literature26, 34, 50, 61, 89, 102, 103. For example, Nádházi and colleagues102 report a plasma endotoxin concentration between the range 0.01–1 EU/mL, with an average value of 0.128±0.215, in 116 healthy donors. Conversely, Lassenius et al.26 report an average concentration 600x these values (60 ±9 EU/mL) in 345 healthy individuals. To complicate matters more, others have reported endotoxin concentrations of septic individuals below 1 EU/mL104. Furthermore, some groups report endotoxin concentrations in pg/mL, with absolute values of endotoxin differing greatly between studies25, 60, 83. Therefore, distinguishing an absolute range of metabolic endotoxemia using the description of metabolic endotoxemia as a condition of chronically elevated plasma LPS at levels 10–50 times lower than during septic conditions is near impossible when comparing between endotoxin concentrations of healthy, obese/T2D, and septic individuals across studies. It is also near impossible to determine an endotoxin concentration at which cardio-metabolic and pro-inflammatory symptoms ensue during metabolic endotoxemia. Conversely, what is consistent in the human studies reviewed herein is that the endotoxin concentration changes between lean and obese individuals, and decreases in circulating endotoxin concentration following weight loss and/or gut modulation therapy27, 83, 84, 86, 87, 89, 105, have been between 0.5–2 fold25, 26, 50, 61,27, 83, 84, 86, 87, 89, 105. Furthermore, the 0.5–2.0-fold change in endotoxin has been accompanied by concomitant changes in metabolism, with or without changes in immune system activation in these studies. Therefore, a 0.5–2.0-fold increase in circulating endotoxin may be a better indicator of metabolic endotoxemia than the traditional definition until standardization methods for endotoxin detection are set forth.
5. Conclusions
Obesity is at epidemic proportions in the United States and worldwide and excess adiposity is related to progression of many cardio-metabolic diseases. The mechanism(s) underpinning the relationship between excess adiposity and cardio-metabolic risk are not fully elucidated, however oxidative stress and inflammation play an important role. Long withstanding evidence has attributed the pro- inflammatory and oxidant milieu in obesity to local adipose issue inflammation and the secretion of activating adipokines. However, strong, evidence in rodents has defined a novel pathway linking gut microbiota derived endotoxin to elevated pro-inflammatory and pro-oxidant activation and subsequent metabolic derangements in overfeeding and obese models. Supporting evidence suggests that elevated systemic LPS, below levels that activate the immune system, alter substrate metabolism in skeletal muscle--a predominate site of substrate disposal whose dysregulation is associated with multiple metabolic disorders. This cumulative evidence in rodents has led to efforts to translate these basic science findings in rodents to human participants.
Not surprisingly, evidence from human studies have less clearly defined the association between metabolic endotoxemia and obesity compared to rodents studies. However, complimentary findings that span cross-sectional, prospective, longitudinal and experimental studies highlight the clinical relevance of metabolic endotoxemia in obesity as it relates to inflammation and cardio-metabolic derangements. The specific bacterial communities responsible for the association between the gut microbiota and metabolic endotoxemia remain controversial.
An important issue that merits future clarification and standardization is endotoxin detection in blood samples. Due to assay and measuring discrepancies, the report of endotoxin values is highly variable and inconsistent between studies. Conversely, relative fold changes in circulating endotoxin are surprisingly consistent between studies, regardless of the absolute values and units provided. Therefore we postulate that 0.5–2.0-fold increase in circulating endotoxin may be a better indicator of metabolic endotoxemia than what has been traditionally defined. Taken together, although difficult to define, it appears that metabolic endotoxemia is both real and relevant in human obesity. Metabolic endotoxemia is related to systemic and local inflammation and therefore may contribute, at least in part to cardio-metabolic disease risk associated with obesity. What remains to be further explored are the mechanism(s) and impact of the pro-inflammatory- independent actions of low levels of LPS on altered substrate metabolism in human obesity and metabolic diseases.
Highlights.
Low-grade elevation in plasma lipopolysaccharide (LPS) has been termed “metabolic endotoxemia” and this state is associated with a heightened pro-inflammatory and oxidant environment often observed in obesity
This review provides supporting evidence of mechanistic associations with cell and animal models, and provides complimentary evidence of the clinical relevance of metabolic endotoxemia in obesity as it relates to inflammation and metabolic derangements in humans.
Discrepancies with endotoxin detection are considered, and an alternate method of reporting metabolic endotoxemia is recommended until a standardized measurement protocol is set forth.
Acknowledgments
Sources of Funding
This work was funded by grants from the American Diabetes Association (1-JF-05-24 and 1-13-CE-16, M. Hulver) and the National Institutes of Health (RO1 DK-078765 and R56 DK-078765, M. Hulver) and from funds from the Fralin Life Sciences Institute at Virginia Tech.
Abbreviations
- LPS
lipopolysaccharide
- TLR4
Toll-Like Receptor 4
- T2D
Type II Diabetes
- GLP2
Glucagon Like Peptide 2
- eCB
endocannabinoid
- ROS
reactive oxygen species
- CD
cluster of differentiation
- IKK
IκB kinase
- NAC
n-acetyl cysteine
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- LBP
LPS binding protein
- Si
insulin sensitivity
- HOMA-IR
homeostatic model assessment of insulin resistance
- RYGB
Roux-en-Y gastric bypass surgery
- LAL
Limulus amebocyte lysate
- EU
endotoxin unit
Footnotes
Conflicts of Interest
The authors report no conflicts of interest. All authors have approved the final version of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nabil E. Boutagy, Email: nabilb@vt.edu.
Ryan P. McMillan, Email: mcmillr@vt.edu.
Madlyn I. Frisard, Email: frisardm@vt.edu.
References
- 1.Hurley JC. Endotoxemia: Methods of detection and clinical correlates. Clinical Microbiology Reviews. 1995;8:268–292. doi: 10.1128/cmr.8.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 5.Bannerman DD, Goldblum SE. Direct effects of endotoxin on the endothelium: Barrier function and injury. Laboratory Investigation; a Journal of Technical Methods and Pathology. 1999;79:1181. [PubMed] [Google Scholar]
- 6.Lu YC, Yeh WC, Ohashi PS. Lps/tlr4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of nf-kappa b. Journal of Immunology (Baltimore, Md: 1950) 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 8.Ryan KA, Smith MF, Jr, Sanders MK, Ernst PB. Reactive oxygen and nitrogen species differentially regulate toll-like receptor 4-mediated activation of nf-kappa b and interleukin-8 expression. Infection and Immunity. 2004;72:2123–2130. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;283:R698–709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 10.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology (Baltimore, Md) 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 11.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochemical and Biophysical Research Communications. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC. The sepsis syndrome: Definition and general approach to management. Clinics in Chest Medicine. 1996;17:175–181. doi: 10.1016/s0272-5231(05)70307-5. [DOI] [PubMed] [Google Scholar]
- 13.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: An update. Annual Reviews in Microbiology. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 14.Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (lps) and lps-binding protein in patients with severe sepsis and septic shock. The Journal of Infectious Diseases. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 15.Ding JL, Ho B. Endotoxins: Structure, Function and Recognition. Springer; 2010. Endotoxin detection–from limulus amebocyte lysate to recombinant factor c; pp. 187–208. [DOI] [PubMed] [Google Scholar]
- 16.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 18.Organization WH. Obesity: Preventing and managing the global epidemic: Report of a who consultation on obesity. Geneva, switzerland: World health organization; 1998. Publication no. World Health Organ Tech Rep Ser.894. [PubMed] [Google Scholar]
- 19.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 20.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends in Immunology. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 22.Control CfD, Prevention. National diabetes statistics report: Estimates of diabetes and its burden in the united states, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 23.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres J, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2015 update: A report from the american heart association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 24.DeMarco VG, Johnson MS, Whaley-Connell AT, Sowers JR. Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Current Hypertension Reports. 2010;12:93–98. doi: 10.1007/s11906-010-0095-5. [DOI] [PubMed] [Google Scholar]
- 25.Kallio KE, Hätönen KA, Lehto M, Salomaa V, Männistö S, Pussinen PJ. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetologica. 2014:1–10. doi: 10.1007/s00592-014-0662-3. [DOI] [PubMed] [Google Scholar]
- 26.Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–1815. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monte SV, Caruana JA, Ghanim H, Sia CL, Korzeniewski K, Schentag JJ, Dandona P. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after roux-en-y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151:587–593. doi: 10.1016/j.surg.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Zeckwer IT, Goodell H. Blood sugar studies : II. Blood sugar changes in fatal bacterial anaphylaxis in the rabbit. The Journal of Experimental Medicine. 1925;42:57–67. doi: 10.1084/jem.42.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delafield M. A comparison of the changes in the blood sugar and blood phosphorus in rabbits following the injection of suspensions of different dead bacteria. The Journal of Pathology and Bacteriology. 1932;35:53–68. [Google Scholar]
- 30.LaNoue KF, Mason AD, Daniels JP. The impairment of glucogenesis by gram negative infection. Metabolism. 1968;17:606–611. doi: 10.1016/0026-0495(68)90019-x. [DOI] [PubMed] [Google Scholar]
- 31.BERRY L, SMITHE D. Effects of bacterial endotoxins on metabolism. VII. Enzyme induction as a protective mechanism against endotoxin toxicity. J Exp Med. 1964 doi: 10.1084/jem.120.5.721. To be submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzer JJ, Bagby GJ, Meszaros K, Lang CH. Altered control of carbohydrate metabolism in endotoxemia. Progress in Clinical and Biological Research. 1988;286:145–165. [PubMed] [Google Scholar]
- 33.Tavakoli H, Mela L. Alterations of mitochondrial metabolism and protein concentrations in subacute septicemia. Infection and Immunity. 1982;38:536–541. doi: 10.1128/iai.38.2.536-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J. Energy intake is associated with endotoxemia in apparently healthy men. The American Journal of Clinical Nutrition. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 35.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving glp-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muccioli GG, Naslain D, Backhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Molecular Systems Biology. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: Role of inflammatory cytokines and reactive oxygen species. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294:R673–R680. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]
- 39.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 40.Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radical Biology & Medicine. 2009;47:213–218. doi: 10.1016/j.freeradbiomed.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Frisard MI, McMillan RP, Marchand J, Wahlberg KA, Wu Y, Voelker KA, Heilbronn L, Haynie K, Muoio B, Li L, Hulver MW. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab. 2010;298:23. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frisard MI, Wu Y, McMillan RP, Voelker KA, Wahlberg KA, Anderson AS, Boutagy N, Resendes K, Ravussin E, Hulver MW. Low levels of lipopolysaccharide modulate mitochondrial oxygen consumption in skeletal muscle. Metabolism. 2015;64:416–427. doi: 10.1016/j.metabol.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: Direct interaction of tlr4 with nad (p) h oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of nf-κb. The Journal of Immunology. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 44.Gloire G, Legrand-Poels S, Piette J. Nf-kappab activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Haimovich B, Zhang Z, Calvano JE, Calvano SE, Kumar A, Macor MA, Corbett S, Coyle SM, Lowry SF. Cellular metabolic regulators: Novel indicators of low-grade inflammation in humans. Annals of Surgery. 2014;259:999–1006. doi: 10.1097/SLA.0b013e31829a4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard J. Systemic low-grade inflammation is related to both circulating and adipose tissue tnfα, leptin and il-6 levels in obese women. International Journal of Obesity. 2004;28:993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 47.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 48.Creely SJ, McTernan PG, Kusminski CM, Da Silva N, Khanolkar M, Evans M, Harte A, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. American Journal of Physiology-Endocrinology And Metabolism. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 49.Reyna SM, Ghosh S, Tantiwong P, Meka CR, Eagan P, Jenkinson CP, Cersosimo E, DeFronzo RA, Coletta DK, Sriwijitkamol A. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity. 2011;19:476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 52.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and scfa in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE. Human gut microbiota in obesity and after gastric bypass. Proceedings of the National Academy of Sciences. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in lactobacillus reuteri and depleted in bifidobacterium animalis and methanobrevibacter smithii. International Journal of Obesity. 2012;36:817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. The American Journal of Clinical Nutrition. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 57.Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, Marti-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. The British Journal of Nutrition. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 58.Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A, Rossman J, Rich G, Dirienzo D, Ogra PL. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in balb/c mice. Dig Dis Sci. 2004;49:579–589. doi: 10.1023/b:ddas.0000026302.92898.ae. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- 60.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. The American Journal of Clinical Nutrition. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 61.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, Sabico S, O’Hare JP, Ceriello A, Saravanan P. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33:991–997. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and toll-like receptor expression. The American Journal of Clinical Nutrition. 2010;91:940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson AS, Haynie KR, McMillan RP, Osterberg KL, Boutagy NE, Frisard MI, Davy BM, Davy KP, Hulver MW. Early skeletal muscle adaptations to short-term high-fat diet in humans before changes in insulin sensitivity. Obesity. 2015;23:4. doi: 10.1002/oby.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laugerette F, Vors C, Géloën A, Chauvin M-A, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M. Emulsified lipids increase endotoxemia: Possible role in early postprandial low-grade inflammation. The Journal of Nutritional Biochemistry. 2011;22:53–59. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Hulston CJ, Churnside AA, Venables MC. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. British Journal of Nutrition. 2015;113:596–602. doi: 10.1017/S0007114514004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101. e1102. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laugerette F, Alligier M, Bastard JP, Drai J, Chanséaume E, Lambert-Porcheron S, Laville M, Morio B, Vidal H, Michalski MC. Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on lps transporters lbp and scd14. Molecular Nutrition & Food Research. 2014;58:1513–1518. doi: 10.1002/mnfr.201400044. [DOI] [PubMed] [Google Scholar]
- 69.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Lu L, Yao P, Ma Y, Wang F, Jin Q, Ye X, Li H, Hu FB, Sun L. Lipopolysaccharide binding protein, obesity status and incidence of metabolic syndrome: A prospective study among middle-aged and older chinese. Diabetologia. 2014;57:1834–1841. doi: 10.1007/s00125-014-3288-7. [DOI] [PubMed] [Google Scholar]
- 71.Patel PN, Shah RY, Ferguson JF, Reilly MP. Human experimental endotoxemia in modeling the pathophysiology, genomics, and therapeutics of innate immunity in complex cardiometabolic diseases. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014 doi: 10.1161/ATVBAHA.114.304455. ATVBAHA. 114.304455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mehta NN, Heffron SP, Patel PN, Ferguson J, Shah RD, Hinkle CC, Krishnamoorthy P, Shah R, Tabita-Martinez J, Terembula K. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J Transl Med. 2012;10:124. doi: 10.1186/1479-5876-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2006;91:2138–2144. doi: 10.1210/jc.2005-2519. [DOI] [PubMed] [Google Scholar]
- 74.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the san antonio heart study. Diabetes Care. 1997;20:1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 75.Buhl M, Bosnjak E, Vendelbo MH, Gjedsted J, Nielsen RR, K-Hafstrøm T, Vestergaard ET, Jessen N, Tønnesen E, Møller AB. Direct effects of locally administered lipopolysaccharide on glucose, lipid, and protein metabolism in the placebo-controlled, bilaterally infused human leg. The Journal of Clinical Endocrinology & Metabolism. 2013;98:2090–2099. doi: 10.1210/jc.2012-3836. [DOI] [PubMed] [Google Scholar]
- 76.Kamisoglu K, Sleight KE, Calvano SE, Coyle SM, Corbett SA, Androulakis IP. Temporal metabolic profiling of plasma during endotoxemia in humans. Shock (Augusta, Ga) 2013;40:519. doi: 10.1097/SHK.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang X-c, Seidman CE, Tremaroli JD, Lai J, Rubin AL. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. Journal of Lipid Research. 2003;44:1489–1498. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 78.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. Journal of Clinical Investigation. 1996;97:2859. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hue L, Taegtmeyer H. The randle cycle revisited: A new head for an old hat. American Journal of Physiology-Endocrinology and Metabolism. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogelzang NJ. Continuous infusion chemotherapy: A critical review. Journal of Clinical Oncology. 1984;2:1289–1304. doi: 10.1200/JCO.1984.2.11.1289. [DOI] [PubMed] [Google Scholar]
- 81.Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Critical Care Medicine. 2009;37:1261–1267. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- 82.Furet J-P, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, Mariat D, Corthier G, Doré J, Henegar C. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ortiz S, Zapater P, Estrada JL, Enriquez P, Rey M, Abad Á, Such J, Lluis F, Francés R. Bacterial DNA translocation holds increased insulin resistance and systemic inflammatory levels in morbid obese patients. The Journal of Clinical Endocrinology & Metabolism. 2014;99:2575–2583. doi: 10.1210/jc.2013-4483. [DOI] [PubMed] [Google Scholar]
- 84.Dehghan P, Gargari BP, Jafar-Abadi MA. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition. 2014;30:418–423. doi: 10.1016/j.nut.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen J-P. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2012 doi: 10.1136/gutjnl-2012-303304. gutjnl-2012–303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dixon A, Valsamakis G, Hanif M, Field A, Boutsiadis A, Harte A, McTernan PG, Barnett AH, Kumar S. Effect of the orlistat on serum endotoxin lipopolysaccharide and adipocytokines in south asian individuals with impaired glucose tolerance. International Journal of Clinical Practice. 2008;62:1124–1129. doi: 10.1111/j.1742-1241.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- 87.Lira FS, Rosa JC, Pimentel GD, Santos RV, Carnier J, Sanches PL, de Piano A, de Souza CT, Tock L, Tufik S. Long-term interdisciplinary therapy reduces endotoxin level and insulin resistance in obese adolescents. Nutrition Journal. 2012;18:74. doi: 10.1186/1475-2891-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao S, Fei N, Pang X, Shen J, Wang L, Zhang B, Zhang M, Zhang X, Zhang C, Li M. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiology Ecology. 2014;87:357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lecerf J-M, Dépeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H. Xylo-oligosaccharide (xos) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while xos alone only shows prebiotic properties. British Journal of Nutrition. 2012;108:1847–1858. doi: 10.1017/S0007114511007252. [DOI] [PubMed] [Google Scholar]
- 90.Das A, Kumar P, Swain S. Recent advances in biosensor based endotoxin detection. Biosensors and Bioelectronics. 2014;51:62–75. doi: 10.1016/j.bios.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Novitsky TJ. Limitations of the limulus amebocyte lysate test in demonstrating circulating lipopolysaccharides. Annals of the New York Academy of Sciences. 1998;851:416–421. doi: 10.1111/j.1749-6632.1998.tb09018.x. [DOI] [PubMed] [Google Scholar]
- 92.Dawson M, Novitsky T, Gould M. Microbes, endotoxins and water. Pharm Engineering. 1988;8:145–148. [Google Scholar]
- 93.Morita T, Nakamura T, Miyata T, Iwanaga S. Biochemical characterization of limulus clotting factors and inhibitors which interact with bacterial endotoxins. Progress in Clinical and Biological Research. 1985;189:53–66. [PubMed] [Google Scholar]
- 94.Sullivan JD, Jr, Watson SW. Inhibitory effect of heparin on the limulus test for endotoxin. Journal of Clinical Microbiology. 1976;2:151. [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen J, McConnell JS. Observations on the measurement and evaluation of endotoxemia by a quantitative limulus lysate microassay. The Journal of Infectious Diseases. 1984;150:916–924. doi: 10.1093/infdis/150.6.916. [DOI] [PubMed] [Google Scholar]
- 96.Ditter B, Becker KP, Urbaschek R, Urbaschek B. Detection of endotoxin in blood and other specimens by evaluation of photometrically registered lal-reaction-kinetics in microtiter plates. Progress in Clinical and Biological Research. 1982;93:385–392. [PubMed] [Google Scholar]
- 97.Novitsky TJ, Roslansky PF. Quantification of endotoxin inhibition in serum and plasma using a turbidimetric lal assay. Progress in Clinical and Biological Research. 1985;189:181–196. [PubMed] [Google Scholar]
- 98.Ding JL, Ho B. A new era in pyrogen testing. TRENDS in Biotechnology. 2001;19:277–281. doi: 10.1016/s0167-7799(01)01694-8. [DOI] [PubMed] [Google Scholar]
- 99.Dawson M. Interference with the lal test and how to address it. LAL Update. 2005;22:1–5. [Google Scholar]
- 100.Novitsky T. Limulus amebocyte lysate (lal) detection of endotoxin in human blood. Journal of Endotoxin Research. 1994;1:253–263. [Google Scholar]
- 101.Munford RS. Invited review: Detoxifying endotoxin: Time, place and person. Journal of Endotoxin Research. 2005;11:69–84. doi: 10.1179/096805105X35161. [DOI] [PubMed] [Google Scholar]
- 102.Nadhazi Z, Takats A, Offenmuller K, Bertok L. Plasma endotoxin level of healthy donors. Acta Microbiologica et Immunologica Hungarica. 2002;49:151–157. doi: 10.1556/AMicr.49.2002.1.15. [DOI] [PubMed] [Google Scholar]
- 103.Al-Attas OS, Al-Daghri NM, Al-Rubeaan K, da Silva NF, Sabico SL, Kumar S, McTernan PG, Harte AL. Changes in endotoxin levels in t2dm subjects on anti-diabetic therapies. Cardiovascular Diabetology. 2009;8:20. doi: 10.1186/1475-2840-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yaroustovsky M, Plyushch M, Popov D, Samsonova N, Abramyan M, Popok Z, Krotenko N. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. Journal of Inflammation (London, England) 2013;10:8. doi: 10.1186/1476-9255-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Volti GL. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Digestive Diseases and Sciences. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]