Abstract
Background
The number of people suffering from balding or hair thinning is increasing, despite the advances in various medical therapies. Therefore, it is highly important to develop new therapies to inhibit balding and increase hair proliferation.
Objective
We investigated the effects of herbal extracts commonly used for improving balding in traditional medicine to identify potential agents for hair proliferation.
Methods
The expression levels of 5α-reductase isoforms (type I and II) were analyzed using quantitative real-time reverse transcription polymerase chain reaction in the human follicular dermal papilla cells (DPCs). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylteterazolium bromide and bromodeoxyuridine tests were used to evaluate the cell proliferation effect of herbal extracts in DPCs. The expression levels of extracellular signal-regulated kinase (ERK), Akt, cyclin D1, cyclin-dependent kinase 4 (Cdk4), B-cell lymphoma (Bcl-2) and Bcl-2-associated X protein (Bax) were measured using western blot analysis.
Results
The 5α-reductase isoform mRNAs and proteins were detected in the cultured DPCs, and the expression level of 5α-R2 in DPCs in the presence of the herbal extracts was gradually decreased. Herbal extracts were found to significantly increase the proliferation of human DPCs at concentrations ranging from 1.5% to 4.5%. These results show that the herbal extracts tested affected the protein expressions of ERK, Akt, cyclin D1, Cdk4, Bcl-2, and Bax in DPCs.
Conclusion
These results suggest that herbal extracts exert positive effects on hair proliferation via ERK, Akt, cyclin D1, and Cdk4 signaling in DPCs; they also suggest that herbal extracts could be a great alternative therapy for increasing hair proliferation.
Keywords: Dermal papilla cell, Hair follicle, Herbal extracts, Proliferation
INTRODUCTION
The turnover of hair follicles occurs in three steady phases, namely proliferation (anagen), involution (catagen), and resting (telogen)1.
Androgens control the proliferation of human hair, which responds to hormones differently depending on the body location2. Dermal papilla cells (DPCs) of the beard, and armpit, and scalp hair of people who are genetically predisposed to baldness were shown to be androgen target cells3.
The binding of androgens to their androgen receptors (ARs) decreases the anagen phase of the hair cycle. DPCs have particularly saturable ARs, and are proliferated from androgen-responsive follicles4. Compared to testosterone (T), 5α-dihydrotestosterone (DHT) has an approximately five-fold higher affinity for the AR. In some androgen sensitive organs such as the prostate, DHT rather than T shows androgenic activity.
The enzyme 5α-reductase (5α-R) converts T to DHT5 and this conversion enhance the androgenic signal via two mechanisms: (i) DHT can not be aromatized to estrogen and, therefor, its effect are solely androgenic and (ii) in vitro DHT binds to the AR with A higher affinity than T does6. There are two 5α-reductase isozymes, type I and II (5α-R1 and 5α-R2, respectively) that are coded by two different genes namely, steroid 5-α-reductase type I (SRD5A1) and SRD5A2 on chromosomes 5 and 2, respectively7. Type I is predominantly expressed in the skin while type II exists in the prostate tissue. It has been established that 5α-R1 and 5α-R2 have pivotal roles in the metabolism of androgen. However, the role of the 5αR and the molecular mechanisms responsible for regulating hair proliferation have not been elucidated.
The number of male and female members of the population suffering from balding or hair thinning is increasing, despite the advances of various medical therapies. Therefor, it is highly necessary to develp new therapies that inhibit balding and increase hair proliferation. Alternative medicine options have not yet been incorporated in mainstream medical practice, because of insufficient scientific data providing proof of their efficacy, and lack of information on the mechanisms involved; however, they have become an extremely attractive option8. The search for new therapeutic approaches has involved the investigations of some drugs of synthetic origin but their associated side effects rendered them unsuitable, and further development was halted. Herbal therapy has now been introduced as a suitable solution to the prevalent problem, and interest in natural products is increasing in the cosmetic industry9. In addition, about 1,000 types of plant extracts have been studied for their potential effects on hair proliferation, and this research trend is rapidly growing, with a considerable potential for expansion in the future9.
Considering the promising benefits of alternative natural therapies, we investigated the potential therapeutics effects of plant extracts that have been commonly used in Asian medicine for curing hair loss. Considering the role of 5α-R and the effect of androgens on the regulation of human hair proliferation, we used follicular DPCs as a model with the following aims: (i) to study the quantitative expressions and activities of 5α-R1 and 5α-R2 and (ii) to investigate the molecular mechanisms and pathways involved in the mediaton of hair proliferation by herbal extracts.
MATERIALS AND METHODS
Plant material
The fruits of Persea americana, flowers of Althaea officinalis, Chamaemelum nobile, Thymus vulgaris, leaves of Rosmarinus officinalis, and Urtica dioica were obtained from the open market and identified by comparing with standard herbarium specimens available in the Food and Drug Control Lab and Research Center, Tehran, Iran. The different plant parts were mixed, and sieved using a 80 sieve and subjected to pharmacognostic evaluations for confirmation.
Characterization and identification of extracts
The different solvent extracts acquired were then subjected to qualitative chemical analysis to determine the different plant constituents using various methods. Thin layer chromatography and paper chromatography using various mixtures of solvent systems were performed to verify the exact identity of the components discovered in the qualitative chemical tests10.
Preparation of herbal hair formulation
The herbs utilized in this study to prepare the herbal extracts were dried, crushed, and sieved through an 80-mesh, stainless steel sieve, using water as the base. The herbal extracts were then evaluated using three methodologies. Firstly, the direct binge method was used in which the crude plants were powdered, weighed and placed in olive oil with constant stirring and heating until the plant material was completely extracted into the base. Then, samples containing 1%, 2%, and 3% of the extracted comounds were prepared.
Secondly,the paste method was used where the fruit, pulp, or the desired plant part were formulated into a paste with a small amount of water and left overnight. Then, the water-moistened plant material was mixed, and blended with continuous stirring at a fixed temperature until the water droplets stop spinning and the plant material had been completely extracted. The extract was then filtered through a muslin cloth, and three different concentrations were prepared so as to contain 4, 5, and 6 g of plant per 100 ml of water (4%, 5%, and 6%, respectively).
The last method was the cloth method in which the dried plant was weighed and tied in a muslin cloth, which was then hung in water as the base, with continuous stirring, the extract was finally filtered. Four different concentrations were prepared, which contained 7, 8, 9, and 10 g of plant extract per 100 ml of water (7%, 8%, 9%, and 10%, respectively).
Preparation of mixed plant herbal hair formulations at different concentrations
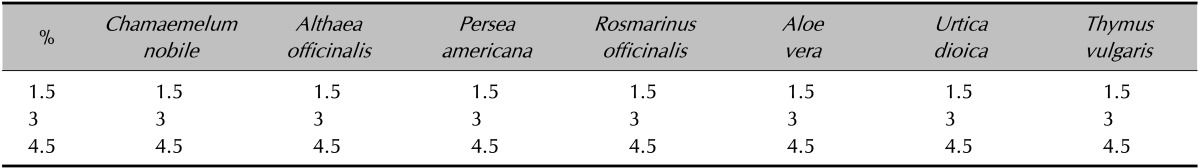
After selecting formulation method, multiple ingredients were combined to prepare the formulation at the effective concentrations based on the previous physical and biological screenings performed. The method selected was the direct binge method and three different plant extract formulations with concentrations of 1.5%, 3%, and 4.5% were prepared to determine the maximum activity (Table 1).
Table 1. Selection of of herbal extract concentrations (amount of drug/100 ml).
Evaluation of herbal extract preparations
The herbal extracts were then subjected to physical and biological evaluation. The physical evaluation included the determination of parameters such as specific gravity, pH, and refractive index were measured and the formulations were subjected to biological evaluations.
Isolation of DPCs and cell culture establishment
Hair follicles in the anagen cycle were isolated from the occipital scalp skin of five males (range, 38~54 years; median, 43 years, respectively) after obtaining informed consent from the participants and ethical approval (IRB No. 903267). Furthermore, we adheared strictly to the guidelines of the Declaration of Helsinki Principles. The microdissection method used was based on the method of Messenger2. Briefly, the hair bulb was excised under a light microscope, and the fibrous sheath with the attached dermal papilla was detached from the epithelium by applying gentle pressure with the tip of a 23 G needle. After the fibrous sheath was opened, the dermal papilla was transected across the stalk and transferred to a culture plate. The isolated papillae were incubated with 10% fetal bovine serum (FBS)-Dulbecco's modified Eagle's medium (DMEM; Gibco, Rockville, Maryland, USA) containing penicillin (100 U/ml) and streptomycin (100 mg/ml). In the primary culture, the DPCs attained confluence in a 35-mm culture plate after a 30-days incubation in a humidified atmosphere of 5% CO2 at at 37℃.
Analysis of 5α-R mRNA expression
Total RNA was extracted from the cells using the RNeasy Mini kit (Qiagen Inc., Valencia, CA, USA) as described previously11. Briefly, the cells were lysed and homogenized in RLT buffer supplemented with 10 µl/ml mercaptoethanol (Sigma-Aldrich, Munich, Germany). The lysate was homogenized using a syringe and a 20-G needle. The sample was placed in a silica column followed by washing and eluting with RNase-free water. The RNA concentration was measured using an ultraviolet spectrophotometry at 260 nm, and the purity and integrity were evaluated using the A260/A280 ratio.
Total RNA was treated with DNase (Fermentas, Burlington, ON, Canada) and then reverse-transcribed using the Revert Aid M-MuLV Reverse Transcriptase (Fermentas) with Oligo dT primers (Invitrogen, Carlsbad, CA, USA) based on the manufacturer's protocol. A semi-quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) assay of 5α-R1 and 5α-R2 cDNA was performed using the SYBR Green kit (Qiagen Inc.) in an ABI 7500 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) based on the manufacturer's recommendations. A melting curve was produced at the end of each PCR reaction to confirm that a single product was amplified. The PCR was performed using identical conditions with 1 µl of cDNA, and the relative expression levels of the genes were normalized to the endogenous housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The relative mRNA expression level was calculated using the 2-ΔΔCT analysis method. The primers used for the qRT-PCR are shown in Table 2.
Table 2. Sequences of primer pairs for human steroid-5-α-reductase and human housekeeping genes used in real-time polymerase chain reaction.
SRD5A1: steroid 5-α-reductase type I, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, Ref-Seq: reference sequence.
DPC culture and MTT assay
The method used for isolating and culturing the DPCs was previously described12. Briefly, the DPCs were cultured in DMEM containing 2 mM L-glutamine, antibiotic-antimycotic solution (1,000 mg/ml streptomycin sulfate, 1,000 units/ml penicillin G sodium, and 2.5 mg/ml of amphotericin B) and 10% FBS. Fourth-passage confluent DPCs were cultured for 24 hours in serum-free DMEM and then treated for 48 hours with different concentrations of herbal extracts (1.5%, 3%, and 4.5%) in the presence and absence of an Akt1/2 kinase inhibitor. As a control, cells treated with finasteride (0.1 µM), a 5-αR2 inhibitor. The cell viability was determined using an MTT assay as previously described11. DPCs (5,000 cells/well) were seeded in 96-well plates, incubated for 24 hours before adding the herbal extracts, and then incubated for 48 hours. The absorbance was measured at 570 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader and the results were expressed as percentages of the untreated controls.
BrdU incorporation cell proliferation assay
The effect of the extracts on cell proliferation was examined using a colorimetric immunoassay based on bromodeoxyuridine (BrdU) incorporation using a BrdU kit (Roche, Mannheim, Germany) following the manufacturer's protocol. Briefly, the DPCs (5,000 cells/well) were seeded in 96-well plates. After 24 hours, the cells were incubated with different concentrations of herbal extracts (1.5%, 3%, and 4.5%), for 48 hours. Then, 20 µl of the BrdU labeling solution was added, and the cells were incubated for an additional 4 hours. During this labeling time the pyrimidine analog, BrdU was incorporated into the DNA of proliferating cells at the location of thymidine. After removing the BrdU labeling solution, the cells were fixed and denatured with the kit's FixDenat solution for 30 min at 25℃. Denaturation of the DNA is necessary to enhance the accessibility of the incorporated BrdU and facilitate its ability to locate the antibody. Samples were incubated for 90 min with a peroxidase-labeled anti-BrdU antibody (anti-BrdU-POD), which binds to the BrdU incorporated into newly synthesized cellular DNA. After washing off the unbound anti-BrdU-POD, the color reaction was developed for 3~5 min with the substrate solution and blocked by adding 25 µl 1 M sulfuric acid, and the optical densities of the samples were measured using a microplate reader at 450 nm (reference value 690 nm).
Western blot analysis
B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), cyclin E, cyclin-dependent kinase 4 (Cdk4), extracellular signal-regulated kinase (ERK), phosphorylated (p)-ERK, Akt, p-Akt, 5-αR1, and 5-αR2 protein content was measured using western blot analysis as described previously13. Cells were serum deprived for 24 hours before extract treatments. Following treatment, the cells were harvested at 4℃ in a lysis buffer containing 20 mM Tris-hydrochloride (HCl, pH 7.5), 0.5% Nonidet P-40, 0.5 mM phenylmethanesulfonyl fluoride, 100 µM β-glycerol 3-phosphate, and 0.5% protease inhibitor cocktail and lysed by sonication and centrifugation (14,000 rpm, 10 min, 4℃). The protein concentration of each lysate was measured using a bicinchoninic acid protein assay kit (Pierce; TEMA Ricerca S.r.l., Bologna, Italy). Each protein sample (30~50 µg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes, which were incubated with blocking buffer consisting of 5% non-fat dry milk in PBS containing 0.1% Tween 20 (PBST) for 1 hour at room temperature Then, the membranes were incubated with monoclonal antibodies against Bcl2, Bax, cyclin E, and Cdk4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and polyclonal antibodies against ERK, p-ERK, Akt, and p-Akt (Cell Signaling Technology Inc., Beverly, MA, USA) overnight at 4℃, and then washed thrice (5 min each) with PBST. The membranes were incubated with complementary secondary antibodies for 1 hour at 25℃. After washing with PBST, the proteins were developed using an enhanced chemiluminescence detection kit (Amersham Corp., Arlington Heights, IL, USA). The expression of GAPDH was used as an internal control.
Statistical analysis
A nonparametric one-way analysis of variance was performed with the Dunnett's test using the GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). Each experiment was performed in triplicate and repeated three to four times independently. A p<0.05 was considered significant, and all the data are expressed as means±standard deviation.
RESULTS
Physical evaluation
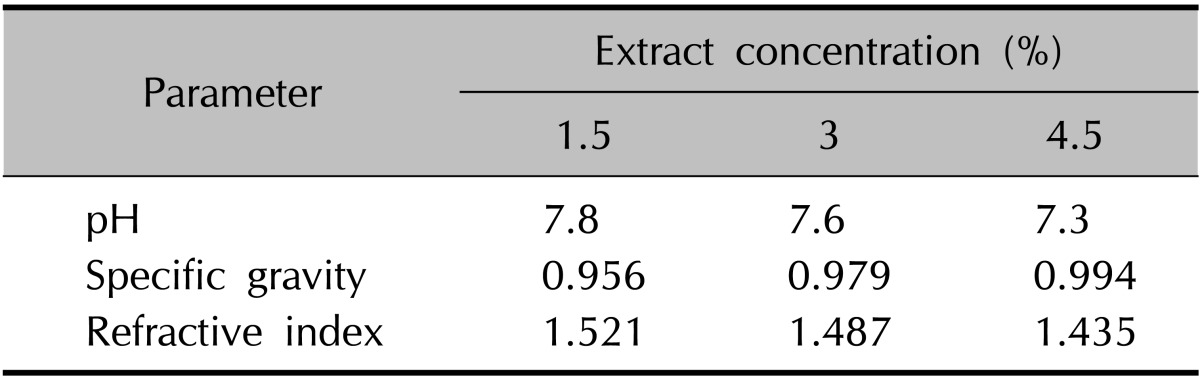
The physical evaluation involved the measurement of parameters such as the specific gravity, pH, and refractive index, and then the formulations were subjected to biological assay. The results of the physical and chemical evaluation are showed in Table 3.
Table 3. Evaluation of chemical and physical parameters.
Expression of 5-αR mRNA in cultured DPCs
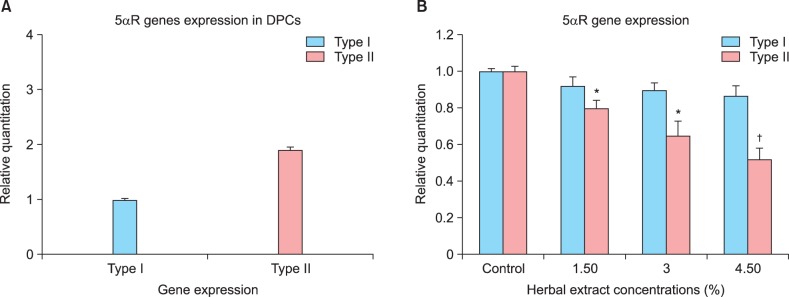
The expression level of the 5-αR isoforms (5-αR1 and 5-αR2) were determined in the cultured human DPCs using a qRT-PCR analysis. The mRNA of both isoforms was identified in the cultured DPCs but at different expression levels as shown in Fig. 1A. The results showed that the expression of 5-αR2 was higher than that of 5-αR1 was. The expression levels of the 5-αR isoforms were normalized to that of GAPDH.
Fig. 1. Relative gene expression of two 5α-reductase (5αR) isoforms in cultured human dermal papilla cells (DPCs). (A) Relative gene expression of 5αR1 and 5αR2 were detected using quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) in DPCs. Data represent three independent experiments and relative expression values were calculated using the equation RQ=2-ΔΔct. (B) qPCR analysis of 5αR1 and 5αR2 mRNA expression in DPCs following herbal extract treatment using graded concentrations. Expression levels were normalized to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Data are mean±standard deviation; *p<0.05 and †p<0.01 compared with untreated control group.
The effect of the herbal extracts on the gene expression of 5-αR1 and 5-αR2 in the cultured DPCs was examined using graded concentrations to determine if it was concentration-dependent. The result indicated that 5-αR2 but not 5-αR1 expression gradually decreased more in the extract-treated group than it did in the control group (p<0.05, Fig. 1B).
Expression of 5-αR protein in cultured DPCs
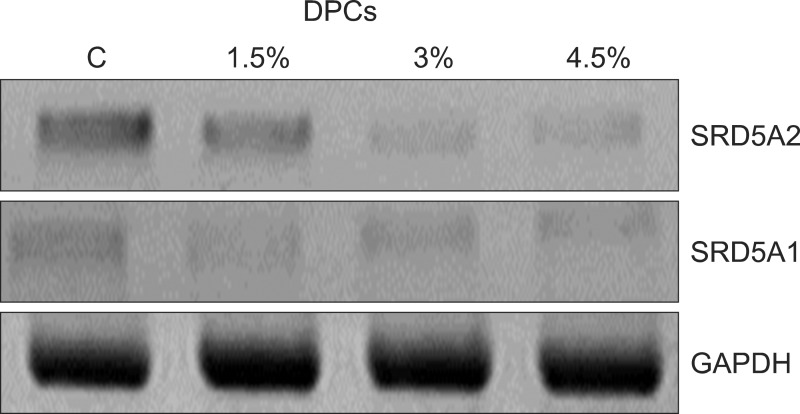
The protein expression of 5-αR1 and 5-αR2 was examined in the cultured human DPCs using western blotting, and both isoforms were detected. The effect of the herbal extracts on the protein expression of 5-αR1 and 5-αR2 in cultured DPCs was evaluated using graded concentrations to determine if there was concentration dependence. The result indicated that the protein expression level of 5-αR2 but not 5-αR1 in the DPCs treated with the herbal extract gradually decreased compared to the control (p<0.05, Fig. 2).
Fig. 2. Effects of herbal extracts on expression of 5α-reductase (5αR) isoform proteins in human dermal papilla cells (DPCs). Cells were treated with indicated concentrations of herbal extracts for 48 hours and then expression of proteins was analyzed using western blotting. SRD5A1: steroid 5αR type I, GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Herbal extracts increased proliferation of human DPCs
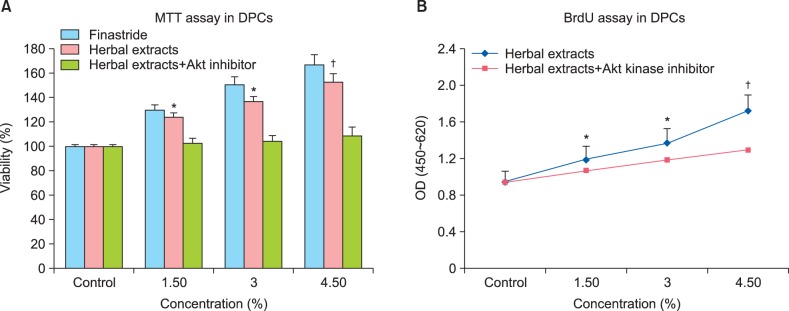
The proliferative effect of the herbal extracts was examined in the human DPCs treated with different concentrations (1.5%~4.5%) in the presence or absence of an Akt1/2 kinase inhibitor for 48 hours. Following treatment, the cell proliferation was detected using MTT and BrdU assays and the 5-aR2 inhibitor, finasteride was used as a control. The MTT viability test showed a concentration-dependent increased in the viability of the DPCs following exposure to herbal extracts. As shown in Fig. 3A, the proliferative effect of the herbal extracts on the DPCs was dose-dependent at concentrations of 1.5%~4.5% (increase, 124%±4.2% to 153%±8.1%, respectively vs. control 100%, p<0.01). Interestingly, the results indicated that finasteride increased the proliferation of DPCs. The results of MTT test were confirmed using the BrdU incorporation method (Fig. 3B), and DPCs exposed to the herbal extracts showed a significant concentration-dependent increase in proliferation (p<0.01).
Fig. 3. Effect of herbal extracts on cell proliferation of human dermal papilla cells (DPCs). Cells were treated with different concentrations of herbal extracts with or without Akt kinase inhibitor for 48 hours, and proliferation was assessed using (A) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and (B) bromodeoxyuridine (BrdU) assays. Herbal extracts increased cell proliferation in DPCs concentration-dependently. Results are mean±standard deviation and were calculated as a percentage of corresponding control values. *p<0.05 and †p<0.01, one-way analysis of variance. Each point represents four repetitions each in triplicate.
To further elucidate the role of Akt in the induction of cell proliferation by the herbal extracts, cells were pretreated with an Akt1/2 kinase inhibitor. The effect of the Akt1/2 kinase inhibitor on the extract-induced inhibition of cell proliferation was evaluated after incubation of DPCs with different extract concentrations. The Akt1/2 kinase inhibitor prevented cell proliferation, as was detected using the MTT and BrdU tests (Fig. 2). Thus, this result demonstrates that cell proliferation was induced by the herbal extracts via the Akt pathway in the DPCs.
Herbal extracts increased cyclin D1 and Cdk4 in cultured DPCs
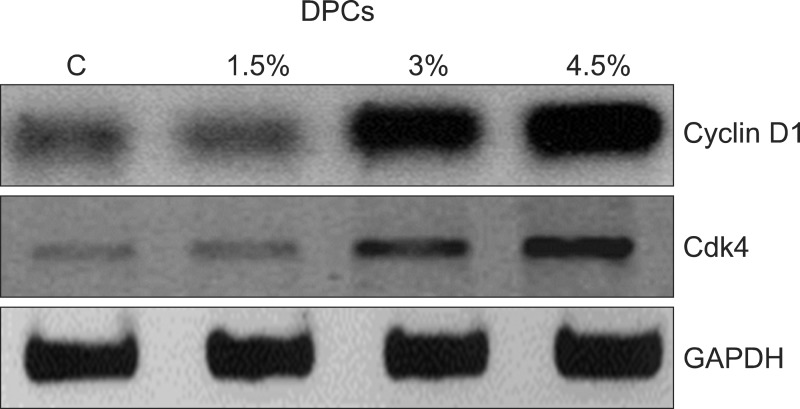
To study the mechanisms underlying the regulation of cell proliferation by the herbal extracts, their effects were evaluated on the expression of cell-cycle regulatory proteins. DPCs were treated with different concentrations (1.5~4.5%) of the herbal extracts for 48 hours, and then the expression levels of related proteins were measured using western blotting. Fig. 3 clearly shows that at 1.5% the herbal extracts had no effect on Cyclin D1 protein expression level, which was similar to that of the untreated controls. However, Cyclin D1 and CDK4, proteins that participate in the G1 phase progression increased in cells treated with 3.0~4.5% of the herbal extracts (Fig. 4). These results suggest that the herbal extracts induced cell proliferation by enhancing the progression of the G1 cell cycle phase.
Fig. 4. Effects of herbal extracts on expression of cell cycle regulatory proteins in human dermal papilla cells (DPCs). Cells were treated with indicated concentrations of herbal extracts for 48 hours and then the expression of proteins was analyzed using western blotting. Cdk4: cyclin-dependent kinase 4, GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Herbal extracts induced phosphorylation of ERK and Akt in cultured DPCs
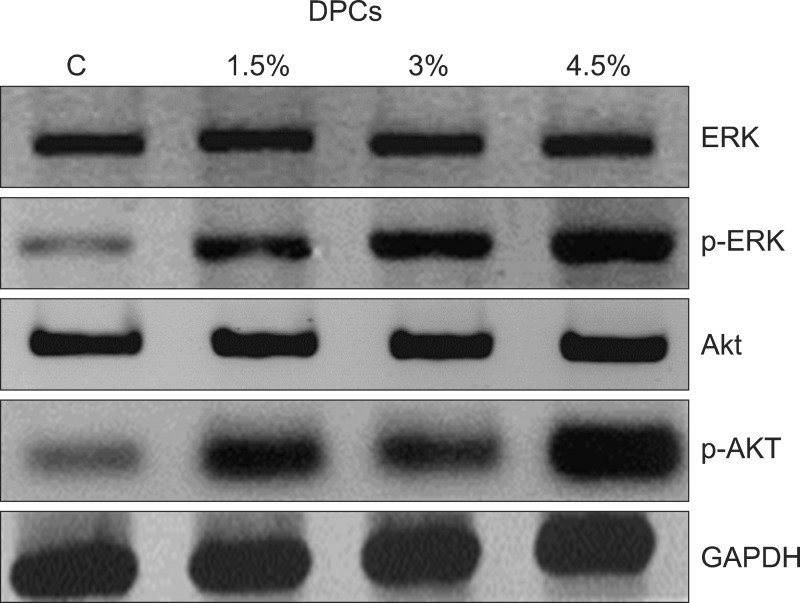
The protein expression of p-ERK was increased concentration-dependently following herbal extract treatment (Fig. 5). The p-ERK protein expression increased after treatment with the herbal extract at 1.5~4.5% compared with control. However, the expression of total ERK was unchanged by the herbal extract treatment. The expression of p-Akt but not total Akt also increased concentration-dependently following herbal extract treatment (1.5~4.5%, Fig. 5) compared with the control.
Fig. 5. Herbal extracts increased phosphorylated extracellular signal-regulated kinase (p-ERK) and p-Akt in cultured dermal papilla cells (DPCs) analyzed using western blot. Level of p-ERK and p-Akt increased after 48 hours treatment concentrationdependently. GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Herbal extracts induced Bcl-2 and inhibited Bax expression in DPCs
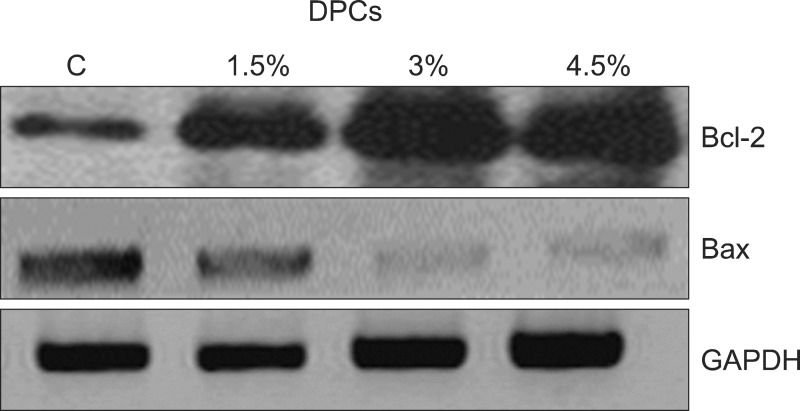
To investigate the possible association between herbal extracts-induced human DPC proliferation and changes in Bcl-2 proteins, the levels of the anti-apoptotic and apoptotic Bcl-2 and Bax proteins respectively were determined in herbal extract-induced cell proliferation of DPCs. The western blot analysis showed that the expression of Bcl-2increased in response to herbal extract treatment while that of Bax steadily decreased (Fig. 6).
Fig. 6. Effects of herbal extracts on expression of apoptosis-related proteins in human dermal papilla cells (DPCs). Cells were treated with indicated concentrations of herbal extracts for 48 hours and then expression of proteins was analyzed using western blotting. Bcl-2: B-cell lymphoma, Bax: Bcl-2-associated X protein, GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
DISCUSSION
The number of males and females currently suffering from balding or hair thinning is increasing. Therefore, it is extremely important to expand the search for new therapies that inhibit balding and increase hair proliferation. In this regard, alternative medicines have become an attractive option; however, they have not yet been incorporated into mainstream medical practice because of the dearth of scientific data to support their efficacy, and inadequate information about the mechanisms involved8.
It is widely accepted that androgens are important regulators of human hair proliferation, but their mechanisms of action are not entirely understood. In this study, we introduced an in vitro follicular cell culture model to elucidate the effects of herbal extracts on hair follicle proliferation.
Androgens are important regulators of human hair proliferation, and they exhibit varying effects on hair follicles depending on the tissue location3. Our data provided evidence that cells derived from the scalp displayed a higher expression of 5α-R2 mRNA than that of 5α-R1. Furthermore, the 5α-R2 mRNA expression level was inhibited concentration-dependently by herbal extract treatment of DPCs (Fig. 1B). In addition, our investigation of 5α-R1 mRNA expression revealed that the level was unchanged following herbal extract treatment of DPCs cells.
The differences in 5α-R2 mRNA expression might partly explain why DPCs are more sensitive to androgens, because in addition to directly binding to the AR, T might be converted into its highly potent metabolite, DHT via 5α-R to further enhance the stimulation of hair proliferation. Consistent with previous reports14, our results demonstrated that high levels of 5α-R mRNA were detected in cultured DPCs. These data support the evidence that 5α-R2 plays a key role in androgen-regulated hair proliferation.
Nakanishi et al.15 and Asada et al.16 demonstrated that the mRNA expression of type II 5α-R is greater in DPCs from other locations while the expression of the type I isoform is low. This observation was further confirmed by the evidence that finasteride, a specific inhibitor of the type II 5 α-R, potently inhibited endogenous 5α-R activity in DPCs while the type I 5α-R inhibitor, MK-386 showed only limited effects17. Therefore, type II 5α-R increases androgen activity in androgenetic alopecia (AGA) by converting T to DHT and, therefore, finasteride is effective in the treatment of AGA. The results of this study suggest that treatment with the herbal extracts decreased 5α-R2 expression and, thereby, protected hair follicles from the balding scalp against excessive androgen action. Moreover, the effect of the herbal extracts on the protein expression of the 5-αR isoforms (5-αR1 and 5-αR2) in cultured DPCs were performed to determine possible concentration-dependence. The results indicated that the protein expression level of 5-αR2 in the DPCs treated with the herbal extracts gradually decreased significantly compared with the untreated group (p<0.05). However, the protein expression of 5-αR1 was unchanged by herbal extract treatment of cultured DPCs compared with the control (Fig. 2).
This study revealed that the herbal extracts significantly increased the proliferation of human DPCs at a concentration range of 1.5~4.5%. Moreover, we demonstrated that Akt kinase blockade inhibits the proliferative effect of the herbal extracts on DPCs. Therefore, this data also demonstrates a role for Akt in the herbal extract-induced cell proliferation (Fig. 3). The present study also demonstrated that the herbal extract affects the expressions of Cyclin D1, Cdk4, ERK, Akt, Bcl-2, and Bax proteins in cultured human DPCs. The herbal extracts increased the proliferation of cultured human DPCs via activation of the ERK and Akt pathways. The role of the ERK signaling pathway in mitogenesis and cell growth has been previously demonstrated18,19.
The mechanism underlying the proliferative action of the herbal extracts in DPCs appeared to be related to the induction of cell cycle progression in the G1 phase (Fig. 4). Moreover, the herbal extracts increased the expression of Cdk4 and cyclin D1 proteins, which play an important role in the G1/S checkpoint as a regulator of cell cycle progression20. Therefore, the herbal extract-mediated G0/G1 phase cell cycle progression is related to the increase in cyclin and Cdk4 expression.
The human DPCs treated with herbal extracts showed an upregulation of the expression of p-ERK compared with the untreated control (Fig. 5). The expression of p-Akt was also upregulated after herbal extract treatment (Fig. 5). It was previously demonstrated that Akt plays a key role in mediating survival signals21,22 and it is also possible that the induction of the Akt pathway by the herbal extracts is involved in regulating the DPC proliferation. The herbal extracts upregulated and downregulated Bcl-2 and Bax exexpression, respectively in cultured DPCs. Specifically, herbal extract treatment for 48 hours increased Bcl-2 protein expression but decreased that of Bax concentration-dependently (Fig. 6). The Bcl-2 family of proteins consists of more than a dozen members, which are either anti- or pro-apoptotic in nature and have been shown to act as gatekeepers of the apoptotic process23. It is widely accepted that Bcl-2 has an anti-apoptotic effect while Bax induces apoptosis. Cyclin D1 and Cdk4 play a key role in promoting G1-to-S phase progression24. The signals related to mitogenesis include the phosphorylation of transcription factors including Akt and ERK as well as the synthesis of cell cycle regulatory proteins such as cyclin D. The herbal extract treatment increased the phosphorylation of Akt and ERK and subsequently increased the expression of cyclin D1 and Cdk4. The increased levels of Bcl-2, Cyclin D1, Cdk4, and the phosphorylation of Akt and ERK might adequately increase the proliferation of DPCs.
Naturally derived compounds can cure androgenic diseases such as alopecia. Roh et al.25 proved that Sophora flavescens, an Asian medicine, was effective for hair loss treatment by demonstrating its promotion of hair proliferation mediated by 5α-R inhibition25.
In another study, Rho et al.26 demonstrated that Asiasari radix extract increased the growth of both HaCaT and human DPCs in vitro via enhancing the expression of vascular endothelial growth factor26. Furthermore, Hay et al.27 studied the efficacy of herbal extracts for the treatment of patients with alopecia, and their data demonstrated that 44% of the 43 patients in the active group showed improvement compared with 15% of the 41 patients in the control group27. In yet another study, Bureau et al.28 demonstrated that the combination of Pimenta racemosa, Myrtus communis, Cedrus atlantica, Laurus nobilis, Pogostemon patchouli, R. officinalis, Salvia officinalis, Salvia sclarea, Thymus satureioides, and Cananga odorata increased the hair density and ratio of anagen hair per total hair in a double-blind randomized, placebo-controlled study in healthy male and female volunteers28. Minoxidil, finasteride, and dutasteride and other synthetic therapeutic drugs are commonly used for alopecia treatment; however, their adverse effects have encouraged the continued search for efficacious alternative treatments with limited side effect, particularly herbs. Therefore, alternative herbal medicines have attracted much interest for use in the treatment of hair loss.
The results of this study demonstrate that herbal extracts might have exerted positive effects on hair proliferation by regulatiing of Erk, Akt, Bcl-2, and Bax in the DPCs. In addition the crucial role of 5α-R in the regulation of hair proliferation was well established. It has been reported that the metabolism of T in hair follicles differs various in tissue locations and depends on their androgen sensitivity, and the type II 5α-reductase plays a critical role in the intra-follicular conversion of T to DHT in DPCs29. Therefore, type II 5α-R is considered one of the most important targets for developing drugs for the treatment of hair loss. In conclusion, we have demonstrated the potential hair proliferative effect and the putative molecular regulatory role of the herbal extracts investigated, which suggests that these herbal extracts might be potential candidates for developing drugs for facilitating hair proliferation.
References
- 1.Paus R, Müller-Röver S, Botchkarev VA. Chronobiology of the hair follicle: hunting "the hair cycle clock". J Investig Dermatol Symp Proc. 1999;4:338–345. doi: 10.1038/sj.jidsp.5640241. [DOI] [PubMed] [Google Scholar]
- 2.Thornton MJ, Messenger AG, Elliott K, Randall VA. Effect of androgens on the growth of cultured human dermal papilla cells derived from beard and scalp hair follicles. J Invest Dermatol. 1991;97:345–348. doi: 10.1111/1523-1747.ep12480688. [DOI] [PubMed] [Google Scholar]
- 3.Randall VA. Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol. 2007;18:274–285. doi: 10.1016/j.semcdb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Randall VA, Hibberts NA, Thornton MJ, Hamada K, Merrick AE, Kato S, et al. The hair follicle: a paradoxical androgen target organ. Horm Res. 2000;54:243–250. doi: 10.1159/000053266. [DOI] [PubMed] [Google Scholar]
- 5.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KM, Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968;219:277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- 7.Andersson S, Russell DW. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc Natl Acad Sci U S A. 1990;87:3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- 9.Rathi V, Rathi JC, Tamizharasi S, Pathak Ak. Plants used for hair growth promotion: a review. Phcog Rev. 2008;2:185–187. [Google Scholar]
- 10.Banerjee PS, Sharma M, Nema RK. Preparation, evaluation and hair growth stimulating activity of herbal hair. J Chem Pharm Res. 2009;1:261–267. [Google Scholar]
- 11.Aghaei M, Karami-Tehrani F, Panjehpour M, Salami S, Fallahian F. Adenosine induces cell-cycle arrest and apoptosis in androgen-dependent and -independent prostate cancer cell lines, LNcap-FGC-10, DU-145, and PC3. Prostate. 2012;72:361–375. doi: 10.1002/pros.21438. [DOI] [PubMed] [Google Scholar]
- 12.Randall VA, Thornton MJ, Hamada K, Redfern CP, Nutbrown M, Ebling FJ, et al. Androgens and the hair follicle. Cultured human dermal papilla cells as a model system. Ann NY Acad Sci. 1991;642:355–375. [PubMed] [Google Scholar]
- 13.Aghaei M, Panjehpour M, Karami-Tehrani F, Salami S. Molecular mechanisms of A3 adenosine receptor-induced G1 cell cycle arrest and apoptosis in androgen-dependent and independent prostate cancer cell lines: involvement of intrinsic pathway. J Cancer Res Clin Oncol. 2011;137:1511–1523. doi: 10.1007/s00432-011-1031-z. [DOI] [PubMed] [Google Scholar]
- 14.Lachgar S, Charveron M, Sarraute J, Mourard M, Gall Y, Bonafe JL. In vitro main pathways of steroid action in cultured hair follicle cells: vascular approach. J Investig Dermatol Symp Proc. 1999;4:290–295. doi: 10.1038/sj.jidsp.5640232. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi S, Adachi IS, Takayasu KS. Expression of androgen receptor, type I and type II 5a-reductase in human dermal papilla cells. In: Neste D, editor. Hair research for the next millennium. Amsterdam: VREPB; 1996. pp. 333–337. [Google Scholar]
- 16.Asada Y, Sonoda T, Ojiro M, Kurata S, Sato T, Ezaki T, et al. 5 alpha-reductase type 2 is constitutively expressed in the dermal papilla and connective tissue sheath of the hair follicle in vivo but not during culture in vitro. J Clin Endocrinol Metab. 2001;86:2875–2880. doi: 10.1210/jcem.86.6.7545. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann R, Happle R. Finasteride is the main inhibitor of 5alpha-reductase activity in microdissected dermal papillae of human hair follicles. Arch Dermatol Res. 1999;291:100–103. doi: 10.1007/s004030050390. [DOI] [PubMed] [Google Scholar]
- 18.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 19.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 20.Jin S, Mazzacurati L, Zhu X, Tong T, Song Y, Shujuan S, et al. Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene. 2003;22:8536–8540. doi: 10.1038/sj.onc.1206907. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad S, Singh N, Glazer RI. Role of AKT1 in 17betaestradiol-and insulin-like growth factor I (IGF-I)-dependent proliferation and prevention of apoptosis in MCF-7 breast carcinoma cells. Biochem Pharmacol. 1999;58:425–430. doi: 10.1016/s0006-2952(99)00125-2. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- 23.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 24.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, et al. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res. 2003;92:e12–e19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 25.Roh SS, Kim CD, Lee MH, Hwang SL, Rang MJ, Yoon YK. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J Dermatol Sci. 2002;30:43–49. doi: 10.1016/s0923-1811(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 26.Rho SS, Park SJ, Hwang SL, Lee MH, Kim CD, Lee IH, et al. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J Dermatol Sci. 2005;38:89–97. doi: 10.1016/j.jdermsci.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349–1352. doi: 10.1001/archderm.134.11.1349. [DOI] [PubMed] [Google Scholar]
- 28.Bureau JP, Ginouves P, Guilbaud J, Roux ME. Essential oils and low-intensity electromagnetic pulses in the treatment of androgen-dependent alopecia. Adv Ther. 2003;20:220–229. doi: 10.1007/BF02850093. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann R, Happle R. Current understanding of androgenetic alopecia. Part I: etiopathogenesis. Eur J Dermatol. 2000;10:319–327. [PubMed] [Google Scholar]