Abstract
Neurosteroid sexert diverse modulatory actions on dopamine neurotransmission and signaling. We previously documented that the enzyme 5α-reductase, which catalyzes the main rate-limiting step in neurosteroid synthesis, is required for the behavioral responses of Sprague-Dawley rats to non-selective dopaminergic agonists, such as the D1–D2 receptor agonist apomorphine. Specifically, systemic and intra-accumbal administrations of the 5α-reductase inhibitor finasteride countered apomorphine-induced deficits of sensorimotor gating, as measured by the prepulse inhibition (PPI) of the startle reflex; the classes of dopamine receptors involved in these effects, however, remain unknown. Prior rodent studies have revealed that the contributions of dopamine receptors to PPI regulation vary depending on the genetic background; thus, we analyzed the effect of finasteride on the PPI deficits induced by selective dopamine receptor agonists in Long-Evans (a strain exhibiting PPI deficits in response to both D1 and D2 receptor agonists) and Sprague-Dawley rats (which display PPI reductions following treatment with D2, and D3, but not D1 receptor agonists). In Long-Evans rats, finasteride opposed the PPI deficits induced by activation of D1, but not D2 receptors; conversely, in Sprague-Dawley rats, finasteride prevented the reductions in %PPI and accumbaldopamine extracellular levels caused by selective stimulation of D3, but not D2 receptors; however, the effects on %PPI were not confirmed by analyses on absolute PPI values. Our findings suggest that 5α-reductase modulates the effects of D1, but not D2 receptor agonists on sensorimotor gating. These data may help elucidate the role of neurosteroids in neuropsychiatric disorders featuring PPI deficits, including schizophrenia and Tourette syndrome.
Keywords: Finasteride, 5α-reductase, dopamine, neurosteroids, prepulse inhibition of the startle
1. Introduction
The prepulse inhibition (PPI) of the acoustic startle reflex is one of the best-validated parameters to measure sensorimotor gating, namely the suppression of a motor response by a sensory stimulus. This endophenotype, consisting in the reduction of the startle response triggered by a weak pre-stimulus immediately preceding the startle-eliciting burst (Hoffman and Ison, 1980), has garnered substantial interest in neuropsychiatric and behavioral research. Indeed, PPI deficits have been observed in several disorders, including schizophrenia and Tourette syndrome (Braff et al., 2001); furthermore, the accessibility of PPI as a valid testing paradigm for humans and experimental animals makes it particularly attractive for translational studies (Swerdlow et al., 1999).
Dopamine plays a key role in the orchestration of PPI. Rich evidence has shown that dopaminergic agonists produce robust PPI deficits in rats and mice (Geyer et al., 2001); the specific contribution of dopaminergic receptors to the modulation of sensorimotor gating, however, varies across different rodent models. In Sprague-Dawley (SD) albino rats, PPI deficits are elicited by agonists for dopamine D2 and D3, but not D1 receptors (Peng et al., 1990; Bristow et al., 1996); nevertheless, D1 receptor activation has been shown to be directly involved in the PPI deficits induced by non-specific dopaminergic agonists, such as apomorphine (APO) (Hoffman and Donovan, 1994). Conversely, we recently found that the selective and independent activation of D1 and D2 receptors produces PPI deficits in the hooded Long-Evans (LE) strain (Mosher et al., 2015).
We previously showed that, in SD rats, the PPI deficits induced by non-selective dopaminergic agonists are countered by inhibition of 5α-reductase (5αR) (Bortolato et al., 2008), the enzyme catalyzing the rate-limiting step in neurosteroid synthesis (i.e. the irreversible saturation of the 4,5 double bond of the A ring of Δ4-3 ketosteroids such as pregnenolone and progesterone) (Martini et al., 1993). Accordingly, systemic and intra-accumbal injections of the selective 5αR inhibitor finasteride (FIN) attenuates the PPI deficits induced by non-selective dopaminergic agonists in SD rats (Bortolato et al., 2008) In parallel with these preclinical results, preliminary results collected by our group suggest that FIN may have elicited therapeutic properties in patients affected by chronic schizophrenia (Koethe et al., 2008) and Tourette syndrome (Bortolato et al., 2007; Muroni et al., 2011). Notably, the anti-dopaminergic effects of FIN and other 5αR blockers were not accompanied by extrapyramidal side effects. While these premises point to these agents as promising options for the therapy of these neuropsychiatric conditions, the specific involvement of dopamine receptors in the PPI-enhancing effects of FIN remains elusive. Based on this background, in the present study we investigated the specific contributions of different dopamine receptor subtypes on sensorimotor gating using SD and LE rats.
2. Material and Methods
2.1. Animals
A total of 390 male SD and 102 LE rats (Harlan; Milan, Italy and Indianapolis, IN) weighing between 250 and 350 g were used for these experiments. Animals were group-housed in cages (n=3–4) with ad libitum access to food and water. Rooms were maintained at 22±0.2°C on reversed 12-hr light/dark cycle (with lights off at 07:00 PM). Each animal was used only once throughout the study and all efforts were made to minimize animal suffering. PPI and microdialysis studies occurred between 11:00 AM and 5:00 PM. Care was taken in ascertaining the uniformity of all husbandry conditions across the two facilities where the experiments were performed (University of Kansas, USA and University of Cagliari, Italy). All experimental procedures were in compliance with the National Institute of Health guidelines and approved by the Institutional Animal Use Committees of the University of Kansas and Cagliari.
2.2. Drugs
The following drugs were used in the present study: finasteride (FIN), (R)-(−)-apomorphine hydrochloride, SKF 82958 hydrobromide, (−)-quinpirole hydrochloride, sumanirole maleate, (+)-PD 128907 hydrochloride, SCH 23390 and GR 103691 (Sigma Aldrich, Milan, Italy). FIN was suspended in a vehicle (VEH) solution containing 5% Tween 80 and 95% saline, while the other drugs were dissolved in saline (SAL) solution. Drug doses are based on mg/kg of salts. All solutions were freshly prepared on the day of testing and administered subcutaneously (SC) and intraperitoneally (IP) in an injection volume of 1 and 2 ml/kg body weight, respectively. The doses and the latency time of the drugs used in these experiments were determined by our previous studies and in accordance with those commonly used in PPI studies on rats (Wan et al., 1996; Geyer et al., 2001; Bortolato et al., 2008; Mosher et al., 2015).
2.3. Acoustic Startle Reflex and PPI
Startle and PPI testing were performed as previously described in Mosher et al. (2015). The apparatus used for detection of startle reflexes (Med Associates, St Albans, VT, USA) consisted of six standard cages placed in sound-attenuated chambers with fan ventilation. Each cage consisted of a Plexiglas cylinder of 9 cm diameter, mounted on a piezoelectric accelerometric platform connected to an analogue-digital converter. Two separate speakers conveyed background noise and acoustic bursts, each one properly placed so as to produce a variation of sound within 1 dB across the startle cage. Both speakers and startle cages were connected to a main PC, which detected and analysed all chamber variables with specific software. Before each testing session, acoustic stimuli and mechanical responses were calibrated via specific devices supplied by Med Associates. Rats were first subjected to a pre-test session, during which they were exposed to a sequence of seventeen trials, consisting of 40-ms, 115-dB burst, with a 70-dB background white noise. Experimental groups were defined based on the average startle amplitude of the rats, so as to maintain comparable values of average startle response across all groups. Three days after the pre-test session, rats were treated and underwent a test session. This session featured a 5-min acclimatization period, with a 70-dB background white noise, which continued for the remainder of the session. The acclimatization period was followed by three blocks, each consisting of a sequence of trials: the first and the third block consisted of five pulse-alone trials of 115 dB (identical to those used in the pre-test session). The second block consisted of a pseudorandom sequence of 50 trials, including 12 pulse-alone trials, 30 trials of pulse preceded by 74, 78, or 82 dB pre-pulses (10 for each level of pre-pulse loudness), and 8 no-pulse trials, where only the background noise was delivered. Inter-trial intervals (i.e, the time between two consecutive trials) were selected randomly between 10 and 15 s.
The % PPI was calculated only on the values relative to the second period, as well, using the following formula:
For both the pre-test and the test session, the interstimulus interval (i.e., the duration between the prepulse and the pulse in each trial) was kept at 100 ms. The selection of this interstimulus interval was based on previously published experiments from our group (Mosher et al., 2015), which showed this parameter to be optimally suited to reveal PPI deficits in response to selective dopamine receptor agonists in LE and SD rats.
A major caveat in %PPI computation is that increases or reductions in startle magnitude can respectively lead to artifacts, due to “ceiling” or “floor” effects (Swerdlow et al., 2000).
In consideration of FIN’s ability to reduce startle magnitude (Bortolato et al., 2008), whenever FIN was found to produce significant effects on both startle magnitude and %PPI, we performed confirmatory analyses of ΔPPI values. This parameter was calculated as the absolute differences between startle magnitudes on pulse-alone and prepulse+pulse trials (Bortolato et al., 2004).
2.4. Microdialysis
Microdialysis experiments were performed as previously described in Devoto et al. (2012). SD rats were deeply anesthetized with Equithesin (containing, per 100 ml, 0.97 g pentobarbital, 2.1 g MgSO4, 4.25 g chloral hydrate, 42.8 ml propylene glycol, 11.5 ml 90% ethanol; 5 ml/kg, i.p.) and placed in a Kopf stereotaxic apparatus. The skull was exposed and a hole was drilled for the implant of vertical microdialysis probes (membrane AN 69-HF, Hospal-Dasco, Bologna, Italy; cut-off 40,000 Daltons, 2 mm active membrane length), in the nucleus accumbens shell [AP +1.7, L ± 0.8, V −7.8 from the bregma, according to the coordinates of Paxinos and Watson (1997)]. The probes were secured to the scull by means of two screw and cranioplastic cement. The day after probe implantation, artificial cerebrospinal fluid (147 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, pH 6–6.5) was pumped through the dialysis probes at a constant rate of 1.1 μl/min via a CMA/100 microinjection pump (Carnegie Medicine, Stockholm, Sweden) in freely moving animals, and dialysate samples were collected every 20 min. Dopamine and DOPAC were immediately analyzed by HPLC with electrochemical detection, by HPLC systems equipped with 3.0 × 150 mm C18 (3.5 μ) Symmetry columns (Waters, Milan, Italy), maintained at 40°C by Series 1100 thermostats (Agilent Technologies, Waldbronn, Germany), and ESA Coulochem II detectors (Chelmford, MA, USA). The mobile phase consisted of 80 mM Na2HPO4, 0.27 mM EDTA, 0.6 mM sodium octyl sulfate, 8% methanol, 3% acetonitrile, pH 2.8 with H3PO4, delivered at 0.3 ml/min; the Coulochem analytical cell first electrode was set at +200 mV, the second one at −200 mV. Quantification was performed recording the second electrode signal. Under these conditions, dopamine detection limit (signal to noise ratio 3:1) was 0.3 pg per injection on column. On completion of testing, rats were sacrificed by Pentothal overdose, the brains removed and sectioned by a cryostat (Leica CM3050 S) in 40 μm thick coronal slices to verify locations of dialysis probes. No animal was found with errant location of the device.
2.5. Data analysis
Normality and homoscedasticity of data distribution were verified by using the Kolmogorov-Smirnov and Bartlett’s tests. Analyses were performed by multiple-way ANOVAs, as appropriate, followed by Student-Newman-Keuls’ test for post hoc comparisons of the means. For %PPI analyses, main effects for prepulse levels were consistently found throughout all the analyses, showing loudness-dependent effects; since no interactions between prepulse levels and other factors were found, data relative to different prepulse levels were collapsed. Significance threshold was set at 0.05.
3. Results
3.1. In LE rats, FIN counters the PPI deficits induced by D1-like, but not D2-like receptor agonists
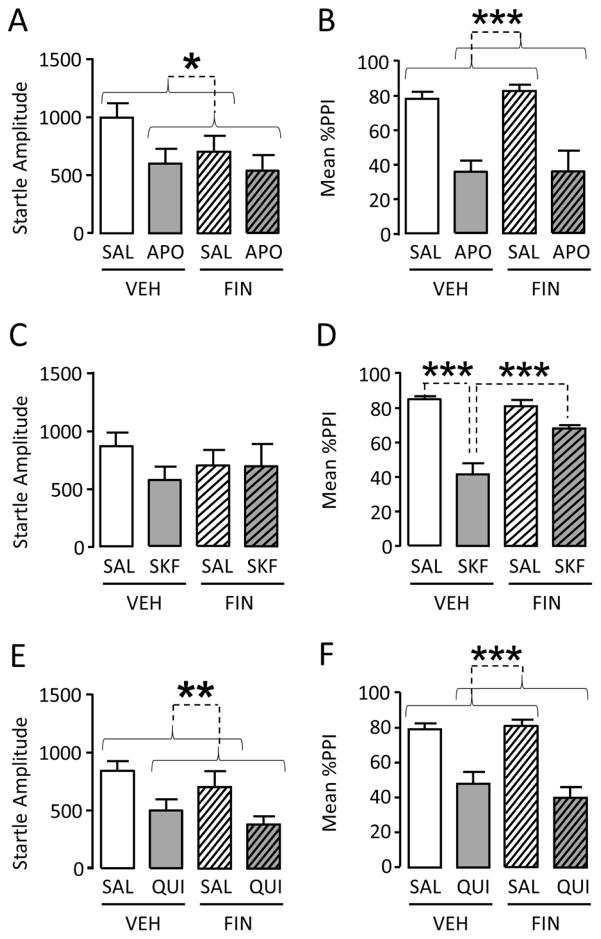
We first investigated the effects of FIN on startle response and PPI in LE rats. Our first experiment was aimed at testing whether FIN may counter APO-induced PPI deficits (n=8–9/treatment group) (Figs. 1A–B). In agreement with our previous results (Mosher et al., 2015), APO (0.5 mg/kg, SC) reduced startle amplitude [(Main effect: F(1, 29)=4.63, P<0.05]. Conversely, FIN (100 mg/kg, IP) did not significantly affect this parameter [Main effect: F(1,29)=1.83, NS]. Furthermore, we found no significant interactions between the two drugs [F(1, 29)=0.79, NS] (Fig. 1A). %PPI analyses indicated that, while APO significantly reduced %PPI in LE rats [Main effect: F(1, 29)=48.47, P<0.001], FIN surprisingly failed to counter this effect [Interaction: F(1, 29)=0.08, NS] (Fig. 1B).
Figure 1.
Effects of finasteride (FIN, 100 mg/kg, IP) on the changes in acoustic startle and prepulse inhibition (PPI) induced by (A–B) the non-selective D1–D2 receptor agonist apomorphine (APO, 0.5 mg/kg, SC), (C–D) the D1 receptor agonist SKF 82958 (SKF, 1 mg/kg, SC), (E–F) the D2-like receptor agonist quinpirole (QUI, 0.6 mg/kg, SC) in male Long-Evans rats. Values represent mean ± SEM for each experimental group. N = 8–10/group. SAL, saline; VEH, vehicle of finasteride; *, P<0.05; **, P<0.01; ***, P<0.001 for comparisons indicated by dotted lines. Curvy brackets are used to indicate main effects.
For more details, see text.
We then examined the effects of FIN on the disruption of PPI induced by the full D1-like receptor agonist SKF 82958 (1 mg/kg, SC) (n=8/treatment group), which we recently documented in LE rats (Mosher et al.., 2015). Neither SKF 82958 nor FIN produced significant effects on startle amplitude [Main SKF 82958 effect: F(1, 28)=1.08, NS; Main FIN effect: F(1, 28)=0.03, NS]. Furthermore, no significant interaction between the two factors was detected [F(1, 28)=1.0, NS] (Fig. 1C). The analysis of %PPI in LE rats revealed that SKF 82958 significantly reduced %PPI [Main effect: F(1, 28)=52.01, P<0.001], but this effect was significantly prevented by FIN [F(1, 28)=15.39, P<0.001; Ps<0.001 for comparisons between VEH-SAL vs VEH-SKF 82958 and VEH-SKF 82958 and FIN-SKF 82958]. (Fig. 1D).
Finally, we tested whether FIN may oppose the effects of the D2-like receptor agonist quinpirole (QUI; 0.6 mg/kg, SC) on PPI (n=9–10/treatment group). As shown in Fig. 1E, QUI markedly reduced startle amplitude [Main effect: F(1,33)=11.27; P<0.01], while FIN did not change this parameter [Main effect: F(1,33)=1.71; NS] and failed to reverse the effects of QUI [Interaction: F(1,33)=0.01; NS] (Fig. 1E). Notably, QUI disrupted %PPI [Main effect: F(1,33)=48.47; P<0.001]; conversely, FIN failed to either affect %PPI [Main effect: F(1,33)=0.34; NS] or counter the effect of QUI [Interaction: F(1,33)=0.90; NS] (Fig. 1F).
3.2. In SD rats, FIN counters the PPI deficits induced by APO, but not D2 receptor agonists
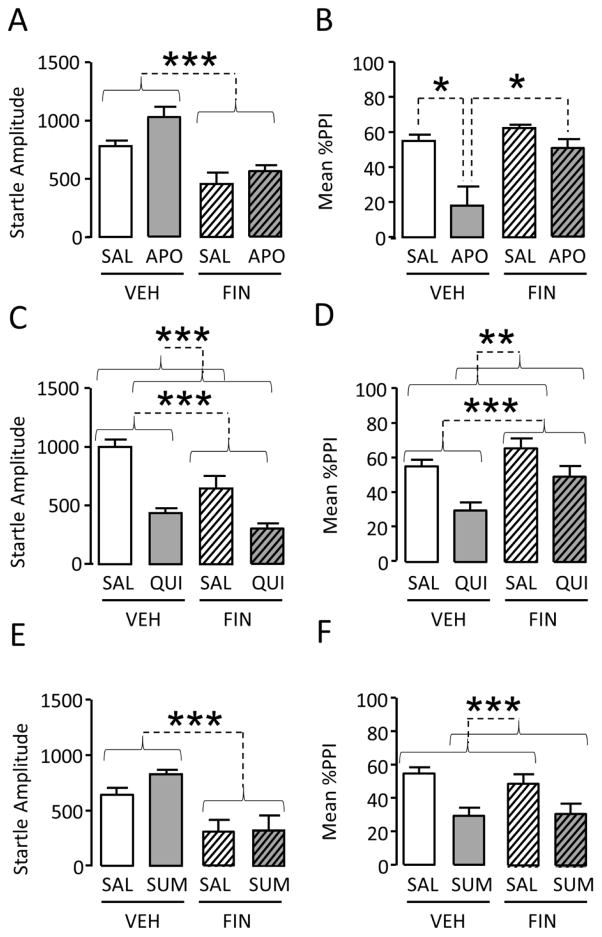
In line with our prior results in SD rats (Bortolato et al., 2008), startle magnitude was significantly reduced by FIN [Main effect: F(1, 35)=32.85, P<0.001], and increased by APO [Main effect: F(1, 35)=6.91, P<0.05] (n=9–10/treatment group); however, ANOVA failed to identify any significant interaction between these two effects [Interaction: F(1, 35)=1.03, NS] (Fig. 2A). %PPI analyses revealed significant main effects for both FIN pretreatment [F(1, 35)=10.93, P<0.01] and APO treatment [F(1, 35)=15.62, P<0.001]. In addition, a significant interaction between these effects was found [F(1, 35)=4.33, P<0.05]; post-hoc analyses revealed that, while APO significantly reduced %PPI (P<0.05), this effect was significantly prevented by FIN pre-treatment (P<0.05) (Fig. 2B). The same effects were detected through the analysis of corresponding ΔPPI values [FIN x APO interaction: F(1,31)=4.55, P<0.05; Ps<0.05 for post-hoc comparisons between VEH+SAL and VEH+APO as well as VEH+APO and FIN+APO] (data not shown).
Figure 2.
Effects of finasteride (FIN, 100 mg/kg, IP) on the changes in acoustic startle and prepulse inhibition (PPI) induced by (A–B) the non-selective D1–D2 receptor agonist apomorphine (APO, 0.25 mg/kg, SC), (C–D) the D2-like receptor agonist quinpirole (QUI, 0.6 mg/kg, SC), (E–F) the D2 selective agonist sumanirole (SUM, 3 mg/kg, SC) in male Sprague-Dawley rats. Values represent mean ± SEM for each experimental group. N = 8–10/group. SAL, saline; VEH, vehicle of finasteride; *, P<0.05; **, P<0.01; ***, P<0.001 for comparisons indicated by dotted lines. Curvy brackets are used to indicate main effects. For more details, see text.
Next, we tested whether the PPI-disrupting effect of the D2-like receptor agonist QUI was reversed by FIN (n=10–11/treatment group) (Fig. 2C–D). Both QUI and FIN significantly reduced startle amplitude [Main QUI effect: F(1,37)=46.68, P<0.001; Main FIN effect: F(1,37)=13.50, P<0.001], yet no significant interaction between the two drugs was found [F(1,37)=2.80, NS]. %PPI analysis detected that this parameter was significantly decreased by QUI [F(1,37)=16.76; P<0.001], and increased by FIN [F(1,37)=8.64; P<0.01]; however, no significant FIN x QUI interaction was detected [F(1,37)=0.81; NS] (Fig. 2D).
Since both D2 and D3 receptor agonists reduce PPI in SD rats, we verified whether the specific contribution of each receptor may be countered by FIN. Thus, we tested whether FIN may counter the effect of the selective D2 receptor agonist sumanirole (SUM; 3 mg/kg, SC) (n=8–9/treatment group). In contrast with QUI, SUM did not affect startle magnitude [F(1,31)=1.16; NS] (Fig. 2E), while FIN significantly reduced this response [F(1,31)=20.50; P<0.001]; however, no significant interaction between these two drugs was detected [F(1,31)=0.81; NS]. Analyses of PPI showed that SUM disrupted PPI [F(1,31)=8.64; P<0.001], but FIN pretreatment failed to prevent this effect [F(1,31)=0.09; NS]. (Fig. 2F).
3.3. In SD rats, FIN counters the %PPI deficits induced by D3 receptor activation
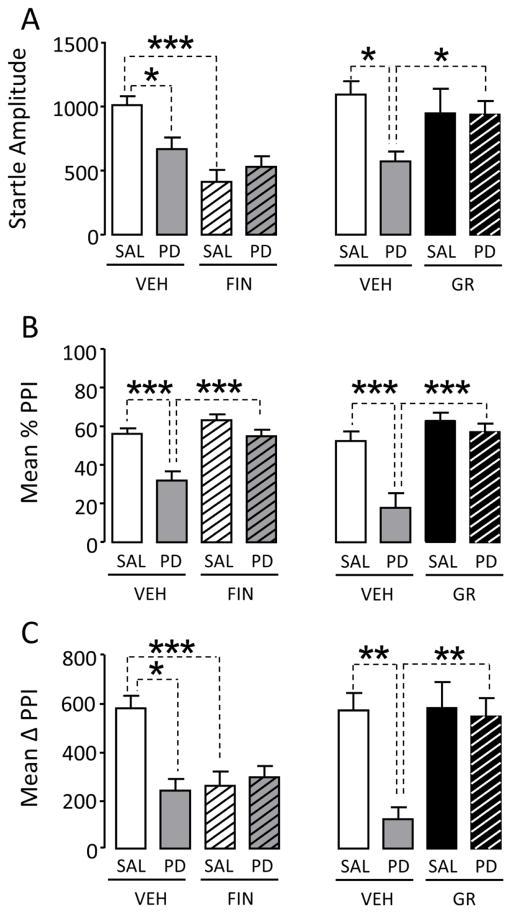
We then tested the effects of FIN on the %PPI reduction induced by D3 receptor agonist PD 128907(n=10–12/treatment group). While FIN reduced acoustic startle amplitude [F(1,40)=19.35; P<0.001], PD 128907(0.1 mg/kg, IP) [F(1,40)=1.83; NS] failed to affect this parameter. In addition, a significant interaction of these two treatments was found [F(1,40)=7.57; P<0.01]; post-hoc analyses revealed that the group treated with VEH and SAL exhibited a significantly higher startle magnitude than those treated with VEH and PD (P<0.05) as well as FIN and SAL (P<0.001) (Fig. 3A). Two-way ANOVA analyses of %PPI parameters showed significant main effects of FIN [F(1,40)=15.86; P<0.001] and PD 128907 [F(1,40)=4.38; P<0.05]. Interestingly, FIN countered the reduction in %PPI induced by PD 128907 [Interaction:F(1,40)=4.38; P<0.001; Ps<0.001 for comparisons between VEH+SAL and VEH+PD 128907 as well as VEH+PD 128907 and FIN+PD 128907] (Fig. 3B). In contrast with these findings, the analysis of ΔPPI values indicated a significant interaction between FIN and PD128907 [F(1,40)=12.92; P<0.001]; however, post-hoc comparisons found a significant difference between VEH+SAL and VEH+PD 128907 (P<0.001) as well as between VEH+SAL and FIN+SAL (P<0.01), but not between VEH+PD 128907 and FIN+PD 128907 (Fig. 3C).
Figure 3.
Effects of finasteride (FIN, 100 mg/kg, IP) and the D3 receptor antagonist GR103691 (0.2 mg/kg, SC) on the changes in (A) acoustic startle and (B) prepulse inhibition (PPI) induced by the D3 receptor agonist PD 128907 (PD, 0.1 mg/kg, IP) in male Sprague-Dawley rats. Values represent mean ± SEM for each experimental group. N = 8–10/group. SAL, saline; VEH, vehicle; *, P<0.05; **, P<0.01; ***, P<0.001 for comparisons indicated by dotted lines. For more details, see text.
To confirm that the observed effects by PD 128907 were mediated by D3 receptors, we tested the effects of the D3 receptor antagonist GR103691 (n=8–9/treatment group). GR103691 (0.2 mg/kg, SC) countered both the reduction of startle amplitude [Interaction: F(1,30)=4.24, P<0.05; Ps<0.05 for comparisons between VEH+SAL and VEH+PD 128907 as well as VEH+PD 128907 and GR103691+PD 128907] (Fig. 3A) and %PPI caused by PD 128907 [Interaction: F(1,30)=6.23; P<0.05; Ps<0.001 for comparisons between VEH+SAL and VEH+PD 128907 as well as VEH+PD 128907 and GR103691+PD 128907] (Fig. 3B). These results were fully confirmed by ΔPPI analyses, which found main effects for both [GR 103691 F(1,28)=7.66; P<0.01] and PD 128907 [F(1,28)=9.51; P<0.01]. Furthermore, a significant interaction between the two treatments [F(1,28)=6.87; P<0.05] was found to reflect significant differences between VEH+SAL and VEH+PD 128907 (P<0.01) as well as between VEH+PD 128907 and GR 103691+PD 128907 (P<0.01)] (Fig. 3C).
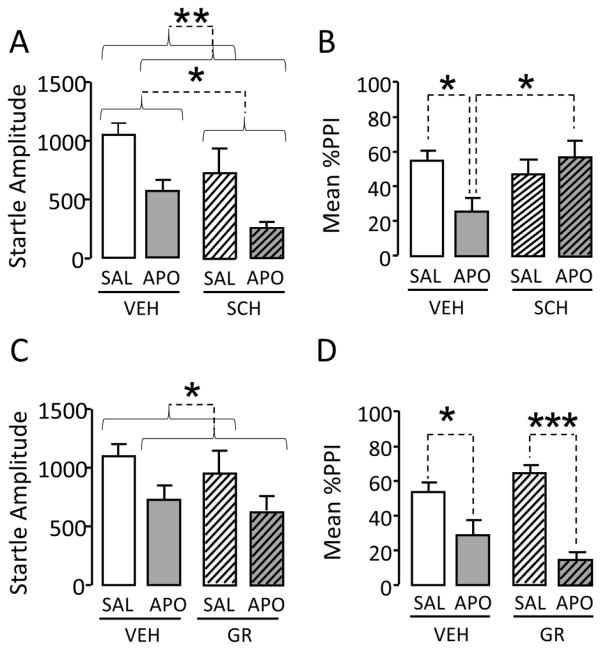
Given that our results qualified the %PPI-ameliorating properties of FIN in relation to the mechanisms of D1 and D3 receptors, we further tested whether APO-induced PPI disruption in SD rats could be countered by D1 and D3 receptor antagonists (Fig. 4). Both the D1 receptor antagonist SCH 23390 (0.1 mg/kg, SC) [F(1,36)=6.61; P< 0.05] and APO [F(1,36)=12.30; P<0.01] reduced acoustic startle amplitude (Fig. 4A) (n=10/treatment group). However, no significant interaction between these two treatments was found. PPI analyses revealed a significant interaction between SCH 23390 and APO [F(1,36)=5.96; P<0.05]. Post-hoc analyses revealed that, while APO caused a significant PPI disruption (P<0.05 for comparison between VEH+SAL and VEH+APO), SCH 23390 reversed this phenomenon (P<0.05 for comparison between VEH+APO vs VEH+SCH) (Fig. 4B), in agreement with previous findings (Wan et al., 1996). We then tested the effects of the D3 receptor antagonist GR103691 on the changes in startle and PPI produced by APO (Fig. 4C–D) (n=8/treatment group). The analysis of acoustic startle response revealed a main effect for APO (Fig. 4C) [F(1,28)=4.20; P=0.05], but not for GR103691 [F(1,28)=2.14; NS]; furthermore, no significant interaction between these two treatments was found [F(1,28)=0.16; NS]. Finally, PPI analyses confirmed that APO disrupted PPI [F(1,28)=42.86; P<0.0001; Main effect for APO] (Fig. 4D), while GR103691 did not affect PPI [F(1,28)=0.11; NS; Main effect for GR103691]. ANOVA also detected a significant interaction between GR103691 and APO [F(1,28)= 4.72; P<0.05]; post-hoc comparisons revealed significant differences between VEH+SAL and VEH+APO (P<0.05) and between GR103691+SAL and GR103691+APO (P<0.001).
Figure 4.
Effects of the D1 receptor antagonist SCH 23390 (SCH, 0.1 mg/kg, SC) and the D3 receptor antagonist GR103691 (GR, 0.2 mg/kg, SC) on the changes in (A–C) acoustic startle and (B–D) prepulse inhibition (PPI) induced by the D1–D2 receptor agonist apomorphine (APO, 0.25 mg/kg, SC) in male Sprague-Dawley rats. Values represent mean ± SEM for each experimental group. N = 8–10/group. SAL, saline; VEH, vehicle; *, P<0.05; **, P<0.01; ***, P<0.001 for comparisons indicated by dotted lines. Curvy brackets are used to indicate main effects. For more details, see text.
3.4. FIN counters the changes in dopamine levels in Nucleus Accumbens shell induced by D3 receptor activation
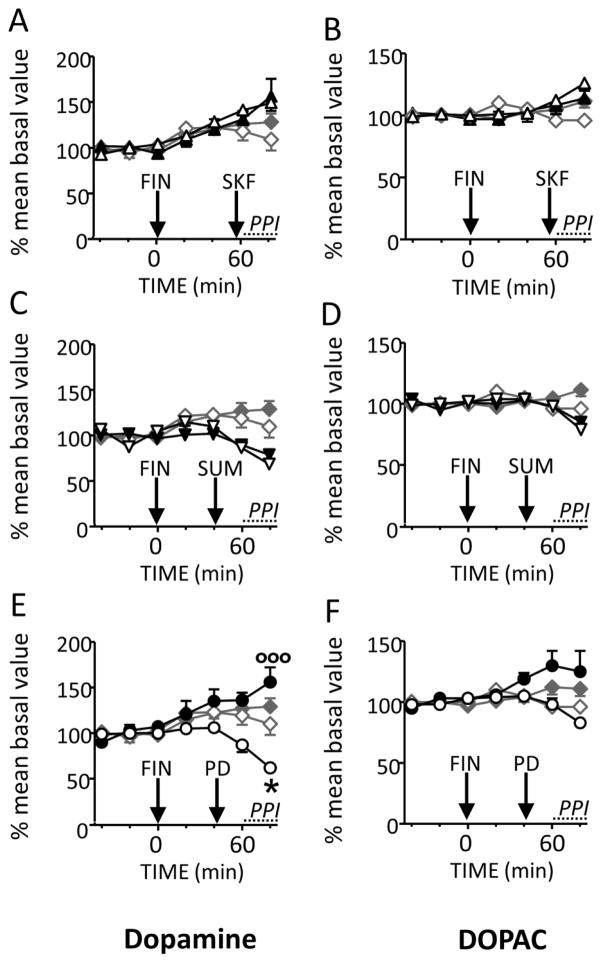
To verify whether the effects of FIN on PPI may be reflective of changes in extracellular dopamine levels in the nucleus accumbens, we tested the effects of selective dopaminergic agonist on levels of the dopamine and DOPAC levels in the nucleus accumbens shell by means of microdialysis in freely moving SD rats (n=7–8/group). Extracellular basal values (mean ± SEM) were: dopamine=2.7±0.2 pg, DOPAC = 1.7±0.1 ng per sample (20 μl dialysate). Confirming our previous study (Devoto et al., 2012), FIN (100 mg/kg, IP) significantly increased extracellular dopamine [F(6,42)=6.05; P<0.0001] and DOPAC [F(6,42)=2.53; P<0.05] above the baseline, starting at 60 and 80 min after FIN injection (100 mg/kg, IP), respectively. Vehicle plus saline administration did not affect dopamine and DOPAC levels (Fig. 5). Dopamine levels were significantly increased by the selective D1 agonist SKF 82958 (1 mg/kg, SC) [F(6,18)=4.09, P<0.01]; however, no interactions between FIN and SKF 82958 were detected [F(1,19)= 0.78, NS; 2-way ANOVA]. The D2 agonist SUM (3 mg/kg, SC) significantly decreased extracellular dopamine levels [F(6,24)= 11.9, P< 0.0001; however, this effect also failed to significantly interact with the effects of FIN on PPI [F(1,24)= 0.38, NS). Conversely, the D3 receptor agonist PD 128907 (0.1 mg/kg, IP) significantly reduced extracellular dopamine levels [F(6,42)=6.05, P< 0.001], but this effect was significantly reversed by FIN [F(1,20)=11.0, P<0.01] (Fig. 5). Temporal analysis showed that this effect was significant at 80 min after PD 128907 injection (Fig. 5E).
Figure 5.
Time-related effects of systemic finasteride (FIN, 100 mg/kg, IP) on extracellular concentrations of dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in the nucleus accumbens shell of Sprague-Dawley rats. FIN was tested in combination with (A–B) D1 agonist SKF 82958 (1 mg/kg, SC); (C–D) D2 agonist sumanirole (SUM, 3 mg/kg, SC); (E–F) D3 agonist PD 128907 (0.1 mg/kg, IP). Arrows represent injection time of FIN or its vehicle (VEH) and the dopaminergic agonist or saline (SAL). The interval corresponding to PPI testing is indicated by a dotted bar alongside the time axis. Values are expressed as mean percent of the baseline (average values of the three first samples) ± S.E.M for each time point. N = 7–8/group. *, P<0.05 compared with baseline values; °°°, P<0.001 in comparison with VEH+PD 128907. Significant within-group (time-dependent) effects are not indicated. For further details, see text.
4. Discussion
In the present study, we showed that the 5αR inhibitor FIN prevented the PPI deficits induced by the activation of D1-like, but not D2 receptor agonists, across different rat strains (Table 1). Specifically, in LE rats, FIN effectively countered the PPI impairment induced by the potent D1 receptor agonist SKF 82958, but failed to significantly prevent the deficits mediated by the D2-like receptor agonist QUI or the non-selective D1–D2 receptor agonist APO. Conversely, in SD rats, FIN countered the PPI-disrupting effects of APO, but not the D2 receptor agonists QUI and SUM. Furthermore, our analyses revealed that, in SD rats, FIN opposed the reduction of %PPI, but not ΔPPI, values induced by the D3 receptor agonist PD 128907. In parallel with these effects on sensorimotor gating, FIN reversed the reduction in extracellular dopamine levels caused by D3, but not D2 receptor activation in the nucleus accumbens of SD rats. The identification of the selective involvement of D1 receptors in FIN’s effects across different ratstrains extends and complements our previous reports on the antipsychotic-like properties of 5αR inhibitors (Bortolato et al., 2008; Devoto et al., 2012; Frau et al., 2013; Frau et al., 2015), and points to a specific mechanism of action for the emerging therapeutic potential of 5αR inhibitors in neuropsychiatric disorders (Paba et al., 2011).
Table 1.
Synoptic table of the combined effects of finasteride and dopaminergic agonists on the prepulse inhibition (PPI) of the startle in Long-Evans (LE) and Sprague-Dawley (SD) rats.
| Effects of finasteride (FIN; 100 mg/kg, IP) in PPI
| |
|---|---|
| Apomorphine; D1–D2 receptor agonist |
FIN opposes %PPI and ΔPPI deficits in SD rats FIN does not oppose %PPI deficits in LE rats |
| SKF 82958; D1 receptor agonist | FIN opposes %PPI and ΔPPI deficits in LE rats |
| Quinpirole; D2–D3 receptor agonist | FIN does not oppose %PPI deficits in SD rats FIN does not oppose %PPI deficits in LE rats |
| Sumanirole; D2 receptor agonist | FIN does not oppose %PPI deficits in SD rats |
| PD 128907; D3 receptor agonist | FIN opposes %PPI, but not ΔPPI, deficits in SD rats |
The implication of D1-like receptors in FIN-induced PPI amelioration was documented both directly in LE rats and indirectly in SD rats. The latter strain does not exhibit PPI impairments in response to administration of D1-like receptor agonists (Wan et al., 1996; Bortolato et al., 2005); however, FIN countered the gating deficits induced by the D1–D2 receptor agonist APO, but not the D2 activators QUI and SUM; furthermore, the actions of FIN mirrored those of the D1 receptor antagonist SCH 23390. The implication of D1 receptor in the antipsychotic-like mechanisms of FIN is in agreement with our previous results on C57BL/6 mice (Frau et al., 2013). Although these animals do not exhibit PPI deficits in response to D2-like receptor agonists (Ralph-Williams et al., 2003), FIN fully prevented the PPI deficits induced by SKF 82958 and paradoxically led to PPI deficits following treatment with the D2 receptor agonist QUI (Frau et al., 2013). Interestingly, key neurosteroids, such as allopregnanolone and dehydroepiandrosterone sulfate, modulate the behavioral effects of D1 receptor activation (Frye et al., 2006; Dong et al., 2007); furthermore, progesterone and allopregnanolone affect the phosphorylation of DARPP-32 (Mani et al., 2000; Frye and Walf, 2010), a key molecule in D1 receptor signaling cascade (Svenningsson et al., 2004).
We previously documented that the mechanism of action of FIN in reversing APO-induced gating deficits is likely reflective of changes in neurosteroid profiles in the nucleus accumbens (Devoto et al., 2012). Interestingly, the intra-accumbal effects of FIN were not accompanied by any significant variations in dopamine extracellular levels, suggesting that the effects are not mediated by changes in dopamine release (Devoto et al., 2012). Those results, together with the lack of significant interactions between FIN and SKF 82958 on dopamine levels in the nucleus accumbens documented in this study, strongly suggest that presynaptic D1 receptors are not directly involved in the modulatory role of 5αR on gating. Accordingly, the effects of APO on PPI are regarded as primarily due to the activation of postsynaptic receptors (Swerdlow et al., 2001). Striatal D1 receptors are predominantly located in extrasynaptic locations of GABAergic medium-spiny neurons (Hersch et al., 1995). This particular localization is posited to enable D1 receptors to be preferentially activated by transient elevations of dopamine levels due to phasic bursts of dopaminergic neuron activity (Gonon, 1997; Wall et al., 2011). Building on this perspective, the modulation of neurosteroidogenesis in the nucleus accumbens may affect sensorimotor gating by altering the response to different dynamics of dopamine neurotransmission. Notably, neurosteroids influence tonic and phasic GABA activity (Matthew and Samba, 2013), whose cross-talk plays a fundamental role in PPI regulation (Curtin and Preuss, 2015). Further studies are necessary to understand whether changes in tonic and phasic activity in GABAergic activity may be directly related to dynamic alterations in accumbaldopamine activity.
A second potentially important finding of this study was that FIN countered the %PPI deficits induced by the D3 receptor agonist PD 128907. However, these results were not validated by parallel ΔPPI analyses, raising the possibility that the observed effects may be due to computational artefacts. Nevertheless, it is worth mentioning that the effects of FIN on PPI were time-locked with a reversal of the PD 128907-mediated reduction in extracellular dopamine levels, which has been linked to the stimulation of presynaptic D3 autoreceptors (Pugsley et al., 1995). The results of these microdialysis studies (and, to a more limited extent, those on %PPI values) suggest that 5αR regulates D3 receptor signaling. Given that FIN does not bind to D3 receptors (S. Ruiu, personal communication), our data suggest that 5αR may regulate their signaling by interfering with the function of one of their downstream effectors, which include Gi/Go proteins and inward rectifying potassium channels (Ahlgren-Beckendorf and Levant, 2004; Hervé and Girault, 2005). Irrespective of the mechanisms, further studies with different testing protocols will be necessary to verify whether the interaction between 5αR and D3 receptors is actually relevant to PPI regulation.
Although FIN opposed the %PPI disruption induced by both APO and PD 128907, the lack of effects of the D3 receptor antagonist GR 103691 on APO-mediated effects suggest that these two effects were likely underpinned by distinct processes, namely the actions of FIN on D1-like and D3 receptors. This difference is in line with the strikingly different properties of these two receptor subtypes: on one hand, D1-like receptors are conducive to excitatory effects, through the concatenated activation of Gαs and Gαolf proteins and their downstream effectors (Romanelli et al., 2010); on the other hand, D3 receptor function appears to be primarily inhibitory (Accili et al., 1996; Menalled et al., 1999). Despite this phenomenological dichotomy, some of the actions of 5αR on D1 and D3 receptor signaling may affect common intracellular substrates. Indeed, these receptor types are highly co-localized in extrasynaptic compartments of the nucleus accumbens (Schwartz et al., 1998), and have been found to interact at multiple levels (Fiorentini et al., 2008), including the formation of heteromers (Marcellino et al., 2008). With respect to this issue, it should be noted that the implication of D1 receptors in the formation of dimers often requires σ1 receptors, which are targeted by several neurosteroids (Cobos et al., 2008). Future studies will be needed to verify the implication of σ1 receptors in the actions of FIN.
In keeping with previous evidence (Bortolato et al., 2008), we found that the dose of FIN used in these studies (100 mg/kg, IP) reduced startle amplitude in SD rats; however, this drug had surprisingly no such effect on LE rats. The difference in the effects of FIN on startle amplitude across these two strains may reflect the diverse properties of this drug with respect to locomotor activity: in fact, the same dose of FIN used in this study produced a generalized decrease in locomotor activity in SD rats (Bortolato et al., 2008); conversely, ongoing studies in our lab are indicating that LE rats exhibit a greater resistance to the locomotor depression induced by high doses of FIN (data not shown). Future studies are warranted to elucidate the neurobiological bases of the different reactivity of SD and LE rats to FIN with respect to startle and locomotor activity.
The marked differences between the effects of FIN in SD and LE rats are in line with previous evidence on the distinct PPI responses in these two strains (Swerdlow et al., 2006). Although the molecular underpinnings of these differences remain unclear, our data suggest a potential role of neurosteroids in these changes. Previous studies have documented that LE rats exhibit higher dopamine turnover in comparison with SD rats (Swerdlow et al., 2005), possibly due to changes in the dopamine-metabolic enzyme catechol-O-methyl transferase (COMT) (Shilling et al., 2008). Notably, this enzyme has been shown to be affected by neuroactive steroids; for example, COMT expression is enhanced by testosterone and DHT (Purves-Tyson et al., 2012) and reduced by estrogens (Xie et al., 1999). These premises suggest that differences in 5αR or other neurosteroids may contribute to the differences in gating regulation between SD and LE rats.
Several limitations of the present study should be acknowledged. First, our analyses did not include analyses of neurosteroid profiles to evaluate the mechanisms underpinning the observed interstrain differences with respect to the role of FIN on the effects of dopaminergic agonists in both strains. Nevertheless, in preliminary studies, we have verified that the analysis of neurosteroid profile in the nucleus accumbens alone is not currently feasible, given the limited size of this region and the detection limits of available systems.
Secondly, our data cannot rule out that the observed effects of FIN may be partially mediated by peripheral effects; in particular, FIN inhibits the conversion of testosterone into the potent androgen hormone dihydrotestosterone (DHT) (Paba et al., 2011). However, this possibility is tempered by our prior finding that the effects of FIN on sensorimotor gating are not affected by gonadectomy (Devoto et al., 2012); in addition, the involvement of testosterone in these effects is unlikely, given that, in separate studies performed on the neurosteroid profile of the combination of striatum and nucleus accumbens, the same dose of FIN used in this study (100 mg/kg, IP) failed to modify the levels of this steroid (Frau et al., 2015).
Thirdly, given the broad scope of our studies, behavioral and microdialysis experiments were restricted to the analysis of the effects of optimal doses of FIN and dopaminergic agonists, based on our prior research and other relevant scientific literature. The lack of dose-response curves, however, limits a comprehensive assessment of the interstrain differences in the dopaminergic regulation of rat PPI, and leaves open the possibility that the actions of different FIN concentrations may result in different effects on PPI regulation in combination with different dopaminergic drugs. In a similar way, PPI was consistently tested with 100-ms interstimulus intervals, as this particular setting allowed us to reveal PPI-disrupting effects of D1 and D2 receptor agonists in LE rats (Mosher et al., 2015), as well as D2 and D3 receptor agonists in SD rats. Different testing conditions and protocols, however, may reveal different effects of FIN with respect to the dopaminergic modulation of PPI.
Finally, although our experiments were performed on equivalent experimental protocols and apparatuses, it is worth noting that the experiments were performed in two different laboratories (SD at the University of Cagliari, and LE at the University of Kansas). Thus, we cannot completely exclude divergences in the colonies from the suppliers. Indeed, differences in PPI can reflect sub-strain variations based on the specific location of the supplier (Swerdlow et al., 2000). Nevertheless, these potential concerns are tempered by the similarity of results obtained in both laboratories on the effects of FIN in modifying PPI preventing, in co-treatment with both APO and QUI in SD rats (see Suppl. Materials).
These limitations notwithstanding, our results highlight a neurobiological link between 5αR, neurosteroids and dopamine receptors, which may be particularly important in the pathophysiology of neuropsychiatric disorders characterized by gating deficits, including schizophrenia and Tourette syndrome. In preliminary clinical observations, we documented that FIN elicits potential therapeutic effects in these disorders (Bortolato et al., 2007; Koethe et al., 2008; Muroni et al., 2011; Bortolato et al., 2013). Furthermore, emerging data indicate the potential of D1 and D3 receptor blockers in the treatment of Tourette syndrome and schizophrenia, respectively (Sokoloff et al., 2013; Gilbert et al., 2014). The selective action of FIN on these receptors, rather than D2, may help explain the lack of extrapyramidal symptoms associated with 5αR inhibitors (Bortolato et al., 2008).
Whereas further research is needed to address these limitations, our findings highlight the critical role of 5αR in the pathophysiology of gating deficits, and point to an important functional link between neurosteroids and D1 and D3 receptors, which may be implicated in the pathophysiology of schizophrenia, Tourette syndrome and other related disorders.
Supplementary Material
Highlights.
We tested finasteride on the PPI deficits induced by selective DA receptor agonists in rats
In Long-Evans, finasteride opposed the PPI deficits induced by D1, but not D2 receptors
In Sprague-Dawley, finasteride prevented the PPI loss and accumbal DA levels caused by D3, but not D2 receptors
Acknowledgments
We would like to thank Alessandra Pardu for her technical assistance.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributors
RF monitored data collection, analyzed behavioral data and performed statistical analyses. VB, performed behavioral tests and performed statistical analyses. LM, GP, RP, PS, SF performed the behavioral tests. PD designed the experiments, analyzed data and discussed the paper. MB designed the experiments, supervised the experimental execution, monitored data collection, performed statistical analyses, wrote and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren-Beckendorf JA, Levant B. Signaling mechanisms of the D3 dopamine receptor. J Recept Signal Transduct Res. 2004;24:117–130. doi: 10.1081/rrs-200029953. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Aru GN, Fa M, Frau R, Orru M, Salis P, Casti A, Luckey GC, Mereu G, Gessa GL. Activation of D1, but not D2 receptors potentiates dizocilpine-mediated disruption of prepulse inhibition of the startle. Neuropsychopharmacology. 2005;30:561–574. doi: 10.1038/sj.npp.1300547. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Godar SC, Mosher LJ, Paba S, Marrosu F, Devoto P. The implication of neuroactive steroids in Tourette’s syndrome pathogenesis: A role for 5alpha-reductase? J Neuroendocrinol. 2013;25:1196–1208. doi: 10.1111/jne.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru M, Bourov Y, Marrosu F, Mereu G, Devoto P, Gessa GL. Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology. 2008;33:3146–3156. doi: 10.1038/npp.2008.39. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette’s syndrome with finasteride. Am J Psychiatry. 2007;164:1914–1915. doi: 10.1176/appi.ajp.2007.07060978. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bristow LJ, Cook GP, Gay JC, Kulagowski JJ, Landon L, Murray F, Saywell KL, Young L, Hutson PH. The behavioural and neurochemical profile of the putative dopamine D3 receptor agonist, (+)-PD 128907, in the rat. Neuropharmacology. 1996;35:285–294. doi: 10.1016/0028-3908(96)00179-7. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Entrena JM, Nieto FR, Cendan CM, Del Pozo E. Pharmacology and therapeutic potential of sigma (1) receptor ligands. Curr Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin PC, Preuss T. Glycine and GABAA receptors mediate tonic and phasic inhibitory processes that contribute to prepulse inhibition in the goldfish startle network. Front Neural Circuits. 2015;9:12. doi: 10.3389/fncir.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Frau R, Bini V, Pillolla G, Saba P, Flore G, Corona M, Marrosu F, Bortolato M. Inhibition of 5alpha-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2012;37:1630–1645. doi: 10.1016/j.psyneuen.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LY, Cheng ZX, Fu YM, Wang ZM, Zhu YH, Sun JL, Dong Y, Zheng P. Neurosteroid dehydroepiandrosterone sulfate enhances spontaneous glutamate release in rat prelimbic cortex through activation of dopamine D1 and sigma-1 receptor. Neuropharmacology. 2007;52:966–974. doi: 10.1016/j.neuropharm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Frau R, Abbiati F, Bini V, Casti A, Caruso D, Devoto P, Bortolato M. Targeting neurosteroid synthesis as a therapy for schizophrenia-related alterations induced by early psychosocial stress. Schizophr Res. 2015 doi: 10.1016/j.schres.2015.04.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Pillolla G, Bini V, Tambaro S, Devoto P, Bortolato M. Inhibition of 5alpha-reductase attenuates behavioral effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology. 2013;38:542–551. doi: 10.1016/j.psyneuen.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Infusions of anti-sense oligonucleotides for DARPP-32 to the ventral tegmental area reduce effects of progesterone- and a dopamine type 1-like receptor agonist to facilitate lordosis. Behav Brain Res. 2010;206:286–292. doi: 10.1016/j.bbr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Petralia SM. In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABA A receptors. Horm Behav. 2006;50:332–337. doi: 10.1016/j.yhbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clin Neuropharmacol. 2014;37:26–30. doi: 10.1097/WNF.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D, Girault JA. Signal transduction of dopamine receptors. In: Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, 21; Dopamine. Elsevier Science; Amsterdam: 2005. pp. 109–151. [Google Scholar]
- Hoffman DC, Donovan H. D1 and D2 dopamine receptor antagonists reverse prepulse inhibition deficits in an animal model of schizophrenia. Psychopharmacology. 1994;115:447–453. doi: 10.1007/BF02245567. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Koethe D, Bortolato M, Piomelli D, Leweke FM. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry. 2008;41:115–116. doi: 10.1055/s-2008-1058110. [DOI] [PubMed] [Google Scholar]
- Mani SK, Fienberg AA, O’Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O’Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Matthew CC, Samba RD. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: reguation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Dziewczapolski G, Garcia MC, Rubinstein M, Gershanik OS. D3 receptor knockdown through antisense oligonucleotide administration supports its inhibitory role in locomotion. Neuroreport. 1999;10:3131–3136. doi: 10.1097/00001756-199910190-00002. [DOI] [PubMed] [Google Scholar]
- Mosher LJ, Frau R, Pardu A, Pes R, Devoto P, Bortolato M. Selective activation of D1 dopamine receptors impairs sensorimotor gating in Long-Evans rats. Br J Pharmacol. 2015 doi: 10.1111/bph.13232. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord. 2011;26:2146–2147. doi: 10.1002/mds.23810. [DOI] [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M. Steroid 5alpha-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des. 2011;17:151–167. doi: 10.2174/138161211795049589. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. Academic Press; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Peng RY, Mansbach RS, Braff DL, Geyer MA. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats. Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikstrom H, Whetzel SZ, Georgic LM, Cooke LW, et al. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther. 1995;275:1355–1366. [PubMed] [Google Scholar]
- Purves-Tyson TD, Handelsman DJ, Double KL, Owens SJ, Bustamante S, Weickert CS. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 2012;13:95. doi: 10.1186/1471-2202-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Romanelli RJ, Williams JT, Neve KA. The dopamine receptors. Humana Press; 2010. Dopamine receptor signaling: intracellular pathways to behavior; pp. 137–173. [Google Scholar]
- Schwartz JC, Ridray S, Bordet R, Diaz J, Sokoloff P. D1/D3 receptor relationships in brain coexpression, coactivation, and coregulation. Adv Pharmacol. 1998;42:408–411. doi: 10.1016/s1054-3589(08)60775-9. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Saint Marie RL, Shoemaker JM, Swerdlow NR. Strain differences in the gating-disruptive effects of apomorphine: relationship to gene expression in nucleus accumbens signaling pathways. Biol Psychiatry. 2008;63:748–758. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Leriche L, Diaz J, Louvel J, Pumain R. Direct and indirect interactions of the dopamine D(3) receptor with glutamate pathways: implications for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:107–124. doi: 10.1007/s00210-012-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci. 1999;877:202–216. doi: 10.1111/j.1749-6632.1999.tb09269.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Krupin AS, Bongiovanni MJ, Shoemaker JM, Goins JC, Hammer RP., Jr Heritable differences in the dopaminergic regulation of behavior in rats: relationship to D2-like receptor G-protein function. Neuropsychopharmacology. 2006;31:721–729. doi: 10.1038/sj.npp.1300877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Kuczenski R, Goins JC, Crain SK, Ma LT, Bongiovanni MJ, Shoemaker JM. Neurochemical analysis of rat strain differences in the startle gating-disruptive effects of dopamine agonists. Pharmacol Biochem Behav. 2005;80:203–211. doi: 10.1016/j.pbb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Martinez ZA, Hanlon FM, Platten A, Farid M, Auerbach P, Braff DL, Geyer MA. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J Neurosci. 2000;20:4325–4336. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall VZ, Parker JG, Fadok JP, Darvas M, Zweifel L, Palmiter RD. A behavioral genetics approach to understanding D1 receptor involvement in phasic dopamine signaling. Mol Cell Neurosci. 2011;46:21–31. doi: 10.1016/j.mcn.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Taaid N, Swerdlow NR. Do D1/D2 interactions regulate prepulse inhibition in rats? Neuropsychopharmacology. 1996;14:265–274. doi: 10.1016/0893-133X(95)00133-X. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.