Abstract
Objective
The present study examined predictors and moderators of treatment response among 165 adults meeting DSM-IV criteria for comorbid posttraumatic stress disorder (PTSD) and alcohol dependence (AD) who were randomized to 24 weeks of naltrexone (NAL), NAL and prolonged exposure (PE), pill placebo, or pill placebo and PE. All participants received supportive counseling for alcohol use.
Method
Six domains of predictors/moderators (23 variables) were evaluated using measures of PTSD (Posttraumatic Stress Symptom Scale Interview; PSS-I) and AD (percent days drinking from the Timeline Follow-Back Interview) collected every four weeks throughout treatment. Multi-level modeling using the Fournier approach was employed to evaluate predictors and moderators of rates of symptom improvement and post-treatment outcomes.
Results
Combat trauma, sexual assault trauma, and higher baseline anxiety sensitivity predicted slower improvement and poorer PTSD outcome. Combat trauma, white race, and higher baseline drinking severity predicted poorer drinking outcome. PTSD severity moderated the efficacy of PE on PTSD outcomes, such that the benefit of PE over no-PE was greater for participants with higher baseline PTSD severity. Baseline depressive severity moderated the efficacy of PE on drinking outcomes, whereby the benefit of PE over no-PE was greater for participants with higher depressive symptoms. NAL effects were most beneficial for those with the longest duration of alcohol dependence.
Conclusions
These results suggest that concurrent, trauma-focused treatment should be recommended for PTSD-AD patients who present with moderate or severe baseline PTSD and depressive symptoms. Future research should examine the mechanisms underlying poorer outcome among identified sub-groups of PTSD-AD patients.
Keywords: prolonged exposure, posttraumatic stress disorder, alcohol dependence, naltrexone, predictors, moderators
Posttraumatic stress disorder (PTSD) and alcohol dependence (AD) are highly co-morbid, with nearly half (42%) of individuals with PTSD also meeting criteria for an alcohol use disorder (Pietrzak, Goldstein, Southwick, & Grant, 2011). Despite this co-occurrence, empirical guidelines regarding treatment of this distressed population are woefully limited. Individuals with co-morbid PTSD and AD show greater severity on both PTSD and alcohol measures (Blanco et al., 2013; Brown, Stout, & Mueller, 1999; Kessler, 2000; Ouimette, Brown, & Najavits, 1998; Ouimette, Goodwin, & Brown, 2006) and relapse sooner following alcohol use treatment than patients with other psychiatry comorbidities (Ouimette, Ahrens, Moos, & Finney, 1997). These findings underscore the importance of identifying effective interventions that address PTSD and alcohol dependence (AD) concurrently.
Exposure-based cognitive behavioral therapies (CBT) are recommended as a front-line treatment for PTSD (Institute of Medicine, 2007), with prolonged exposure (PE) gaining the most empirical evidence for its efficacy (see for review: Cahill, Rothbaum, Resick & Follette, 2009). A growing number of studies support the use of exposure-based CBT for co-morbid substance dependence and PTSD, either in addition to traditional alcohol treatments or as an integrated component (Mills et al., 2012; Najavits, Schmitz, Gotthardt, & Weiss, 2005; Triffleman, 2000). Only two randomized controlled trials (RCTs) have looked specifically at PTSD and AD. Sannibale et al. (2013) compared an integrated PTSD-AD treatment that included in-vivo and imaginal exposure to address PTSD symptoms (n = 33) to an AD-only treatment that did not address PTSD symptoms (n = 29). At follow-up, participants who received exposure sessions were twice as likely to achieve clinically significant change in PTSD symptoms. Participants who received AD-only treatment showed superior drinking outcomes; however, the authors note that this finding was confounded by significantly greater use of additional alcohol-related treatment services during follow-up in the AD-only group. In a larger RCT (n = 165), Foa et al. (2013) examined the efficacy of the opioid antagonist naltrexone (NAL) for alcohol dependence and prolonged exposure therapy (PE) for PTSD. Participants received supportive counseling focusing on alcohol use and were randomized to NAL, NAL and PE, pill placebo, or pill placebo and PE. All four groups showed large reductions in both alcohol use and PTSD symptoms. NAL was associated with a lower percentage of days drinking than placebo, and PE was associated with lower rates of relapse over the follow-up period, especially when combined with NAL. The results of this study suggest that concurrent treatment is not only safe, but also may be of particular benefit to individuals with both disorders to promote long-term maintenance of treatment gains.
Research is needed to determine what factors best predict response to concurrent PTSD-AD treatment and whether moderators can be used to inform treatment selection. Indeed, the examination of predictors and moderators is central to the goal of individualizing treatment (Kazdin, 2007). Non-specific predictors refer to baseline characteristics that are associated with symptom change, irrespective of the treatment used (i.e., not specific to one treatment or another; Kraemer, Wilson, Fairburn, & Agras, 2002). Non-specific predictors can thus be used to identify treatment refractory patients that may require refined or augmented interventions. In the context of clinical trials, moderators refer to characteristics that predict differential response to one treatment over another. Thus, as noted by Kraemer, Frank, and Kupfer (2006), moderator research helps us understand which treatments work best for which patients, and has important implications for clinical decision-making.
Studies that have investigated the relationship between PTSD treatment response and demographic and psychological baseline characteristics have produced inconsistent results. Several studies have linked lower income and education to drop out in CBT for PTSD (Difede et al., 2007; Rizvi, Vogt, & Resick, 2009). Isolated studies show poorer outcomes among men (Karatzias et al., 2007) and those who live alone (Tarrier, Sommerfield, Pilgrim, & Faragher, 2000). No studies to our knowledge have shown an effect of race or ethnicity on PTSD treatment outcome, although black racial membership has been associated with higher risk of drop out in CBT for anxiety disorders (Chambless & Williams, 1995). Among clinical characteristics, initial PTSD severity has predicted poorer outcome in some studies (Karatzias et al., 2007; Taylor et al., 2001; Van Minnen, Arntz, & Keijsers, 2002) but not in others (Foa, Riggs, Massie, & Yarczower, 1995; Forbes, Creamer, Hawthorne, Allen, & McHugh, 2003). Likewise, some studies have identified comorbid depression as a predictor of poorer outcome (Forbes et al., 2003; Taylor et al., 2001) while others have found no relationship between depressive symptoms and outcome (Hagenaars, van Minnen, & Hoogduin, 2010; Karatzias et al., 2007), and one study found higher depressive symptoms to predict better outcome (Rizvi et al., 2009). Research on moderators of PTSD outcome among different treatment options is scarce, in part because traditional moderator analyses require large sample sizes to ensure adequate power.
The most consistent predictors of alcohol treatment outcome are baseline alcohol consumption and dependence severity (see for review: Adamson, Sellman, & Frampton, 2009). With respect to NAL efficacy, high levels of baseline alcohol craving have been found to moderate the effects of NAL versus placebo in some studies (Jaffe et al, 1996; Monterosso et al., 2001; Volpicelli, Clay, Watson, & O'Brien, 1995). In contrast to craving, high risk alcohol consumption and regular drinking patterns at baseline have been associated with poorer NAL response when provided in combination with cognitive behavioral therapy (Vuoristo-Myllys, Lipsanen, Lahti, Kalska, & Alho, 2014). Some studies have found high baseline depression to predict better response to NAL (Kiefer et al., 2005), while others have reported the inverse (Morley et al., 2006; Morley, Teesson, Sannibale, Baillie, & Haber, 2010). Finally, among demographic predictors, NAL has been found to be more effective than placebo for men but not women in some studies (Garbutt et al., 2005; Hernandez-avila et al., 2006), but not others (Baros, Latham, & Anton, 2008; Morley et al., 2010). The very mixed picture that emerges regarding predictors of AD and PTSD outcome may be due to variable methodologies and trauma samples across studies, as well as limitations in the statistic approaches employed – which have typically been hierarchical regressions co-varying for a small number of putative confounding variables (e.g., baseline severity).
The present study used data from a randomized controlled trial (Foa et al., 2013) to evaluate predictors and moderators of treatment improvement during concurrent treatment of AD and PTSD. To this end, we adopted an advanced analytic approach developed by Fournier (Fournier et al., 2009) that has been employed in recent predictor research (e.g., Amir et al., 2011; Powers et al., 2014; Smits et al., 2013). The present analysis differs from much of the previous research in several ways: First, multilevel modeling (MLM) was employed rather than multiple regression. Since MLM retains all subjects regardless of missing data, it is more powerful and does not require imputation of outcome data. Second, the Fournier approach to moderator analyses allows for the simultaneous entry of numerous putative predictors and moderators, thus providing a relatively thorough array of control variables, to ensure that predictors/moderators that are significant are not better accounted for by other correlated constructs. Third, the present study uses monthly assessment time points to test for the impact of baseline predictors/moderators on both post-treatment outcomes and rates of change during treatment. Variables of interest were grouped into six categories: demographics, socio-economic factors, comorbid psychopathology, trauma features, PTSD features, and AD features. In addition to PTSD features common to the literature (e.g., trauma type; PTSD duration), we have included anxiety sensitivity as a PTSD feature, given evidence that anxiety sensitivity is highly correlated with PTSD diagnosis and symptom severity (Federoff, Taylor, Asmundson, & Koch, 2000; Taylor, Koch, & McNally, 1992). No previous investigations, to our knowledge, have assessed moderators and predictors of drinking and PTSD outcomes in a comorbid sample.
Methods
Participants
Table 1 presents participant baseline characteristics. Participants (n = 165) were adults meeting DSM-IV criteria for current AD and PTSD who were enrolled in a randomized, single-blinded clinical trial at the University of Pennsylvania's Center for the Treatment and Study of Anxiety and the Philadelphia Veterans' Affairs Hospital. Exclusion criteria were: 1) current substance dependence other than nicotine or cannabis, 2) current psychotic disorder (e.g., bipolar disorder, schizophrenia), 3) active suicidal or homicidal ideation, 4) opiate use in the month prior to study entry, 5) medical illnesses that could interfere with treatment (e.g., AIDS, active hepatitis), or 6) pregnancy or nursing. At baseline, the average PSS-I score was 28.5 (SD=6.5), indicating moderately severe PTSD, and mean percentage days drinking over the preceding month was 74.8%.
Table 1. Baseline Characteristics (n = 165).
| Variable | Number (%) |
|---|---|
| Gender | |
| Female | 57 (34.5) |
| Male | 108 (65.5) |
| Race/Ethnicity | |
| Black/African-American | 105 (63.6) |
| White/Caucasian | 50 (30.3) |
| Hispanic/Latino | 7 (4.2) |
| Other | 3 (1.8) |
| Living Alone | 36 (21.8) |
| Employed | 57 (34.5) |
| Median Income Range | $15,000-20,000 |
| Education | |
| Some College or More | 84 (50.9) |
| High School or Less | 81 (49.1) |
| Types of Trauma | |
| Sexual Assault | 42 (25.5) |
| Physical Assault | 62 (37.6) |
| Combat | 19 (11.5) |
| Other | 42 (25.5) |
| Number of additional Axis I diagnoses | |
| 0 | 95 (57.6) |
| 1 | 44 (26.7) |
| 2 | 19 (11.5) |
| 3 | 3 (1.8) |
| 4 | 3 (1.8) |
| 5 | 1 (.6) |
| Other Substance Use Disorder | 35 (21.2) |
| Current Personality Disorder | 41 (24.8) |
|
| |
| Variable | M (SD) |
|
| |
| Age | 42.78 (9.76) |
| Alcohol Dependence Duration | 13.36 (11.04) |
| PTSD Duration | 14.55 (15.26) |
| Baseline PSS-I | 28.14 (7.86) |
| % Drinking Days | 74.82 (25.26) |
| Alcohol Craving | 18.38 (6.91) |
| ASI | 27.44 (13.95) |
| BDI | 26.31 (11.54) |
Note: The precise n per variable differed due to missing data on some variables. ASI: Anxiety Sensitivity Index, BDI: Beck Depression Inventory, PSS-I: PTSD Symptom Scale Interview
Procedure
Potential participants completed an intake assessment comprised of a psychiatric evaluation, physical examination, and laboratory assessments. All participants meeting study eligibility criteria completed outpatient detoxification (defined as 3 or more consecutive days of alcohol abstinence as measured by self-report and breathalyzer testing) prior to randomization. Oxazepam was administered as needed to patients who presented during detoxification with elevated withdrawal symptoms requiring medical management, and to those deemed to be high risk for poor response based on a history of elevated withdrawal symptoms. Eligible participants were then consented and randomly assigned to 1 of 4 treatment conditions: NAL + PE, placebo + PE, NAL + no PE, or placebo + no PE. Patients in all conditions received concurrent supportive counseling focusing on alcohol use and medication management. During treatment, blind assessments and self-report questionnaires were completed every four weeks (from week 0 to week 24). All study procedures were approved by the University of Pennsylvania institutional review board.
Measures
Structured Clinical Interview for DSM-IV (SCID-IV; First & Gibbon, 2004)
The SCID is a 60-minute, semi-structured interview that yields current and lifetime DSM-IV Axis I diagnoses for the major psychiatric disorders. The SCID was used to confirm diagnosis of AD and PTSD and to evaluate the presence of other Axis I disorders at baseline and post-treatment. This interview is a widely used and reliable measure of psychopathology, with joint inter-rater reliability coefficients ranging from 0.60 to 0.83, depending on the disorder (Lobbestael, Leurgans, & Arntz, 2010).
The Psychiatric Research Interview for Substance and Mental Disorders (PRISM; Hasin et al., 1996) is a semi-structural interview used to assess disorders that are commonly co-morbid with substance use disorders. In the current study, the PRISM was used to assess for the presence of anti-social personality disorder and borderline personality disorder. The PRISM has shown good diagnostic validity for Axis II personality disorders, high concordance with other established diagnostic measures such as the SCID-II, good-excellent internal consistency, and good inter-rater reliability (κ=0.66-0.75) for the disorders assessed in the current study (Hasin et al., 2006; Torrens, Serrano, Astals, Pérez-Domínguez, & Martín-Santos, 2004).
PTSD Symptom Scale Interview (PSS-I; Foa, Riggs, Dancu, & Rothbaum, 1993)
The PSS-I is a 17-item clinician-rated interview that assesses the severity of PTSD symptoms according to DSM-IV criteria over the preceding two weeks. The PSS-I yields a total score with a possible range from 0 to 51, with higher scores indicating more severe PTSD symptoms. A psychometric study of this measure using the current sample (Powers, Gillihan, Rosenfield, Jerud, & Foa, 2012) demonstrated excellent internal consistency (e.g., α=.90 for the full scale), very good one-month test-retest reliability (r=.80), good inter-rater reliability (ICC=0.73 for total severity score), and good convergent validity with SCID-IV PTSD diagnoses (κ=.75).
Timeline Follow-Back Interview (TFBI; Sobell & Sobell, 1992)
The TFBI interview utilizes a calendar method to assess for frequency and degree of alcohol consumed on a daily basis. In the current study, the TFBI provided information about the percentage of days over the past month spent drinking (PDD). The TLFB has demonstrated good test-retest reliability (α=.79-.94) and concurrent validity (r=.84-.95 with collateral reports of drinking) (Maisto, Sobell, & Sobell, 1982; Sobell, Maisto, Sobell, & Cooper, 1979).
The Penn Alcohol Craving Scale (Flannery, Volpicelli, & Pettinati, 1999)
The Penn Alcohol Craving Scale is a 5-item self-report measure that assesses degree of alcohol craving during the preceding week. The total scores on this measure range from 0 to 30, with higher scores indicative of higher craving. The PACS has excellent reliability (α=.92), high item-total correlations (r=.80-.92), and good concurrent validity (r=.55) with the Obsessive Compulsive Drinking Scale (Modell, Glaser, Mountz, Schmaltz, & Cyr, 1992), another validated measure of alcohol craving. Cronbach's alpha in the current sample was .91.
Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986)
The ASI assesses for fear of anxiety-related sensations and beliefs about the negative consequences of anxiety. The scale consists of 16 items rated on a 5-point Likert Scale which yield a total score ranging from 0 to 64. The ASI has strong documented psychometric properties including good discriminant and predictive validity (Taylor, Koch, & Crockett, 1991), adequate test-retest reliability and good internal consistency (Reiss et al., 1986). Cronbach's alpha in the current sample was .92.
Beck Depression Inventory II (BDI-II; Beck, Steer, & Brown, 1996)
The BDI-II is a well validated and widely used measure of depressive symptoms. The BDI-II consists of 21 items scored on a 4-point Likert scale (0-3), resulting in a total score ranging from 0 to 63, with higher scores indicating higher levels of depressive symptoms in the past week. Cronbach's alpha in the current sample was .92.
Treatments
Prolonged Exposure (PE)
PE consisted of 18-sessions provided over 24 weeks (12 weekly sessions following by 6 bi-weekly sessions). Each session was 90 minutes long and contained the key components of PE: imaginal exposure (repeated revisiting of traumatic memories), processing (discussing thoughts and feelings arising from the recounting of the trauma memory), and assignment of in-vivo exposure (confronting trauma-reminders in daily life). PE was provided by doctoral-level psychologists. Overall treatment adherence rate, assessed using a random sample (15%) of video-recorded sessions, was 96%. Participants completed a mean of 6.18 (SD = 3.86) exposure sessions in the PE + NAL group and 6.48 (SD = 3.49) sessions in the PE + placebo group (p=0.73).
Naltrexone
Naltrexone is an opiate antagonist treatment for AD approved by the U.S. Food and Drug Administration. Participants were started on 50 mg/d for a minimum of three days and titrated within one week to the target dose of 100 mg/d. Compliance was assessed via weekly pill counts in the first 3 months and biweekly counts for the next 3 months. Most participants tolerated this dosing regimen; a small number (N=3) were titrated back down to 50 mg/d due to side effects.
Supportive Counseling
All participants received 18, 30-40 minute sessions of supportive counseling using the BRENDA model (Starosta, Leeman, & Volpicelli, 2006), which consisted of medication management combined with techniques aimed at enhancing compliance through motivational interviewing (Miller & Rollnick, 1991). Specifically, these sessions entailed the dispensation of medication, compliance monitoring, education regarding AD, and support/advice around drinking. BRENDA sessions were conducted by the study nurse and were provided on the same schedule as PE sessions. Eighty five percent of the sample met criteria for adherence to medication and supportive counseling (i.e., ≥80% adherence and attendance).
Treatment Retention
Fifty three (32.1%) participants dropped out of the study. Dropout rates did not significantly differ across treatment groups (χ23=1.55; P =.67).
Data Analysis
The Fournier approach (see Amir et al., 2011; Fournier et al., 2009; Smits et al., 2013) was employed to identify significant predictors and moderators of change in PTSD symptoms (PSS-I) and percentage days drinking (PDD) across treatment. In this approach, potential predictors/moderators are grouped into domains of related variables (e.g., a demographics domain, a comorbid disorders domain, etc.). Significant predictors/moderators are identified within each domain, and then the significant predictors/moderators from each domain are all entered into a final model. Grouping predictors into domains from which significant predictors are identified and entered into a final model allows the investigation of a large number of predictors without substantially increasing either Type I or Type II error. Type I error is minimized because variables are identified that are predictive over and above others in their domain, and over and above significant predictors from the other domains. Type II error is minimized because the moderation analysis does not include all potential predictors/moderators in a single, very large model. Multilevel modeling (MLM), an intent-to-treat analysis, was used to analyze PSS-I scores and PDD, which were collected every 4 weeks from baseline to post-treatment (week 24).
Putative predictors and moderators were grouped in six domains: 1) demographics (age, gender, white vs. minority race), 2) socio-economic factors (co-habitation status, employment status, education level, income),3) comorbid disorders (number of comorbid Axis I disorders, presence vs. absence of additional substance use disorders, presence vs. absence of a personality disorder, depressive symptom severity), 4) trauma features (index trauma type [sexual assault, combat, physical assault, other trauma], number of other traumatic events), 5) PTSD features (baseline PSS-I, age of trauma onset, PTSD duration, anxiety sensitivity), and 6) alcohol features (baseline percentage days drinking, craving, age of AD onset, duration of AD). Post-hoc power analyses were performed for the final model using the program PinT 2.12 (Power in Two-Level Models; Snijders & Bosker, 1993). This model included 27 predictors, but had 1003 data points from 165 participants. PintT indicated greater than a .95 power to detect a medium effect size for a moderator or predictor.
The stepwise Fournier procedure for each domain was conducted as follows: In Step 1, all potential moderator variables within the domain are included in the analysis. In Step 2, only the variables with a significance level p<.20 in Step 1 are included in a second MLM analysis. Step 3 includes all terms from Step 2 that were p<.10. The analysis in Step 4 is then comprised of the terms from Step 3 that were significant at p<.05. This stepwise procedure using these a priori criteria is performed for each domain of predictors, and identifies significant predictors/moderators from each domain. Then, each term that is significant at p<.05 in Step 4 from each domain is included in the final MLM model, allowing the testing of the effects of each variable while controlling for the effects of the other variables. Variables coding treatment condition and the interactions of treatment condition and Time were included in all MLM models regardless of their significance level. Since treatment condition was comprised of a PE main effect (PE), NAL main effect (NAL), and their interaction, the treatment condition variables that were included in all models were: PE, NAL, PE × NAL, Time, PE × Time, NAL × Time, and PE × NAL × Time. Subcomponents of interactions that were included in Step 4 were also necessarily included in the final model. For example, if the combat trauma × PE × Time interaction was significant in Step 4 of the trauma features domain, its subcomponents (Combat, Combat × Time, and Combat × PE) were also included in the final model (the other subcomponents, PE, Time, and PE × Time, were included in all analyses).
To investigate moderators, we added each potential moderator and its interactions with the treatment condition and Time variables. To understand the nature of the moderator interactions that were found to be significant, we followed the approach developed by Aiken and West (1991), calculating the effect of the treatments at high and low levels of the moderator (usually defined as 1 SD above and 1 SD below the mean, respectively). This technique, which uses all the data in the MLM model to calculate model predicted parameters for different levels of the moderator, allows one to understand how the relationship between treatment and outcome varies for high and low values of the moderator.
As reported previously (Foa et al., 2013), PSS-I and PDD decreased rapidly over time and then leveled off in this study sample. Foa et al. found that, for PSS-I, the change over time was modeled most accurately by using the log of time. Thus, our Time variable for the analysis of PSS-I was ln (week+1). For PDD, the change over time was most accurately modeled by a hyperbolic function. Thus, our Time variable in the PDD analysis was coded as: (1-1/[week+1]). We then centered the Time variable at post-treatment. All variables in the models were converted to z-scores to facilitate comparison among them and to center them at their means for the interactions.
Five of our variables of interest were missing greater than 5% of their data: income (7%), alcohol craving (13%), depressive symptoms (15%), anxiety sensitivity (18%), and presence of a personality disorder (28%). To avoid dropping cases, multiple imputation was employed to impute the missing moderators. Twenty datasets were imputed using the multiple imputation routine in SPSS 21.0. All MLM analyses were then performed on all 20 datasets. The results from these analyses were “pooled” statistically across the 20 datasets according to the appropriate algorithm in SPSS 21.0.
Results
Missing Data
Because MLM assumes that data is missing at random, we examined whether participants who had missing outcome data at some assessments differed from those for whom we had complete data. A MANOVA examining differences on our continuous measures at baseline (e.g., anxiety sensitivity, depressive symptoms, PTSD symptoms, age, etc.) showed no differences between those with and without missing data (p=.89). Similarly, Fisher Exact Tests showed that there were no differences between the groups on any of the baseline dichotomous measures (e.g., gender, ethnicity, cohabitation, etc.), ps>.21. Thus, there was no evidence that those with no missing data differed from those with missing data at baseline.
Predictor and Moderator Analyses for PTSD Outcome (PSS-I)
Below we report the statistics for all significant predictors identified in Step 4 of each domain, followed by statistics for the variables that remained significant in Step 4 of the final model. A number of complex interactions were significant in the analyses of separate domains but were no longer significant when combined with other variables in the final model. Since these interactions were non-significant when fully controlling for all other variables of interest, we present these interactions in Step 4 of each domain but do not discuss the direction of effects in detail until they are verified as significant in the final model.
Stepwise Analyses within each Domain
Demographics
The only variable from the Demographic domain that was significant in Step 4 was age. There was a significant PE × NAL × age interaction, b=-1.60, t(171)=2.44, p=.015 (as stated above, the form of interactions are only discussed if they are significant in the final model, and are only discussed under the “Final Model”). No other Demographic variables were significantly related to PSS-I.
Socio-economic Factors
Step 4 of the Socio-economic Factors domain showed that participants who were employed had lower PSS-I scores at post-treatment than those who were not employed, b=-2.20, t(157)= -3.96, p<.001. No other socio-economic variable was significantly related to PSS-I.
Comorbid Disorders
Step 4 of the Comorbid Disorders domain indicated that depressive symptoms moderated the PE × NAL × Time interaction, b=.55, t(115)=2.02, p=.043.
Trauma Features
Step 4 of the Trauma Features domain indicated that those who had trauma due to sexual assault improved more slowly during treatment than those who had other types of trauma, b=.79, t(135)=2.79, p=.005, and they had higher PSS-I at post-treatment, b=2.19, t(144)=2.51, p=.012. Step 4 of this domain also showed a significant combat trauma × PE × Time interaction, b=.62, t(138)=2.18, p=.029.
PTSD Features
Step 4 of the PTSD Features domain showed that higher anxiety sensitivity was related to slower rates of improvement in PSS-I over time, b=.78, t(594)=3.59, p<.001, and to higher post-treatment PSS-I scores, b=2.05, t(188)=3.45, p=.001. In addition, baseline PSS-I was a moderator of the PE × NAL × Time interaction, b=.47, t(603)=2.34, p=.019.
Alcohol Features
Step 4 of the Alcohol Features domain showed that those with higher baseline alcohol craving had higher post-treatment PSS-I scores, b=1.69, t(129)=3.29, p=.001. Also, Step 4 revealed a significant duration of AD × NAL × Time interaction, b=-.62, t(110)=-2.21, p=.027, along with a similar duration of AD × NAL interaction affecting PSS-I scores at post-treatment, b=-1.96, t(117)=-2.27, p=.024.
Final Model for PSS-I
The final model included the simultaneous entry of the predictor and moderator variables found to be significant in Step 4 of the previous sets of analyses (plus the treatment condition and Time variables, and their interactions). Results from the final model are presented in Table 2 and Figures 1 and 2.
Table 2. Predictors and moderators of PTSD outcome (PSS-I) in Step 4 and the Final Model.
| Post-Treatment Main Effects | Slope Effects | |||||
|---|---|---|---|---|---|---|
| Domain/Predictor | b | t | p | b | t | p |
|
|
||||||
| Demographic variables | ||||||
|
| ||||||
| Gender | 1.04 | 1.65 | .098 | - | - | - |
| Age | -.24 | -.36 | .716 | - | - | - |
| Age × NAL | -1.17 | -1.79 | .073 | - | - | - |
| Age × PE | .83 | 1.26 | .210 | - | - | - |
| Age × NAL × PE | -1.60 | -2.44 | .015 | - | - | - |
|
| ||||||
| Socio-economic factors | ||||||
|
| ||||||
| Employment | -2.20 | -3.96 | <.001 | - | - | - |
|
| ||||||
| Comorbid disorders | ||||||
|
| ||||||
| Depressive Symptoms (BDI) | 1.33 | 1.71 | .087 | -1.00 | -3.73 | <.001 |
| Depressive Symptoms (BDI) × NAL | -.19 | -.25 | .805 | -.07 | -.26 | .798 |
| Depressive Symptoms (BDI) × PE | -.80 | -1.00 | .323 | -.48 | -1.75 | .080 |
| Depressive Symptoms (BDI) × PE × NAL | 1.45 | 1.80 | .073 | .55 | 2.02 | .043 |
|
| ||||||
| Trauma features | ||||||
|
| ||||||
| Sexual Assault | 2.19 | 2.51 | .012 | .79 | 2.79 | .005 |
| Sexual Assault × PE | .75 | 1.28 | .202 | - | - | - |
| Combat | 1.71 | 1.93 | .054 | .46 | 1.60 | .109 |
| Combat × PE | 1.24 | 1.42 | .156 | .62 | 2.18 | .029 |
|
| ||||||
| PTSD features | ||||||
|
| ||||||
| Baseline PSS-I | 2.77 | 4.60 | <.001 | -1.54 | -7.20 | p<.001 |
| Baseline PSS-I × NAL | -.40 | -.71 | .479 | -.19 | -.95 | .341 |
| Baseline PSS-I × PE | -1.46 | -2.61 | .009 | -.50 | -2.52 | .012 |
| Baseline PSS-I × NAL × PE | 1.40 | 2.47 | .013 | .47 | 2.34 | .019 |
| Anxiety Sensitivity (ASI) | 2.05 | 3.45 | .001 | .78 | 3.59 | p<.001 |
|
| ||||||
| Alcohol features | ||||||
|
| ||||||
| Craving | 1.69 | 3.29 | .001 | - | - | - |
| Duration of AD | .20 | .23 | .816 | -.14 | -.48 | .633 |
| Duration of AD × NAL | -1.96 | -2.27 | .024 | -.62 | -2.21 | .027 |
|
| ||||||
| Final model predictors | ||||||
|
| ||||||
| Baseline PSS-I | 2.89 | 5.00 | <.001 | -1.50 | -7.08 | <.001 |
| Anxiety Sensitivity (ASI) | 2.13 | 3.78 | <.001 | .81 | 3.76 | <.001 |
| Duration of AD | -.38 | -.70 | .487 | -.18 | -.92 | .357 |
| Combat | 1.81 | 3.24 | .001 | .54 | 2.68 | .007 |
| Sexual Assault | 2.53 | 4.56 | <.001 | .75 | 3.71 | <.001 |
|
| ||||||
| Final model moderators | ||||||
|
| ||||||
| Baseline PSS-I × PE | -1.63 | -3.02 | .003 | -.54 | -2.76 | .006 |
| Duration of AD × NAL | -2.22 | -4.01 | <.001 | -.71 | -3.55 | <.001 |
Note. All variables were z-scored before computing interactions. Hence, main effects for all predictors/moderators reflected their effects for the mean of the present sample. ASI = Anxiety Sensitivity Index; AD = alcohol dependence; BDI = Beck Depression Inventory; NAL = naltrexone vs. placebo; PE = prolonged exposure therapy vs. supportive counseling; PTSD = post-traumatic stress disorder; PSS-I = PTSD Symptom Scale Interview
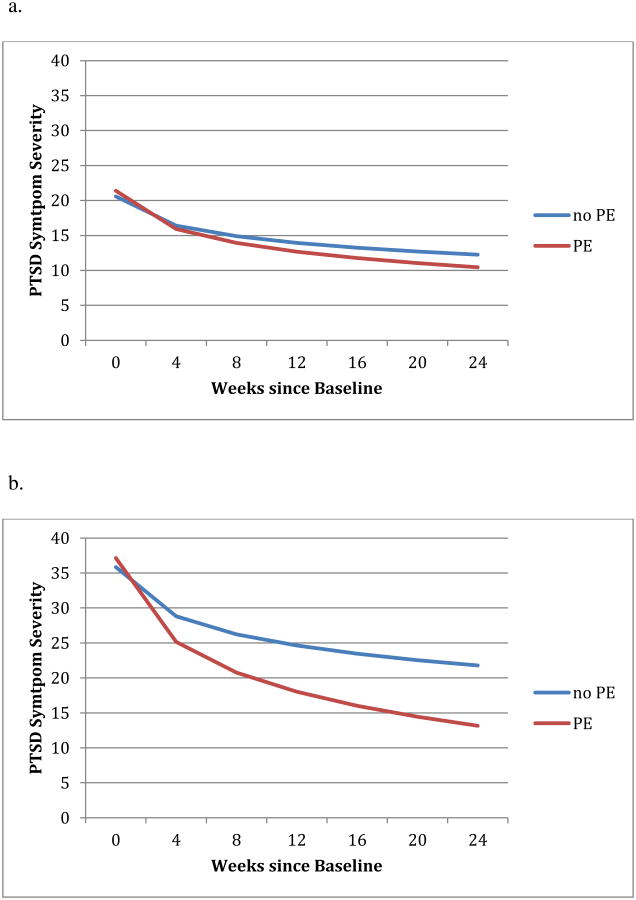
Figure 1. The effect of PE on PTSD outcome moderated by baseline PTSD severity.
a. Effect of PE on PSS-I for Low Baseline PSS-I (1 SD below mean)
b. Effect of PE on PSS-I for High Baseline PSS-I (1 SD above mean)
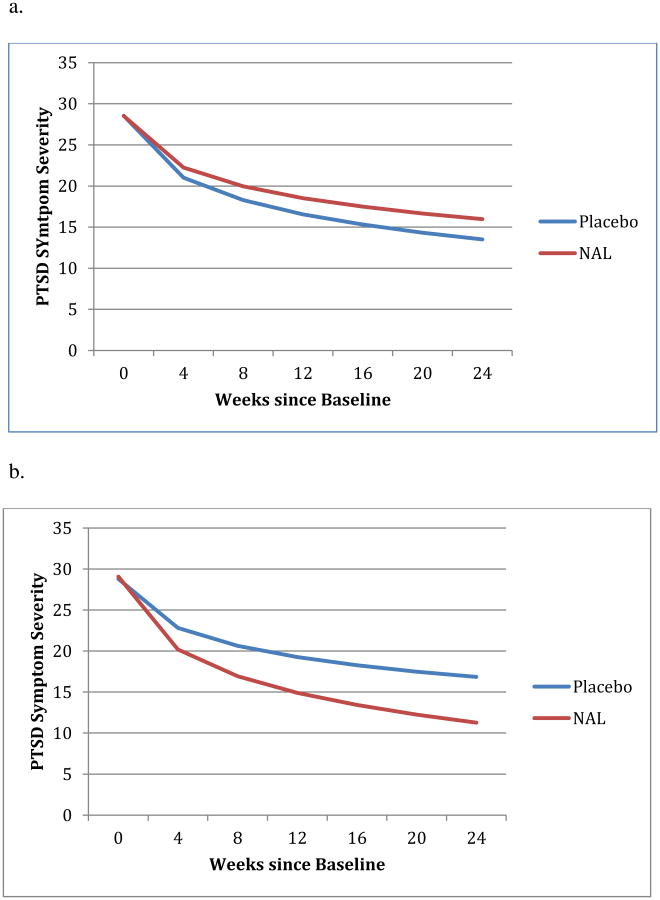
Figure 2. The effect of NAL on PTSD outcome moderated by duration of alcohol dependence.
a. The effect of NAL on PSS-I for participants with short duration of alcohol dependence
b. The effect of NAL on PSS-I for participants with long duration of alcohol dependence
Step 4 of the final model showed that participants receiving PE had faster rates of improvement and lower PSS-I at post-treatment than those not receiving PE, b=-.98, t(498)= -5.00, p<.001, and b=-2.65, t(154)=-4.92, p<.001, respectively. There were no significant effects for NAL (ps>.10) nor for the PE × NAL interaction (ps>.18).
Predictors
Three variables were significant predictors of PSS-I, regardless of treatment condition: sexual assault trauma, combat trauma, and baseline anxiety sensitivity. Sexual assault, combat trauma, and higher anxiety sensitivity were all associated with slower rates of improvement during treatment and with higher levels of PSS-I at post-treatment: for sexual assault trauma, b=.75, t(488)=3.71, p<.001 and b=2.53, t(146)=4.56, p<.001 for the slope and post-treatment effects, respectively; for combat trauma, b=.54, t(499)=2.68, p=.007 and b=1.81, t(155)=3.24, p=.001; and for anxiety sensitivity: b=.81, t(482)=3.76, p<.001 and b=2.13, t(144)=3.78, p<.001.
Moderators
Baseline PSS-I was a significant moderator of the effect of PE on both of the slopes of change over time, b=-.54, t(493)=-2.76, p=.006, and on outcome at post-treatment, b=-1.63, t(150)=-3.02, p=.003 (See Figure 1). Among participants who did not receive PE, participants with high baseline PSS-I (i.e., 1 SD above the mean, PSS-I=35.98) had much higher PSS-I at post-treatment, b=4.46, t(150)=5.22, p<.001, than those with low baseline PSS-I (i.e., 1 SD below the mean, PSS-I=20.3). However, among participants who did receive PE, those with high baseline PSS-I improved much faster than those with low baseline PSS-I, bΔ=2.06, t(493)=7.89, p<.001, such that they did not have statistically higher PSS-I at post-treatment than those with low baseline PSS-I, b=1.22, t(150)=1.71, p=.087. Another way to look at the moderating effects of baseline PSS-I is that PE did not have a significant effect on post-treatment PSS-I for those with low baseline PSS-I (PSS-I=20.3), b=-2.04, t(150)=-1.37, p=.171 (Figure 1a), but did confer significant benefit for those with high baseline PSS-I (PSS-I=35.98), b=-8.52, t(150)=-5.49, p<.001 (Figure 1b). Calculating the “region of significance” for the effect of PE (i.e., the range of PSS-I values for which PE has a significant effect), PE had a significant benefit on post-treatment outcome for participants with a baseline PSS-I score over 21.
Duration of AD moderated the effect of NAL on PSS-I, b=-.71, t(493)=-3.55, p<.001 for the AD duration × NAL × Time interaction, and b=-2.22, t(148)=-4.01, p<.001 for the AD duration × NAL interaction affecting post-treatment PSS-I (see Figure 2). For those with a shorter duration of AD (i.e., 1 SD below the mean, duration=2.35 years), NAL did not improve post-treatment outcome nor did it lead to faster improvement in PSS-I during treatment (see Figure 2a). But for participants with a longer history of AD (i.e., 1 SD above the mean, duration=24.37; Figure 2b), NAL significantly improved outcome at post-treatment, b=-3.09, t(148)=-4.09, p<.001 and significantly increased the rate of improvement during treatment, b=-1.00, t(148)=-3.63, p<.001.
Predictors and Moderators of Drinking Outcomes (Percentage Days Drinking; PDD)
The significant results from Step 4 of the Fournier procedure for each domain are presented in Table 3. In the text, we report only the significant results of Step 4 for each domain, and the results from the final model. The nature of the interactions between moderators and treatments are presented in the latter section if the finding retained significance in the final model.
Table 3. Predictors and moderators of drinking outcome (percent days drinking) in Step 4 and the Final Model.
| Post-Treatment Main Effects | Slope Effects | |||||
|---|---|---|---|---|---|---|
| Domain/Predictor | b | t | p | b | t | p |
|
|
||||||
| Demographic variables | ||||||
|
| ||||||
| Race (white vs. non-white) | .37 | 4.47 | <.001 | .33 | 3.33 | .001 |
|
| ||||||
| Socio-economic factors | ||||||
|
| ||||||
| Education | -.12 | -1.45 | .147 | - | - | - |
| Education × NAL | -1.49 | -1.84 | .066 | - | - | - |
| Education × PE | .13 | 1.66 | .096 | - | - | - |
|
| ||||||
| Comorbid disorders | ||||||
|
| ||||||
| Depressive Symptoms (BDI) | -.05 | -.65 | .517 | -.06 | -.64 | .521 |
| Depressive Symptoms (BDI) × PE | -.26 | -3.07 | .002 | -.33 | -3.32 | .001 |
|
| ||||||
| Trauma features | ||||||
|
| ||||||
| Sexual Assault | .08 | .84 | .400 | .09 | .79 | .428 |
| Sexual Assault × PE | .03 | .36 | .720 | - | - | - |
| Combat | .25 | 2.80 | .005 | .30 | .2.87 | .004 |
| Combat × PE | .16 | 1.87 | .061 | - | - | - |
| Other Trauma | .18 | 1.97 | .048 | .23 | 2.15 | .031 |
| Other Trauma × PE | .20 | 2.30 | .022 | - | - | - |
|
| ||||||
| PTSD features | ||||||
|
| ||||||
| Anxiety Sensitivity (ASI) | -.02 | -.19 | .846 | .03 | .32 | .751 |
| Anxiety Sensitivity × PE | -.19 | -2.08 | .040 | -.29 | -2.84 | .005 |
| Age of Trauma Onset | -.04 | -.27 | .785 | - | - | - |
| Age of Trauma Onset × NAL | -.31 | -2.35 | .019 | - | - | - |
| PTSD Duration | -.02 | -.18 | .861 | - | - | - |
| PTSD Duration × NAL | -.27 | -2.01 | .044 | - | - | - |
|
| ||||||
| Alcohol features | ||||||
|
| ||||||
| Craving | .12 | 1.50 | .136 | .19 | 1.93 | .054 |
| Craving × PE | -.20 | -2.53 | .012 | -.28 | -2.78 | .005 |
| Age of AD Onset | .05 | .61 | .541 | - | - | - |
| Age of AD Onset × NAL | -.08 | -1.05 | .296 | - | - | - |
| % Days Drinking at Baseline | .21 | 2.62 | .009 | -.22 | -2.25 | .024 |
|
| ||||||
| Final model predictors | ||||||
|
| ||||||
| % Days Drinking at Baseline | .23 | 3.03 | .002 | -.20 | -2.12 | .034 |
| Depressive Symptoms (BDI) | -.06 | -.77 | .440 | -.05 | -.55 | .581 |
| Sexual Assault | .08 | .93 | .351 | .10 | .93 | .353 |
| Combat | .24 | 2.86 | .004 | .24 | 2.27 | .023 |
| Other (General Trauma) | .11 | 1.31 | .192 | .14 | 1.28 | .202 |
| Race (white vs. non-white) | .32 | 4.12 | <.001 | .31 | 3.12 | .002 |
|
| ||||||
| Final model moderators | ||||||
|
| ||||||
| Depressive Symptoms (BDI) × PE | -.25 | -3.13 | .002 | -.31 | -3.10 | .002 |
Note. All variables were z-scored before computing interactions. Hence, main effects for all predictors/moderators reflected their effects for the mean of the present sample. ASI = Anxiety Sensitivity Index; AD = alcohol dependence; BDI = Beck Depression Inventory; NAL = naltrexone vs. placebo; PE = prolonged exposure therapy vs. supportive counseling; PTSD = post-traumatic stress disorder.
Stepwise Procedure within each Domain
Demographics
Step 4 of the Fournier analyses revealed that white participants reported slower improvement in PDD over time than minority participants, b=.33, t(870)=3.33, p=.001, and higher post-treatment PDD, b=.37, t(194)=4.47, p<.001.
Socio-economic Factors
None of the socioeconomic variables were significantly related to PDD.
Comorbid Disorders
In Step 4 of the Comorbid Disorders domain, depressive symptoms moderated the effect of PE on the rate of PDD reduction over time, b=-.33, t(740)=-3.32, p=.001, and on PDD at post-treatment, b=-.26, t(155)=-3.07, p=.002.
Trauma Features
Step 4 of the Trauma Features domain revealed that those with combat trauma had slower improvement in PDD over time, b=.30, t(865)=2.87, p=.004, and higher PDD at post, b=.25, t(195)=2.80, p=.005. “Other” trauma type was also associated with slower improvement over time, b=.23, t(862)=2.15, p=.031, and higher PDD at post-treatment, b=.18, t(188)=1.97, p=.048. Finally, “other” trauma type moderated the effect of PE on PDD at post-treatment, b=.20, t(188)=2.30, p=.022.
PTSD Features
In Step 4 of the PTSD Features domain, anxiety sensitivity was a significant moderator of the effect of PE on both the slope of PDD over time, b=-.29, t(672)=-2.84, p=.005, and on post-treatment PDD, b=-.19, t(143)=-2.08, p=.040. Step 4 also showed that age at which the trauma occurred and the amount of time since that trauma were both moderators of the effect of NAL on PDD at post-treatment, b=-.31, t(135)=-2.35, p=.019 and b=-.27, t(135)=-2.01, p=.044, respectively.
Alcohol Features
In Step 4, higher baseline PDD was related to faster improvement in PDD over time, b=-.22, t(730)=-2.25, p=.024, but to higher post-treatment PDD, b=.21, t(156)=2.62, p=.009. Step 4 also showed that baseline alcohol craving significantly moderated the effect of PE on both the rate of PDD reduction over time, b=-.28, t(731)=-2.78, p=.005, and on post-treatment PDD, b=-.20, t(157)=-2.53, p=.012.
Final Model for Percentage Days Drinking
Results from the final model are presented in Table 2 and Figures 1 and 2. Step 4 of the final model showed that participants given NAL had faster rates of improvement in PDD over the course of the treatment, b=-.07, t(587)=-2.10, p=.035, and lower PDD at post, b=-.28, t(129)=-3.53, p<.001. There were no significant effects for PE (ps>.410) nor was there a significant NAL × PE interaction (ps>.495).
Predictors
Three significant predictors of PDD were found. White race was associated with slower rates of improvement in PDD over time, b=.31, t(589)=3.12, p=.002, and to higher PDD at post-treatment, b=.32, t(128)=4.12, p<.001. Combat trauma was also related to slower rates of improvement in PDD over time, b=.24 t(584)=2.27, p=.023, and to higher post-treatment PDD, b=.24, t(126)=2.86, p=.004. Higher baseline PDD was related to faster rates of improvement over time, b=-.20, t(584)=-2.12, p<.034, but higher post-treatment PDD, b=.23, t(123)=3.03, p=.002.
Moderators
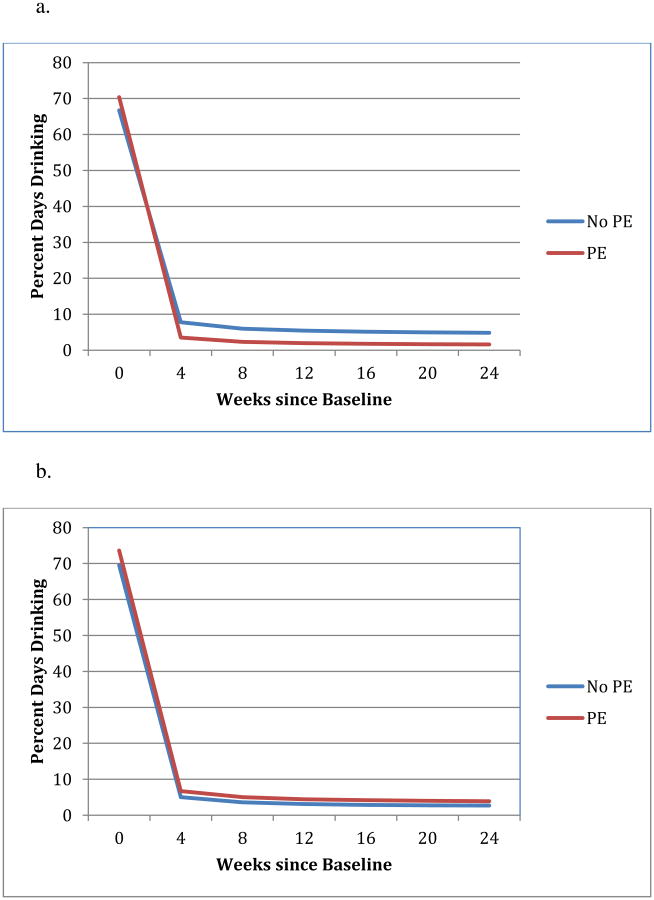
Baseline depressive symptom severity was a significant moderator of the effect of PE on both the slope of change over time, b=-.31, t(583)=-3.10, p=.002, and on PDD at post-treatment, b=-.25, t(126)=-3.13, p=.002 (Figure 3). To better understand the effect of depressive symptoms on PE, we again used the Aiken and West technique to estimate the predicted effect of PE on PDD at high levels of baseline depressive symptoms (for illustration, we used BDI=40, in the “severe depression” range) and the effect of PE on PDD for those with lower baseline depressive symptoms (for illustration, we used BDI=19, the high end of the range for “mild depression”). Participants with high baseline depressive symptoms (BDI=40) had significantly lower post-treatment PDD when provided PE than when not provided PE, b=-.40, t(126)=-2.99, p=.003. On the other hand, for participants with lower levels of depression (BDI=19), PDD did not differ for those in PE vs. no PE groups (p>.12). No other predictor/moderator variables retained significance in the final model.
Figure 3. The effect of PE on percent days drinking moderated by baseline depressive symptoms.
a. The effect of PE on PDD for participants with severe baseline depression (BDI = 40)
b. The effect of PE on PDD for participants with mild baseline depression (BDI = 19)
Post-hoc Analyses
We used pattern mixture modeling (see Hedeker and Gibbons, 2006; Enders, 2011) to determine if the predictor or moderator effects in the final models for PDD or PSS-I differed for dropouts compared to non-dropouts. These analyses found no significant interactions between dropout status and predictor/moderator effects.
Discussion
The present study examined predictors and moderators of symptom improvement during concurrent treatment of PTSD and alcohol dependence (AD), with prolonged exposure (PE) for PTSD and naltrexone (NAL) for alcohol dependence. Our aim was to identify baseline characteristics that can signal patients at risk of poor response regardless of treatment condition (i.e., predictors) and those that might be used guide treatment selection (i.e., moderators). Overall, provision of PE (compared to no-PE) was associated with faster improvement and lower PTSD severity at post-treatment. However, patients who received PE did not achieve better drinking outcomes than patients who received supportive counseling-only. Medication (NAL) was associated with faster reductions in drinking and better post-treatment drinking outcomes, but did not have a significant impact on PTSD outcomes relative to placebo. These findings support the overall efficacy of PE and NAL on the specific symptoms that they targeted.
Predictors of PTSD Outcome
Two characteristics were associated with slower improvement and poorer PTSD outcomes across treatment groups: trauma type and anxiety sensitivity. Participants with sexual assault and combat trauma showed slower improvement than those with other types of trauma (e.g., physical assaults; natural disasters; accidents) and had higher PTSD severity at post-treatment. This was true when controlling for co-occurring characteristics such as baseline PTSD symptoms, drinking severity, and age of trauma onset. Previous research has generally not found trauma type to impact treatment outcome; however, efficacy studies frequently focus on a single trauma population (e.g., all female assault survivors; all combat veterans) or may have limited variance regarding trauma type. It should be noted that there is a strong evidence supporting PE efficacy with female sexual assault survivors (e.g., Foa et al., 2005; Resick, Nishith, Weaver, Astin, & Feuer, 2002), and rigorous clinical studies have shown excellent response to PTSD treatment among combat veterans, with effect sizes comparable to civilian populations (e.g., Tuerk et al., 2011). Thus, the present results may reflect features specific to our co-morbid and predominantly male sample. Indeed, many studies of PE for sexual assault have included only women; whereas in the current study, 43% of patients reporting sexual assault were male. Future research should examine corresponding therapy processes in PTSD-AD treatment that may account for inferior outcomes among sexual assault and combat trauma survivors compared to other trauma types.
Higher anxiety sensitivity was associated with slower improvement and higher post-treatment PTSD symptoms across all conditions. This finding mirrors previous research showing correlations between anxiety sensitivity reduction and PTSD treatment outcome (Federoff et al. 2000). Anxiety sensitivity is defined as a fear of physical sensations associated with anxiety (Reiss et al., 1986) and thus can amplify the intensity of emotional reactions. To illustrate, a person with PTSD and high anxiety sensitivity may experience fear of trauma reminders compounded by fear of his/her own anxious reactions. In the treatment of PTSD, anxiety sensitivity could influence important treatment processes. For example, individuals with high anxiety sensitivity may be more avoidant and less willing to engage emotionally during exposure exercises. While patients with high ASI showed significant symptom change during treatment, they may benefit from a higher dose of treatment to reach comparable outcomes. In addition, it may be helpful to incorporate interceptive exposures or other treatments for anxiety sensitivity into trauma-focused treatment in order to improve outcomes for patients with high baseline ASI.
Several previously identified predictors of PTSD outcomes were not significant in the current study (e.g., gender, age, education level, depressive symptoms). Of note, age and depressive symptoms were both identified as predictors of PTSD outcomes in the initial model, but neither remained significant in the final model when controlling for co-occurring factors (e.g., anxiety sensitivity; initial PTSD severity). Discrepancies with previous studies could be due to differences in the treatment sample (PTSD-AD) or due to differences in statistical approach. The present analyses included a wide range of potential predictors, thus providing a relatively thorough array of control variables. Thus, the failure of age and depressive symptoms to be significantly related to PTSD in the final model here may be due to our more thorough control for alternative predictors.
Moderators of PTSD Outcomes
Two moderators of PTSD outcome were identified. First, baseline PTSD severity was found to moderate the efficacy of PE versus no-PE. Specifically, PE was superior to supportive counseling in reducing PTSD symptoms for participants with higher pre-treatment PTSD severity. Indeed, higher initial severity in the non-PE groups was associated with much higher PTSD severity at post-treatment; whereas, in the PE groups, this relationship was not found. Conversely, among those with lower baseline PTSD scores (i.e., PSS-I less than 21), PE was not superior to supportive counseling-only. This finding suggests that non-specific therapy factors (e.g., attention, support, alliance) may be sufficient to reduce PTSD severity in patients with mild PTSD symptoms. On the other hand, patients with moderate-to-severe PTSD symptoms show greater benefit from exposure-based treatments that specifically target trauma symptoms. If replicated, these results have great practical implications for matching patients to therapies. Given that therapists trained in exposure-based treatments with in community settings are often in short supply, the current findings might be used by organizations to most efficiently allocate clinical resources.
Second, duration of alcohol dependence moderated the efficacy of NAL versus placebo on PTSD outcomes. NAL enhanced the rate of PTSD improvement in patients with long alcohol dependence history (e.g., 24 years or more), but did not improve PTSD outcomes among those with a shorter duration of alcohol dependence (e.g., 2-3 years or less). This finding may be accounted for by reduction in alcohol use. Lower levels of drinking during treatment would be expected to improve PTSD outcomes, as drinking is hypothesized to be a means of avoiding trauma-related thoughts and, thus, may block emotionally processing of the trauma. Overall, those who received NAL in the current study exhibited faster decreases in drinking behavior and lower post-treatment alcohol use. A post-hoc comparison indicated that, among participants with longer alcohol duration, those receiving placebo had a higher number of drinking days than those on NAL (p< .05). While precise investigation of the mediating effect of within-treatment drinking is beyond the scope of the current paper, this an important issue to pursue in future research. For patients whose alcohol dependence is more entrenched, NAL may be particularly beneficial for enhancing PTSD treatment outcomes in concurrent alcohol dependence and PTSD treatment.
Predictors of Drinking Outcome
Three factors emerged as negative predictors of drinking outcomes across treatment conditions: higher baseline severity of drinking, combat trauma, and white race. Participants with higher baseline drinking showed poorer post-treatment drinking outcomes, but faster improvement in alcohol use during treatment. This suggests that with additional time, this subset of patients may achieve comparable outcomes, a hypothesis that would be beneficial to examine empirically. Baseline alcohol consumption has been identified as one of the most consistent predictors of alcohol dependence treatment outcome in previous research (Adamson et al., 2009).
Patients with combat-related trauma showed slower reductions in drinking behavior and poorer post-treatment drinking outcomes. Thus, in the present study, combat trauma emerged as a negative predictor of both PTSD and drinking outcomes. Prevalence of heavy drinking in military personnel is substantial (15-20%; Bray & Hourani, 2007) and significantly higher than that of age-matched civilian samples (Ames & Cunradi, 2004). It is possible that the dominant culture of alcohol use in the military might make this maladaptive coping strategy more resistant to change in combat-trauma survivors. The current results suggest that, compared to other trauma types, individuals with combat-trauma may require additional support or treatment modification to improve the efficacy of concurrent PTSD-AD treatment.
Finally, white race predicted poorer drinking outcomes in the current study. Large-scale epidemiological studies such as the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) show both a higher lifetime prevalence of alcohol dependence in individuals identifying as white compared to black or Hispanic, and a distinctly shorter average time from first drink to dependence trajectory (whites: 8 years; blacks and Hispanics: 16 years) (Lopez-Quintero et al., 2011). The current findings suggest that white patients may also be more resistant to treatment. This mirrors the results of one of the largest clinical trials of alcohol use treatments, Project MATCH (Project Match Research Group, 1997). Secondary analyses from this study found that, among those receiving treatment in an outpatient setting, white participants reported significantly lower rates of monthly abstinence relative to black participants at both six and twelve month follow-up (Tonigan, 2003).
Moderators of Drinking Outcome
Severity of depressive symptoms emerged as the only significant moderator of PE efficacy. Specifically, patients with higher baseline depression (i.e., in the moderate-severe range) had lower percentage days drinking at post-treatment if they received PE (versus no-PE). For those with lower depressive severity, receiving PE did not influence drinking at post-treatment. The effects of PE on drinking outcomes among patients with high baseline depression may have been mediated by reduction of depressive symptoms. Standard PE incorporates behavioral activation for patients with depression and has been shown to reduce depressive symptoms, guilt, and general anxiety in addition to PTSD symptoms (e.g., Keane, Marshall, & Taft, 2006; Rauch et al., 2010). Research strongly supports negative affect as a cue for alcohol cravings (e.g., Nosen et al., 2012; Sinha et al., 2009) and relapse (Greenfield et al., 1998; Marlatt & Gordon, 1980). Thus, it is plausible that PE promotes better drinking outcomes for patients with moderate-to-severe depressive symptoms by reducing negative affect. Additional research is required to evaluate this hypothesis.
Limitations
Several limitations should be noted. First, participants in the current study showed lower treatment attendance than is typical in PTSD treatment studies. Thirty two percent of the sample dropped out of the study, and the average number of sessions attended in PE was six out of a possible 18 sessions. Comparable rates of adherence to therapy have been found in other studies involving comorbid substance dependence and PTSD samples (e.g., Hien et al., 2009; Sannibale et al., 2013), suggesting that poor adherence to treatment is a challenge inherent to this population requiring additional study. Germane to the present results, however, MLM is capable of handling this level of missing data, producing unbiased estimates of regression coefficients in data sets with dropout as high as 90% (Hedeker & Gibbons, 2006). Moreover, no differences were found between dropouts and non-dropouts with respect to baseline characteristics, and pattern-mixture modeling revealed that predictor/moderator effects in the final model did not differ based on dropout status.
Second, due to safety concerns, participants in all four treatment conditions received supportive counseling. As such, it is difficult to isolate the separate contribution of this intervention or to assess the generalizability of the current findings to settings where adjunctive supportive counseling is not provided. This limitation is particularly relevant for moderator findings, as the inclusion of supportive counseling may have obscured potential moderators of the effectiveness of PE or NAL compared to a no-treatment group. Third, regarding NAL adherence, pill counts were used to determine medication compliance in the present study, and use of other methods (e.g., blood samples) in future studies would provide a more reliable measure of adherence. Conclusions regarding NAL effects are thus limited by this feature. Finally, some demographic features in the current sample were restricted. For example, only a small proportion of participants (6%) represented racial memberships other than white/Caucasian or black/African American, and – as such – we were limited in our ability to investigate more nuanced differences among racial or ethnic groups.
Conclusions
This study is the first to evaluate predictors and moderators of outcome in concurrent treatment for AD and PTSD. The non-specific predictors identified here – particularly combat trauma, anxiety sensitivity, and white race – warrant replication and additional research to better understand the mechanisms underlying poorer response to treatment. The present findings suggest that pre-treatment severity of PTSD, depressive symptoms, and duration of alcohol dependence may be useful in determining the most effective combination of therapies for this population. Specifically, concurrent trauma-focused exposure therapy appears to be of additive benefit to PTSD-AD patients who present with moderate or severe PTSD and moderate or severe depressive symptoms; while NAL confers most benefit for those with longer durations of alcohol dependence.
Public Impact.
This study suggests that, when treating comorbid PTSD and alcohol dependence, prolonged exposure therapy significantly improves PTSD outcomes for patients with moderate or severe PTSD symptoms.
Individuals with mild PTSD symptoms may not derive additional benefit from prolonged exposure compared to supportive counseling alone.
The opioid antagonist naltrexone showed greatest effects among patients with longer histories of alcohol dependence.
Acknowledgments
This study was funded by National Institute on Alcohol Abuse and Alcoholism grant R01 AA 012428 (PI: Dr. Foa). Dr. Foa received this grant support during the course of this study (09/19/01-1/31/09). She receives royalties from the sale of Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide, and Reclaiming your Life from a Traumatic Experience Workbook by Oxford University Press.
References
- Adamson SJ, Sellman JD, Frampton C. Patient predictors of alcohol treatment outcome: a systematic review. Journal of Substance Abuse Treatment. 2009;36:75–86. doi: 10.1016/j.jsat.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Ames G, Cunradi C. Alcohol use and preventing alcohol-related problems among young adults in the military. Alcohol Research & Health. 2004;28:252–257. [Google Scholar]
- Amir N, Taylor CT, Donohue MC. Predictors of response to an attention modification program in generalized social phobia. Journal of Consulting and Clinical Psychology. 2011;79:533–541. doi: 10.1037/a0023808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Anton RF. Naltrexone and cognitive behavioral therapy for the treatment of alcohol dependence: Do sex differences exist? Alcoholism: Clinical and Experimental Research. 2008;32:771–776. doi: 10.1111/j.1530-0277.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: Results from National Epidemiological Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;132:630–638. doi: 10.1016/j.drugalcdep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the united states department of defense health related behavior surveys, 1980-2005. Addiction. 2007;102:1092–1101. doi: 10.1111/j.1360-0443.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stout RL, Mueller T. Substance use disorder and posttraumatic stress disorder comorbidity: Addiction and psychiatric treatment rates. Psychology of Addictive Behaviors. 1999;13:115–122. [Google Scholar]
- Cahill S, Rothbaum B, Resick P, Follette V. Cognitive-behavioural therapy for adults. In: Foa E, Keane T, Friedman M, Cohen J, editors. Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies. New York, NY: Guilford Press; 2009. pp. 139–223. [Google Scholar]
- Chambless DL, Williams KE. A preliminary study of African Americans with agoraphobia: Symptom severity and outcome of treatment with in vivo exposure. Behavior Therapy. 1995;26:501–515. [Google Scholar]
- Difede J, Malta LS, Best S, Henn-Haase C, Metzler T, Bryant R, Marmar C. A randomized controlled clinical treatment trial for World Trade Center attack-related PTSD in disaster workers. Journal of Nervous and Mental Disease. 2007;195:861–865. doi: 10.1097/NMD.0b013e3181568612. [DOI] [PubMed] [Google Scholar]
- Enders CK. Analyzing longitudinal data with missing values. Rehabilitation Psychology. 2011;56:267–288. doi: 10.1037/a0025579. [DOI] [PubMed] [Google Scholar]
- Federoff IC, Taylor S, Asmundson GJ, Koch WJ. Cognitive factors in traumatic stress reactions: Predicting PTSD symptoms from anxiety sensitivity and beliefs about harmful events. Behavioural and Cognitive Psychotherapy. 2000;28:5–15. [Google Scholar]
- First MB, Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcoholism: Clinical and Experimental Research. 1999;23:1289–1295. [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Cahill SP, Rauch SAM, Riggs DS, Feeny NC, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. Journal of Consulting and Clinical Psychology. 2005;73:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. [Google Scholar]
- Foa EB, Riggs DS, Massie ED, Yarczower M. The impact of fear activation and anger on the efficacy of exposure treatment for posttraumatic stress disorder. Behavior Therapy. 1995;26:487–499. [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr, Oslin D, et al. Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA: Journal of the American Medical Association. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Hawthorne G, Allen N, McHugh T. Comorbidity as a predictor of symptom change after treatment in combat-related posttraumatic stress disorder. Journal of Nervous and Mental Disease. 2003;191:93–99. doi: 10.1097/01.NMD.0000051903.60517.98. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology. 2009;77:775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfried DR, Pettinati HM, Silverman BL, et al. Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA: Journal of the American Medical Association. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: A prospective study. Archives of General Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hagenaars MA, van Minnen A, Hoogduin KAL. The impact of dissociation and depression on the efficacy of prolonged exposure treatment for PTSD. Behaviour Research and Therapy. 2010;48:19–27. doi: 10.1016/j.brat.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hasin D, Samet S, Nunes E, Meydan J, Matseoane K, Waxman R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. The American Journal of Psychiatry. 2006;163:689–696. doi: 10.1176/ajp.2006.163.4.689. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability for substance abusers. The American Journal Of Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Hernandez-Avila C, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR. Targeted versus daily naltrexone: Secondary analysis of effects on average daily drinking. Alcoholism: Clinical and Experimental Research. 2006;30:860–865. doi: 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Hien DA, Wells EA, Jiang H, Suarez-Morales L, Campbell ANC, Cohen LR, et al. Nunes EV. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. Journal of Consulting and Clinical Psychology. 2009;77:607–619. doi: 10.1037/a0016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Treatment of PTSD: An assessment of the evidence. Washington, DC: Author; 2007. [Google Scholar]
- Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O'Malley SS. Naltrexone, relapse prevention, and supportive therapy with alcoholics: An analysis of patient treatment matching. Journal of Consulting and Clinical Psychology. 1996;64:1044–1053. doi: 10.1037//0022-006x.64.5.1044. [DOI] [PubMed] [Google Scholar]
- Karatzias A, Power K, McGoldrick T, Brown K, Buchanan R, Sharp D, Swanson V. Predicting treatment outcome on three measures of post-traumatic stress disorder. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:40–46. doi: 10.1007/s00406-006-0682-2. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: Etiology, epidemiology, and treatment outcome. Annual Review of Clinical Psychology. 2006;2:161–197. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61:4–14. [PubMed] [Google Scholar]
- Kiefer F, Helwig H, Tarnaske T, Otte C, Jahn H, Wiedemann K. Pharmacological relapse prevention of alcoholism: Clinical predictors of outcome. European Addiction Research. 2005;11:83–91. doi: 10.1159/000083037. [DOI] [PubMed] [Google Scholar]
- Kraemer H, Frank E, Kupfer D. Moderators of treatment outcomes: Clinical, research, and policy importance. Journal of the American Medical Association. 2006;296:1285–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clinical Psychology & Psychotherapy. 2010;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Sobell MB, Sobell LC. Reliability of self-reports of low ethanol consumption by problem drinkers over 18 months of follow-up. Drug and Alcohol Dependence. 1982;9:273–278. doi: 10.1016/0376-8716(82)90066-7. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. New York, NY: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York, NY: Guilford Press; 1991. [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, et al. Ewer PL. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized controlled trial. JAMA: Journal of the American Medical Association. 2012;308:690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Modell JG, Glaser FB, Mountz JM, Schmaltz S, Cyr L. Obsessive and compulsive characteristics of alcohol abuse and dependence: Quantification by a newly developed questionnaire. Alcoholism: Clinical and Experimental Research. 1992;16:266–271. doi: 10.1111/j.1530-0277.1992.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: The influence of craving and family history. The American Journal on Addictions. 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, et al. Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Morley KC, Teesson M, Sannibale C, Baillie A, Haber PS. Clinical predictors of outcome from an Australian pharmacological relapse prevention trial. Alcohol and Alcoholism. 2010;45:520–526. doi: 10.1093/alcalc/agq068. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Schmitz M, Gotthardt S, Weiss RD. Seeking safety plus exposure therapy: An outcome study on dual diagnosis men. Journal of Psychoactive Drugs. 2005;37:425–435. doi: 10.1080/02791072.2005.10399816. [DOI] [PubMed] [Google Scholar]
- Nosen E, Nillni YI, Berenz EC, Schumacher JA, Stasiewicz PR, Coffey SF. Cue-elicited affect and craving: Advancement of the conceptualization of craving in co-occurring posttraumatic stress disorder and alcohol dependence. Behavior Modification. 2012;36:808–833. doi: 10.1177/0145445512446741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette PC, Ahrens C, Moos RH, Finney JW. Posttraumatic stress disorder in substance abuse patients: Relationship to 1-year post treatment outcomes. Psychology of Addictive Behaviors. 1997;11:34–47. [Google Scholar]
- Ouimette PC, Brown PJ, Najavits LM. Course and treatment of patients with both substance use and posttraumatic stress disorders. Addictive Behaviors. 1998;23:785–796. doi: 10.1016/s0306-4603(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Ouimette PC, Goodwin E, Brown PJ. Health and well-being of substance use disorder patients with and without posttraumatic stress disorder. Addictive Behaviors. 2006;31:1415–1423. doi: 10.1016/j.addbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders. 2011;25:456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Gillihan SJ, Rosenfield D, Jerud AB, Foa EB. Reliability and validity of the PDS and PSS-I among participants with PTSD and alcohol dependence. Journal Of Anxiety Disorders. 2012;26:617–623. doi: 10.1016/j.janxdis.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Powers MB, Warren AM, Rosenfield D, Roden-Foreman K, Bennett M, Reynolds MC, Davis ML, Foreman ML, Petrey LB, Smits JAJ. Predictors of PTSD symptoms in adults admitted to a Level I trauma center: A prospective analysis. Journal of Anxiety Disorders. 2014;28:301–309. doi: 10.1016/j.janxdis.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Project Match Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Rauch SAM, Favorite T, Giardino N, Porcari C, Defever E, Liberzon I. Relationship between anxiety, depression, and health satisfaction among veterans with PTSD. Journal of Affective Disorders. 2010;121:165–168. doi: 10.1016/j.jad.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. Journal of Consulting and Clinical Psychology. 2002;70:867–879. doi: 10.1037//0022-006x.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SL, Vogt DS, Resick PA. Cognitive and affective predictors of treatment outcome in cognitive processing therapy and prolonged exposure for posttraumatic stress disorder. Behaviour Research and Therapy. 2009;47:737–743. doi: 10.1016/j.brat.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannibale C, Teesson M, Creamer M, Sitharthan T, Bryant RA, Sutherland K, Peek-O'Leary M. Randomized controlled trial of cognitive behaviour therapy for comorbid post-traumatic stress disorder and alcohol use disorders. Addiction. 2013;108:1397–1410. doi: 10.1111/add.12167. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Hofmann SG, Rosenfield D, DeBoer LB, Costa PT, Simon NM, et al. Pollack MH. D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: Prognostic and prescriptive variables. Journal of Consulting and Clinical Psychology. 2013;81:1100–1112. doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Standard errors and sample sizes in two-level research. Journal of Educational Statistics. 1993;18:237–260. [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. Timeline follow-back: A technique for assessing self- reported alcohol consumption; pp. 41–72. [Google Scholar]
- Tonigan JS. Project match treatment participation and outcome by self-reported ethnicity. Alcoholism: Clinical and Experimental Research. 2003;27:1340–1344. doi: 10.1097/01.ALC.0000080673.83739.F3. [DOI] [PubMed] [Google Scholar]
- Torrens M, Serrano D, Astals M, Pérez-Domínquez GS, Martín-Santos R. Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. The American Journal of Psychiatry. 2004;161:1231–1237. doi: 10.1176/appi.ajp.161.7.1231. [DOI] [PubMed] [Google Scholar]
- Starosta AN, Leeman RF, Volpicelli JR. The BRENDA model: Integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. Journal of Psychiatric Practice. 2006;12:80–89. doi: 10.1097/00131746-200603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrier N, Sommerfield C, Pilgrim H, Faragher B. Factors associated with outcome of cognitive-behavioural treatment of chronic post-traumatic stress disorder. Behaviour Research and Therapy. 2000;38:191–202. doi: 10.1016/s0005-7967(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Taylor S, Koch WJ, Crockett DJ. Anxiety sensitivity, trait anxiety, and the anxiety disorders. Journal of Anxiety Disorders. 1991;5:293–311. [Google Scholar]
- Taylor S, Koch WJ, McNally RJ. How does anxiety sensitivity vary across the anxiety disorders? Journal of Anxiety Disorders. 1992;6:249–259. [Google Scholar]
- Taylor S, Fedoroff IC, Koch WJ, Thordarson DS, Fecteau G, Nicki RM. Posttraumatic stress disorder arising after road traffic collisions: Patterns of response to cognitive-behavior therapy. Journal of Consulting and Clinical Psychology. 2001;69:541–551. [PubMed] [Google Scholar]
- Triffleman E. Gender differences in a controlled pilot study of psychosocial treatments in substance dependent patients with post-traumatic stress disorder: Design considerations and outcomes. Alcoholism Treatment Quarterly. 2000;18:113–126. [Google Scholar]
- Tuerk PW, Yoder M, Grubaugh A, Myrick H, Hamner M, Acierno R. Prolonged exposure therapy for combat-related posttraumatic stress disorder: An examination of treatment effectiveness for veterans of the wars in Afghanistan and Iraq. Journal of Anxiety Disorders. 2011;25:397–403. doi: 10.1016/j.janxdis.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Minnen A, Arntz A, Keijsers GPJ. Prolonged exposure in patients with chronic PTSD: Predictors of treatment outcome and dropout. Behaviour Research and Therapy. 2002;40:439–457. doi: 10.1016/s0005-7967(01)00024-9. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Clay KL, Watson NT, Volpicelli LA. Naltrexone and the treatment of alcohol dependence. Alcohol Health & Research World. 1995;18:272–278. [PMC free article] [PubMed] [Google Scholar]
- Vuoristo-Myllys S, Lipsanen J, Lahti J, Kalska H, Alho H. Outcome predictors for problem drinkers treated with combined cognitive behavioral therapy and naltrexone. The American Journal of Drug and Alcohol Abuse. 2014;40:103–110. doi: 10.3109/00952990.2013.853074. [DOI] [PubMed] [Google Scholar]