Abstract
Nitric oxide (NO) is endogenously released in the airways, and the fractional concentration of NO in exhaled breath (FeNO) is now recognized as a surrogate marker of eosinophilic airway inflammation that can be measured using a noninvasive technique suitable for young children. Although FeNO levels are affected by several factors, the most important clinical determinants of increased FeNO levels are atopy, asthma, and allergic rhinitis. In addition, air pollution is an environmental determinant of FeNO that may contribute to the high prevalence of allergic disease. In this review, we discuss the mechanism for airway NO production, methods for measuring FeNO, and determinants of FeNO in children, including host and environmental factors such as air pollution. We also discuss the clinical utility of FeNO in children with asthma and allergic rhinitis and further useful directions using FeNO measurement.
Keywords: Nitric oxide, children, asthma, allergic rhinitis, air pollution
INTRODUCTION
Nitric oxide (NO) is recognized as a biological messenger.1 The role of NO in the lungs as an inflammatory mediator and/or biomarker is supported by an increasing amount of evidence. 1,2,3 NO is noninvasively measured, and devices and protocols for the measurement of fractional nitric oxide concentration in exhaled breath (FeNO) have been developed and refined since 1991. FeNO is currently used as a noninvasive biomarker for assessment of airway inflammatory status in respiratory diseases such as asthma.2
Airway inflammation plays a pivotal role in asthma and a number of other respiratory diseases.2 Accordingly, FeNO has gained substantial clinical and scientific interest since it is seen as a safer and less invasive alternative tool to bronchoscopy or induced sputum for diagnosis, monitoring, or predicting the response to the treatment of airway inflammation, especially in children.3 Therefore, this review focuses on the clinical application of FeNO in children with allergic airway disease and the environmental and other factors that need to be considered when interpreting the results from FeNO measurements.
What is FeNO?
Biology of nitric oxide in the airways
NO is endogenously generated through the oxidation of the amino acid ʟ-arginine by 3 nitric oxide synthases (NOS) that are expressed in several cell types, such as epithelial cells, inflammatory cells (macrophages, neutrophils, and mast cells), airway nerves, and vascular endothelial cells.1 There are 3 distinct isoforms of NOS: 2 constitutive isoforms, neural NOS (nNOS) and endothelial NOS (eNOS), and 1 inducible isoform, inducible NOS (iNOS). The production of NO depends on many local factors, including genetic and epigenetic variants, the amount and activity of the enzymes responsible for producing NO, the concentration of substrates, the level of oxidative stress and its rate of uptake by antioxidant molecules (Fig. 1). In the lung, low concentrations of NO derived by nNOS and eNOS mediates a variety of physiological responses, including lung development, airway smooth muscle relaxation, bronchoprotection, and ciliary motility, whereas high concentrations of NO produced by iNOS appear to be involved in nonspecific host defense mechanisms and chronic inflammatory disease.1,2
Fig. 1. Schematic overview on metabolism of ʟ-arginine and regulation in the airways. ʟ-Arginine is transported into the cell via the cationic amino acid transport (CAT) system and can be metabolized by two groups of enzymes: nitric oxide synthases [constitutive NOS (cNOS) and inducible NOS (iNOS)] and arginases (I and II). NOS converts ʟ-arginine in two steps to NO and ʟ-citrulline with NG-hydroxy-ʟ-arginine as an intermediate. cNOS is activated by an increase in the intracellular concentration of Ca2+, which catalyzes NO synthesis that can bind either thiol groups leading to S-nitrosothiols (R-SNO) or the iron of soluble guanylyl cyclase (sGC) that stimulate the conversion of GTP to cGMP, then both have a variety of physiological effects in the airway. Pro-inflammatory cytokines [interleukin-4 (IL-4), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α)] activate transcription factors and induce iNOS expression, which leads to the prolonged release of high amounts of NO. iNOS-derived NO react with a broad spectrum of molecules such as superoxide (O2-) radicals and transition metals, which can lead to nitration of most classes of biomolecules (nitrative stress). ʟ-citrulline can be converted to ʟ-arginine by argininosuccinate. Lipopolysaccharide (LPS) and Th2 cytokines might lead to over-expression of arginase, then leads to the increased generation of proline and polyamines from ʟ-ornithine.
Measurement of FeNO
There are several analyzers to measure FeNO. Chemiluminescence analyzers determine NO concentrations by measuring the light generated by a chemical reaction of NO with ozone.4 This technique has high sensitivity, and NO can be accurately measured in the parts per billion (ppb) range.5 Several FeNO analyzers have been commercially available, such as NIOX (Aerocrine, Stockholm, Sweden), NOA 280i (Sievers, GE Analytical Instruments, Boulder, CO, USA), and CLD 88 (Eco Medics, Duernten, Switzerland). Although chemiluminescence analyzers provide the highest quality data, they require regular quality control checks and are rather expensive, large, and poorly portable. Recently, smaller, cheaper, and more portable electrochemical sensor devices have been developed and introduced as alternatives to chemiluminescence analyzers. Commercially, NIOX MINO and NIOX VERO (Aerocrine, Stockholm, Sweden) and NObreath (Bedfont Scientific Ltd, Kent, UK) are available. These have limitations in sensitivity compared to chemiluminescence analyzers.
During the FeNO exhalation maneuver, it is important to maintain a constant expiratory flow rate, since NO levels vary as a function of flow rate. Fast flow rates reduce the contribution from airway wall diffusion, resulting in lower FeNO levels, while slow rates increase FeNO.4 According to the ATS/ERS recommendation, the standard expiratory flow rate for FeNO measurement is 50 mL/s.5 Subjects should sit in an upright position, exhale to residual volume, insert a mouth piece, inhale to total lung capacity-preferably using NO free air-and then exhale immediately against a standard backpressure to close the velum for 10 seconds at a constant flow rate of 50 mL/s.
For young children, adequate practice with well-trained staff, audiovisual aids, and dynamic flow restrictors are recommended. Reports of FeNO measurement should include time of day, age, sex, race, height, weight, smoking status, prior diagnosis of airway disease, reason for testing, current corticosteroid medication, FeNO analyzer model used, and room air NO concentration, if possible.
Determinants of FeNO levels
Subject-related factors
Several host factors are associated with FeNO levels. The most important factors are age, gender, race/ethnicity, and atopy (Table). Reports of normal ranges of FeNO from studies in a variety of countries considered the effects of these factors across all ages of children.6,7,8,9 Many studies have shown that FeNO increases with age in children at a rate of 5% per year, and the ATS/ERS guidelines state that FeNO levels in children ≤12 years of age are associated with age.5,6,7,8 However, there are still some inconsistencies in reported associations of FeNO with age in children.9 FeNO levels have been found to be higher in males than in females.7,8 Anthropometric factors, such as height, weight, and body surface area, also need to be considered when interpreting FeNO levels.6,7 FeNO is significantly correlated with height in children as well as in adults, due to the increased lung size with taller height.6 Additionally, FeNO levels have been reported to differ by race/ethnicity. For example, Asians have higher levels of FeNO compared to whites.5,6 Some Hispanic children have been found to have higher mean FeNO than non-Hispanic white children.7
Table. Determinants of FeNO levels in children.
| Factors | Effect on FeNO levels |
|---|---|
| Age | Age-dependent increase10,12,13,14,15 |
| Gender | Boys >Girls13,15,19 |
| Race/ethnicity | Asians higher than whites10,12,13,19 |
| Hispanic children higher than non-Hispanic white children13 | |
| Height | Rise by height gained12 |
| Nitrate containing food | Increase21 |
| Caffeine | Conflicting results24,25 |
| Respiratory tract infection | Unclear26,27 |
| Circadian rhythm | Unclear10 |
| Exercise | Transient reduction23 |
| Spirometry, bronchial challenge test, sputum induction | Transient reduction30,31 |
| Smoking | |
| Active | Reduction22,55 |
| Passive | Transient reduction56 |
| Atopy | Increase17,32,33,34,38 |
| Outdoor air pollution | |
| Ozone, NO2, PM2.5, PM10, elemental carbon | Increase by acute and chronic exposure43,44,45,46 |
| Indoor air pollution | |
| Electric baseboard heating | Increase49,50,51 |
| Woodstoves, candles, gas cooker | Not associated52 |
| Polyvinyl chloride material | Unclear53,54 |
| Medication | |
| Inhaled or systemic corticosteroid | Reduction28 |
| Leukotriene receptor antagonist | Reduction29 |
Prior to FeNO measurement, strenuous physical exercise, eating, drinking, or smoking must be avoided for at least 1 hour.10,11,12 Some foods containing nitrates may increase FeNO levels,10 and caffeinated food and beverages showed conflicting associations with FeNO.13,14 Smoking causes acute and chronic reduction in FeNO levels.11 Caution should be used when assessing FeNO levels in children with respiratory tract infections because FeNO levels have been shown to vary by phase of the infection and to be different between studies.15,16 Medications, including inhaled or systemic corticosteroids or leukotriene receptor antagonists, need to be considered as they significantly reduce FeNO levels. 17,18 Serial measurements should be performed at approximately the same time of the day since circadian rhythms may affect FeNO levels.5 Additionally, FeNO should be measured prior to spirometry or bronchial challenge tests. These respiratory maneuvers transiently reduce FeNO levels.19
Atopy
Children with allergic sensitization generally have significantly higher levels of FeNO compared to nonatopic children, with some studies showing the association to be the same regardless of asthma symptoms,9,20 and another showing the association to be enhanced by asthma (Table).20 In addition, atopy and eosinophilia had an additive effect on FeNO levels in schoolchildren, independent of a history of respiratory symptoms.21 Other studies found a positive, linear dose-response relation between skin test grades and FeNO levels.21,24 FeNO levels are also increased by the presence of respiratory symptoms, especially wheeze, suggesting that FeNO measurement could be a simple and noninvasive method to identify the subjects at risk for developing asthma.22 However, the relationship between atopy and FeNO may not be apparent in all populations as some authors did not find any difference in FeNO between atopic and nonatopic individuals.23
Environmental factors
1) Outdoor air pollution
Air pollutants, such as ozone (O3), nitrogen dioxide (NO2), particulate matter with an aerodynamic diameter of less than 10 µm (PM10), particulate matter with an aerodynamic diameter of less than 2.5 µm (PM2.5), and inorganic acid vapor, have chronic adverse effects on the development of lung function in children.25
Although mechanisms by which exposure to air pollutants causes airway inflammation are not yet fully clarified, one hypothesis is that reactive oxygen species (ROS) induce oxidative stress, and activate transcriptional factors and cytokines to cause inflammation, which may vary by the type of air pollution. Ultimately, high levels of NO are produced from epithelial cells by the induction of iNOS.1 Therefore, the effect of air pollution on airway inflammation can be detected by FeNO measurement.
Emerging evidence indicates that air pollution is associated with FeNO.26,27 Therefore, FeNO could be used as an indirect biomarker of airway inflammation in population-based epidemiological research to assess the impact of ambient air pollution on children's respiratory health. Short-term increases in ambient air pollutants, such as PM2.5, PM10, and O3, have been associated with higher levels of FeNO in children, independent of asthma and allergic status (Table).26 Additionally, data from the Southern California Children's Health Study have shown that increases in annual average exposure to ambient air pollutants, such as NO2 and PM2.5, are significantly associated with increased FeNO in children, independent of short-term exposure to ambient air pollution and asthma status.27
FeNO levels have been correlated with personal and ambient air pollution in children with asthma.28 In 2 pollutant models, FeNO levels were strongly positively associated with personal and ambient elemental carbon and NO2, and with personal, but not with ambient PM2.5. Therefore, to evaluate the effects of air pollution on airway inflammation using FeNO, the type of air pollutants and measurement methods need to be carefully selected.
Most of the literature shows that FeNO assessment is a useful measurement to evaluate the effects of air pollutant exposure in children. There are some inconsistent results with regard to the study population (i.e., atopic vs nonatopic and asthmatic vs nonasthmatic), treatment (i.e., naïve asthma vs after corticosteroid treatment), asthma condition (i.e., asthma controlled vs uncontrolled and stable asthma vs during asthma exacerbation), and various levels of exposure (i.e., personal vs ambient exposure, level of exposure, and short term vs long term exposure). 27 Therefore, further research on the usefulness of FeNO to identify the adverse respiratory effects of the exposure to pollutants in children is needed.
2) Indoor air pollution
Indoor pollutants that are related to housing conditions have been associated with airway inflammation in children. Because people generally spend most of their time indoors, indoor air pollution plays an important role in their health problems, especially in early childhood. Indoor pollutants affect airway inflammation through complex interrelationships with outdoor pollution as well as triggering allergic responses in asthmatic patients.29 Therefore, FeNO is potentially useful for measuring adverse effects of indoor air pollution on respiratory health.
Electric baseboard heating is reported to be associated with higher FeNO levels compared to forced air and hot water radiant heating (Table).30 The authors speculated that forced air heating is associated with lower indoor dust mite levels, whereas electric heating increases indoor dust mite levels and is related to higher formaldehyde concentration, thus increasing the allergic sensitization and levels of FeNO.31,32 In addition, there are 2 conflicting studies reporting the association between polyvinyl chloride material and FeNO levels.33,34
3) Smoking
There are many studies investigating the effects of smoking on FeNO. In healthy and asthmatic adults, both active smoking and acute passive smoke exposure lead to a transient decrease in FeNO levels.11 There are limited data documenting the association between passive smoke and FeNO levels in healthy children. The reported associations between smoking exposure and FeNO levels in asthmatic children have been discordant, probably due to methodological biases, such as small sample sizes, heterogeneous study populations, and lack of control for potential confounding factors.30,35,36 Some studies have not found a significant effect of passive smoking on FeNO in children, 30,36 whereas others suggested that environmental tobacco smoke lowers FeNO levels (Table).27,35 According to the type of smoke exposure, acute exposure may induce a marked, but transient reduction in FeNO levels related to a negative feedback of iNOS activity, since tobacco smoke contains high concentrations of NO.27 The mechanism for the effect of daily exposure to tobacco smoke could be hypothesized as a progressive negative feedback leading to the inhibition of iNOS gene expression. These data suggest that low-level exposure to second hand tobacco smoke may be an important factor to consider in interpreting FeNO as a biomarker of airway inflammation.
There are genetic variants modulating the effect of nicotine exposure on FeNO. Children with at least 1 T allele in NO synthase gene (NOS3) G894T showed decreased levels of FeNO when exposed to high concentrations of nicotine, possibly caused by decreased enzyme activity.37 In contrast, nicotine exposure did not affect FeNO levels in children with GG genotype. The conflicting results of the effect of smoking studies on FeNO levels may be explained in part by genetic differences in study participants.
Clinical application of FeNO measurement
The use of FeNO in the clinical setting is an emerging area. There is limited literature for the use of FeNO as an assessment tool in respiratory diseases, such as chronic obstructive pulmonary disease, pulmonary hypertension, primary ciliary dyskinesia, and cystic fibrosis.2 However, there are many reports on the use of FeNO to evaluate airway inflammation in allergic airway disease.
Asthma
1) FeNO and asthma phenotype
The decision to treat asthmatic children with inhaled corticosteroids (ICS) or other medications for asthma control can be informed by assessing phenotypes of wheezing and asthma. FeNO is a useful biomarker to measure the degree of eosinophilic airway inflammation when evaluating the effectiveness of chosen therapies. The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study, a prospective birth cohort study in the Netherlands, evaluated the association of 5 wheezing phenotypes with FeNO.38 FeNO measured at 4 years of age was higher in children with intermediate-onset wheezing and persistent wheezing compared to children with never and transient wheezing. FeNO at 8 years of age differed between all phenotypes, with the highest FeNO levels for persistent, intermediate-onset, and late-onset wheezing. The increase in FeNO from 4 to 8 years in children with intermediate- and late-onset wheeze was higher compared to the increase in FeNO in children who never wheezed and with transient wheezing.
Another phenotype of asthma was defined by obesity in nonatopic asthma and adult-onset asthma.39 In the INTERGENE cohort study, wheezing is negatively associated with FeNO in obese adults but positively associated with FeNO in nonobese adults.40 In late-onset asthma (>12 years old), FeNO had an inverse relationship with body mass index, which was explained by the plasma ratio of L-arginine to asymmetric dimethyl arginine (ADMA).41 Therefore, obesity may be a factor that worsens asthma, but reduces FeNO levels.
2) The use of FeNO for asthma diagnosis
FeNO levels are typically higher in asthmatic patients than in healthy subjects, but the respective distributions of FeNO have substantial overlap. Recent studies have reported central values for the distribution of FeNO in healthy children, but the upper limit has varied widely depending on study populations. Significant factors that affected normal FeNO ranges were age, gender, and race/ethnicity.7,8,9 However, FeNO levels have been shown to be elevated in selected groups of children with asthma compared to healthy children.1,2 Based on these findings, FeNO has been used for the assessment of airway inflammation in asthma.
Among inflammatory phenotypes in asthma, the eosinophilic phenotype is associated with FeNO.42 FeNO was higher in patients with the persistent eosinophilic phenotype of severe refractory asthma compared to those with the noneosinophilic phenotype.43 Among many tests to diagnose and/or monitor asthma, FeNO is a useful tool for evaluating eosinophilic airway inflammation.
A cutoff point is useful to interpret FeNO measurements for clinical diagnostic decision. Many studies have investigated the usefulness of FeNO to distinguish asthmatics from nonasthmatics. Cutoff values for FeNO have been calculated based on receiving operator characteristic (ROC) curves with a wide range of sensitivities and specificities.44 When using FeNO as a diagnostic tool for asthma, a high value of FeNO alone does not equate to asthma. Cutoff values for FeNO that define asthma still do not clearly differentiate asthmatics from healthy children. However, based on previous reports, the ATS guidelines recommend that cutoff points of FeNO levels at <20 ppb indicate a decreased likeliness of eosinophilic inflammation and >35 ppb can be used to indicate eosinophilic inflammation in steroid-naïve children with respiratory symptoms.8 Compared to conventional tests like spirometry, broncho-provocation and induced sputum, using a cutoff point of >20 ppb for FeNO has greater accuracy in the diagnosis of childhood asthma.45 Further research is needed for establishing a single cut point to improve the utility of FeNO in asthma diagnosis.
3) FeNO for predicting asthma onset
Considering the natural history of asthma, predicting the development of new-onset asthma in children may be important for targeting prevention efforts. FeNO is a practical noninvasive measure for children to perform during the period of greatest asthma incidence-the preschool age. In a cohort of preschool children, FeNO levels were elevated in those who developed asthma at school age compared to children who did not develop asthma.46 For schoolchildren, without a parental history of asthma, elevated FeNO was associated with an increased risk for new-onset asthma in a 3-year follow-up study.22 Irrespective of a history of respiratory allergy, similar patterns of increasing risk of new-onset asthma were shown by increasing quartiles of FeNO.
4) Measuring FeNO to guide asthma management and monitor asthma control
Biomarkers that can measure the responsiveness to the therapy are urgently needed to guide decisions on the use of specific medications for childhood asthma. Recently, FeNO has been used as a biomarker for assessing clinical asthma control of the underlying inflammatory disease process.3 FeNO has been correlated with indicators of asthma control, such as asthma symptoms, dyspnea score, the use of rescue medications, and the reversibility of airflow obstruction.47 Some reports suggest that FeNO predicts the likelihood of ICS responsiveness more consistently than spirometry, bronchodilator response or methacholine bronchial challenge tests.8
In the ATS/ERS guidelines, FeNO <25 ppb in children is suggested as a strong indicator of unlikely ICS responsiveness, whereas FeNO >35 ppb in children as a strong indicator of likely ICS responsiveness.8 Several studies have presented FeNO as a sensitive predictor of exacerbation or relapse of asthma after steroid withdrawal in children.48,49 In one study, a cutoff value of 49 ppb for FeNO produced a sensitivity/specificity for future relapse of 71%/93%. When FeNO was <22 ppb, it was useful for predicting successful steroid reduction.48 Another study in children presented 22.9 ppb as a FeNO cutoff with a sensitivity of 80% and a specificity of 60% for predicting exacerbation.50 Emerging information indicates that assessment of within-individual changes in FeNO may be a more accurate method for evaluating responses to therapy.
In general for children, elevated eosinophil counts in sputum may be more sensitive for predicting asthma exacerbations or loss of asthma control after steroid reduction than elevated FeNO.49 This is because high FeNO is not always pathological, whereas induced sputum eosinophil counts >1% is abnormal. An induced sputum test with no eosinophils is very useful in predicting that exacerbations will not occur.49 However, an induced sputum test is more difficult to perform than FeNO measurements in young children, especially in those with poor lung function. In addition, less than half of children can produce an adequate sputum sample. For these reasons, decisions in asthma management in young children were previously based on symptoms and lung function tests. FeNO measurements can provide additional information for asthma therapy, since steroid therapy contributes to increased airway neutrophilia as well as eosinophilia. For example, even when asthma patients do not show sputum eosinophilia, FeNO levels are highly predictive of ICS response.51 Additionally, FeNO levels have been shown to decrease in response to asthma treatments, including systemic steroids,17 ICS,52 and montelukast.18
Despite the evidence that FeNO or changes in FeNO can predict asthma exacerbation, there are conflicting results showing a negative correlation between FeNO levels and exacerbation rates/prediction of future asthma risk.53 For example, some patients have persistently high FeNO levels despite treatment. This implies that FeNO alone should not be incorporated into current treatment guidelines.
Although some studies suggest a beneficial effect of the use of FeNO as a guide of asthma treatment,52 in general the use of FeNO measurement has not resulted in improved asthma control. One meta-analysis found that adjusting ICS dose using FeNO was not different compared to conventional methods.54 Another meta-analysis questioning the utility of FeNO to tailor the dose of ICS in children showed that it cannot be recommended for routine clinical practice because of the risk of increasing ICS doses without any effect on asthma exacerbations. 55 This report emphasized the need to consider many disease- and nondisease-related factors that could confound the interpretation of FeNO levels. The role of FeNO in the management of childhood asthma is not yet defined, hence further investigations are needed.
Allergic rhinitis
Allergic rhinitis (AR) is well known as the common form of rhinitis with cardinal symptoms of nasal itching, sneezing, rhinorrhea, and nasal congestion. Although the prevalence of AR varies among countries, the prevalence has been continuously increasing worldwide. In addition, the age of onset is decreasing. In a European study of children aged 3-5 years, the prevalence of AR was found to be 16.8%.56 It is difficult to differentiate between AR and other common diseases, such as nonallergic rhinitis (NAR) and chronic rhinosinusitis, since those are common and have similar symptoms to AR in young children. FeNO could be used in the assessment of childhood rhinitis.
FeNO analysis is well known for measuring eosinophilic airway inflammation in bronchial asthma. Many studies have shown increased FeNO in adults with AR. Although the literature on FeNO measurement in children with AR is less extensive, FeNO is increased in children with AR.57,58
A considerable body of evidence suggests that rhinitis and asthma represent the manifestations of one airway disease of common pathogenic pathway with a wide spectrum of severity. 59 Increased FeNO in patients with AR probably reflects the extension of inflammation throughout the airways, a feature in the well-known concept of the 'united airway.' It was reported that diffusing capacity of NO in the bronchial wall of patients with AR at the symptomatic stage was higher than that of healthy controls. This might reflect changes in the physical properties of bronchial mucosa, which is induced by subclinical lower airway inflammation in AR.60
There are some conflicting data of FeNO among 3 groups: AR, AR with allergic asthma, and asthma. FeNO levels are higher in the asthma group than in the AR group.57 A study with children has shown that median and interquartile range of FeNO levels are significantly different in the 3 groups of combined asthma with rhinitis, asthma, and rhinitis.61 On the other hand, another study has shown similarly elevated FeNO in children with only AR and in those with combined AR and asthma.62 Extended NO analysis has found that the airway transfer factor is increased in the AR only group, similar to the asthma only group, while airway wall concentration of NO is increased in the asthma only group, but not in the AR only group.60 The main factors that need to be taken into consideration when interpreting FeNO among groups are asthma phenotype (atopic vs nonatopic or eosinophilic vs neutrophilic), asthma severity or control status, and AR severity in each study. Further studies with well-defined subgroups with large sample size will clarify these issues.
AR is a risk factor for developing clinical asthma. Moreover, AR is often associated with bronchial hyperresponsiveness (BHR). The prevalence of BHR in nonasthmatic children with AR ranges from about 30% to 60%. It has been suggested that patients with AR and BHR are at higher risk of developing asthma. 63 Some recent studies have highlighted that FeNO is highly correlated with degree of BHR.60,61 In a children's study, FeNO was higher in the AR group with BHR than in the AR group without BHR. A moderate inverse correlation was found between FeNO levels and methacholine PC20 in both patients with asthma and those with asthma and rhinitis (r=-0.63 and r=-0.61, respectively).61 On the other hand, there were some conflicting results possibly due to different study subjects, small sample size, and lack of control for confounding factors. To evaluate whether FeNO is helpful to detect BHR in AR, further additional studies with larger sample sizes are needed.
A study showed that FeNO was higher in the persistent AR group, while nasal NO was higher in the intermittent AR group. Similarly, FeNO was higher in the moderate-severe AR group than in the mild group, whereas nasal NO was higher in the mild AR group than in the moderate-severe group.58
A population-based cohort study of school children in southern California using an innovative quantile regression approach showed a significantly larger effect of active rhinitis on FeNO in children with high FeNO concentrations than those with low concentrations. The difference in the 80th percentiles of FeNO for children with and without active rhinitis in the last 7 days was more than 6 times larger than the difference in the 20th percentiles of the 2 groups (unpublished data). Nasal symptoms and FeNO have been shown to be moderately correlated.62 Another study found a weakly positive correlation between FeNO and nasal symptoms of watery rhinorrhea and sneezing in children with AR.58 While FeNO decreased after treatment with intranasal corticosteroid and/or montelukast, there was no change in FeNO after treatment with antihistamines in children with perennial AR.64
A previous study also found that higher FeNO levels at baseline were associated with increased risk for new-onset and persistent rhinitis after analyzing follow-up data of 959 randomly selected adolescents (13-14 years) for 4 years.6
Further uses for FeNO measures
Unlike other exhaled gases, NO is produced in the airway wall and has a relatively low concentration in the distal alveolar region. FeNO measured at a single constant expiratory flow rate cannot differentiate between proximal and distal sources of NO. Measurements of FeNO at multiple flow rates ("extended NO" analysis) can be used to estimate parameters that quantify both proximal and distal sources of NO.27 Mathematical models of varying complexity have been developed to describe the production and dynamics of NO in the lower respiratory tract.66,67,68 During exhalation, air passing through the lower respiratory tract is enriched with NO from the bronchial walls. Since the conventional 50 mL/s flow rate is relatively slow, FeNO at 50 mL/s (FeNO50) predominantly reflects NO from the proximal airway.68 In contrast, exhaled NO from a high constant expiratory flow rate is more reflective of NO from the distal alveolar region because the air has had less time to become enriched with NO from the bronchial walls on its transit through the airways.
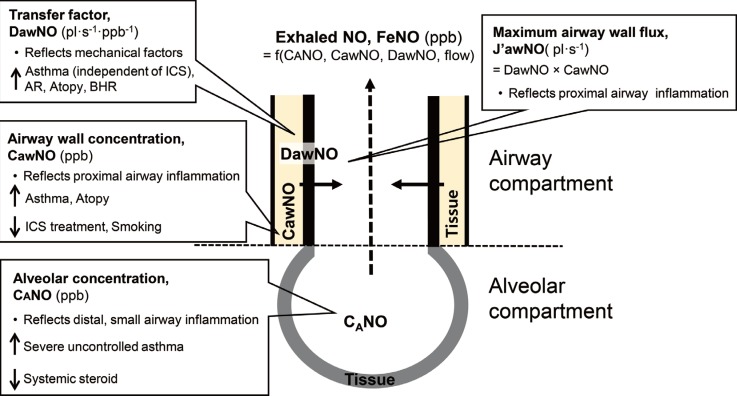
As shown in Fig. 2, three flow-independent parameters from the two-compartment models can be estimated, 1 describing the alveolar region and 2 describing the airway region. The primary parameter of interest is the concentration of NO in the alveolar region, CANO (ppb). CANO is typically <5 ppb and has been found to be elevated in severe asthma and potentially reduced by oral prednisone, since it reflects distal airway inflammation. 66,69 Increased CANO had significantly worse asthma control and morbidity in children with asthma.
Fig. 2. Schematic diagram of two compartments of alveolar NO and airway NO. During exhalation, gas with an NO in the alveolar region, CANO (ppb) passes through the airways. During its passage, NO diffuses from the airway walls to the bronchial lumen. Thus, the concentration of exhaled NO, FeNO is a function of J'awNO and CANO. The maximal flux of NO from the airway wall into the lumen, J'awNO (pl·s-1) is the product of the airway wall concentration of NO, CawNO (ppb) and airway tissue diffusing capacity, DawNO (pl·s-1·ppb-1).
Other parameters from extended NO analysis in the airway region include the airway tissue diffusing capacity (or "transfer factor" for NO from the airway wall to the gas stream), DawNO (pl·s-1·ppb-1), and either the maximal flux of NO from the airway wall into the lumen, J'awNO (pl·s-1) or the airway wall concentration of NO, CawNO (ppb).66 DawNO depends on both the physical features of the airway wall (airway surface area or tissue thickness) and the rate of production and consumption.70 DawNO showed a significant relationship with the caliber of the airways. DawNO was inversely correlated with both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) in asthma patients.70 DawNO has been found to be elevated in children with atopy, AR, and allergic asthma, and not to be affected by inhaled corticosteroids.60,68 J'awNO is simply the product of DawNO×CawNO, the maximal flux which would occur if the concentration of NO in the airway gas was zero.66 Because J'awNO is highly correlated with FeNO at 50 mL/s (typically, Pearson's correlation >0.95), it offers little additional information.69 CawNO is considered to reflect proximal airway inflammation. CawNO increases in asthma and decreases in treatment with inhaled corticosteroid.70 IgE sensitization has been related to increases in both DawNO and CawNO.
Extended NO requires an instrument capable of measuring FeNO at multiple flow rates (e.g., CLD88-SP from EcoMedics, Duernten, Switzerland). Some of the instruments in most widespread use clinically (e.g., NIOX MINO or NIOX VERO, Aerocrine, Sweden) assess FeNO at only 50 mL/s. No consensus has been reached on the sampling protocol for extended NO analysis, but most researchers use 2-3 measurements per flow rate at 2-4 flow rates, ranging from 10 mL/s to ~350 mL/s, with a narrower range for children.66,67 Numerous statistical methods have been developed to estimate parameters from the simple and robust 2-compartment model. Simple linear approximation methods require only 2 high flow rates (e.g. >50 mL/s) and produce estimates only of CANO and J'awNO, while more refined methods require at least 3 flow rates (low, medium, and high) to estimate all 3 NO parameters.68 Although extended NO analysis is a promising method for noninvasive assessment of localized airway inflammation, its assessment should be standardized, like FeNO at 50 mL/s, to ensure successful translation to clinical use. Further studies are needed to develop and evaluate the utility of more accurate models of NO dynamics and to identify the role of extended NO analysis as a tool for monitoring of asthma.
Although numerous studies have investigated demographic and disease-related determinants of FeNO, considerable between-subject heterogeneity remains unexplained. In particular, some subjects have high FeNO with no apparent cause. Quantile regression, which estimates the difference in a specific quantile of FeNO (rather than the mean) associated with a potential determinant, may be a useful statistical tool for better understanding sources of variation at high levels of FeNO.
CONCLUSIONS
FeNO measurements are simple and easy to perform for most populations, especially for young children, and are therefore useful in evaluating airway inflammation. FeNO is helpful in research settings to study the etiology of respiratory symptoms, especially to identify the eosinophilic asthma phenotype. Increased FeNO is associated with new-onset asthma. FeNO can predict wheeze onset and also can be used to monitor the airway condition or treatment effect in allergic diseases. However, there is limited evidence that FeNO measured at a single point in time or at a constant expiratory flow rate provides an additional benefit for assessment of asthma control. Longitudinal assessment of FeNO in patients and/or extended NO analysis may play more roles in monitoring asthma control and guiding treatment.
Additionally, physicians should know whether patients have AR when they use FeNO as a diagnostic tool for asthma or monitoring tool for treatment efficacy. FeNO may be elevated in AR patients who do not have concomitant asthma. In addition, for population-based epidemiological research, this noninvasive biomarker allows evaluation of airway inflammation caused by air pollutants. Therefore, FeNO level could be a useful intermediate marker to detect the risk of adverse respiratory health outcomes in susceptible children.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Ricciardolo FL. Revisiting the role of exhaled nitric oxide in asthma. Curr Opin Pulm Med. 2014;20:53–59. doi: 10.1097/MCP.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 2.Pijnenburg MW, De Jongste JC. Exhaled nitric oxide in childhood asthma: a review. Clin Exp Allergy. 2008;38:246–259. doi: 10.1111/j.1365-2222.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- 3.Verini M, Consilvio NP, Di Pillo S, Cingolani A, Spagnuolo C, Rapino D, et al. FeNO as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy (Cairo) 2010;2010:691425. doi: 10.1155/2010/691425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristescu SM, Mandon J, Harren FJ, Meriläinen P, Högman M. Methods of NO detection in exhaled breath. J Breath Res. 2013;7:017104. doi: 10.1088/1752-7155/7/1/017104. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 6.Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2008;133:169–175. doi: 10.1378/chest.07-1177. [DOI] [PubMed] [Google Scholar]
- 7.Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res. 2009;10:28. doi: 10.1186/1465-9921-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HJ, Jung YH, Yang SI, Lee E, Kim HY, Seo JH, et al. Reference values and determinants of fractional concentration of exhaled nitric oxide in healthy children. Allergy Asthma Immunol Res. 2014;6:169–174. doi: 10.4168/aair.2014.6.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olin AC, Aldenbratt A, Ekman A, Ljungkvist G, Jungersten L, Alving K, et al. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir Med. 2001;95:153–158. doi: 10.1053/rmed.2000.1010. [DOI] [PubMed] [Google Scholar]
- 11.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 12.Kippelen P, Caillaud C, Robert E, Masmoudi K, Préfaut C. Exhaled nitric oxide level during and after heavy exercise in athletes with exercise-induced hypoxaemia. Pflugers Arch. 2002;444:397–404. doi: 10.1007/s00424-002-0816-y. [DOI] [PubMed] [Google Scholar]
- 13.Taylor ES, Smith AD, Cowan JO, Herbison GP, Taylor DR. Effect of caffeine ingestion on exhaled nitric oxide measurements in patients with asthma. Am J Respir Crit Care Med. 2004;169:1019–1021. doi: 10.1164/rccm.200310-1473OC. [DOI] [PubMed] [Google Scholar]
- 14.Bruce C, Yates DH, Thomas PS. Caffeine decreases exhaled nitric oxide. Thorax. 2002;57:361–363. doi: 10.1136/thorax.57.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadish T, Soferman R, Merimovitch T, Fireman E, Sivan Y. Exhaled nitric oxide in acute respiratory syncytial virus bronchiolitis. Arch Pediatr Adolesc Med. 2010;164:727–731. doi: 10.1001/archpediatrics.2010.128. [DOI] [PubMed] [Google Scholar]
- 16.Peña Zarza JA, Osona B, Gil-Sanchez JA, Figuerola J. Exhaled nitric oxide in acute phase of bronchiolitis and its relation with episodes of subsequent wheezing in children of preschool age. Pediatr Allergy Immunol Pulmonol. 2012;25:92–96. doi: 10.1089/ped.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax. 2000;55:232–234. doi: 10.1136/thorax.55.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisgaard H, Loland L, Oj JA. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am J Respir Crit Care Med. 1999;160:1227–1231. doi: 10.1164/ajrccm.160.4.9903004. [DOI] [PubMed] [Google Scholar]
- 19.Deykin A, Halpern O, Massaro AF, Drazen JM, Israel E. Expired nitric oxide after bronchoprovocation and repeated spirometry in patients with asthma. Am J Respir Crit Care Med. 1998;157:769–775. doi: 10.1164/ajrccm.157.3.9707114. [DOI] [PubMed] [Google Scholar]
- 20.Cibella F, Cuttitta G, La Grutta S, Passalacqua G, Viegi G. Factors that influence exhaled nitric oxide in Italian schoolchildren. Ann Allergy Asthma Immunol. 2008;101:407–412. doi: 10.1016/S1081-1206(10)60318-3. [DOI] [PubMed] [Google Scholar]
- 21.Barreto M, Villa MP, Monti F, Bohmerova Z, Martella S, Montesano M, et al. Additive effect of eosinophilia and atopy on exhaled nitric oxide levels in children with or without a history of respiratory symptoms. Pediatr Allergy Immunol. 2005;16:52–58. doi: 10.1111/j.1399-3038.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 22.Bastain TM, Islam T, Berhane KT, McConnell RS, Rappaport EB, Salam MT, et al. Exhaled nitric oxide, susceptibility and new-onset asthma in the Children's Health Study. Eur Respir J. 2011;37:523–531. doi: 10.1183/09031936.00021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouaville LF, Annesi-Maesano I, Nguyen LT, Bocage AS, Bedu M, Caillaud D. Interrelationships among asthma, atopy, rhinitis and exhaled nitric oxide in a population-based sample of children. Clin Exp Allergy. 2003;33:1506–1511. doi: 10.1046/j.1365-2222.2003.01800.x. [DOI] [PubMed] [Google Scholar]
- 24.van Amsterdam JG, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, et al. The relationship between exhaled nitric oxide and allergic sensitization in a random sample of school children. Clin Exp Allergy. 2003;33:187–191. doi: 10.1046/j.1365-2222.2003.01597.x. [DOI] [PubMed] [Google Scholar]
- 25.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 26.Berhane K, Zhang Y, Linn WS, Rappaport EB, Bastain TM, Salam MT, et al. The effect of ambient air pollution on exhaled nitric oxide in the Children's Health Study. Eur Respir J. 2011;37:1029–1036. doi: 10.1183/09031936.00081410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berhane K, Zhang Y, Salam MT, Eckel SP, Linn WS, Rappaport EB, et al. Longitudinal effects of air pollution on exhaled nitric oxide: the Children's Health Study. Occup Environ Med. 2014;71:507–513. doi: 10.1136/oemed-2013-101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect. 2006;114:1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viegi G, Simoni M, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L, et al. Indoor air pollution and airway disease. Int J Tuberc Lung Dis. 2004;8:1401–1415. [PubMed] [Google Scholar]
- 30.Kovesi TA, Dales RE. Effects of the indoor environment on the fraction of exhaled nitric oxide in school-aged children. Can Respir J. 2009;16:e18–e23. doi: 10.1155/2009/954382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert NL, Gauvin D, Guay M, Héroux ME, Dupuis G, Legris M, et al. Housing characteristics and indoor concentrations of nitrogen dioxide and formaldehyde in Quebec City, Canada. Environ Res. 2006;102:1–8. doi: 10.1016/j.envres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Burge HA, Friedman W, et al. House dust mite allergen in US beds: results from the First National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2003;111:408–414. doi: 10.1067/mai.2003.16. [DOI] [PubMed] [Google Scholar]
- 33.Tuomainen A, Stark H, Seuri M, Hirvonen MR, Linnainmaa M, Sieppi A, et al. Experimental PVC material challenge in subjects with occupational PVC exposure. Environ Health Perspect. 2006;114:1409–1413. doi: 10.1289/ehp.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolarik B, Lagercrantz L, Sundell J. Nitric oxide in exhaled and aspirated nasal air as an objective measure of human response to indoor air pollution. Indoor Air. 2009;19:145–152. doi: 10.1111/j.1600-0668.2008.00572.x. [DOI] [PubMed] [Google Scholar]
- 35.Laoudi Y, Nikasinovic L, Sahraoui F, Grimfeld A, Momas I, Just J. Passive smoking is a major determinant of exhaled nitric oxide levels in allergic asthmatic children. Allergy. 2010;65:491–497. doi: 10.1111/j.1398-9995.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 36.Dinakar C, Lapuente M, Barnes C, Garg U. Real-life environmental tobacco exposure does not affect exhaled nitric oxide levels in asthmatic children. J Asthma. 2005;42:113–118. [PubMed] [Google Scholar]
- 37.Spanier AJ, Kahn RS, Hornung RW, Wang N, Sun G, Lierl MB, et al. Environmental exposures, nitric oxide synthase genes, and exhaled nitric oxide in asthmatic children. Pediatr Pulmonol. 2009;44:812–819. doi: 10.1002/ppul.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Valk RJ, Caudri D, Savenije O, Koppelman GH, Smit HA, Wijga AH, et al. Childhood wheezing phenotypes and FeNO in atopic children at age 8. Clin Exp Allergy. 2012;42:1329–1336. doi: 10.1111/j.1365-2222.2012.04010.x. [DOI] [PubMed] [Google Scholar]
- 39.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg CM, Thelle DS, Rosengren A, Lissner L, Torén K, Olin AC. Decreased fraction of exhaled nitric oxide in obese subjects with asthma symptoms: data from the population study INTERGENE/ADONIX. Chest. 2011;139:1109–1116. doi: 10.1378/chest.10-1299. [DOI] [PubMed] [Google Scholar]
- 41.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187:153–159. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–387. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–1255. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Majid H, Kao C. Utility of exhaled nitric oxide in the diagnosis and management of asthma. Curr Opin Pulm Med. 2010;16:42–47. doi: 10.1097/MCP.0b013e328332ca46. [DOI] [PubMed] [Google Scholar]
- 45.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, et al. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004;169:473–478. doi: 10.1164/rccm.200310-1376OC. [DOI] [PubMed] [Google Scholar]
- 46.Singer F, Luchsinger I, Inci D, Knauer N, Latzin P, Wildhaber JH, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy. 2013;68:531–538. doi: 10.1111/all.12127. [DOI] [PubMed] [Google Scholar]
- 47.Sippel JM, Holden WE, Tilles SA, O'Hollaren M, Cook J, Thukkani N, et al. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000;106:645–650. doi: 10.1067/mai.2000.109618. [DOI] [PubMed] [Google Scholar]
- 48.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–218. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zacharasiewicz A, Wilson N, Lex C, Erin EM, Li AM, Hansel T, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171:1077–1082. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 50.Fritsch M, Uxa S, Horak F, Jr, Putschoegl B, Dehlink E, Szepfalusi Z, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol. 2006;41:855–862. doi: 10.1002/ppul.20455. [DOI] [PubMed] [Google Scholar]
- 51.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65:384–390. doi: 10.1136/thx.2009.126722. [DOI] [PubMed] [Google Scholar]
- 52.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 53.Menzies D, Jackson C, Mistry C, Houston R, Lipworth BJ. Symptoms, spirometry, exhaled nitric oxide, and asthma exacerbations in clinical practice. Ann Allergy Asthma Immunol. 2008;101:248–255. doi: 10.1016/S1081-1206(10)60489-9. [DOI] [PubMed] [Google Scholar]
- 54.Petsky HL, Cates CJ, Li A, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2009:CD006340. doi: 10.1002/14651858.CD006340.pub3. [DOI] [PubMed] [Google Scholar]
- 55.Jartti T, Wendelin-Saarenhovi M, Heinonen I, Hartiala J, Vanto T. Childhood asthma management guided by repeated FeNO measurements: a meta-analysis. Paediatr Respir Rev. 2012;13:178–183. doi: 10.1016/j.prrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Peroni DG, Piacentini GL, Alfonsi L, Zerman L, Di Blasi P, Visona G, et al. Rhinitis in pre-school children: prevalence, association with allergic diseases and risk factors. Clin Exp Allergy. 2003;33:1349–1354. doi: 10.1046/j.1365-2222.2003.01766.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim YH, Park HB, Kim MJ, Kim HS, Lee HS, Han YK, et al. Fractional exhaled nitric oxide and impulse oscillometry in children with allergic rhinitis. Allergy Asthma Immunol Res. 2014;6:27–32. doi: 10.4168/aair.2014.6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee KJ, Cho SH, Lee SH, Tae K, Yoon HJ, Kim SH, et al. Nasal and exhaled nitric oxide in allergic rhinitis. Clin Exp Otorhinolaryngol. 2012;5:228–233. doi: 10.3342/ceo.2012.5.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–1183. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- 60.Makinen T, Lehtimäki L, Kinnunen H, Nieminen R, Kankaanranta H, Moilanen E. Bronchial diffusing capacity of nitric oxide is increased in patients with allergic rhinitis. Int Arch Allergy Immunol. 2009;148:154–160. doi: 10.1159/000155746. [DOI] [PubMed] [Google Scholar]
- 61.Ciprandi G, Tosca MA, Capasso M. Exhaled nitric oxide in children with allergic rhinitis and/or asthma: a relationship with bronchial hyperreactivity. J Asthma. 2010;47:1142–1147. doi: 10.3109/02770903.2010.527026. [DOI] [PubMed] [Google Scholar]
- 62.de Bot CM, Moed H, Bindels PJ, van Wijk RG, Berger MY, de Groot H, et al. Exhaled nitric oxide measures allergy not symptoms in children with allergic rhinitis in primary care: a prospective cross-sectional and longitudinal cohort study. Prim Care Respir J. 2013;22:44–50. doi: 10.4104/pcrj.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulrik CS, Backer V, Hesse B, Dirksen A. Risk factors for development of asthma in children and adolescents: findings from a longitudinal population study. Respir Med. 1996;90:623–630. doi: 10.1016/s0954-6111(96)90021-9. [DOI] [PubMed] [Google Scholar]
- 64.Hung CH, Hua YM, Hsu WT, Lai YS, Yang KD, Jong YJ, et al. Montelukast decreased exhaled nitric oxide in children with perennial allergic rhinitis. Pediatr Int. 2007;49:322–327. doi: 10.1111/j.1442-200X.2007.02375.x. [DOI] [PubMed] [Google Scholar]
- 65.Malinovschi A, Alving K, Kalm-Stephens P, Janson C, Nordvall L. Increased exhaled nitric oxide predicts new-onset rhinitis and persistent rhinitis in adolescents without allergic symptoms. Clin Exp Allergy. 2012;42:433–440. doi: 10.1111/j.1365-2222.2011.03947.x. [DOI] [PubMed] [Google Scholar]
- 66.George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol (1985) 2004;96:831–839. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 67.Högman M. Extended NO analysis in health and disease. J Breath Res. 2012;6:047103. doi: 10.1088/1752-7155/6/4/047103. [DOI] [PubMed] [Google Scholar]
- 68.Eckel SP, Linn WS, Berhane K, Rappaport EB, Salam MT, Zhang Y, et al. Estimation of parameters in the two-compartment model for exhaled nitric oxide. PLoS One. 2014;9:e85471. doi: 10.1371/journal.pone.0085471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sardón O, Corcuera P, Aldasoro A, Korta J, Mintegui J, Emparanza JI, et al. Alveolar nitric oxide and its role in pediatric asthma control assessment. BMC Pulm Med. 2014;14:126. doi: 10.1186/1471-2466-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin HW, Rose-Gottron CM, Cooper DM, Newcomb RL, George SC. Airway diffusing capacity of nitric oxide and steroid therapy in asthma. J Appl Physiol (1985) 2004;96:65–75. doi: 10.1152/japplphysiol.00575.2003. [DOI] [PubMed] [Google Scholar]