Summary
Condensin is a conserved chromosomal complex necessary to promote mitotic chromosome condensation and sister chromatid resolution during anaphase. Here, we report that yeast condensin binds to replicated centromere regions. We show that centromeric condensin relocalizes to chromosome arms as cells undergo anaphase segregation. We find that condensin relocalization is initiated immediately after the bipolar attachment of sister kinetochores to spindles and requires Polo kinase activity. Moreover, condensin localization during anaphase involves a higher binding rate on DNA and temporally overlaps with condensin’s DNA overwinding activity. Finally, we demonstrate that topoisomerase 2 (Top2) is also recruited to chromosome arms during anaphase in a condensin-dependent manner. Our results uncover a functional relation between condensin and Top2 during anaphase to mediate chromosome segregation.
Graphical Abstract

Highlights
-
•
Condensin recruitment to centromeric regions requires DNA replication
-
•
Centromeric condensin spreads to chromosome arms during anaphase
-
•
Condensin promotes recruitment of Top2 during anaphase
-
•
Condensin localization requires Polo kinase and correlates with DNA overwinding
Chromosome condensation requires condensin and topoisomerase 2 (Top2) and is necessary for segregation. Leonard et al. show that condensin localizes to yeast centromeres following DNA replication and spreads to chromosome arms during anaphase when Top2 is also recruited. Condensin and Top2 activities are coordinated to shape mitotic chromosomes.
Introduction
Mitotic chromosome condensation involves a dramatic reorganization of chromatin strands into compact chromosomes. Chromosome condensation is mediated by the condensin complex (Hirano, 2005) and is necessary to prevent sister chromatids from being entangled during segregation. In budding yeast, condensin is a five-subunit complex containing a pair of SMC ATPases (Smc2 and Smc4) and several non-SMC proteins (Brn1, Ycg1, and Ycs4) (Freeman et al., 2000). In addition to condensin (condensin I), most eukaryotic species contain a second condensin complex, condensin II, with different non-SMC protein subunits (Ono et al., 2003). Importantly, in higher eukaryotes both condensin complexes collaborate to shape mitotic chromosomes (Shintomi and Hirano, 2011).
One of the functions attributed to chromosome condensation is the removal of entanglements between replicated chromatids (Koshland and Strunnikov, 1996) that arise as a consequence of DNA replication (Sundin and Varshavsky, 1980). These entanglements or sister chromatid intertwines (SCI) can only be resolved by type IIA topoisomerases (such as Top2 in yeast) because of their ability to transiently break DNA strands and pass double helices through the break before religation (Wang, 2002). Therefore chromosome condensation, and condensin itself, has been thought to facilitate the role of Top2 in removing SCIs (Nasmyth, 2001).
However, the functional relation between Top2 and condensin is not fully understood. The consequences of Top2 and condensin ablation impairing yeast chromosome organization and segregation carry significant resemblance (Freeman et al., 2000, Holm et al., 1985, Saka et al., 1994, Uemura et al., 1987) thus suggesting functional cooperation between these two factors. Inactivation of condensin has been shown to prevent the timely decatenation of yeast minichromosomes (Charbin et al., 2014). Early in vitro studies demonstrated that condensin overwinds DNA generating positive supercoiling in the presence of type IB topoisomerases (Bazett-Jones et al., 2002, Kimura and Hirano, 1997), and this activity was recently described in vivo on yeast minichromosomes (Baxter et al., 2011). Moreover, TopoIIα (one of the eukaryotic type IIA topoisomerases) shows a bias towards decatenation on catenated plasmids that are positively supercoiled but not on negatively supercoiled substrates (Baxter et al., 2011), raising the possibility that condensin-dependent overwinding indirectly promotes decatenation by Top2 through alteration of substrate topology (Baxter and Aragón, 2012). In mammals, TopoIIα and condensin are present on chromosome axes (Maeshima and Laemmli, 2003), and recent data from chicken DT40 cell lines demonstrated that inactivation of condensin function prevents TopoIIα localization (Samejima et al., 2012).
Sister chromatid intertwines (SCIs) persist on cohesin sites until biorientation on mitotic spindles takes place (Farcas et al., 2011). Direct visualization of SCIs on yeast minichromosomes using sucrose gradient fractionation and southern analysis demonstrated that SCIs are present on minichromosomes in a metaphase arrest without spindles (nocodazole arrests) but they disappear as chromosomes become bioriented in a metaphase arrest with spindles (cdc20-depletion arrests) (Farcas et al., 2011). Coincidentally, this mirrors the overwinding activity observed for condensin, which is absent in nocodazole arrests but present in cdc20-depletion arrests (Baxter et al., 2011). During early yeast mitosis (metaphase) condensin localizes to centromeres (D’Ambrosio et al., 2008, Verzijlbergen et al., 2014) and plays specific roles in sensing kinetochore tension (Yong-Gonzalez et al., 2007) and biorientation (Verzijlbergen et al., 2014). In late mitosis (anaphase), condensin subunits become hyperphosphorylated by Cdc5 polo-kinase (St-Pierre et al., 2009), a modification shown to enhance condensin’s overwinding activity in vitro (St-Pierre et al., 2009). Importantly, this anaphase role of condensin has been linked to a late-condensation step during chromosome segregation (Lavoie et al., 2004, Machín et al., 2005) necessary to remove residual cohesin complexes (Renshaw et al., 2010) and prevent chromosome breakage (Cuylen et al., 2013).
Here, we show that condensin binds to centromere regions only after DNA replication and it is transiently relocalized to chromosome arms during yeast anaphase. We also characterize the requirements and consequences of condensin's anaphase relocalization.
Results
Chromosome Biorientation Promotes Reduced Condensin Binding at Centromeres
Previous work has demonstrated that condensin is enriched at the ribosomal gene array and centromeres sequences in metaphase arrests mediated by nocodazole, where cells lack mitotic spindles (D’Ambrosio et al., 2008, Verzijlbergen et al., 2014). Condensin-dependent DNA overwinding is also absent in nocodazole-arrested cells (Baxter et al., 2011). Therefore, biorientation on the spindles not only requires condensin (Verzijlbergen et al., 2014), but it also triggers condensin-dependent overwinding on minichromosomes (Baxter et al., 2011). We set out to investigate whether the centromeric localization of condensin in nocodazole-arrested cells is altered upon chromosome biorientation. To this aim, we compared centromeric localization of Smc2 by chromatin immunoprecipitation sequencing (ChIP-seq) analysis on cells arrested in metaphase by depletion of the APC activator cdc20 in the presence or absence of mitotic spindles (Figure 1A). Cdc20-depleted cells were first arrested in metaphase in the presence of nocodazole (where the spindles are absent) (Figure 1A; CDC20 arrests-plus nocodazole). As previously described, Smc2 was enriched at centromere cores and pericentromeric regions (D’Ambrosio et al., 2008, Verzijlbergen et al., 2014) (Figure 1A). Next, nocodazole was removed from the culture to allow spindle formation in the cdc20-depleted cells. This led to a significant reduction in Smc2 binding around centromeres (Figure 1A; CDC20 arrests—minus nocodazole) suggesting that condensin association is reduced when chromosomes achieve biorientation. To test whether lack of chromosome biorientation is responsible for the binding of condensin around centromeres, we added new nocodazole to Cdc20-depleted cells (Figure 1A; CDC20 arrests—nocodazole addback). High Smc2 binding around centromeres was re-established in these cells (Figure 1A). In contrast to metaphase arrests treated with nocodazole, Smc2 enrichment around centromeres was completely abolished in G1-arrested cells (Figure 1B). We were surprised by this result because previous studies reported unaltered chromosomal binding of condensin to centromeres throughout the cell cycle (D’Ambrosio et al., 2008). To confirm our results, we decided to use ChIP-qPCR analysis at various time points following a synchronized culture release from a G1 arrest (Figure 2A). We used two primer pairs covering the regions around the centromere of chromosome 4 and three locations in the long chromosome arm at different distances from the centromere (15, 50, and 100 kb away) (Figure 2A). Consistent with our ChIP-seq data, we observed that Smc2 binding occurred at centromere regions only after genome replication (Figure 2A). From these results, we conclude that condensin binds to replicated chromosomes mainly around centromere regions (Figure 1; Data S1, S2, S3, and S4).
Figure 1.
Chromosome Biorientation Promotes Reduced Condensin Binding at Centromeres
(A) Association of condensin subunit Smc2 by ChIP-seq analysis to chromosomes in metaphase arrested cells in the presence and absence of mitotic spindles. cdc20-td SMC2-3HA cells were arrested in metaphase with nocodazole under Cdc20 depleting conditions (CDC20 metaphase arrest-plus nocodazole). After the arrest, nocodazole was removed and samples collected at 120 min (CDC20 metaphase arrest-minus nocodazole). Finally, nocodazole was added back and samples collected after 120 min (CDC20 metaphase arrest- nocodazole addback). Note that Smc2 centromere localization is inversely correlated to the presence of spindles.
(B) Cells as in (A) were synchronized in G1 and released into the cell cycle in the presence of nocodazole. Samples were taken for analysis at the G1 block and 120 min after release when cells reached metaphase arrest. Note that Smc2 centromeric localization is absent in cells arrested in G1.
Figure 2.
Condensin Localizes to the Chromosome upon Chromosome Biorientation
(A) SMC2-6HA cells were synchronized in G1 and released into the cell cycle. Samples were taken at the indicated time points and processed for ChIP-qPCR analysis. Graphs show the mean ± SD.
(B) Met-CDC20 SMC2-9MYC cells were arrested in metaphase with nocodazole under Cdc20 depleting conditions. Cells were transferred to new media lacking nocodazole at 37°C and samples collected for ChIP-qPCR analysis at the indicated times. Graphs show the mean ± SD.
(C) Cells were treated as in (B). Chromosomal association at selected positions was determined by ChIP-qPCR. Graphs show the mean ± SD.
Condensin Relocalizes to Chromosome Arms
During our ChIP-seq analysis, we observed the localization of condensin to centromere regions in the presence of nocodazole but not in cells arrested in metaphase by Cdc20 depletion (Figure 1A), suggesting that biorientation causes condensin dissociation. We decided to further investigate this using ChIP-qPCR analysis on various chromosome regions around the vicinity of the CEN sequence of chromosome 4. We arrested cdc20-depleted cells in metaphase in the presence of nocodazole, removed the spindle poison, and took samples for analysis at various time points after nocodazole removal. Cytological analysis of mitotic spindles confirmed their formation after nocodazole removal (data not shown). Analysis of Smc2 localization by ChIP-qPCR revealed an increase in binding to the core centromere (CEN4-0) upon nocodazole removal (10 min) (Figure 2B) followed by a steady decrease overtime leading to significantly lower levels to those observed in the previous nocodazole arrests (Figure 2B). This is consistent with our observations using ChIP-seq, where condensin localization decreased significantly after 120 min of nocodazole removal (Figure 1A). Next, we wondered whether the dissociation of condensin from centromeres (Figure 2B) leads to relocalization of the complex to other chromosomal regions. To investigate this, we extended our analysis to chromosomal regions 15, 50, and 100 kb away from CEN4 (Figure 2C). As centromere binding of Smc2 decreased (Figure 2C; from 20 min onward), binding on arm regions increased following nocodazole removal (Figure 2C; 40 and 60 min). These results demonstrate that, following biorientation of sister chromatids on the mitotic spindles, condensin binding around centromeres is decreased while its binding at chromosome arm regions increases.
Condensin Relocalization Depends on Polo-Kinase Cdc5 and Correlates with Its Activity Promoting DNA Overwinding
Condensin activity during mitosis is regulated by several kinases, including Aurora B Ipl1 (Lavoie et al., 2004), Cdk1 (Kimura et al., 1998, Robellet et al., 2015, Sutani et al., 1999), and polo kinase Cdc5 (St-Pierre et al., 2009). Importantly, Cdc5 phosphorylation affects several subunits of condensin and causes condensin to overwind DNA in vitro (St-Pierre et al., 2009). Moreover, Cdc5 is necessary for anaphase-chromosome condensation and segregation (St-Pierre et al., 2009). First, we decided to confirm that Cdc5 is required for condensin’s DNA overwinding activity in vivo. Mitotic overwinding by condensin is associated with a change in electrophoretic mobility of yeast minichromosomes in the absence of Top2 activity (Baxter et al., 2011). The shift in gel mobility is caused by a transition from catenated dimers that are negatively supercoiled (CatC) to catenated dimers that are positively supercoiled (CatC∗) (Baxter et al., 2011). To test a potential requirement for Cdc5 in condensin-dependent overwinding, we compared minichromosome migration in cells released from G1 in the absence of Top2 (top2-td) or both Top2 and Cdc5 (cdc20-td cdc5-1) (Figure 3A). CatC∗ (overwound catenanes- positively supercoiled) were only observed in cells with Cdc5 activity but not in cdc5-1 samples (Figure 3A) demonstrating that this kinase is indeed required for DNA overwinding. A similar analysis in cells lacking Aurora B demonstrated that this kinase also contributes to DNA overwinding (Figure 3A). Inactivation of Ipl1 led to defects in the formation of CatC∗ (overwound catenanes—positively supercoiled) similar to those observed in conditional mutants of the condensin subunit Brn1 (Figure 3A). This is consistent with previous studies linking Ipl1 function to condensin phosphorylation (St-Pierre et al., 2009). In addition, we found that tension across bioriented sister kinetochores is also necessary for overwinding, as we did not detect CatC∗ formation when spindle motors, Dyn1, Kip1, and Cyn8-3 were inactivated (Figure 3A), despite the fact that kinetochore-spindle attachments are intact in these cells (Saunders et al., 1995). Next, we investigated whether condensin overwinding occurs normally during anaphase. We looked for the formation of positively supercoiled plasmid topoisomers during a release from metaphase (nocodazole block) to telophase (mediated by cdc15-2 conditional mutant) using 2D chloroquine gels to reveal the supercoiling distribution of the monomer plasmids (Figure 3B). We observed the formation of positively supercoiled monomers as the cells proceeded through anaphase (Figure 3B), demonstrating that condensin overwinding activity occurs during anaphase. Next, we decided to investigate the potential correlation between condensin overwinding and the relocalization from centromeres to chromosome arms we had observed since both events occur in anaphase. To this aim, we tested whether in the absence of Cdc5 activity, which is required for condensin-mediated overwinding (Figure 3A), condensin relocalization to chromosome arms occurs. We used the conditional allele cdc5-1 and looked at the localization of Smc2 to the arms of chromosome 12 during a metaphase release in the presence and absence of Cdc5 activity. Similar to what we had observed for chromosome 4 in cdc20-depleted conditions (Figure 2C), Smc2 showed enrichment at centromeres upon nocodazole removal followed by binding to chromosome 12 arms regions during anaphase when Cdc5 activity was present (Figure 3C). In contrast, binding to arm regions was not observed in the absence of Cdc5 (Figure 3C). This result demonstrates that Cdc5 is essential for the relocalization of condensin to arm regions during anaphase and establishes a temporal correlation between condensin-dependent overwinding and its localization to chromosome arm regions.
Figure 3.
Condensin-Mediated DNA Supercoiling and Localization to Chromosome Arms Temporally Overlap during Anaphase and Require Polo Kinase
(A) Cdc20-td top2-td, cdc20-td top2-td cdc5-1, cdc20-td top2-td ipl1-321, cdc20-td top2-td brn1-60, and cdc20-td top2-td dyn1Δ kip1Δ cyn8-3 strains bearing a circular centromeric minichromosome (pRS316) were synchronized in G1, split in two, and released into the cell cycle under conditions of Cdc20 and Top2 depletion at 37°C. Nocodazole was added to one of the samples. DNA was resolved in agarose gels and probed for the circular minichromosome. Electrophoretic mobility of monomers (OCm, relaxed monomer; Lm, linear monomer; CCCm, supercoiled monomer) is indicated. Dimeric forms CatC type catenanes (negatively supercoiled) and CatC∗ type catenanes—(positively supercoiled) are indicated.
(B) A top2-td cdc15-2 strain carrying a minichromosome (pRS316) was synchronously arrested in mitosis using nocodazole to prevent formation of mitotic spindles. Top2 protein was then degraded before washing off the nocodazole to allow mitotic spindles to form and cells were allowed to proceed to a telophase arrest at 37°C mediated by cdc15-2. DNA was analyzed in 2D chloroquine gels to reveal the supercoiling distribution of the monomer plasmids. To assign the mobility of topoisomers (as positive or negative) we treated minichromosomes with eukaryotic topoisomerase I and run the fully relaxed plasmids under the same gel conditions. A cartoon representation of how the plasmid distribution relates to supercoiling status is shown (right). OC, open circles; SM, supercoiled monomer; CatC, type C catenane negatively supercoiled; CatC∗, type C catenane positively supercoiled.
(C) SMC2-6HA and cdc5-1 SMC2-6HA cells were synchronized in G1 and released into the cell cycle in the presence of nocodazole. Following metaphase arrest, cells were transferred to new media lacking nocodazole at 37°C and samples were collected at the indicated time points. Association to chromosome 12 was determined by ChIP-qPCR. Graphs show the mean ± SD.
Condensin Behavior during Anaphase Involves a Higher Binding Rate on DNA
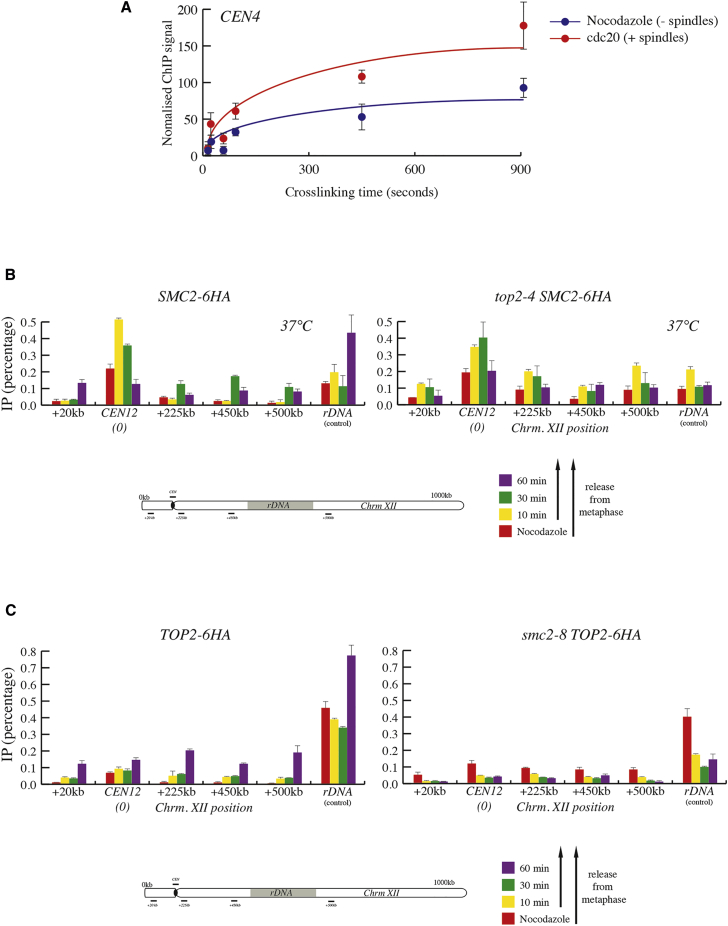
Photobleaching analysis of fluorescently labeled condensin subunits has shown that the binding of the complex (condensin I) to mammalian chromosomes is highly dynamic (Gerlich et al., 2006). Our results demonstrate that Smc2 is differentially enriched at centromeres in the presence of nocodazole and on chromosome arms during anaphase in a Cdc5-dependent manner (Figure 3C), we therefore wondered whether differences in dynamic binding of condensin exist before and after activation by Cdc5. To investigate this, we used cross-linking kinetic (CLK) analysis (Poorey et al., 2013) of Smc2 to the centromere of chromosome 4 (CEN4). This approach uses time-dependence of formaldehyde crosslinking to extract on- and off-rates for chromatin binding in vivo (Poorey et al., 2013). We compared CEN4 ChIP dataset, spanning a range of crosslinking times, between cells arrested in nocodazole and cells just released from nocodazole (at 10 min of release), when increased binding to centromeres was observed in a Cdc5-dependent manner (Figure 3C). The CLK profiles observed for Smc2 best fitted those described for transcription factors with a fast on and off rate and low occupancy (Figure 4A) (Poorey et al., 2013). This is in good agreement with the highly dynamic behavior reported for condensin on mammalian chromosomes (Gerlich et al., 2006), flies (Oliveira et al., 2007), and yeast (Robellet et al., 2015). In addition, the binding rate for Smc2 was increased 2-fold in cells just released from nocodazole (Figure 4A; 10 min after nocodazole removal—post spindles). We conclude from these results that yeast condensin, like mammalian condensin I, is highly dynamic and binds faster after Cdc5-dependent activation in anaphase.
Figure 4.
Condensin Localization in Anaphase Involves a Higher Binding Rate on DNA and Promotes Top2 Binding to Chromosome Arms
(A) CLK analysis for SMC2-3HA binding to CEN4 in cells arrested in nocodazole (−spindles; blue) or released to a cdc20 block (+spindles; red). Conditions were as in Figure 2B. Smc2 binding to CEN4 was determined by ChIP-qPCR for each crosslinking time. Data and fits are shown. Graphs show the mean ± SD.
(B) SMC2-6HA and top2-4 SMC2-6HA cells were synchronized in G1 and released into the cell cycle in the presence of nocodazole. Following metaphase arrest, cells were transferred to new media lacking nocodazole at 37°C and samples were collected at the indicated time points. Chromosomal association at selected positions was determined by ChIP-qPCR. Graphs show the mean ± SD.
(C) TOP2-6HA and smc2-8 TOP2-6HA cells were treated and analyzed as in (B). Graphs show the mean ± SD.
Condensin Promotes Recruitment of Top2 to Anaphase Chromosomes
Recent work demonstrated that inactivation of condensin in chicken DT40 cell lines prevents TopoIIα localization to mitotic chromosomes (Samejima et al., 2012). Similarly, inactivation of yeast condensin has been shown to prevent the timely decatenation of minichromosomes (Charbin et al., 2014). Moreover, condensin mutants insensitive to Cdc5 phosphorylation show condensation/segregation defects (St-Pierre et al., 2009). We therefore set out to investigate whether the Cdc5-dependent relocalization of condensin to chromosome arms is important for Top2 recruitment to chromosomes. To this aim, we initially tested whether Top2 could be detected on chromosome arms during a release from nocodazole. We used ChIP-qPCR to several regions covering the long arm of chromosome 12 in cells expressing Top2 tagged with HA epitopes. We detected Top2 recruitment to chromosome arms during late time points of the release (Figure 4C; 60 min), similar to what we had observed for Smc2 (Figure 3C). Unlike Smc2, we did not detect an increase in Top2 binding to core centromere regions upon nocodazole removal (Figure 4C). Next, we investigated whether Top2 binding to chromosomes required condensin. We used the conditional allele smc2-8 to inactivate Smc2 in a nocodazole release. We did not detect any Top2 enrichment at late time points in smc2-8 samples (Figure 4C) demonstrating that Top2 requires condensin function for localization to chromosome arms. The functional relation between Top2 and condensin prompted us to further investigate whether inactivation of Top2 also affected condensin localization to chromosomes arms. Time course analysis of Smc2 localization in metaphase releases lacking Top2 function (using the conditional allele, top2-4) revealed an accumulation of Smc2 binding on chromosome arms (Figure 4B). These results demonstrate that condensin recruitment does not depend on Top2; however, the persistence in condensin binding observed in top2-4 mutants suggests that the transient localization of condensin to chromosome arms during anaphase is affected by Top2 activity.
Discussion
Our work provides insights into the coordination of condensin and topoisomerase II functions during chromosome segregation. Although a role for condensin in anaphase condensation had been proposed earlier (Cuylen et al., 2013, Lavoie et al., 2004, Machín et al., 2005, Renshaw et al., 2010, St-Pierre et al., 2009), neither its detailed regulation and activation nor its consequences had been fully elucidated. First, we have demonstrated that condensin binding around centromere regions occurs after DNA replication. Then, upon bipolar attachment to the spindles, condensin activation by Cdc5 (St-Pierre et al., 2009) causes it to become localized to chromosome arms with increased dynamics. Moreover, this step in condensin activation coincides with the time of the DNA overwinding activity described earlier (Baxter et al., 2011, Kimura and Hirano, 1997) and the recruitment of Top2 to anaphase chromosomes as they are being segregated. We previously proposed a model where the role of topoisomerases in mitotic chromosome architecture was to respond to the enzymatic activity of condensin by relaxing condensin-dependent supercoiling (caused by condensin’s overwinding activity) and in addition to such role in supercoil relaxation to remove any remaining chromosomal intertwinings (SCI) (Baxter and Aragón, 2012). On the other hand, condensin’s molecular function was to promote overwinding-mediated compaction of the DNA that would attract Top2. In such a model, varying the rates of condensin overwinding or Top2 relaxation activities could lead to distinct changes in the shape and mechanical properties of chromosomes (Baxter and Aragón, 2012). Interestingly, recent studies looking at mammalian mitotic chromosome structure in the absence of condensin and topoisomerase IIα function demonstrated opposing activities for condensin and topoisomerase IIα (Samejima et al., 2012) consistent with this model.
Our data suggest that at the anaphase onset, condensin-dependent overwinding is activated and the rate of condensin association with DNA changes, as we detected a 2-fold increase in binding rates (Figure 4A). We show that Top2 binding responds to condensin’s overwinding activity by being enriched on chromosome arms. Furthermore, when cellular supercoil relaxation activities are decreased in the absence of Top2 (note that Top1 can partially compensate relaxation), condensin binding as predicted in this model is altered (Figure 4B). Therefore, our results are fully consistent with a model where the rates of condensin and Top2 molecular activities are coordinated to shape condensed chromosomes and to remove intertwinings in the replicated genome.
Experimental Procedures
Yeast strains used in this study are fully listed in Table S1. Detailed experimental procedures are provided in the Supplemental Experimental Procedures. Specific cell growth and synchronization conditions used for each experiment are described in the corresponding figure legend. Analysis of minichromosome topology was done as described earlier (Baxter et al., 2011) with minor changes indicated in the Supplemental Experimental Procedures.
For ChIP-seq analysis, reads were aligned to the Saccharomyces cerevisiae genome sequence using Bowtie (Langmead et al., 2009). Mapping results were processed and converted to genome-wide protein distribution profiles (ChIP-seq profiles) using parse2wig and DROMPA software as described (Sutani et al., 2015).
ChIP-qPCR conditions are described in detail in the Supplemental Experimental Procedures. CLK analysis was done as in Poorey et al. (2013). To eliminate non-specific enrichment due to transcription in our ChIP analysis, as it has been recently demonstrated (Teytelman et al., 2013), we used tagged and untagged strains and compared the enrichment in tagged strains relative to the untagged controls. First, ChIP-qPCR experiments signals obtained for both tagged and isogenic untagged strains were each normalized to their respective input samples. Then signals for each primer pair from untagged strains were subtracted from those obtained for the tagged samples. The results obtained are presented as the percentages of the recovery over the input.
Acknowledgments
We would like to thank present and former members of the L.A. laboratory for discussions and critical reading of the manuscript. This work was funded by the Intramural Research Programme of the Medical Research Council UK (J.L.) and a Wellcome Trust Award (100955-“Functional dissection of mitotic chromatin”) to L.A. (N.S. and R.T.) as well as a Grant-in-Aid for Scientific Research on Innovative Areas “Chromosome Orchestration System” from MEXT to K.S.
Published: December 10, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, two tables, and four data files and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.11.041.
Supplemental Information
References
- Baxter J., Aragón L. A model for chromosome condensation based on the interplay between condensin and topoisomerase II. Trends Genet. 2012;28:110–117. doi: 10.1016/j.tig.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Baxter J., Sen N., Martínez V.L., De Carandini M.E., Schvartzman J.B., Diffley J.F., Aragón L. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science. 2011;331:1328–1332. doi: 10.1126/science.1201538. [DOI] [PubMed] [Google Scholar]
- Bazett-Jones D.P., Kimura K., Hirano T. Efficient supercoiling of DNA by a single condensin complex as revealed by electron spectroscopic imaging. Mol. Cell. 2002;9:1183–1190. doi: 10.1016/s1097-2765(02)00546-4. [DOI] [PubMed] [Google Scholar]
- Charbin A., Bouchoux C., Uhlmann F. Condensin aids sister chromatid decatenation by topoisomerase II. Nucleic Acids Res. 2014;42:340–348. doi: 10.1093/nar/gkt882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuylen S., Metz J., Hruby A., Haering C.H. Entrapment of chromosomes by condensin rings prevents their breakage during cytokinesis. Dev. Cell. 2013;27:469–478. doi: 10.1016/j.devcel.2013.10.018. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio C., Schmidt C.K., Katou Y., Kelly G., Itoh T., Shirahige K., Uhlmann F. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008;22:2215–2227. doi: 10.1101/gad.1675708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcas A.M., Uluocak P., Helmhart W., Nasmyth K. Cohesin’s concatenation of sister DNAs maintains their intertwining. Mol. Cell. 2011;44:97–107. doi: 10.1016/j.molcel.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L., Aragon-Alcaide L., Strunnikov A. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol. 2000;149:811–824. doi: 10.1083/jcb.149.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich D., Hirota T., Koch B., Peters J.M., Ellenberg J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr. Biol. 2006;16:333–344. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Hirano T. Condensins: organizing and segregating the genome. Curr. Biol. 2005;15:R265–R275. doi: 10.1016/j.cub.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J.C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Kimura K., Hirano T. ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell. 1997;90:625–634. doi: 10.1016/s0092-8674(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Kimura K., Hirano M., Kobayashi R., Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282:487–490. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- Koshland D., Strunnikov A. Mitotic chromosome condensation. Annu. Rev. Cell Dev. Biol. 1996;12:305–333. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B.D., Hogan E., Koshland D. In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 2004;18:76–87. doi: 10.1101/gad.1150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machín F., Torres-Rosell J., Jarmuz A., Aragón L. Spindle-independent condensation-mediated segregation of yeast ribosomal DNA in late anaphase. J. Cell Biol. 2005;168:209–219. doi: 10.1083/jcb.200408087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima K., Laemmli U.K. A two-step scaffolding model for mitotic chromosome assembly. Dev. Cell. 2003;4:467–480. doi: 10.1016/s1534-5807(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- Oliveira R.A., Heidmann S., Sunkel C.E. Condensin I binds chromatin early in prophase and displays a highly dynamic association with Drosophila mitotic chromosomes. Chromosoma. 2007;116:259–274. doi: 10.1007/s00412-007-0097-5. [DOI] [PubMed] [Google Scholar]
- Ono T., Losada A., Hirano M., Myers M.P., Neuwald A.F., Hirano T. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 2003;115:109–121. doi: 10.1016/s0092-8674(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Poorey K., Viswanathan R., Carver M.N., Karpova T.S., Cirimotich S.M., McNally J.G., Bekiranov S., Auble D.T. Measuring chromatin interaction dynamics on the second time scale at single-copy genes. Science. 2013;342:369–372. doi: 10.1126/science.1242369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M.J., Ward J.J., Kanemaki M., Natsume K., Nédélec F.J., Tanaka T.U. Condensins promote chromosome recoiling during early anaphase to complete sister chromatid separation. Dev. Cell. 2010;19:232–244. doi: 10.1016/j.devcel.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robellet X., Thattikota Y., Wang F., Wee T.L., Pascariu M., Shankar S., Bonneil É., Brown C.M., D’Amours D. A high-sensitivity phospho-switch triggered by Cdk1 governs chromosome morphogenesis during cell division. Genes Dev. 2015;29:426–439. doi: 10.1101/gad.253294.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y., Sutani T., Yamashita Y., Saitoh S., Takeuchi M., Nakaseko Y., Yanagida M. Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 1994;13:4938–4952. doi: 10.1002/j.1460-2075.1994.tb06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K., Samejima I., Vagnarelli P., Ogawa H., Vargiu G., Kelly D.A., de Lima Alves F., Kerr A., Green L.C., Hudson D.F. Mitotic chromosomes are compacted laterally by KIF4 and condensin and axially by topoisomerase IIα. J. Cell Biol. 2012;199:755–770. doi: 10.1083/jcb.201202155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W.S., Koshland D., Eshel D., Gibbons I.R., Hoyt M.A. Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J. Cell Biol. 1995;128:617–624. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintomi K., Hirano T. The relative ratio of condensin I to II determines chromosome shapes. Genes Dev. 2011;25:1464–1469. doi: 10.1101/gad.2060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J., Douziech M., Bazile F., Pascariu M., Bonneil E., Sauvé V., Ratsima H., D’Amours D. Polo kinase regulates mitotic chromosome condensation by hyperactivation of condensin DNA supercoiling activity. Mol. Cell. 2009;34:416–426. doi: 10.1016/j.molcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980;21:103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Sutani T., Yuasa T., Tomonaga T., Dohmae N., Takio K., Yanagida M. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 1999;13:2271–2283. doi: 10.1101/gad.13.17.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutani T., Sakata T., Nakato R., Masuda K., Ishibashi M., Yamashita D., Suzuki Y., Hirano T., Bando M., Shirahige K. Condensin targets and reduces unwound DNA structure associated with transcription in mitotic chromosome condensation. Nat. Commun. 2015;6:7815. doi: 10.1038/ncomms8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teytelman L., Thurtle D.M., Rine J., van Oudenaarden A. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. Proc. Natl. Acad. Sci. USA. 2013;110:18602–18607. doi: 10.1073/pnas.1316064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987;50:917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Verzijlbergen K.F., Nerusheva O.O., Kelly D., Kerr A., Clift D., de Lima Alves F., Rappsilber J., Marston A.L. Shugoshin biases chromosomes for biorientation through condensin recruitment to the pericentromere. eLife. 2014;3:e01374. doi: 10.7554/eLife.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.C. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- Yong-Gonzalez V., Wang B.D., Butylin P., Ouspenski I., Strunnikov A. Condensin function at centromere chromatin facilitates proper kinetochore tension and ensures correct mitotic segregation of sister chromatids. Genes Cells. 2007;12:1075–1090. doi: 10.1111/j.1365-2443.2007.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.