Abstract
Astrocytes regulate neuronal homeostasis and have been implicated in affecting the viability and functioning of surrounding neurons under stressed and injured conditions. Previous data from our lab suggests indirect actions of estrogen through ERα in neighboring astroglia to protect dopamine neurons against 1-methyl-4-phenylpyridinium (MPP+) toxicity in mouse mesencephalic cultures. We further evaluate estrogen signaling in astrocytes and the mechanism of estrogen’s indirect neuroprotective effects on dopamine neurons. Primary mesencephalic cultures pre-treated with 17β-estradiol and the membrane impermeable estrogen, E2-BSA, were both neuroprotective against MPP+ -induced dopamine neuron toxicity, suggesting membrane-initiated neuroprotection. ERα was found in the plasma membrane of astrocyte cultures and colocalized with the lipid raft marker, flotillin-1. A 17β-estradiol time course revealed a significant increase in Akt, which was inhibited by the PI3 kinase inhibitor, LY294004. Estrogen conditioned media collected from pure astrocyte cultures rescued glial deficient mesencephalic cultures from MPP+. This indirect estrogen-mediated neuroprotective effect in mesencephalic cultures was significantly reduced when PI3 kinase signaling in astrocytes was blocked prior to collecting estrogen-conditioned media using the irreversible PI3 kinase inhibitor, Wortmannin. Estrogen signaling via astrocytes is rapidly initiated at the membrane level and requires PI3 kinase signaling in order to protect primary mesencephalic dopamine neurons from MPP+ neurotoxicity.
Keywords: estrogen, neuroprotection, astrocytes, dopamine neurons
Introduction
Of all the non-neuronal cell types in the brain, astrocytes are the most abundant, outnumbering neurons 10:1 [23]. Astrocytes play a major role in glucose metabolism, extracellular ion homeostasis, synaptic transmission and neuroprotection and the disruption of neuron-astrocyte interactions result in abnormal CNS signaling and neuronal death [1, 2, 5, 6, 18]. The synthesis and secretion of soluble factors that are released into the extracellular space are the major neuroprotective mechanisms of astrocytes. During oxidative stress when neuronal glutathione (GSH) levels become depleted, astrocytes produce and release high levels of GSH, which act as antioxidants or increase the synthesis of neuronal GSH [12, 36]. In response to neuronal damage, astrocytes induce insulin-like growth factor I (IGF-I) expression and release transforming growth factor-beta1 (TGF-β1), thereby regulating neuronal survival [10, 15]. The up-regulation of glial cell line-derived neurotrophic factor (GDNF) following ischemic hypoxic injury and 6 hydroxydopamine insult is also well documented [27, 28]
Astrocytes also respond to the steroid, estrogen, causing the cells to modulate both the expression and release of various neurotrophic factors. Dhandapani et al. (2005) showed that estrogen treatment increased the expression and release of both TGF-β1 and TGF-β2 in cortical astrocytes through the phosphotidylinositol 3-kinase (PI3K)/Akt signaling pathway [11]. Such rapid signaling suggests actions by a membrane ER, which has been described in mouse midbrain astroglial cultures [26]. Similarly, Chaban et al. (2004) demonstrated that treating astrocytes with the membrane impermeable estrogen conjugate, E2-BSA, induces [Ca2+] flux, which is dependent on PLC/IP3 signaling [9].
The focus of this study was to investigate potential rapid estrogen membrane signaling in midbrain astrocytes and to determine whether these signaling mechanisms result in the previously observed neuroprotective actions of estrogen on DA neurons against 1-methyl-4-phenylpyridinium (MPP+) neurotoxicity. We utilize an in vitro model of neurodegeneration in which glial deficient primary mouse mesencephalic cultures were pretreated with 17β-estradiol directly or indirectly via estrogen-conditioned astrocyte media followed by exposure to the dopaminergic neurotoxin, MPP+. We report that estrogen indirectly protects DA neurons by a mechanism that involves ER-alpha (ERα)-mediated PI3K signaling in astrocytes and subsequent neuroprotection of DA neurons against MPP+ toxicity.
Material and Methods
Primary mesencephalic cultures
Animal procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio and were conducted in accordance with policies for the ethical treatment of animals established by the National Institutes of Health.
Progenitor cells were harvested from E13.5 embryos from time-mated C57BL/6 mice (Harlan Sprague-Dawley; Indianapolis, IN) as previously described [4]. The ventral mesencephalon was dissected as previously described by Studer (1997) [33]. Cells were plated directly onto poly-L-ornithine and fibronectin pretreated glass coverslips in 24-well plates. Cells were grown in chemically defined media and incubated at 37°C in 5% CO2 [4]. TH+ neurons represent 4–5% of the total number of neurons in culture, as determined by TH (1:1000, Novus Biologicals; Littleton, CO) vs. NeuN (1:1000 Chemicon; Temecula, CA) immunocytochemistry. For neuron-enriched cultures, mesencephalic cells were treated with the mitotic inhibitor, 5-Fluoro-2′-deoxyuridine (dFUR) at a concentration of 15 μg/mL beginning on day two for 48 hr. Neuronal enrichment was verified by immunocytochemistry using glial fibrillary acidic protein (GFAP) (1:200, Boehringer Mannheim Corp.; Indianapolis, Ind.), MAC-1 (1:500, Serotec, Raleigh; North Carolina) and TH (1:1000 Novus Biologicals; Littleton, CO). dFUR treatment reduced glial expression by >95% without affecting TH expressing neurons. All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Astroglial monolayers
Astroglial cultures were prepared from the midbrain of E13.5 embryos from time-mated C57BL/6 mice (Harlan Sprague-Dawley; Indianapolis, IN). A modified protocol from Ho and Blum (1997) was used for the preparation of astroglial cultures [19]. Tissues were incubated in 5 mL KRB containing 20 μL trypsin (Gibco/Invitrogen; Carlsbad, CA) for 5 min at 37°C, centrifuged (3000 × g, 10 min), decanted and triturated in 5 mL of calcium and magnesium-free KRB buffer containing 50 μL DNase (Gibco/Invitrogen). The triturated cells were centrifuged again, decanted and the pellet was resuspended in 20 mL Dulbecco’s minimal essential medium (Gibco/Invitrogen) supplemented with 10% fetal bovine serum (FBS). The cell suspension was filtered through a 70 μm nylon mesh screen (Falcon; Franklin Lakes, NJ). Cells were grown in 5% CO2 at 37°C. Confluent monolayers were shaken at 37°C for 2 hr at 250 rpm to remove microglia. The purity of the cells was assessed using antibodies directed against MAC-1 and GFAP for identification of microglia and astrocytes, respectively and MAC-1 positive cells represented less than 5% of the total cell population.
Chemicals and Treatments
17β-Estradiol or E2-BSA (~30 mol E2: mol BSA) were added to mesencephalic cultures for 24 hr, or at indicated times prior to MPP+ treatment and removed by replacing cultures with fresh media containing MPP+ for 24 hr. 1 μM ICI182,780 (Tocris; Ellisville, MO) was added to 17β-Estradiol conditioned astroglia media 1 hr prior to application of media on mesencephalic cultures. LY294004 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one] (20 μM) and Wortmannin (100 nM) were added to cultures 30 min before the addition of 17-β-estradiol. Stock solutions of MPP+ (0.1 M), 17-β-Estradiol (encapsulated; 1 mM), and dFUR (25 mg/mL) were dissolved in water, E2-BSA (10 μM) was dissolved in Tris-HCL, ICI182,780 was dissolved in ethanol at 1 mM and LY294002 and Wortmannin were dissolved in DMSO at 200 mM and 1mM respectively before use and diluted in defined media to the final concentrations. Control cultures received the appropriate amount of vehicle, which did not alter DA neuronal survival as measured by tyrosine hydroxylase (TH) immunocytochemistry. All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise stated.
Cell Fractionation and Gradient Analysis
Subcellular fractionation analysis was performed as previously described [20]. As a reference, an aliquot of the crude soluble/cytoplasmic fraction was resolved adjacent to the lipid raft fractions. Flotillin-1, a known lipid raft marker, served as a control to identify lipid raft containing fractions.
SDS-Page and Western Blotting
Basic Western blotting methods were followed as previously described [4] using the following primary antibodies: ERα (Santa Cruz Biotechnology, Santa Cruz, CA), Flotillin-1 (BD Transduction Laboratories, Lexington, KY), anti-pERK1/2 (Santa Cruz Biotechnology), total ERK1/2 (Santa Cruz Biotechnology), anti-pAKT (Santa Cruz Biotechnology), and total Akt (Santa Cruz Biotechnology). Uncalibrated, optical density measurements were made using NIH Image 1.62 (National Institutes of Health, Bethesda, MD, USA) from scanned films.
Tyrosine Hydroxylase Immunocytochemistry
TH immunocytochemistry methods were followed as previously described [4]. Stained cells were viewed on a Zeiss Axioplan 2 microscope and images captured using Axiovision imaging software (Carl Zeiss Inc.; Göttingen, Germany). TH-ir cells were manually counted with the assistance of Stereo Investigator’s meander scan function (MicroBrightField Inc.; Williston, VT) on the Zeiss Axioplan 2 microscope. The number of TH positive cells were counted from n=4 coverslips for each condition and each experiment was performed in triplicate.
Statistical analysis
Estrogen signaling and neuroprotective effects were analyzed using one-way ANOVA followed by Tukey-Kramer post-hoc comparisons. All statistical procedures were performed using GraphPad Prism v. 4 (GraphPad Software Inc., San Diego CA). In all tests, p<0.05 was defined as statistically significant.
Results
Estrogen-mediated neuroprotection of DA neurons involves cell membrane receptor activation
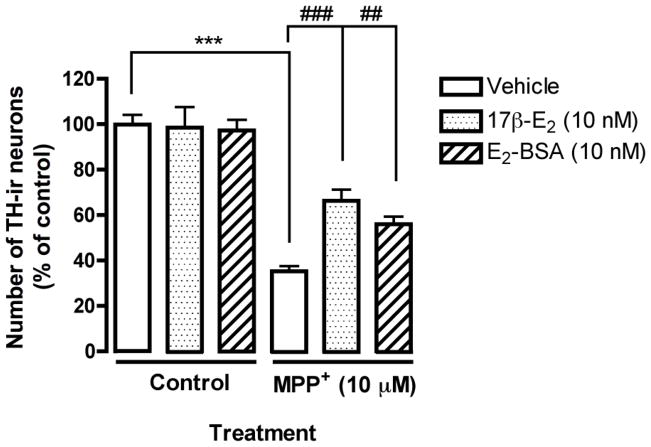
To examine membrane initiated neuroprotection, we treated primary mesencephalic neurons with 17β-estradiol and the membrane impermeable E2-BSA. The treatment paradigm included a 4 hr pre-treatment with 17β-estradiol or E2-BSA followed by MPP+ (10 μM) exposure for 24 hr. Primary cultures were then fixed and analyzed for TH immunoreactivity (TH-ir). MPP+ exposure induced a significant decrease in TH-ir cells, which was partially reversed by 17β-estradiol pre-treatment (Fig. 1, ***p<0.001 compared to vehicle control, and ###p<0.001 compared to MPP+ treatment). Pre-treatment with E2-BSA also showed significantly more TH-ir cells (Fig. 1, ##p<0.01 compared to MPP+ treatment).
Fig. 1.
Estrogen-mediated neuroprotection is initiated at the membrane level in mesencephalic cultures. Mouse mesencephalic cultures were pre-treated with 17β-estradiol, or E2-BSA prior to the addition of MPP+ (10μM). Results are presented as number of TH-ir cells, percent of control and represent the mean SEM of three experiments. ***p<0.001 compared to vehicle-treated control, ###p<0.001 and ##p<0.01 compared to MPP+ treatment.
ERα is expressed in the plasma membrane of astrocytes
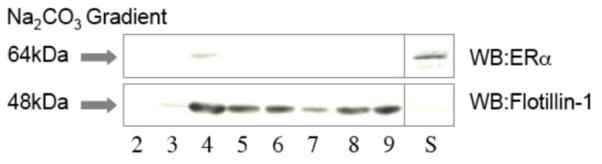
We have previously demonstrated ERα expression in both DA neurons and astrocytes using immunofluorescence [4]. Here we investigated plasma membrane ERα expression in midbrain astrocyte cultures (>95% pure) by Western blot as a possible initiator of an indirect neuroprotective signaling response. Cells were separated into nuclear, cytosol and membrane fractions and the crude membrane pellet was further analyzed by discontinuous (45/35/5%) sucrose density gradient centrifugation and detergent treatment as described in the methods to enrich the plasma membrane elements. Upon collection of the centrifuged fractions, individual membrane fractions were incubated with 1% Triton X-100 to solubilize non-lipid raft membrane proteins. Western blot analysis of the insoluble material of each of the nine fractions was performed (Fig. 2). ERα was detected within the lipid raft fractions (fractions 4 & 5) and co-fractionated with flotillin-1, a lipid raft marker.
Fig. 2.
Plasma membrane expression of ERα in pure astrocyte cultures. Western blot analysis of Na2CO3 sucrose density gradient fractions (2–9) insoluble in 1% Triton X-100. Insoluble product was collected by centrifugation, and resolved by SDS–PAGE and Western blot along with the crude soluble fraction (S). Flotillin-1 served as a control for fraction contamination and was appropriately expressed in fractions 4–6.
17β-estradiol activates the PI3K/Akt pathway in astrocytes
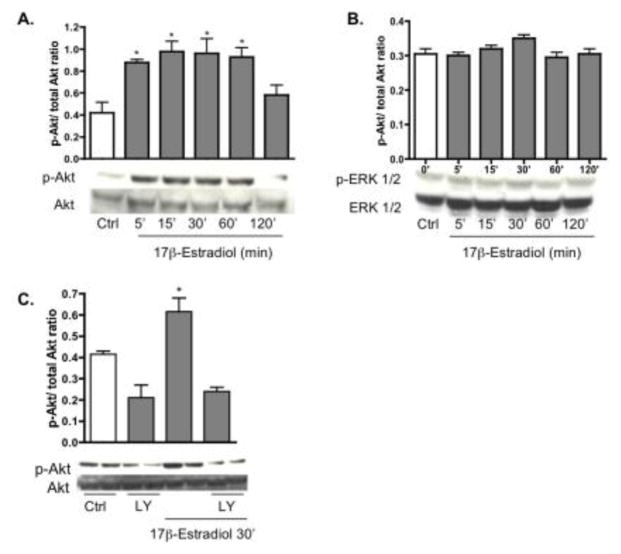
The mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) signaling cascades were analyzed for estrogen responsiveness. Pure astrocyte cultures were treated with 17β-estradiol (10 nM) at various time points (0, 5, 15, 30, 60 and 120 min) and protein was harvested for Western blot analysis of pAkt and pERK1/2. Activation of pAkt was detected at 5 min, which peaked at 30 min and returned to control levels by 120 min (Fig. 3A). pERK1/2, on the other hand, was not significantly altered by 17β-estradiol (Fig. 3B). Expression of total Akt and ERK1/2 was unchanged throughout the treatment (Fig. 3A,B). No significant change in expression level of p38 and JNK was observed suggesting that the neuroprotective effect of estrogen is MAPK-independent (data not shown).
Fig. 3.
17β-estradiol time dependently induces Akt, but not ERK1/2 phosphorylation. Astrocyte cultures were treated with 17β-estradiol (10 nM) for 0, 5, 15, 30, 60, and 120 min after which protein was collected. A: Western blot analysis of pure astrocyte cultures shows an increase in Akt phosphorylation starting at 5 min and extending through 60 min. B: 17β-estradiol did not significantly activate pERK1/2 at any of the time points. C: 17β-estradiol induced activation of pAkt at 30 min was blocked by co administration of the PI3K inhibitor LY294002. No change was observed in total AKT or total ERK1/2, which served as loading controls. Results are from a single experiment and are representative of at least three independent experiments. *p < 0.05 versus control astrocytes.
To determine whether the 17β-estradiol induced increase of pAkt was specific to PI3K activation, the PI3K inhibitor, LY294002 (20 μM) was applied alone or with 17β-estradiol (10 nM) for 30 min. Blockade of PI3K signaling completely inhibited 17β-estradiol activation of pAkt at 30 min (Fig. 3C). LY294002 alone also altered the baseline phosphorylation level of pAKT at 30 min, while total Akt remained the same.
Estrogen mediated neuroprotection of DA neurons requires PI3K signaling in mesencephalic cultures
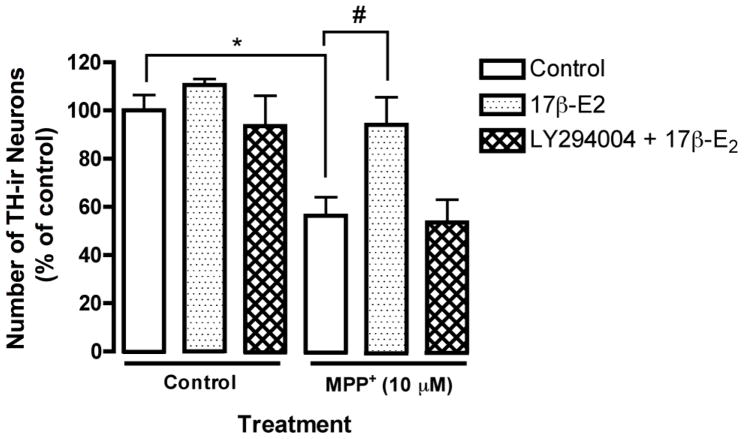
Mesencephalic cultures were treated with 17β-estradiol alone or in combination with LY294002 (20 μM) for 24 hr and then exposed to MPP+ (10 μM) for another 24 hr. As before, MPP+ exposure induced a significant decrease in the number of TH-ir neurons, which was reversed by 17β-estradiol pre-treatment (Fig. 4 *p< 0.05 compared to vehicle control and #p<0.05 compared to MPP+ treatment). Co administration of LY294002 with 17β-estradiol completely abolished estrogen’s neuroprotective effect against MPP+ toxicity, implicating a neuroprotective mechanism in these cultures that is dependent on PI3K signaling (Fig. 4 #p<0.05 compared to MPP+ treatment).
Fig. 4.
The neuroprotective effect of estrogen in mesencephalic cultures is PI3K dependent. Mouse mesencephalic cultures were pre-treated with 17β-estradiol (10 nM) and/or LY294002 for 24 hr prior to the addition of MPP+ (10μM) for another 24 hr. Results are presented as number of TH-ir cells, percent of control and represent the mean ± SEM of three experiments. *p<0.05 compared to vehicle-treated control, #p<0.05 compared to MPP+ treatment.
Estrogen conditioned astrocyte media protects glial deficient mesencephalic cultures from MPP+ toxicity
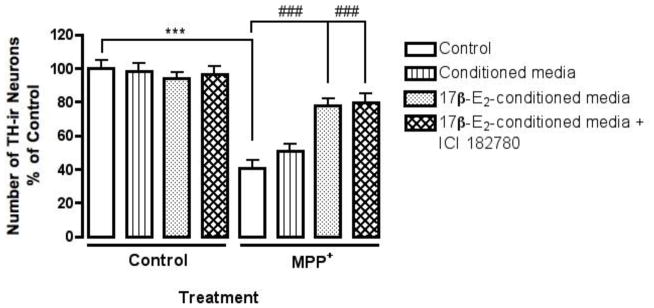
We treated glial deficient mesencephalic cultures with 17β-estradiol conditioned media collected from astrocyte cultures. 5-Fluoro-2′-deoxyuridine (dFUR), a mitotic inhibitor, was used to remove glial cells from primary mesencephalic cultures. We have shown previously that dFUR treated mesencephalic cultures are no longer responsive to 17β-estradiol as pre-treatment no longer protects against MPP+ toxicity [4]. Astrocyte cultures were exposed to 17β-estradiol or vehicle for 24 hr after which the media was collected and applied directly to dFUR treated mesencephalic cultures (Fig. 5). Cultures were incubated for 24 hr with conditioned media and then exposed to MPP+; however cultures were treated with a lower concentration of MPP+ (5μM) since in the absence of glia, the sensitivity to MPP+-induced toxicity increases (Bains et al., 2007). Vehicle conditioned astrocyte media did not protect against MPP+ toxicity and was similar to MPP+ treatment alone (Fig. 5 ***p< 0.001 compared to vehicle control). 17β-estradiol conditioned media significantly protect against MPP+ induced depletion of TH-ir neurons (Fig. 5 ###p<0.001 compared to MPP+ treatment), suggesting that estrogen modulates astrocytes resulting in the indirect protection of dopamine neurons.
Fig. 5.
Estrogen conditioned astrocyte media protects glial deficient mesencephalic cultures from MPP+ toxicity. dFUR treated mouse mesencephalic cultures were pre-treated with vehicle, 17β-estradiol conditioned astrocyte media or 17β-estradiol conditioned astrocyte media plus ICI 182,780 for 24 hr prior to the addition of MPP+ (5μM). ICI 182,780 was added to 17β-estradiol conditioned media 1 hr prior to adding the media to dFUR treated mesencephalic cultures. Results are presented as number of TH-ir cells, percent of control and represent the mean ± SEM of three experiments. ***p<0.001 compared to vehicle-treated control, and ###p<0.001 compared to MPP+ treatment.
To address a possible direct protective action of estrogen on dopamine neurons in conjunction with indirect signaling, we added the ER antagonist, ICI 182,780 to 17β-estradiol conditioned media collected from astrocyte cultures prior to treating dopamine neurons. Mesencephalic cultures were then incubated for 24 hr with the conditioned media and exposed to MPP+ as previously described. ICI 182,780 treated 17β-estradiol conditioned medium did not inhibit or reduce the observed protective effect of 17β-estradiol conditioned media against MPP+ toxicity (Fig. 5 ###p<0.01 compared to MPP+ treatment), suggesting that estrogen-mediated neuroprotection of DA neurons is completely indirect.
Estrogen mediated neuroprotection of DA neurons involves PI3K signaling in astrocytes
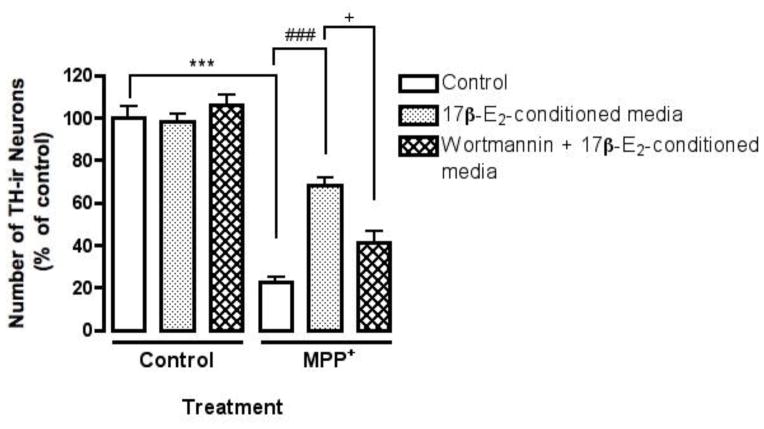
Since estrogen-mediated neuroprotection was indirect, we wanted to determine if the previously observed PI3K dependent neuroprotective effect in mesencephalic cultures required PI3K signaling in astrocytes. We utilized another PI3K inhibitor, Wortmannin, which unlike LY294002, irreversibly blocks PI3-kinase by covalent modification of Lys 802 of the p110 catalytic subunit. Wortmannin (100 nM) was added to primary astrocyte cultures for one hour after which the media was replaced with fresh media for 1 hr to wash out unbound Wortmannin. 17β-estradiol (10 nM) was then added to the astrocyte cultures for 24 hr and the media was then collected and applied directly to the dFUR treated mesencephalic cultures. Control experiments measuring pAKT levels in astrocyte cultures confirmed Wortmannin dependent inhibition of PI3K signaling in the presence of 17β-estradiol (data not shown). As previously observed, 17β-estradiol conditioned media significantly protected against MPP+ induced loss of TH-ir neurons (Fig. 6 ###p<0.001 compared to MPP+ treatment). Inhibiting PI3K signaling in astrocytes by application of Wortmannin (100 nM) significantly blocked the observed protective effect of 17β-estradiol conditioned media against MPP+ toxicity (Fig. 6 +p<0.05 compared to estrogen-conditioned media treatment).
Fig. 6.
The neuroprotective effect of estrogen in mesencephalic cultures involves PI3K signaling via astrocytes. dFUR treated mouse mesencephalic cultures were pre-treated for 24 hr with 17β-estradiol conditioned astrocyte media, or 17β-estradiol conditioned astrocyte media plus Wortmannin (100 nM). MPP+ (5μM) was added for 24 hr. Wortmannin was applied to astrocyte cultures for 1 hr, washed out with fresh media for another hour and then cultures were treated with 17β-estradiol. Results are presented as number of TH-ir cells expressed as percent of control and represent the mean± SEM of three experiments. ***p<0.001 compared to vehicle-treated control, ###p<0.05 and +p<0.001 compared to MPP+ treatment.
To control for unbound Wortmannin contamination in collected astrocyte conditioned media, separate astrocyte cultures were incubated in astrocyte conditioned media that had been collected from astrocytes treated with Wortmannin using the treatment paradigm described above. These astrocyte cultures were then treated with 17β-estradiol (10 nM) to analyze Akt phosphorylation at 30 min. Western blot analysis revealed that pAkt was significantly elevated in astrocytes stimulated with 17β-estradiol (10 nM) in media collected from both vehicle and Wortmannin treated astrocyte cultures, confirming that Wortmannin was appropriately washed out prior to application of 17β-estradiol (10 nM) conditioned media to mesencephalic cultures (data not shown).
Discussion
The aim of this study was to further examine estrogen signaling in astrocytes and to determine whether the astrocyte signaling pathways responsive to estrogen mediated the neuroprotection of DA neurons against MPP+ neurotoxicity. These studies were proposed in response to data collected in primary mesencephalic cultures, which demonstrated the requirement of astroglia in 17β-estradiol mediated neuroprotection of DA neurons. We show that estrogen modulates astrocytes through a mechanism that involves rapid membrane ER initiated PI3K/Akt signaling and that this signaling must be initiated first to elicit neuroprotection against MPP+.
Our results support previous findings of indirect estrogen-mediated neuroprotection via astrocyte signaling, which have been similarly investigated in models of camptothecin and beta-amyloid (Aβ)-induced neuronal death in cortical cultures [11, 29]. We have extended these studies to include estrogen signaling through astrocytes as a neuroprotective mechanism against MPP+ induced DA neuronal death in primary mesencephalic cultures. Similar to Dhandapani et al. (2005), activation of the PI3K/Akt pathway by estrogen was necessary to elicit neuroprotection as inhibition of PI3K signaling via LY294002 completely prevented estrogen-induced protection, which also suggested a membrane-initiated mechanism [11]. LY294002 has previously been reported to demonstrate anti-estrogenic effects by binding directly to the ER and inhibiting 17β-estradiol-mediated transactivation of reporter genes [25]. Results obtained using LY294002 to confirm estradiol-mediated PI3K signaling could be misinterpreted if LY294002 is indeed binding to the ER to inhibit estradiol signaling. This is unlikely the case in our model as 17β-estradiol and PI3K-mediated neuroprotection of DA neurons from MPP+ toxicity was confirmed using Wortmannin.
ERα and ERβ expression has been reported in astrocytes by several investigators [3, 7, 30, 31, 34] including membrane ER expression, further supporting the idea of non-classical estrogen signaling [9]. We show that plasma membrane ERα co localized with the lipid raft marker, flotillin-1 by sucrose density analysis in mesencephalic astrocytes. ERα may associate with lipid rafts in astrocytes to concentrate the ER for signal transduction activation. ERβ was not further investigated as our earlier work, using ERβ specific agonists, suggested that it was not involved in the neuroprotection from MPP+ and it’s expression in cultured astrocytes was very low [4]. Astrocyte expression of the G protein-coupled estrogen receptor (GPR30) has been previously observed in primary rat astrocyte cultures [21]. Activation of GPR30 in response to estrogen increased glutamate transporter (GLT-1 and GLAST) expression and glutamate uptake. Although we demonstrated ERα expression in the lipid rafts of primary mouse astrocyte cultures, we have not ruled out the expression and activity of GPR30 in these cultures.
A caveat in utilizing embryonic or postnatal brain primary cultures for estrogen neuroprotection studies is that they represent the developing brain, raising the issue of differences in ER expression levels in the developing versus adult brain. However, alterations in ER expression levels during central nervous system (CNS) damage are well documented. Kanic acid treatment and stab-wound lesions in the rat hippocampus induces ERα expression in astrocytes [16]. Similarly, middle cerebral artery occlusion produced a phasic response of the ER, which included an early induction of ERα in the cerebral cortex followed by a late phase induction of ERβ [13]. Thus, regions of the adult brain, which under normal conditions express low to undetectable levels of ER, remain responsive to estrogen and may be up-regulated under conditions of neuronal damage.
Secretion of estrogen-regulated astrocyte-derived soluble factors may be responsible for the observed neuroprotection of DA neurons against MPP+ toxicity. Both Sortino et al (2004) and Dhandapani et al (2005) demonstrated using neutralizing antibodies that estrogen neuroprotective effects occurred via the release of astrocyte-derived transforming growth factor-beta (TGF-β1) [11, 29]. Other likely candidates previously demonstrated to be regulated by estrogen actions on astrocytes include insulin like growth factor-1 (IGF-1), GDNF and apolipoprotein E (ApoE) [8, 14, 27, 28, 32]. In addition to trophic factors, estrogen may regulate the release of antioxidants. A synergistic antioxidant effect of low estrogen treatment and GSH has been observed in SK-N-SH human neuroblastoma cells as well as spinal motor neurons [17, 24]. Furthermore, estrogen has been shown to modulate astrocytes via the down-regulation of pro-inflammatory cytokines and up-regulation of glutamate transporters [22, 31]. The PI3K-Akt pathway has been previously shown to regulate the expression of TGF-β1, TGF-β1 and GDNF in astrocyte and rat glioma cultures [11, 35]. The mechanism by which the observed PI3K-depdenent astrocyte-estrogen signaling protected DA neurons is likely a combination of regulating the expression of trophic factors, pro-inflammatory mediators, glutamate regulation and/or antioxidant mechanisms.
Here we demonstrated that estrogen mediated dopamine neuroprotection is indirect, membrane initiated, ERα and PI3 kinase dependent and requires pre-exposure to estrogen prior to inducing neuronal damage. Our data support two separate, but linked estrogen-regulated pathways, which collectively provide neuroprotection of DA neurons against MPP+ toxicity. The age-related decline in estrogen and neurotrophic factor expression including astrocytic specific expression of trophic factors leads to diminished endogenous neuroprotective mechanisms and the subsequent acceleration of late stages of neurodegenerative disease. Thus, studies directed towards potentiation of such astrocyte-estrogen indirect pathways may advance our understanding of estrogen’s neuroprotective mechanisms and possibly identify new therapeutic approaches for the treatment of DA neurodegeneration in Parkinson’s Disease.
Highlights.
Estrogen receptor alpha is expressed in the membrane of midbrain astrocyte cultures
Estrogen rapidly actives PI3 kinase signaling in mouse midbrain astrocyte cultures
Estrogen neuroprotection of neurons requires PI3 kinase signaling in astrocytes
Estrogen neuroprotection of neurons is indirect through estrogen action in astrocytes
Acknowledgments
This work was supported by NIH grants AG-08538, NS-42080 and the Duvosin Award from the American Parkinson’s Disease Foundation to JLR. We would like to thank Yollanda V. Acosta for technical help and Dr. Nathan Jeske for training in lipid raft isolation.
Abbreviations
- ERα
estrogen receptor alpha
- GSH
glutathione
- IGF-1
insulin-like growth factor 1
- TGF-β1
transforming growth factor beta 1
- ApoE
apolipoprotein
- GDNF
glial cell line-derived neurotrophic factor
- MPP+
1-methyl-4-phenylpyridinium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci. 2003;23:5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- 4.Bains M, Cousins JC, Roberts JL. Neuroprotection by estrogen against MPP+-induced dopamine neuron death is mediated by ERalpha in primary cultures of mouse mesencephalon. Exp Neurol. 2007;204:767–776. doi: 10.1016/j.expneurol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- 6.Blanc EM, Bruce-Keller AJ, Mattson MP. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J Neurochem. 1998;70:958–970. doi: 10.1046/j.1471-4159.1998.70030958.x. [DOI] [PubMed] [Google Scholar]
- 7.Blurton-Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol. 2001;433:115–123. doi: 10.1002/cne.1129. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan CD, Mahesh VB, Brann DW. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1) Biol Reprod. 2000;62:1710–1721. doi: 10.1095/biolreprod62.6.1710. [DOI] [PubMed] [Google Scholar]
- 9.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 10.Dhandapani KM, Hadman M, De Sevilla L, Wade MF, Mahesh VB, Brann DW. Astrocyte protection of neurons: role of transforming growth factor-beta signaling via a c-Jun-AP-1 protective pathway. J Biol Chem. 2003;278:43329–43339. doi: 10.1074/jbc.M305835200. [DOI] [PubMed] [Google Scholar]
- 11.Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 12.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 13.Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 14.Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59:528–538. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Estrada J, Garcia-Segura LM, Torres-Aleman I. Expression of insulin-like growth factor I by astrocytes in response to injury. Brain Res. 1992;592:343–347. doi: 10.1016/0006-8993(92)91695-b. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Ovejero D, Veiga S, Garcia-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 17.Gridley KE, Green PS, Simpkins JW. A novel, synergistic interaction between 17 beta-estradiol and glutathione in the protection of neurons against beta-amyloid 25-35-induced toxicity in vitro. Mol Pharmacol. 1998;54:874–880. doi: 10.1124/mol.54.5.874. [DOI] [PubMed] [Google Scholar]
- 18.Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- 19.Ho A, Blum M. Regulation of astroglial-derived dopaminergic neurotrophic factors by interleukin-1 beta in the striatum of young and middle-aged mice. Exp Neurol. 1997;148:348–359. doi: 10.1006/exnr.1997.6659. [DOI] [PubMed] [Google Scholar]
- 20.Jeske NA, Glucksman MJ, Roberts JL. Metalloendopeptidase EC3.4.24.15 is constitutively released from the exofacial leaflet of lipid rafts in GT1-7 cells. J Neurochem. 2004;90:819–828. doi: 10.1111/j.1471-4159.2004.02557.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Lee L, Aschner M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem. 2012;287:26817–26828. doi: 10.1074/jbc.M112.341867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. J Neurochem. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Nakamizo T, Urushitani M, Inoue R, Shinohara A, Sawada H, Honda K, Kihara T, Akaike A, Shimohama S. Protection of cultured spinal motor neurons by estradiol. Neuroreport. 2000;11:3493–3497. doi: 10.1097/00001756-200011090-00019. [DOI] [PubMed] [Google Scholar]
- 24.Pasapera Limon AM, Herrera-Munoz J, Gutierrez-Sagal R, Ulloa-Aguirre A. The phosphatidylinositol 3-kinase inhibitor LY294002 binds the estrogen receptor and inhibits 17beta-estradiol-induced transcriptional activity of an estrogen sensitive reporter gene. Mol Cell Endocrinol. 2003;200:199–202. doi: 10.1016/s0303-7207(02)00421-5. [DOI] [PubMed] [Google Scholar]
- 25.Pawlak J, Karolczak M, Krust A, Chambon P, Beyer C. Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia. 2005;50:270–275. doi: 10.1002/glia.20162. [DOI] [PubMed] [Google Scholar]
- 26.Saavedra A, Baltazar G, Santos P, Carvalho CM, Duarte EP. Selective injury to dopaminergic neurons up-regulates GDNF in substantia nigra postnatal cell cultures: role of neuron-glia crosstalk. Neurobiol Dis. 2006;23:533–542. doi: 10.1016/j.nbd.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Dis. 2009;33:405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Sortino MA, Chisari M, Merlo S, Vancheri C, Caruso M, Nicoletti F, Canonico PL, Copani A. Glia mediates the neuroprotective action of estradiol on beta-amyloid-induced neuronal death. Endocrinology. 2004;145:5080–5086. doi: 10.1210/en.2004-0973. [DOI] [PubMed] [Google Scholar]
- 29.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci. 2013;33:10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Struble RG, Nathan BP, Cady C, Cheng X, McAsey M. Estradiol regulation of astroglia and apolipoprotein E: an important role in neuronal regeneration. Exp Gerontol. 2007;42:54–63. doi: 10.1016/j.exger.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Studer L. Culture of substantia nigra neurons. Curr Protoc Neurosci. 2001;Chapter 3(Unit 3):3. doi: 10.1002/0471142301.ns0303s00. [DOI] [PubMed] [Google Scholar]
- 34.Su JD, Qiu J, Zhong YP, Chen YZ. Expression of estrogen receptor -alpha and -beta immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. Neuroreport. 2001;12:1955–1959. doi: 10.1097/00001756-200107030-00036. [DOI] [PubMed] [Google Scholar]
- 35.Tanabea K, Natsushima-Nishiwaki R, Iida M, Kozawa O, Iida H. Involvement of phosphatidylinositol 3-kinase/Akt on basic fibroblast growth factor-induced glial cell line-derived neurotrophic factor release from rat glioma cells. Brain Res. 2012;1627:41–51. doi: 10.1016/j.brainres.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 36.Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]